Abstract
Factor XII (FXII) is the principal initiator of the plasma contact system and has proinflammatory and prothrombotic activities. This single‐center, first‐in‐human phase I study aimed to assess the safety and tolerability of single escalating doses of garadacimab, a monoclonal antibody that specifically inhibits activated FXII (FXIIa), in healthy male volunteers. Volunteers were randomized to eight cohorts, with intravenous (i.v.) doses of 0.1, 0.3, 1, 3, and 10 mg/kg and subcutaneous (s.c.) doses of 1, 3, and 10 mg/kg. Six volunteers in each cohort received garadacimab or placebo in a ratio of 2:1. Follow‐up for safety lasted 85 days after dosing. Blood samples were collected throughout for pharmacokinetic/pharmacodynamic analysis. Forty‐eight volunteers were enrolled: 32 received garadacimab and 16 received placebo. Most volunteers experienced at least one treatment‐emergent adverse event (TEAE), predominantly grade 1. No serious TEAEs, deaths, or TEAEs leading to discontinuation were reported. No volunteers tested positive for garadacimab antidrug antibodies. Garadacimab plasma concentrations increased in a dose‐dependent manner. Sustained inhibition of FXIIa‐mediated kallikrein activity beyond day 28 resulted from 3 and 10 mg/kg garadacimab (i.v. and s.c.). A dose‐dependent increase in activated partial thromboplastin time with no change in prothrombin time was demonstrated. Garadacimab (single‐dose i.v. and s.c.) was well‐tolerated in healthy volunteers. Dose‐dependent increases in plasma concentration and pharmacodynamic effects in relevant kinin and coagulation pathways were observed. These results support the clinical development of garadacimab, including in phase II studies in hereditary angioedema and coronavirus disease 2019 (COVID‐19).
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Plasma protein factor XII (FXII) is the principal initiator of the contact system. Activated FXII (FXIIa) leads to the production of the proinflammatory mediator bradykinin via the kallikrein–kinin system. The production of bradykinin results in increased vascular permeability, vasodilation, and chemotaxis. FXIIa is being investigated as a potential target in hereditary angioedema. Garadacimab is a fully human recombinant antibody that specifically inhibits FXIIa. Preclinical in vitro and in vivo studies of garadacimab showed it to inhibit FXIIa, produce anti‐inflammatory effects, and prevent the formation of bradykinin as well as effectively reduce edema and block proinflammatory cytokine production.
WHAT QUESTION DID THIS STUDY ADDRESS?
This phase I, single‐center study assessed the safety, tolerability, pharmacokinetics, and pharmacodynamics of escalating doses of garadacimab after a single intravenous (i.v.) infusion or subcutaneous (s.c.) injection in healthy volunteers.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The data in this study show that s.c. and i.v. administration of garadacimab in healthy male volunteers is well‐tolerated with no serious treatment‐emergent adverse events (TEAEs) reported during the study, and no discontinuations of garadacimab due to TEAEs. Moreover, the data show that garadacimab plasma concentrations and inhibition of FXII‐mediated kallikrein activity were increased in a dose‐dependent manner. A dose‐dependent increase in activated partial thromboplastin time with no change in prothrombin time and no associated bleeding was also observed.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study provides the first evidence of the safety and pharmacodynamic impact in FXIIa blockade in humans using a monoclonal antibody. These results support the investigation of garadacimab in a variety of disease states.
