Abstract
Background
Myostatin (MSTN) is a key negative regulator of muscle mass in humans and animals, having direct and indirect influences on molecular regulators of atrophy and hypertrophy, thus potentially impacting fitness and physical function. We have shown that myostatin is elevated in conditions of chronic disability (e.g. paretic limb of stroke). Our hypothesis is that myostatin would be elevated in older adults with sarcopenia. The purpose of this study was to examine the role of skeletal muscle myostatin in sarcopenia.
Methods
Sixty-four overweight to obese aged 45–81 years underwent a maximal aerobic capacity (VO2max) test, dual-energy X-ray absorptiometry (DXA) scan to determine appendicular lean tissue (ALM), and vastus lateralis muscle biopsy to determine myostatin mRNA expression by quantitative real time PCR (Q-RT-PCR). Rates of sarcopenia were determined using (ALM/BMI), and sarcopenia was defined as <0.789 in men and <0.512 in women. Subjects had low fitness (VO2max: 22.7 ± 0.7 mL/kg/min) and on average 40.9 ± 1% body fat.
Results
The prevalence of sarcopenia in this cohort was 16%. BMI, % body fat, and fat mass were higher in adults with sarcopenia than those without sarcopenia (all P < 0.001). Myostatin mRNA expression was lower in those without sarcopenia than those with sarcopenia (P < 0.05) and higher in men than women (P < 0.001). Myostatin expression was associated with BMI (r = 0.36, P < 0.01) and mid-thigh intramuscular fat (r = 0.29, P < 0.05).
Conclusion
Given that myostatin is important in muscle atrophy, fat accumulation, and sarcopenia, further work could address its implication in other aging cohorts of disability and chronic disease.
Keywords: Sarcopenia, Myostatin, Skeletal muscle atrophy, Fat accumulation, Obesity, Aging
Introduction
Sarcopenia, the age-related loss in skeletal muscle mass and strength, is a leading contributor to the development of frailty. Sarcopenic obesity is described as the combination of loss of muscle mass, function and elevated body mass index1 and also increases the risk for metabolic disturbances.2 Over 15 years ago, Baumgartner and colleagues3 reported that adults with sarcopenic obesity are two to three times more likely to report an onset of Instrumental Activities of Daily Living (IADL) disability than adults with normal body composition, lean sarcopenic, and obese nonsarcopenic adults, indicating an independent association of sarcopenic obesity and the development of disability.3 Furthermore, this >2.5 relative risk for incident disability in sarcopenic obese adults occurs after adjustment for confounding factors of age, sex, physical activity level, length of follow-up, and prevalent morbidity. Although several other groups have nicely reviewed sarcopenia obesity4,5 and potential biological pathways that could explain its pathology,6 the skeletal muscle mechanisms linking sarcopenia with obesity in adults is not completely understood.
Myostatin is a member of the transforming growth factor beta family of secreted growth factors and a significant regulator of skeletal muscle development and size.7 In fact, anti-myostatin antibodies are potential therapeutic options for sarcopenia.8,9 Myokines, including myostatin, play a role in the pathogenesis of sarcopenic obesity.10 Furthermore, ectopic fat deposition, obesity, and aging are implicated in impaired skeletal muscle protein synthesis leading to muscle lipid accumulation.11,12
We have shown greater muscle atrophy, increased intramuscular fat, and higher skeletal muscle myostatin in conditions of chronic disability (e.g. paretic limb of stroke).13-15 We hypothesized that skeletal muscle myostatin would be elevated in adults with sarcopenia. Therefore, the aim of the study was to compare skeletal muscle myostatin expression in middle-aged and older overweight and obese adults with and without sarcopenia.
