Abstract
We have cloned Staphylococcus aureus DNA gyrase and topoisomerase IV and expressed them in Escherichia coli as polyhistidine-tagged proteins to facilitate purification and eliminate contamination by host enzymes. The enzyme preparations had specific activities similar to previously reported values. Potassium glutamate (K-Glu) stimulated the drug-induced DNA cleavage activity and was optimal between 100 and 200 mM for gyrase and peaked at 100 mM for topoisomerase IV. Higher concentrations of K-Glu inhibited the cleavage activities of both enzymes. Using a common buffer system containing 100 mM K-Glu, we tested the enzyme-mediated DNA cleavage activities of both gyrase and topoisomerase IV with oxolinic acid, norfloxacin, ciprofloxacin, trovafloxacin, clinafloxacin, and the 2-pyridone ABT-719. As expected, all drugs tested demonstrated greater potency against topoisomerase IV than against gyrase. In addition, cleavage activity was found to correlate well with antibacterial activity.
DNA gyrase and topoisomerase IV are the biological targets of the quinolones in bacterial cells. These enzymes are heterotetrameric (A2B2), type II DNA topoisomerases that play essential roles in bacterial DNA replication, chromosome segregation, recombination, repair, and transcription. In addition to inhibiting the catalytic activities of the enzymes, quinolones induce the formation of stable, covalent protein-DNA complexes (5, 9, 29). The antibacterial activity of quinolones is thought to be derived, in part, from their ability to induce enzyme-mediated double-strand DNA breaks, which ultimately lead to lethal DNA damage (3). The 2-pyridones, of which ABT-719 is an example, are a related class of agents that also target DNA gyrase and topoisomerase IV. They are a potent series of compounds which demonstrate a broad spectrum of antibacterial activity. For instance, ABT-719 is highly active against ciprofloxacin-resistant Staphylococcus aureus, including ciprofloxacin-resistant methicillin-resistant S. aureus (8).
Interesting differences in the mechanisms of quinolone resistance have been observed in gram-positive and gram-negative bacteria. In general, the majority of first-step mutations conferring quinolone resistance in gram-negative organisms arise in the gyrA gene encoding the A subunit of gyrase (4, 30). First-step mutations in gram-positive species are generally found in the gene encoding the corresponding subunit of topoisomerase IV (e.g., grlA in S. aureus and parC in Streptococcus pneumoniae) (6, 7, 17, 19, 22). Thus, while gyrase appears to be the primary target of quinolones in gram-negative organisms (3, 15), topoisomerase IV is the primary target in gram-positive organisms (6, 7, 17, 19, 22).
Biochemical analyses also suggest that the molecular basis of these differences in resistance mechanisms likely lies in the relative sensitivities of the enzymes from various species to any given quinolone. In Escherichia coli, gyrase is more sensitive than topoisomerase IV to inhibition of catalytic activity by quinolones (1, 13, 15), as well as to induction of cleavage complex formation (1). Conversely, in S. aureus, topoisomerase IV is more sensitive than gyrase to both inhibition of catalytic activity (1, 27) and induction of cleavage complex formation (1).
In an effort to further characterize the effect of selected quinolones and 2-pyridones, we have cloned, expressed, and purified individual subunits of S. aureus gyrase and topoisomerase IV. During characterization of the enzyme preparations, we discovered conditions whereby cleavage complex formation with S. aureus gyrase could be readily detected by using plasmid DNA as the substrate. We also examined the cleavage complex-stimulating activities of selected quinolones and the 2-pyridone ABT-719 against both S. aureus DNA gyrase and topoisomerase IV.
MATERIALS AND METHODS
Abbreviations.
CC50, drug concentration at which half-maximal cleavage (DNA linearization) was attained, relative to the maximal cleavage shown by ciprofloxacin, which was dosed at up to 200 μg/ml; IPTG, isopropyl-β-d-thiogalactopyranoside; K-Glu, potassium glutamate; Ni-NTA, nickel-nitrilotriacetic acid; DTT, dithiothreitol; BSA, bovine serum albumin.
Materials.
Plasmids pET-19b, pET-21(+), and pET-28a and the E. coli hosts HMS174(λDE3)(pLysS) and NovaBlue(λDE3) were from Novagen. The TA cloning kit containing plasmid pCR2.1 was from Invitrogen. The pCR-Script Cam SK(+) cloning kit was from Stratagene. AmpliTaq DNA polymerase was from Perkin-Elmer. Carbenicillin and IPTG were from Sigma. Complete EDTA-free protease inhibitor cocktail tablets were from Boehringer Mannheim. Ni-NTA resin was from Qiagen. Centricon units were from Millipore.