INTRODUCTION
Plasma protein factor XII (FXII) is the principal initiator of the contact system, a protease cascade involving FXII, high‐molecular‐weight kininogen, and plasma kallikrein. 1 , 2 Activated FXII (FXIIa) leads to the production of the proinflammatory mediator bradykinin via the kallikrein–kinin system. The production of bradykinin results in increased vascular permeability, vasodilation, and chemotaxis. 1 , 3 FXIIa also triggers fibrin formation through activation of the intrinsic coagulation pathway. 1 The intrinsic coagulation pathway is thought to support the formation of a stable thrombus but, unlike the tissue factor‐initiated extrinsic coagulation pathway, appears to have no critical function in fibrin formation during normal hemostasis at the site of injury. This finding is supported by the observation that patients who have a congenital deficiency of FXII do not exhibit a bleeding phenotype, despite having a prolonged activated partial thromboplastin time (aPTT). 4 Cleavage of FXIIa releases the 30‐kDa light chain containing the catalytic domain (βFXIIa), which can activate the complement pathway. 5
An increasing body of evidence implicates FXII in a range of inflammatory states. 6 FXIIa is being investigated as a potential target in hereditary angioedema (HAE), a rare genetic disorder with three known disease types. 1 , 6 , 7 HAE types I and II are associated with a deficiency in functional C1‐esterase inhibitor (C1‐INH). 6 The underlying pathophysiology of the third type, HAE with normal C1‐INH levels (nC1‐INH‐HAE), is poorly understood, but it has been reported that dysregulation of the plasma contact system leads to bradykinin‐mediated inflammation. 7 , 8 A subset of patients with nC1‐INH‐HAE has been identified with mutations in FXII (HAE‐FXII) and recent studies have shown that the mutant forms of FXII (T309>K/R) are more susceptible to contact activation 9 and possess a novel plasmin cleavage site that facilitates bradykinin formation. 10 Clinically, all types of HAE are characterized by edema of the extremities, face, and genitals; abdominal pain and swelling; and occasionally life‐threatening attacks of laryngeal edema. 9 , 11
Blockade of FXII may offer a novel therapeutic approach in several inflammatory disorders associated with the lungs. Bradykinin‐mediated inflammation and vascular leakage have been postulated as having a pathophysiologic role in coronavirus disease 2019 (COVID‐19) associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. 12 , 13 In addition, patients with COVID‐19 exhibit a hypercoagulable state. 14 Independent of kinin system activation, FXII has been shown to induce the production and release of inflammatory mediators in human lung slices. 15 There is increasing evidence for a role of FXII in idiopathic pulmonary fibrosis (IPF). Increased expression and local activation of FXII was observed in the lungs of patients with IPF. 16 In addition, targeted deletion of the FXII gene and blockade of FXII activity decreased fibrogenesis in a bleomycin model of lung injury in mice. 16 An antibody to FXII has been shown to block βFXIIa‐induced production of interleukin (IL)‐6 in lung fibroblasts. 17
Garadacimab is a fully human recombinant antibody that specifically inhibits FXIIa. Preclinical in vitro and in vivo studies of garadacimab showed it to inhibit FXIIa, produce anti‐inflammatory effects, and prevent the formation of bradykinin as well as effectively reduce edema and block proinflammatory cytokine production. 17 , 18
The aim of this phase I, single‐center study was to assess the safety, tolerability, pharmacokinetics (PKs), and pharmacodynamics (PDs) of escalating doses of garadacimab after a single intravenous (i.v.) infusion or subcutaneous (s.c.) injection in healthy volunteers.
Methods
Study design
A phase I, single‐center, first‐in‐human, randomized, double‐blind, placebo‐controlled study of garadacimab in healthy male adult volunteers was carried out between October 28, 2016, and September 22, 2017, in Adelaide, Australia (ACTRN 12616001438448). The study design is outlined in Figure S1. Planned enrollment was six healthy male volunteers per each of the eight cohorts, with a total study population of 48 volunteers.
Garadacimab i.v. doses were 0.1, 0.3, 1, 3, and 10 mg/kg administered via infusion over 60 minutes. Garadacimab s.c. doses were 1, 3, and 10 mg/kg administered via s.c. injection into the abdomen. The doses in this study were selected based on data obtained from in vitro nonclinical pharmacologic and toxicology studies.
