Germinal centers can be induced by T-independent antigens. Liu et al. show that, surprisingly, these germinal centers can produce long-lived memory B cell and plasma cell output.
Abstract
Liu et al. (2022. J. Exp. Med. https://doi.org/10.1084/jem.20210527) in this issue show that T cell–independent germinal centers (GCs) can produce long-lived memory and plasma cell output. This may help explain how polysaccharide antigens provide long-term protection.
A study in this month’s issue of JEM by Liu et al. (2022) shows surprising evidence that B cells can form long-lived memory B and plasma cells while differentiating in germinal centers (GCs) without the need for T cell help. According to past dogma differentiation of B cells in GCs, the generation of B cell memory and of long-lived plasma cells are all dependent on T cell help. Around 20 yr ago, it was published in this journal (de Vinuesa et al., 2000) that the strong stimulus generated by T-independent type 2 (TI-II) antigens can induce GCs in the absence of T help. While these T-independent GCs were deemed sterile, later studies showed that immunization with TI-II antigens can induce B cell memory (Obukhanych and Nussenzweig, 2006) as well as long-lived plasma cells (Hsu et al., 2006). This is thought to happen independently of GC differentiation, because it is also known that there is a GC-independent pathway of memory B cell development (Taylor et al., 2012).

Insights from Yang Zhang and Kai-Michael Toellner.
The team of Hai Qi in this issue (Liu et al., 2022) have revisited these experiments, making use of gene-manipulated animal models that allow for better tracing of lineages and differentiation events. Similar to what had been shown earlier (de Vinuesa et al., 2000), antigen-specific B cells transferred into adoptive hosts that were immunized with TI-II antigens, proliferated vigorously, and formed large GCs. Like in earlier experiments, GCs involuted after 6 d. However, while the disappearance of GCs earlier had been attributed to extensive apoptosis, the current study shows that most GC cells stayed at the centroblast stage and generated output of long-lived memory B and plasma cells that could survive for at least 19 d. Indeed, the numbers of memory B and plasma cells produced in these TI-II responses could surpasses those produced by T-dependent GCs. Their formation was dependent on BCL6, the main transcription factor directing GC fate. B cells from GC fate–mapping S1pr2-CreERT2 mice show that the long-lived cells generated indeed have a history of differentiation inside GCs.
Knowledge at the time may explain why this was missed in the earlier study (de Vinuesa et al., 2000). Liu et al. (2022) point out that histology data presented earlier show that B cell follicles are depleted of antigen-specific B cells at the height of the GC response, but these cells seem to reappear after GCs have waned. The same figures also show that large numbers of plasma cells are seen in areas where we now know plasma cells are exiting GCs (Zhang et al., 2018).
There are also some major differences in the experimental setup of the two studies. While the earlier study shows very strong apoptosis 1 d before GCs involute, this was not seen in the present study. This may be due to apoptosis being detected by flow cytometry, while the earlier study was based on histology. Histology is far superior in detecting apoptotic B cells, which are rapidly ingested by tingible body macrophages prevalent in GCs. Further, the earlier study transferred B cells, which express a low-affinity 4-hydroxy-3-nitrophenyl–specific B cell receptor (BCR) with a canonical VDJ combination from BALB/c mice (Cascalho et al., 1996). B18hi cells used in the current study have a 10× higher affinity than the canonical germline response (Shih et al., 2002). Are stronger antigen–BCR interactions responsible for some of the differences seen, and if so, is there a role for BCR signaling in the selection of GC B cells?
Currently, the most important signals thought to regulate GC B cell selection are signals from T follicular helper (Tfh) cells (Victora et al., 2010). Signals from Tfh cells induce Myc induction (Dominguez-Sola et al., 2012), upregulation of CXCR4, and migration of light zone B cells to the GC dark zone (Allen et al., 2004), which will trigger further proliferation and immunoglobulin hypermutation, keeping the cycle of affinity maturation going (Gitlin et al., 2014). But what about the selection of B cells into other fates?