Construction of S. aureus DNA gyrase and topoisomerase IV A and B subunit expression vectors.
The gyrA, gyrB, grlA, and grlB genes were amplified from S. aureus ISP8 (also known as 8325-4 or RN450; kindly provided by J. J. Iandolo) genomic DNA by PCR (23) with the following oligonucleotide primers: gyrA, 5′-ctatactctaactcgagGCTGAATTACCTCAATC-3′ and 5′-cattacacatcctcgagTTATTATTCTTCATCTG-3′; gyrB, 5′-cgcggatccaattttgtttaactttaagaaggagatatagcATGGTGACTGCATTGTCAGAT-3′ and 5′-atatatgcgtatgcgctcgagagaacccatggtGAAGTCTAAGTTTGCATAAACTGC-3′; grlA, 5′-accgtctcaAGTGAAATAATTCAAGAT-3′ and 5′-accgtctcaGCTAATATACATGTCTATTAC-3′; and grlB, 5′-gaattcatcgaaggtcgtATGAATAAACAAAAT-3′ and 5′-gtcgacCTAGATTTCCTCCTCATCAAATTG-3′. (Gene sequences are denoted by capital letters, and restriction sites are underlined.)
The resulting gyrA and gyrB PCR products were cloned into the TA vector, pCR2.1, and sequenced. The sequences obtained were identical to the S. aureus gyrA and gyrB sequences available from GenBank (accession no. D10489). The gyrA gene was subcloned into the XhoI site of pET-19b, creating pACS50 for the production of 10xHis-gyrase A protein. The gyrB gene was subcloned into the BamHI and XhoI sites of pET-21+, creating pACS60 for the production of gyrase B-6xHis protein. The resulting grlA and grlB PCR products were cloned into pCR-Script Cam SK(+) vector and pCR2.1, respectively, and multiple clones of each were sequenced. The primary amino acid sequence for grlA differed by 4 amino acids from the sequence available from GenBank (accession no. L25288), while the amino acid sequence obtained for grlB was as expected (GenBank accession no. D10489). The grlA gene was subcloned with BsmBI into UpET (12), creating pLS1 for the production of 6xHis-ubiquitin-GrlA protein. The grlB gene was subcloned into the EcoRI and SalI sites of pET-28a, creating pLS2 for the production of 6xHis-GrlB protein.
Expression and purification of S. aureus DNA gyrase and topoisomerase IV subunits.
Transformants of E. coli HMS174(λDE3, pLysS, pACS50), HMS174(λDE3, pLysS, pACS60), NovaBlue(λDE3, pLS1), and BL21(λDE3, pLS2) were cultured in Luria-Bertani medium containing 100 μg of carbenicillin/ml at 30°C and induced at mid-exponential phase by the addition of IPTG to a final concentration of 1 mM. After incubation for an additional 5 h, the cells were harvested by centrifugation, resuspended in 2.5 ml of buffer A (50 mM Na-PO4 [pH 8.0], 300 mM NaCl, and 1 mM β-mercaptoethanol) per g (wet weight), and frozen at −80°C until they were ready for lysis.
All subsequent steps were performed at 4°C, according to the basic purification protocol for Ni-NTA affinity chromatography provided by Qiagen, with the following exceptions. Complete EDTA-free protease inhibitor cocktail was added to cell suspensions containing pACS50 and pACS60, which were lysed by thawing. Cell suspensions containing pLS1 and pLS2 were lysed on ice for 30 min in the presence of phenylmethylsulfonyl fluoride (1 mM) and lysozyme (200 μg/ml), followed by addition of tergitol NP-40 to 1%, sonication, and then centrifugation at >10,000 × g. For purification of 10xHis-gyrase A, 6xHis-ubiquitin-GrlA, and 6xHis-GrlB, the clear lysates were applied to Ni-NTA columns and washed with buffer A, followed by buffer B (buffer A plus 10% glycerol, pH 6.0). For purification of gyrase B-6xHis, following the buffer A wash, the column was washed with buffer B with 1 M NaCl, followed by 6 column volumes of buffer B. The 10xHis-gyrase A, gyrase B-6xHis, 6xHis-ubiquitin-GrlA, and 6xHis-GrlB proteins were eluted with buffer B containing 400, 300, 100, and 300 mM imidazole, respectively. The 10xHis-gyrase A and gyrase B-6xHis protein eluates were treated identically as follows. Both were dialyzed separately at 4°C against a solution of 100 mM Tris-HCl (pH 7.5), 10% glycerol, 2 mM EDTA, 2 mM DTT, and 100 mM NaCl and were concentrated with Centricon-50 units. Concentration of the GrlA and GrlB proteins and buffer exchange were carried out with Centricon-30 units with 50 mM K-PO4 (pH 7.5), 10% glycerol, 0.5 mM EDTA, and 1 mM DTT. Before storage at −80°C, glycerol was added to a final concentration of 50%.