Methods
Participants
Male and female adults aged 45–80 years from the Baltimore/Washington area were eligible to participate if generally healthy, weight stable (<2.0 kg weight change in past year), sedentary (<20 min of aerobic exercise 2× per week), overweight or obese (BMI 29–50 kg/m2), and without the presence of heart disease, diabetes, cancer, anaemia, dementia, untreated dyslipidaemia, or other unstable or chronic diseases affecting the liver, lungs, or kidneys. Women were included only if they had undergone menopause for at least 1 year. Potential participants were screened and underwent a physical examination including a comprehensive past medical history, fasting blood profile, and a graded exercise treadmill test as part of another longitudinal investigation. Each study participant provided written consent. All methods and procedures were approved by the Institutional Review Board at University of Maryland School of Medicine and the VA Research and Development Committee.
Tests
Height (cm) and weight (kg) were measured to calculate body mass index (BMI). Body fat mass, lean mass of the arms and legs (to calculate appendicular lean mass) were measured by dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare). Sarcopenia was defined as appendicular lean mass/height2, ALM/ht2 < 7.26 kg/m2 in men and <5.45 kg/m2 in women.16 Measurements of grip strength and gait speed were not part of the original longitudinal study goals and so were not available for use in the definition of sarcopenia. Computed tomography (CT) scans (Siemens Somatom Sensation 64 Scanner, Fairfield, CT) at L4-L5 region was used to determine visceral adipose tissue area, subcutaneous adipose tissue area, and a scan at the mid-thigh to quantify muscle area, subcutaneous fat area, and intramuscular adipose tissue (IMAT). Scans were analysed using Medical Image Processing, Analysis and Visualization, v.7.0.0. Ten individuals were missing measurement of visceral fat and 25 were missing thigh scans for measurement of IMAT. VO2max was measured using a continuous treadmill test protocol. Vastus lateralis percutaneous needle muscle biopsies were taken from each participant under local anaesthesia using a 5 mm Bergstrom needle (Stille, Solna, Sweden). Muscle was immediately freeze-clamped and stored at −80°C. Approximately 50–80 mg of muscle was used for RNA isolation and myostatin gene expression. Total RNA extraction, cDNA synthesis and quantitative real-time PCR (qPCR) were done by our standard laboratory methods.17 To be included in this research question, each participant had to have sufficient muscle sample for myostatin analysis. All testing and lab analysis were blinded as to sarcopenia status of either the participant and the study samples to eliminate any bias.
Statistical analyses
Descriptive means were calculated on 14 variables. One-way ANOVAs were used to test differences between groups. Pearson correlations and partial correlations were used to assess relationships between key variables. Statistical significance was set at a two-tailed P < 0.05. Data were analysed using SPSS Statistics 24 (SPSS Inc., Chicago); results are expressed as mean ± SEM.
Results
Study sample
Descriptive characteristics of the participants (n = 64) are provided in Table 1. They were 78% Caucasian and 22% African American (n = 14) and included 31 men (48%) and 33 women (52%). On average, participants had low fitness levels with a wide range of total body fat.
Table 1.
Subject characteristics
| Variables | Mean ± SEM | Range |
|---|---|---|
| Age (yrs) | 61 ± 1 | 45–81 |
| Weight (kg) | 94.3 ± 2.2 | 63.6–142.2 |
| Height (m) | 1.70 ± 0.01 | 1.53–1.88 |
| BMI (kg/m2) | 32.7 ± 0.6 | 24.9–46.1 |
| VO2max (mL/kg/min) | 22.7 ± 0.7 | 8.9–36.4 |
| VO2max (L/min) | 2.2 ± 0.1 | 0.8–3.7 |
| Body fat (%) | 40.9 ± 1 | 24.5–59.3 |
| Fat mass (kg) | 38.9 ± 1.4 | 20.0–78.3 |
| Fat-free mass (kg) | 55.9 ± 1.5 | 38.2–75.2 |
| Appendicular lean mass (kg) | 24.2 ± 0.7 | 14.57–34.1 |
| Mid-thigh muscle area (cm2) | 91.2 ± 5.1 | 47.8–169.6 |
| Mid-thigh subcutaneous fat area (cm2) | 118.6 ± 9.8 | 36.2–313.9 |
| Mid-thigh intramuscular fat area (cm2) | 27.3 ± 1.8 | 12.0–56.1 |
| Visceral fat area (cm2) | 185.2.0 ± 10.7 | 66.4–393.8 |
| Subcutaneous abdominal fat area (cm2) | 414.2 ± 17.2 | 216.2–684 |
| Myostatin gene expression (AU) | 68.4 ± 3.9 | 9.4–172.5 |
Mean ± SEM.