A total of six volunteers received garadacimab or placebo in a ratio of 2:1 in each cohort. Sentinel dosing was implemented for each i.v. cohort and the first (1 mg/kg) s.c. cohort, and enrollment was staggered between cohorts (Figure S1). The first two volunteers in each cohort undergoing sentinel dosing were randomized 1:1 to receive garadacimab or placebo (formulation buffer). If there were no safety concerns 48 h post‐administration, the remaining four volunteers were randomized in a 3:1 ratio to receive garadacimab or placebo. Volunteers from each cohort were monitored for 14 days after drug administration; safety, PK, and select PD data were reviewed by the safety review committee before the next cohort was administered the next dose.
The study consisted of a screening period, a study drug administration period, and safety follow‐up, which lasted 85 days after dosing. The protocol and amendment were approved by the independent Bellberry Human Research Ethics Committee, and the study was conducted in accordance with standards of Good Clinical Practice (as defined by the International Council for Harmonisation), ethical principles that have their origin in the Declaration of Helsinki, and all applicable national and local regulations. Written informed consent was obtained and documented for all volunteers before study participation.
Eligibility criteria
To be eligible for this study, volunteers had to be capable of providing written informed consent, willing and able to adhere to all protocol requirements, healthy, male, aged between 18 and 45 years inclusive, and with a body mass index of 18.0 to less than 30.0 kg/m2. Volunteers with a clinically significant medical condition, disorder, or disease, including clinically significant arterial or venous thrombosis, bleeding disorder, or any abnormal coagulation test result, were excluded from the study. Full inclusion and exclusion criteria can be found in the Supplementary Table S1.
Study population
The full analysis set (FAS) comprised all volunteers who provided informed consent and who were eligible for inclusion in the study after screening. The intention‐to‐treat (ITT) population comprised all volunteers in the FAS who were randomized. Overall analyses included i.v. and s.c. administration. The safety analysis set comprised all volunteers in the ITT population who received one dose of garadacimab. Safety analyses were overall and by route of administration and dose. The PK analysis set comprised all volunteers in the safety analysis set who received one dose of garadacimab and for whom at least one concentration value was reported. The PD analysis set comprised all volunteers in the safety analysis set for whom results were obtained for at least one of the exploratory biomarkers of aPTT or FXIIa‐mediated kallikrein activity. PK/PD analyses were by route of administration.
Assessments
Safety was assessed by the frequency, severity, and relatedness of treatment‐emergent adverse events (TEAEs) and the evaluation of changes in clinical laboratory tests, cardiac monitoring, vital signs, physical examination, and immunogenicity. Laboratory tests included hematology, biochemistry, coagulation, complement activity, and urinalysis. Cytokine activity was assessed, including interferon‐γ, tumor necrosis factor‐α, IL‐6, IL‐8, IL‐13, IL1β, IL‐2, IL‐4, IL‐10, and IL‐12p70. Immunogenicity was assessed as the proportion of volunteers with antidrug antibodies (ADAs) to garadacimab at the end‐of‐study visit.
A validated clinical assay for the detection, confirmation, and titration of ADAs was used. The method utilizes a bridging format where acid‐dissociated samples are incubated in the presence of both biotinylated and sulfo‐tagged garadacimab, which form complexes with any ADAs present. An anti‐garadacimab antibody (4D3.11) isolated from a human antibody phage library at CSL Limited was used as a positive control. The complexes are captured onto a mesoscale discovery streptavidin plate, enabling an electrochemiluminescent signal to develop, which is proportional to the amount of ADA bound. The assay was validated according to guidance from the US Food and Drug Administration, European Medicines Agency, and Shankar et al. 2008. 19 In the validation, sensitivity was determined to be 20 ng/ml with drug tolerance of 75 µg/ml for the high positive control (750 ng/ml), 50 µg/ml for medium (250 ng/ml), and low (100 ng/ml) positive controls. General acceptance criteria across performance parameters were set at less than or equal to 20% coefficient of variation. The validation exercise used a data set generated from the assay of 50 drug‐naïve healthy donors to determine a screening assay cut point signal and likewise 50 donors were assayed with spiking of 100 µg/ml of garadacimab to determine the confirmatory assay cut point.