Topoisomerase reactions.
Both S. aureus DNA gyrase and topoisomerase IV holoenzymes were reconstituted by mixing equimolar amounts of the A and B subunits of the respective enzymes (GyrA-GyrB or GrlA-GrlB) and incubating them at room temperature for 10 min. The activities of the holoenzymes were found to be stable for at least 2 months when stored at −20°C. Reaction termination, electrophoresis, DNA quantitation, and calculation of CC50 values were performed as described previously (24).
(i) Catalytic activity assay.
S. aureus DNA gyrase was incubated at 37°C for 1 h in a total reaction volume of 20 μl containing 75 mM Tris-HCl (pH 7.5), 7.5 mM MgCl2, 7.5 mM DTT, 2 mM ATP, 75 μg of BSA/ml, 30 mM KCl, 500 mM K-Glu, 0.1 μg of relaxed ColE1 DNA, and 2 μg of tRNA. DNA relaxation and decatenation reactions catalyzed by S. aureus topoisomerase IV were carried out at 37°C for 1 h in a total reaction volume of 20 μl containing 50 mM Tris-HCl (pH 7.7), 5 mM MgCl2, 5 mM DTT, 1.5 mM ATP, 50 μg of BSA/ml, 20 mM KCl, 5 mM spermidine, a specified amount of K-Glu, and 0.1 μg of supercoiled ColE1 or 0.15 μg of catenated kinetoplast DNA (TopoGen). Agarose gel electrophoresis without ethidium bromide was used to separate relaxed species from supercoiled species. One U of supercoiling or relaxation activity was defined as the amount of enzyme needed to supercoil or relax 50% of the ColE1 substrate in the reaction under the above-mentioned conditions.
(ii) DNA cleavage assay.
Topoisomerase-mediated DNA cleavage reactions with both S. aureus gyrase and topoisomerase IV were performed under identical conditions as follows. Cleavage reactions were carried out at 37°C for 30 min in a 20-μl reaction volume containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 5 mM DTT, 1.5 mM ATP, 50 μg of BSA/ml, 100 mM K-Glu, 5 mM spermidine, 0.15 μg of supercoiled ColE1 DNA, topoisomerase (130 ng of gyrase or 38 ng of topoisomerase IV), and drug.
RESULTS AND DISCUSSION
Purification and catalytic activities of S. aureus gyrase and topoisomerase IV.
S. aureus gyrase is known to be a difficult enzyme to isolate from bacterial cell cultures, and the results of its testing against quinolones are largely variable (2, 11, 25, 28). We proceeded to clone and express the S. aureus gyrase and topoisomerase IV subunits in E. coli as polyhistidine-tagged proteins to facilitate enzyme purification by immobilized metal affinity chromatography and to eliminate both the likelihood of contamination by endogenous topoisomerases and exposure to harsh solvent conditions.
Polyhistidine-tagged GyrA, GyrB, GrlA, and GrlB subunits were purified to about 80, 70, 50, and 60% apparent homogeneity, respectively, as judged by Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels (data not shown). The reconstituted enzymes had specific catalytic activities similar to or better than the values reported in the literature (1, 18, 20). For topoisomerase IV, the specific activity for both the decatenation and relaxation reactions was 27,000 U per mg of the reconstituted enzyme (at 250 mM K-Glu), in agreement with the published values of 20,000 and 30,000 U/mg, respectively (1). For gyrase, the specific activity was found to be approximately 4,000 U per mg of the reconstituted enzyme (at 500 mM K-Glu), similar to what has been reported for the enzyme purified from S. aureus (1,100 to 2,200 U/mg [20] and 2,900 to 8,700 U/mg [18]) but lower than the value of 500,000 U/mg reported by Blanche et al. (1).