A few years back, we showed that output of affinity-matured plasma cells from the GC happens in the interphase between dark zone and is dependent on signals from Tfh cell–CD40 ligation and IL-21, presumably delivered in the light zone (Zhang et al., 2018). At the time, we were speculating that the main effect of IL-21 on B cells might be the induction of migration of Tfh-selected high-affinity B cells from the light zone into the dark zone. The present study confirms that plasma cell generation itself may be independent of Tfh signals and could be just due to dark zone B cells making random contact with stroma surrounding the dark zone (Zhang et al., 2018).
Memory B cells generated from GCs are not efficiently selected for affinity and have undergone no or suboptimal interactions with Tfh cells (Suan et al., 2017). Strong interaction with TI antigen on follicular dendritic cells may be sufficient to keep cells alive and allow them to differentiate into memory B cells. The fourth differentiation pathway, apoptosis, is the default pathway GC B cells are programmed to take (Liu et al., 1989). Stronger BCR stimulation may induce some survival independently of Tfh signals, which may explain why less apoptosis was detected in the current study.
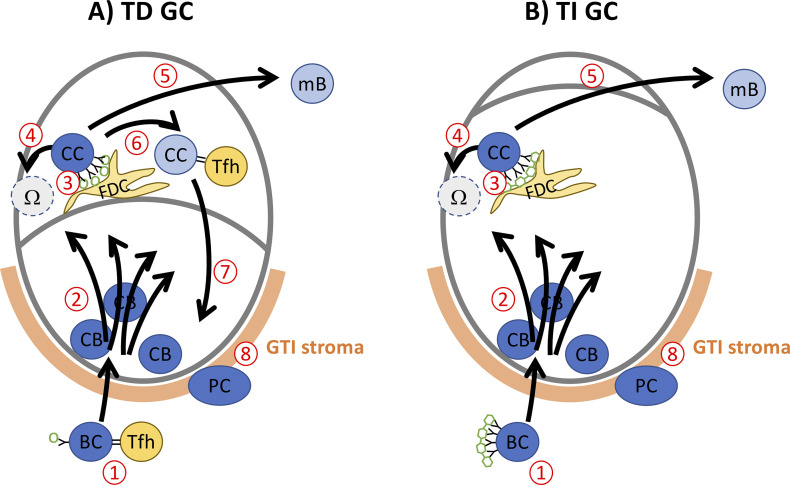
How things might work. (A) T-dependent (TD) GC after protein immunization. (1) B cells (BC) are activated by antigen contact and Tfh help. (2) They enter B cell follicles, expand, and mutate as dark zone centroblasts (CB). (3) Centrocytes (CC) receive BCR stimulation with antigen on follicular dendritic cells (FDC) in the light zone. (4) Cells failing to receive efficient BCR signals go into apoptosis (Ω). (5) CC failing to make efficient interaction with Tfh cells leave as memory B cells (mB). (6) Repeated stimulation by Tfh cells maintains CC. (7) Sufficient Tfh help (CD40L and IL-21) triggers recycling into the dark zone CB pool (Zotos et al., 2021). (8) CB making contact with GC–T zone interphase (GTI) stroma get triggered to become plasma cells (PC). (B) T-independent GC. Increased proliferation maintains CB for several days. Lack of Tfh interactions (6) prevents maintenance of a CC pool. Only apoptosis (4) or mB output (5) occur. PC continue to be generated from CB, making contact with GTI stroma (8). Without step 7, the process peters out after a few days.
That would leave the selection of high-affinity variants as the only function of Tfh interaction. Tfh selection is thought to be a slow process involving repetitive interactions (Wan et al., 2019), and may contribute to keeping cells temporarily in the light zone stage. Ultimately, recycling into the dark zone will be induced, allowing accumulation of further mutations, something not seen in T-independent GCs (Toellner et al., 2002).