Blood samples for PK evaluation were collected during in‐house days (at predose and 0.5, 1, 6, 12, 24, and 48 h post‐i.v. administration, and at predose and 1, 6, 12, 24, 32, and 48 h post‐s.c. administration) and up to 85 ± 5 days after the end of garadacimab administration. Garadacimab plasma concentrations were measured using a validated, clinical enzyme‐linked immunosorbent assay. Garadacimab present in the samples was captured by a microtiter plate coated with an anti‐garadacimab antibody (4D3.7) isolated from a human antibody phage library at CSL Limited. Captured garadacimab was detected with a mouse monoclonal anti‐human lambda chain antibody conjugated with horseradish peroxidase (Abcam, Cambridge, MA, USA), which, when exposed to an enzymatic substrate, results in a color change directly proportional to the amount of garadacimab in the sample. The assay uses an 11‐point (plus blank) calibration curve of 200–0.56 ng/ml. During validation of this assay, a lower limit of detection of 100 ng/ml and a minimal required sample dilution of 1:100 were defined. Validation parameters, such as precision (intra‐assay, interassay, and interoperator), accuracy, dilutional linearity, selectivity, and robustness were defined within acceptance criteria limits of 20% for coefficient of variation and relative error (30% relative error for dilutional linearity). These criteria are applied to the validity of clinical test data.
PK assessments included the maximum plasma concentration (Cmax), area under the plasma concentration–time curve (AUC) from the time of dosing up to collection time t (AUC0–t), AUC extrapolated to infinity (AUC0–inf), time to maximum concentration in plasma, terminal elimination half‐life (t 1/2), total systemic clearance (CL for i.v. infusion and CL/F for s.c. injection), volume of distribution during the elimination phase (V z for i.v. infusion and V z/F for s.c. injection), and drug bioavailability after s.c. dosing. The acceptable percentage AUC that was extrapolated for the AUC0‐inf calculation was less than 20%, as per an internal standard operating procedure. Dose proportionality was assessed separately for i.v. and s.c. doses for the PK parameters Cmax, AUC0–inf, and AUC0–t. Dose proportionality was explored with the Hummel power model analysis. 20
Exploratory biomarkers of the PD effects of garadacimab were assessed, including aPTT and FXIIa‐mediated kallikrein activity. The aPTT was measured by standard methodology using the Siemens system with Dade Actin reagent as activator of intrinsic coagulation. To measure FXIIa‐mediated kallikrein activity, 40 µl of sodium citrate plasma was mixed with 10 µl of a reaction buffer containing 10 mM HEPES, 137 mM NaCl, 4 mM KCl, 11 mM d‐glucose, pH 7.4, and 40 µl of the same buffer containing dextran sulphate MW40,000 to a final assay concentration of 2.5 µg/ml in 96‐well microtiter plates. The plate was placed in a 37°C warmed reader and read for 3 min before addition of 10 µl chromogenic substrate H‐D‐Pro‐Phe‐Arg‐pNA•2HCl (S‐2302) to 0.6 mM. Amidolytic activity, which reflects the kallikrein activity generated, was monitored in the sample by absorbance at 405 nm over 30 min and the maximal rate of cleavage derived from the kinetic curves.
Statistical analysis
Dose proportionality was assessed for Cmax, AUC0–inf, and AUC0–t for the garadacimab i.v. and s.c. doses separately. Exploratory dose proportionality was analyzed using a power model by the Hummel et al. method. 20 After ln‐transformation of the power model, the slope and its 90% confidence interval (CI) were estimated with a linear mixed‐effect model in SAS (PROC MIXED). When the 90% CI for the slope is within the predefined critical interval, based on the ratio between the highest and lowest dose levels of garadacimab, dose proportionality can be declared. The critical interval to declare dose proportionality was 0.85–1.15 for the 90% CI of the slope estimate for i.v. infusion PK parameters and 0.7–1.3 for the 90% CI of the slope estimate for s.c. administration.