Similar to previous reports (1), the supercoiling activity of S. aureus gyrase was stimulated by K-Glu over a wide range of concentrations, from 300 mM to 1 M K-Glu, with maximal stimulation at approximately 500 mM K-Glu. Unlike S. aureus gyrase, the stimulation of relaxation and decatenation activity of S. aureus topoisomerase IV by K-Glu occurred over a narrower concentration range: between 150 and 400 mM for relaxation activity and between 100 and 500 mM for decatenation activity (data not shown). At the optimum concentration of 250 mM K-Glu, topoisomerase IV displayed similar specific activities for both relaxation and decatenation reactions. This result differed from previous observations that decatenation activity required K-Glu while relaxation activity was inhibited by K-Glu (1).
Quinolone- and 2-pyridone-stimulated DNA cleavage activities of S. aureus gyrase and topoisomerase IV.
Previous publications which tested quinolone activity against S. aureus DNA gyrase and topoisomerase IV were limited to the use of catalytic inhibition assays (18, 20, 26, 27). Similar results with quinolones were reported for other gram-positive bacterial type II DNA topoisomerases (10, 21). Blanche et al. (1) examined the ability of S. aureus gyrase to form cleavage complexes but were unable to detect induction of plasmid DNA cleavage at any K-Glu concentration with 25 μg of either ciprofloxacin or sparfloxacin/ml. As reported previously, and also confirmed in our assays, the enzyme required high concentrations of K-Glu for optimal catalytic activity (1). However, for other topoisomerases, including the human type I and type II enzymes, high salt concentrations are known to cause reversal of drug-induced cleavage complex formation (14, 16). Therefore, we explored the possibility that high concentrations of K-Glu could be interfering with the formation of quinolone-induced S. aureus gyrase cleavage complexes. By performing cleavage reactions in buffer containing little or no K-Glu, the DNA cleavage activity of S. aureus gyrase could be readily detected.
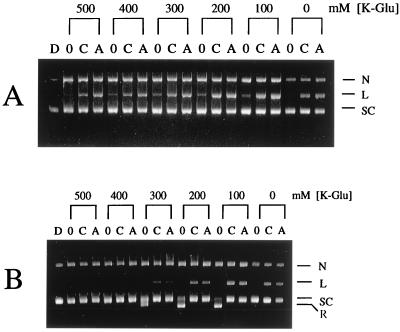
The effect of K-Glu on the induction of gyrase- and topoisomerase IV-mediated DNA cleavage was tested with the fluoroquinolone ciprofloxacin and the 2-pyridone ABT-719. With either enzyme, at the lower range of K-Glu concentrations tested, both drugs induced substantial conversion of supercoiled ColE1 substrate into linear DNA product. For gyrase, drug-induced cleavage activity was observed between 0 and 500 mM K-Glu. Strong stimulation of gyrase cleavage activity occurred between 100 and 200 mM K-Glu, with a maximum at 100 mM (Fig. 1A). For topoisomerase IV, the drug-induced cleavage activity was stimulated over a narrower concentration range, from 0 to 200 mM K-Glu, with a maximum at 100 mM (Fig. 1B). Drug-stimulated cleavage activity of topoisomerase IV was essentially nonexistent at 300 mM K-Glu, whereas the catalytic activity was still observable. In fact, the dependence of both DNA cleavage and catalytic inhibition on the K-Glu concentration was seen on the gel shown in Fig. 1B. Based on these observations, 100 mM K-Glu was selected as the standard reaction condition used for both enzymes in testing additional compounds for cleavage activity.
FIG. 1.
Effect of K-Glu on S. aureus DNA gyrase (A)- and topoisomerase IV (B)-mediated DNA cleavage by ciprofloxacin and ABT-719. Each lane is marked as 0, C, or A to denote reactions containing no drug, ciprofloxacin at 100 μg/ml, and ABT-719 at 100 μg/ml, respectively. The concentration of K-Glu is indicated above each set of lanes. The relative migrations of nicked (N), linear (cleaved) (L), relaxed (R), and supercoiled (SC) DNA are indicated.
A plausible explanation for differences in the ability to detect cleavage complexes with S. aureus gyrase could be the methods used in enzyme preparation. Blanche et al. (1) employed novobiocin affinity chromatography with elution by 5 M urea followed by renaturation, whereas we isolated individual gyrase subunits by Ni-NTA affinity chromatography with imidazole elution, thereby eliminating the denaturation and renaturation steps.