Subject characteristics between sarcopenic (n = 10) and non-sarcopenic (n = 54) adults are presented in Table 2. The prevalence of sarcopenia in this cohort was 16%. Age, height, VO2max, or FFM did not differ between groups defined by sarcopenia status. Adults with sarcopenia had higher body weight (P < 0.05), BMI (P < 0.05), per cent body fat (P < 0.05), and body fat mass (P < 0.01). Myostatin mRNA expression was lower (30.4%) in those without sarcopenia than those with sarcopenia (P < 0.05, Figure 1). Myostatin expression was higher in men than women in the total group (83.4 ± 5.7 vs. 54.3 ± 4.2 AU, P < 0.0001). It was also higher in men (n = 6) than women (n = 4) with sarcopenia (120.6 ± 13.7 vs. 48.8 ± 11.6 AU, P < 0.01) and men (n = 25) than women (n = 29) without sarcopenia (74.4 ± 4.9 vs. 55.1 ± 4.5 AU, P < 0.01).
Table 2.
Comparison between sarcopenic and non-sarcopenic groups
| Variables | Sarcopenia (n = 10) | Non-sarcopenic (n = 54) |
|---|---|---|
| Age (years) | 60 ± 2 | 62 ± 1 |
| Weight (kg) | 110.4 ± 6.9* | 91.3 ± 2.1 |
| Height (m) | 1.71 ± 0.02 | 1.69 ± 0.01 |
| BMI (kg/m2) | 37.6 ± 1.9 ** | 31.8 ± 0.6 |
| VO2max (mL/kg/min) | 20.9 ± 1.1 | 22.8 ± 0.8 |
| Body fat (%) | 46.1 ± 2.1* | 40.0 ± 1.1 |
| Fat mass (kg) | 50.5 ± 4.0** | 36.8 ± 1.3 |
| Fat-free mass (kg) | 59.1 ± 4.2 | 55.3 ± 1.6 |
Mean ± SEM. Sarcopenia versus non-Sarcopenia.
P < 0.05.
P < 0.001.
Figure 1.
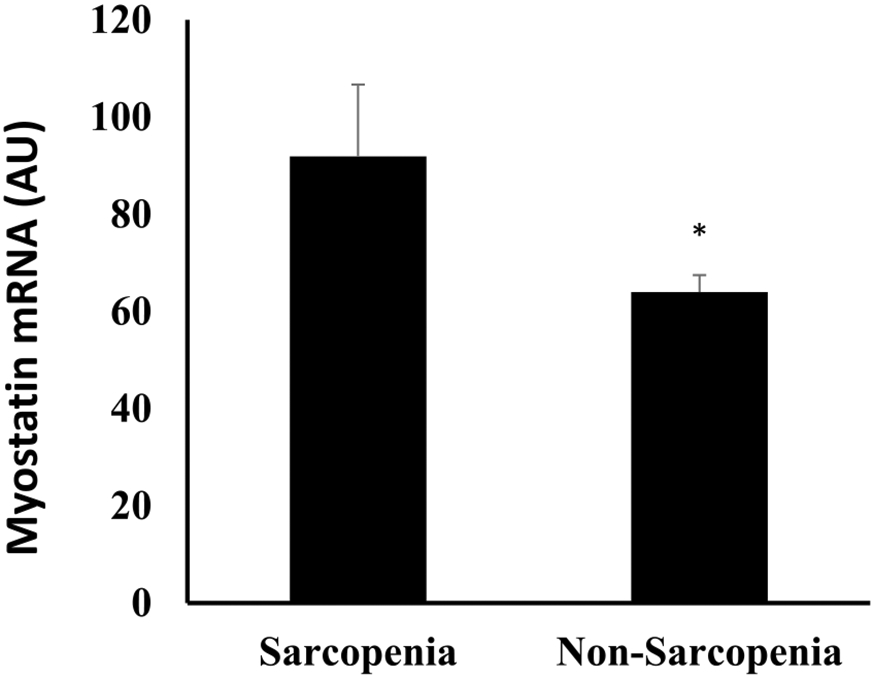
Skeletal muscle MSTN level between sarcopenia and non-sarcopenia. *P < 0.05.