Are TI-II antigens relevant to study GC responses? They can certainly disentangle effects of BCR signaling from signals delivered by Tfh cells. Further, follicular dendritic cells in GCs are known to generate repetitive patterns that may deliver similar stimuli as TI-II antigens (El Shikh et al., 2009). More importantly, TI-II antigens are relevant, as they are widely used in human vaccination. While there has been concern that polysaccharide antigens could deplete antigen-specific cells by inducing only short-lived antibody formation, they do produce long-term immune protection in human adults (Defrance et al., 2011). Young infants, however, benefit from protein conjugate vaccines efficiently recruiting Tfh help (O’Brien et al., 2007). Liu et al. (2022) add to our understanding here as well. Different from what is happening in aging, in which defective antibody responses are caused by a loss of fitness of T cells, and not the aging B cell (Lee and Linterman, 2022), Liu et al. (2022) show that in infants, the lack of GC formation to TI-II antigen is B cell intrinsic and not caused by the infant environment. It will be interesting to see whether these B cell–intrinsic effects involve BCR signaling, and how Tfh cell–derived signals overcome these defects in the very young.
Acknowledgments
Y. Zhang is funded by Biotechnology and Biological Sciences Research Council grant BB/S003800/1.
References
- Allen, C.D., et al. 2004. Nat. Immunol. 10.1038/ni1100 [DOI] [PubMed] [Google Scholar]
- Cascalho, M., et al. 1996. Science. 10.1126/science.272.5268.1649 [DOI] [Google Scholar]
- Defrance, T., et al. 2011. Curr. Opin. Immunol. 10.1016/j.coi.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Dominguez-Sola, D., et al. 2012. Nat. Immunol. 10.1038/ni.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Shikh, M.E., et al. 2009. J. Immunol. 10.4049/jimmunol.0802317 [DOI] [Google Scholar]
- Gitlin, A.D., et al. 2014. Nature. 10.1038/nature13300 [DOI] [Google Scholar]
- Hsu, M.C., et al. 2006. Proc. Natl. Acad. Sci. U S A. 10.1073/pnas.0601502103 [DOI] [Google Scholar]
- Lee, J.L., and Linterman M.A.. 2022. Immunol. Lett. 10.1016/j.imlet.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., et al. 2022. J. Exp. Med. 10.1084/jem.20210527 [DOI] [Google Scholar]
- Liu, Y.J., et al. 1989. Nature. 10.1038/342929a0 [DOI] [Google Scholar]
- O’Brien, K.L., et al. 2007. Lancet Infect. Dis. 10.1016/S1473-3099(07)70210-4 [DOI] [Google Scholar]
- Obukhanych, T.V., and Nussenzweig M.C.. 2006. J. Exp. Med. 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, T.A., et al. 2002. Nat. Immunol. 10.1038/ni803 [DOI] [PubMed] [Google Scholar]
- Suan, D., et al. 2017. Immunity. 10.1016/j.immuni.2017.11.022 [DOI] [Google Scholar]
- Taylor, J.J., et al. 2012. J. Exp. Med. 10.1084/jem.20111696 [DOI] [Google Scholar]
- Toellner, K.-M., et al. 2002. J. Exp. Med. 10.1084/jem.20011112 [DOI] [Google Scholar]
- Victora, G.D., et al. 2010. Cell. 10.1016/j.cell.2010.10.032 [DOI] [Google Scholar]
- de Vinuesa, C.G., et al. 2000. J. Exp. Med. 10.1084/jem.191.3.485 [DOI] [Google Scholar]
- Wan, Z., et al. 2019. Immunol. Rev. 10.1111/imr.12747 [DOI] [Google Scholar]
- Zhang, Y., et al. 2018. J. Exp. Med. 10.1084/jem.20160832 [DOI] [Google Scholar]
- Zotos, D., et al. 2021. Nat Commun. 10.1038/s41467-021-27477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]