The CC50 values for gyrase- and topoisomerase IV-mediated DNA cleavage and the S. aureus antibacterial activities (MICs) of ciprofloxacin, ABT-719, clinafloxacin, trovafloxacin, norfloxacin, and oxolinic acid are listed in Table 1. The CC50 values for DNA gyrase represent the first report of quantitative DNA cleavage data obtained with this enzyme. The results show that, for the compounds tested, topoisomerase IV was more susceptible than gyrase, consistent with previously published data (1). The CC50 values of ciprofloxacin and norfloxacin against S. aureus topoisomerase IV were 0.18 and 0.43 μg/ml, respectively, and were close to the respective values of 0.1 and 0.25 previously determined with end-labeled linear pBR322 DNA as the cleavage assay substrate (1).
TABLE 1.
Topoisomerase-dependent DNA cleavage assay resultsa
| Drug | CC50 (μg/ml) with S. aureus gyrase | CC50 (μg/ml) with S. aureus topo IV | CC50 ratio (gyrase/topo IV) | MIC for S. aureusb |
|---|---|---|---|---|
| ABT-719 | 0.05 | 0.024 | 2.1 | 0.01 |
| Clinafloxacin | 0.1 | 0.03 | 3.3 | 0.02 |
| Trovafloxacin | 1.6 | 0.19 | 8.4 | 0.2 |
| Ciprofloxacin | 3.5 | 0.18 | 19 | 0.2 |
| Norfloxacin | 20 | 0.43 | 47 | 0.78 |
| Oxolinic acid | 115 | 77 | 1.5 | 3.2 |
CC50 is defined as the half-maximal cleavage (DNA linearization) relative to the maximal level shown by ciprofloxacin at concentrations up to 200 μg/ml. Topo, topoisomerase.
S. aureus ATCC 6538P.
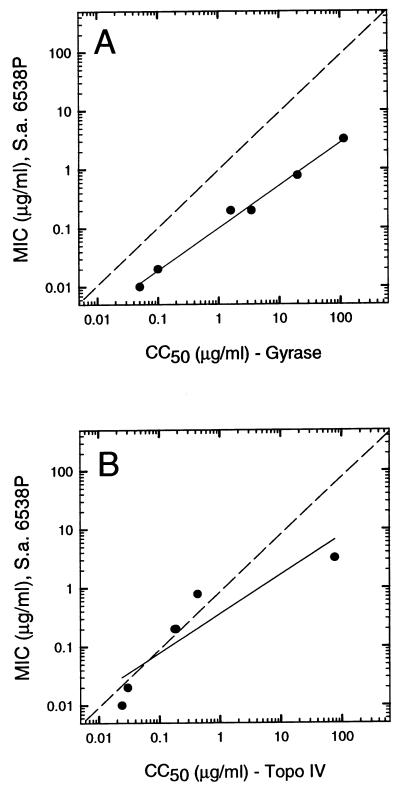
There was a direct correlation between the potencies of the compounds against the two enzymes (Table 1). Topoisomerase IV was more sensitive to all compounds tested than gyrase, with gyrase being 1.5- to 47-fold less sensitive than topoisomerase IV. The potencies of these compounds against both enzymes also directly paralleled their antibacterial activities (Fig. 2). For the 2-pyridone and fluoroquinolones tested, there was a distinct trend, indicating that the more highly potent the in vitro cleavage-stimulating activity, the greater the antibacterial activity and the less selective the compound for topoisomerase IV. The curve for gyrase was about 1 order of magnitude higher than the MIC values. For topoisomerase IV, the CC50 values were very similar to the MIC values, with the exception of oxolinic acid (CC50 = 115 μg/ml), which is a weak archetype of the quinolone class. The antistaphylococcal activity of the quinolone-like compounds tested here was likely due to their degree of potency against topoisomerase IV, but it may also have been due, in part, to their activity against gyrase. This observation suggests that yet-to-be-discovered agents with exquisite potency against S. aureus gyrase may be highly active against the majority of ciprofloxacin-resistant clinical isolates.
FIG. 2.
Correlation of antibacterial potency (expressed as the MIC values against S. aureus ATCC 6538P) and DNA cleavage activity of quinolones and ABT-719 mediated by S. aureus gyrase (r = 0.998) (A) and topoisomerase IV (r = 0.903) (B). The dotted line represents 1:1 correlation. The data were taken from Table 1.