Relationships
Skeletal muscle myostatin expression was associated with mid-thigh IMAT (r = 0.29, P < 0.05), mid-thigh muscle area (r = 0.43, P < 0.05), BMI (r = 0.36, P < 0.01), and WHR (r = 0.43, P < 0.01) but not related to age, VO2max, total body fat mass, or visceral fat in the total group. Skeletal muscle myostatin mRNA level tends to be positively correlated with fat mass in both men and women (men: r = 0.33, P = 0.07; women: r = 0.32, P = 0.07) and was positively correlated with subcutaneous abdominal fat in men (r = 0.40, P < 0.05) but not in women (r = 0.30, P = 0.11). Myostatin levels were not related to mid-thigh muscle area in either men or women alone.
Discussion
Sarcopenia is an age-related condition of loss of skeletal muscle mass, physical function, and strength. Participants in our study were overweight and obese who were on average 60 years of age. The mechanisms linking sarcopenia with obesity in adults are still not completely clear. Our data show that myostatin mRNA expression was significantly higher in adults with sarcopenia than those without sarcopenia. Myostatin as a myokine is expressed in skeletal muscle and is a negative regulator of skeletal muscle growth in animals and human and is associated with body fatness and aerobic capacity.18-20 Our data suggest that myostatin levels in the skeletal muscle might be one of the drivers for sarcopenia.
Muscle and fat are the two major metabolic tissues secreting cytokines, myokines, and adipokines that regulate metabolism and body composition. Myostatin is encoded by the myostatin gene in humans and is a myokine produced and released by myocytes that acts on muscle cells’ autocrine function to inhibit muscle cell growth and differentiation.21 The full length gene encodes a preproprotein that has 375 amino acids.21 This preproprotein is inactive until a protease cleaves the NH2-terminal, or “pro-domain” portion of the molecule, forming the active COOH-terminal dimer with a total molecular weight of 25.0 kDa. Each monomer of the protein dimer has 109 amino acid residues.7,22 Thus, myostatin detected in skeletal muscle by the biopsy technique can provide insight into muscle loss.
There are several lines of evidence to support that myostatin levels would differ between those with and without sarcopenia. Myostatin acts in two ways: by inhibiting either muscle differentiation or Akt-induced protein synthesis. When myostatin binds to the activin type II receptor, it results in a recruitment of either coreceptor Alk-3 or Alk-4. This coreceptor then initiates a cell signalling cascade in the muscle including the activation of transcription factors, SMAD2 and SMAD3. These factors then induce myostatin-specific gene regulation. When applied to myoblasts, myostatin inhibits their differentiation into mature muscle fibres.9 Myostatin also inhibits Akt, a kinase that causes muscle hypertrophy through the activation of protein synthesis.23 Additional studies could examine muscle transcription factors or Akt levels and their relationship to sarcopenia in adults with obesity.