The bioavailability of s.c. garadacimab relative to that of i.v. garadacimab infusion was assessed. Comparisons were completed using an analysis of variance model and log transformed PK parameters AUC0–inf/total dose. The geometric mean ratio of dose normalized AUC0–inf of garadacimab administered s.c. to i.v. was estimated by exponentiating the difference in least‐squares mean, resulting in absolute bioavailability with corresponding 90% CI. Eight subjects were included in the analysis of each dose and 24 subjects were included in the analysis of all doses. SAS version 9.3 was used for statistical analysis. All statistical tests were exploratory and nonconfirmatory. No formal hypothesis was tested in this study, and all results were descriptive. The sample size was considered appropriate for assessing safety.
RESULTS
Subjects
Forty‐eight healthy male volunteers were enrolled: 32 volunteers received garadacimab and 16 received placebo (Figure S2). All 48 volunteers completed the study as planned and none were withdrawn. No volunteers were excluded from any of the analyses. All 48 subjects who received garadacimab or placebo were included in the FAS, ITT, safety, and PD analyses. All 32 volunteers who received garadacimab were included in the PK analysis. There were no major differences in demographics and baseline characteristics across the different cohorts (Table S2). Volunteers were aged between 19 and 44 years with a mean body mass index of 24 kg/m2 and were predominantly White.
Safety
At least one TEAE was experienced by the majority of volunteers, with a higher proportion in those who received garadacimab compared with placebo. The majority of TEAEs were grade 1 and no serious TEAEs, deaths, TEAEs leading to discontinuation, thromboembolic, bleeding, or anaphylaxis events were reported (Table 1). The frequency and severity of TEAEs were not dose dependent. One grade 3 TEAE of bipolar disorder was reported for one volunteer in the s.c. 10 mg/kg cohort. The volunteer failed to disclose his medical history of bipolar disorder at screening and the TEAE was not thought to be study‐drug‐related.
TABLE 1.
Summary of treatment‐emergent adverse events in intravenous and subcutaneous cohorts (safety analysis set)
| TEAE, n (%) |
I.V. garadacimab (n = 20) |
I.V. placebo (n = 10) |
S.C. garadacimab (n = 12) |
S.C. placebo (n = 6) |
|---|---|---|---|---|
| Any TEAE | 18 (90.0) | 8 (80.0) | 12 (100.0) | 5 (83.3) |
| Grade 1 TEAE | 18 (90.0) | 8 (80.0) | 12 (100.0) | 5 (83.3) |
| Grade 2 TEAE | 4 (20.0) | 4 (40.0) | 1 (8.3) | 0 |
| Grade 3 TEAE | 0 | 0 | 1 (8.3) | 0 |
| Any study‐treatment‐related TEAE | 8 (40.0) | 5 (50.0) | 11 (91.7) | 2 (33.3) |
| Serious TEAE | 0 | 0 | 0 | 0 |
Abbreviation: TEAE, treatment‐emergent adverse event.
The most commonly reported TEAEs according to dose and route of administration are presented in Table 2. Headache was the most common TEAE in the garadacimab i.v. cohorts (30% of volunteers; Table 2). Injection‐site reactions were the most common TEAEs in the s.c. cohorts and contributed to the higher report of TEAEs exhibited by the s.c. garadacimab cohorts compared with placebo cohorts. Injection‐site reactions included injection‐site erythema (75% and 33.3% of volunteers administered garadacimab and placebo, respectively), pain (25% and 0% of volunteers administered garadacimab and placebo, respectively), and pruritus (25% and 16.7% of volunteers administered garadacimab and placebo, respectively; Table 2). All injection‐site reactions were grade 1 and resolved without the need for treatment. There were no clinically relevant differences across cohorts for blood pressure, heart rate, respiratory rate, and body temperature.