ACKNOWLEDGMENTS
We thank Earl Gubbins for providing universal cloning site vectors and methodology, as well as Bob Flamm, Angela Nilius, Mai-Ha Bui, and Patti Raney for MIC data.
REFERENCES
- 1.Blanche F, Cameron B, Bernard F X, Maton L, Manse B, Ferrero L, Ratet N, Lecoq C, Goniot A, Bisch D, Crouzet J. Differential behaviors of Staphylococcus aureus and Escherichia coli type II DNA topoisomerases. Antimicrob Agents Chemother. 1996;40:2714–2720. doi: 10.1128/aac.40.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockbank S M, Barth P T. Cloning, sequencing, and expression of the DNA gyrase genes from Staphylococcus aureus. J Bacteriol. 1993;175:3269–3277. doi: 10.1128/jb.175.11.3269-3277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi T, Fukuoka A, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Ishihara S, Ban Y, Kawada Y. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV in quinolone-resistant clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1997;41:699–701. doi: 10.1128/aac.41.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drlica K, Zhao X L. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 8.Flamm R K, Vojtko C, Chu D T, Li Q, Beyer J, Hensey D, Ramer N, Clement J J, Tanaka S K. In vitro evaluation of ABT-719, a novel DNA gyrase inhibitor. Antimicrob Agents Chemother. 1995;39:964–970. doi: 10.1128/aac.39.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- 10.George J T, Morrissey I. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Activity of levofloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against DNA gyrase from S. pneumoniae C3LN4, abstr. C-176; p. 120. [Google Scholar]
- 11.Georgopapadakou N H, Dix B A. Abstracts of the 86th Annual Meeting of the American Society for Microbiology 1986. Washington, D.C: American Society for Microbiology; 1986. Purification of Staphylococcus aureus DNA gyrase and inhibition by quinolones, abstr. A-33. [Google Scholar]
- 12.Gubbins E J, Pohlman K L, Lee J A, Smith H T, Parry J M, Simmer R L. The universal cloning site system for designing gene expression cassettes and vectors. Protein Eng. 1997;10:27. [Google Scholar]
- 13.Hoshino K, Kitamura A, Morrissey I, Sato K, Kato J, Ikeda H. Comparison of inhibition of Escherichia coli topoisomerase IV by quinolones with DNA gyrase inhibition. Antimicrob Agents Chemother. 1994;38:2623–2627. doi: 10.1128/aac.38.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiang Y-H, Hertzberg R, Hecht S, Liu L F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 15.Khodursky A B, Zechiedrich E L, Cozzarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L F, Rowe T C, Yang L, Tewey K M, Chen G L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 17.Munoz R, De La Campa A G. ParC subunit of DNA topoisomerase-IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi N, Yoshida S, Wakebe H, Inoue M, Yamaguchi T, Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2562–2567. doi: 10.1128/aac.35.12.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda J, Okamoto S, Takahata M, Nishino T. Inhibitory effects of ciprofloxacin and sparfloxacin on DNA gyrase purified from fluoroquinolone-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2288–2293. doi: 10.1128/aac.35.11.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onodera Y, Uchida Y, Tanaka M, Sato K. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Dual inhibitory activity of DU-6859a against DNA gyrase and topoisomerase IV of Streptococcus pneumoniae, abstr. C-175; p. 119. [Google Scholar]
- 22.Pan X S, Ambler J, Mehtar S, Fisher L M. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2321–2326. doi: 10.1128/aac.40.10.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 24.Shen L L, Baranowski J, Fostel J, Montgomery D A, Lartey P A. DNA topoisomerases from pathogenic fungi: targets for the discovery of antifungal drugs. Antimicrob Agents Chemother. 1992;36:2778–2784. doi: 10.1128/aac.36.12.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahata M, Nishino T. DNA gyrase of Staphylococcus aureus and inhibitory effect of quinolones on its activity. Antimicrob Agents Chemother. 1988;32:1192–1195. doi: 10.1128/aac.32.8.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka M, Onodera Y, Uchida Y, Sato K. Quinolone resistance mutants in the GyrB protein of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:3044–3046. doi: 10.1128/aac.42.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka M, Sato K, Kimura Y, Hayakawa I, Osada Y, Nishino T. Inhibition by quinolones of DNA gyrase from Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:1489–1491. doi: 10.1128/aac.35.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]