Sarcopenia can affect up to one-fourth of older adults24 whereas sarcopenic obesity using muscle mass in the definition of sarcopenia has a lower prevalence ranging from 4% to 12%.25-27 Thus, the 16% prevalence observed in our study population is slightly higher than that previously reported. Moreover, our participants with sarcopenia would be considered sarcopenic obesity. Specifically, all the men were obese by BMI and the two women whose BMI were below the obesity cut-point (e.g. 28.6 and 29.9 kg/m2), and body fat percentage were high (51% and 45%, respectively). Obesity is associated with metabolic disorders and implies a connection between muscle and adipose tissue. Both aging and an unhealthy lifestyle such as physical inactivity and over nutrition contribute to the development of sarcopenic obesity.28 When people age, the capability for protein synthesis decreases while protein degradation increases.28-30 The proliferation and self-renewal of satellite cells as well as the differentiation of myocytes decreases, causing a loss in muscle mass and decline in physical function. In adults with obesity, mitochondrial biogenesis tends to decrease, causing an increase in oxidative stress and inflammation.28,31 Myostatin has also been found to be expressed in fat tissues and plays a key role in adipogenesis and potential control of body fat mass.32 One study shows that myostatin is associated with abdominal obesity in women with polycystic ovary syndrome.33 In a pre-clinical study that compared the myostatin-null mice with wild-type littermates, the former had a significant reduction in fat accumulation with increasing age.34 Myostatin deletion or inhibition leads to a decrease in fat mass in mice.32 Further, there is no effect on body composition on the inhibition of myostatin signals within adipose tissue in mice fed a standard diet or a high-fat diet whereas in contrast, inhibition of myostatin signalling in skeletal muscle results in an increase in lean mass, a decrease in fat mass, and improved glucose metabolism on standard and high-fat diets, and resistance to diet-induced obesity.35 In a pre-clinical aging model, protein synthesis is reduced in diet-induced obesity and the lipid redistribution in lean tissues or ectopic fat reduces protein turnover.12 Our data would support these pre-clinical studies and aligns with our finding that ectopic fat deposition in the muscle was associated with skeletal muscle myostatin. In addition, our findings that myostatin levels and BMI and waist-hip ratio are positively associated suggest that skeletal muscle myostatin may be important in the development of both central obesity and IMAT. The higher myostatin levels in men than women likely drive its relationship with mid-thigh muscle area in the total group. In myostatin-knockout mice, muscle hypertrophy is augmented and in androgen receptor-knockout mice, skeletal muscle growth is inhibited.36 Thus, the higher myostatin expression observed in men could speculatively reduce the actions of androgens including the regulation of muscle mass, strength, and fibre-type distribution. Nevertheless, myostatin is also related to central obesity (subcutaneous abdominal fat) in men demonstrating the role of this myokine in adiposity.
Plasma or serum myostatin has been suggested to be implicated in the muscle-wasting in sarcopenia.37 Strengths of this study include the conduct of skeletal muscle biopsies and measurement of myostatin in the skeletal muscle, careful characterization of the study sample with fitness and body composition measures, sample size, and novelty of the question. Our study may not be generalizable to older adults with chronic disease or multi-morbidities. Study limitations are the inability to discern sex differences in sarcopenia and myostatin and the lack of measurement of grip strength or gait speed to use in other definitions of sarcopenia, and additional factors in skeletal muscle that could contribute to the sarcopenia of loss of muscle mass and function.
Conclusion
Skeletal muscle myostatin appears to be important in muscle atrophy, fat accumulation and sarcopenic obesity. Further work could address its implication in other aging cohorts of disability and chronic disease.
Acknowledgements
This research was supported funds from a Senior Research Career Scientist Award (ASR) from the United States Department of Veterans Affairs (VA) Rehabilitation R&D (Rehab RD) Service, VA Merit Award 1153486 (ASR) Clinical Service R&D, VA Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC), National Institutes of Health Grant P30-AG028747, and P30 DK072488.
Footnotes
Conflict of interest
The authors have no competing interests. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.38
References
- 1.Cauley JA. An overview of sarcopenic obesity. J Clin Densitom 2015;18:499–505. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 2008;18:388–395. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004. [DOI] [PubMed] [Google Scholar]
- 4.Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, and therapeutic perspectives. Curr Obes Rep 2019;8:458–471. [DOI] [PubMed] [Google Scholar]
- 5.Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—a narrative review. Clin Interv Aging 2015;10:1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature 1997;387:83–90. [DOI] [PubMed] [Google Scholar]
- 8.Scimeca M, Piccirilli E, Mastrangeli F, Rao C, Feola M, Orlandi A, et al. Bone morphogenetic proteins and myostatin pathways: key mediator of human sarcopenia. J Transl Med 2017;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TA, LeBrasseur NK. Myostatin and sarcopenia: opportunities and challenges—a mini-review. Gerontology 2014;60:289–293. [DOI] [PubMed] [Google Scholar]
- 10.Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones 2018;17:321–331. [DOI] [PubMed] [Google Scholar]
- 11.Katsanos CS, Mandarino LJ. Protein metabolism in human obesity:a shift in focus from whole-body to skeletal muscle. Obesity (Silver Spring) 2011;19:469–475. [DOI] [PubMed] [Google Scholar]
- 12.Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier JF, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through EIF2α activation. Aging Cell 2014;13:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair 2011;25:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 2002;83:1703–1707. [DOI] [PubMed] [Google Scholar]
- 15.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke 2011;42:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in new mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 17.Ryan AS, Li G, Blumenthal JB, Ortmeyer HK. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity 2013;21:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Li Y, Duan Y, Hu CA, Tang Y, Yin Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev 2017;33:73–82. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho LP, Basso-Vanelli RP, Di Thommazo-Luporini L, Mendes RG, Oliveira-Junior MC, Vieira RP, et al. Myostatin and adipokines: the role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults. Cytokine 2018;107:118–124. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol 2019;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, et al. Organization of the human myostatin gene and expression in healthy men and hiv-infected men with muscle wasting. Proc Natl Acad Sci U S A 1998;95:14938–14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favia M, Fitak R, Guerra L, Pierri CL, Faye B, Oulmouden A, et al. Beyond the big five: investigating myostatin structure, polymorphism and expression in camelus dromedarius. Front Genet 2019;10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartori R, Gregorevic P, Sandri M. Tgfbeta and bmp signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab 2014;25:464–471. [DOI] [PubMed] [Google Scholar]
- 24.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 2002;57:M772–M777. [DOI] [PubMed] [Google Scholar]
- 25.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc 2002;50:1802–1809. [DOI] [PubMed] [Google Scholar]
- 26.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord 2004;28:234–241. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci 2000;904:437–448. [DOI] [PubMed] [Google Scholar]
- 28.Guo A, Li K, Xiao Q. Sarcopenic obesity: myokines as potential diagnostic biomarkers and therapeutic targets? Exp Gerontol 2020;139:111022. [DOI] [PubMed] [Google Scholar]
- 29.Cretoiu SM, Zugravu CA. Nutritional considerations in preventing muscle atrophy. Adv Exp Med Biol 2018;1088:497–528. [DOI] [PubMed] [Google Scholar]
- 30.Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol 2017;122:1336–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cisterna B, Giagnacovo M, Costanzo M, Fattoretti P, Zancanaro C, Pellicciari C, et al. Adapted physical exercise enhances activation and differentiation potential of satellite cells in the skeletal muscle of old mice. J Anat 2016;228:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng B, Zhang F, Wen J, Ye S, Wang L, Yang Y, et al. The function of myostatin in the regulation of fat mass in mammals. Nutr Metab 2017;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MJ, Han DS, Yang JH, Yang YS, Ho HN, Yang WS. Myostatin and its association with abdominal obesity, androgen and follistatin levels in women with polycystic ovary syndrome. Hum Reprod 2012;27:2476–2483. [DOI] [PubMed] [Google Scholar]
- 34.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 2002;109:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 2009;4:e4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubois V, Laurent MR, Sinnesael M, Cielen N, Helsen C, Clinckemalie L, et al. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J 2014;28:2979–2994. [DOI] [PubMed] [Google Scholar]
- 37.Baczek J, Silkiewicz M, Wojszel Z. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 2020;12:2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