TABLE 2.
Most common treatment‐emergent adverse events experienced by volunteers in the intravenous and subcutaneous cohorts (safety analysis set).
| Preferred term | Garadacimab |
Placebo a n (%) |
|||||
|---|---|---|---|---|---|---|---|
|
0.1 mg/kg (n = 4) n |
0.3 mg/kg (n = 4) n |
1 mg/kg (n = 4) n |
3 mg/kg (n = 4) n |
10 mg/kg (n = 4) n |
All doses a n (%) |
||
| Intravenous | |||||||
| Headache | 0 | 2 | 1 | 0 | 3 | 6 (30.0) | 3 (30.0) |
| Dermatitis contact | 1 | 1 | 0 | 0 | 1 | 3 (15.0) | 1 (10.0) |
| Upper respiratory tract infection | 2 | 0 | 0 | 1 | 0 | 3 (15.0) | 3 (30.0) |
| Cough | 0 | 0 | 0 | 1 | 1 | 2 (10.0) | 0 |
| Hepatic enzyme increased | 2 | 0 | 0 | 0 | 0 | 2 (10.0) | 0 |
| Infusion‐site bruising | 0 | 0 | 0 | 1 | 1 | 2 (10.0) | 0 |
| Dry lip | 0 | 0 | 0 | 1 | 1 | 2 (10.0) | 0 |
| Medical device‐site dermatitis | 0 | 0 | 0 | 0 | 2 | 2 (10.0) | 1 (10.0) |
| Medical device‐site reaction | 1 | 0 | 0 | 1 | 0 | 2 (10.0) | 0 |
| Rhinitis | 0 | 0 | 1 | 0 | 1 | 2 (10.0) | 0 |
| Toothache | 0 | 0 | 0 | 1 | 1 | 2 (10.0) | 0 |
| Vessel puncture‐site bruise | 0 | 0 | 0 | 2 | 0 | 2 (10.0) | 0 |
| Subcutaneous | |||||||
| Injection‐site erythema | – | – | 3 | 4 | 2 | 9 (75.0) | 2 (33.3) |
| Injection‐site pain | – | – | 2 | 1 | 0 | 3 (25.0) | 0 |
| Injection‐site pruritus | – | – | 1 | 0 | 2 | 3 (25.0) | 1 (16.7) |
| Fatigue | – | – | 2 | 0 | 0 | 2 (16.7) | 1 (16.7) |
| Vessel puncture‐site bruise | – | – | 0 | 0 | 2 | 2 (16.7) | 1 (16.7) |
Treatment‐emergent adverse events reported in greater than or equal to 2 volunteers in the combined garadacimab intravenous and subcutaneous cohorts.
The “all doses” data refers to 20 volunteers in the intravenous cohort and 12 in the subcutaneous cohort. Similarly, the placebo data consists of 10 volunteers in the intravenous cohort and six in the subcutaneous cohort.
Laboratory test results
There were no clinically relevant findings in hematology, biochemistry, urinalysis, coagulation, or complement activity results. Although abnormal laboratory values were observed in individual volunteers, no safety concerns were identified. In two subjects who received 0.1 mg/kg i.v. garadacimab, increased hepatic enzyme was reported as a TEAE. In the first subject, abnormal alanine aminotransferase (ALT) values (1–2 × upper limit of normal [ULN]) were reported from day 8 to day 22 post dosing. During this time, other hepatic biomarkers, such as alkaline phosphatase, aspartate transaminase (AST), and bilirubin, were within the normal ranges. This event was grade 1 in severity and assessed as related to garadacimab. In the second subject, abnormal ALT (from day 36 to day 57) and AST (on day 36) values, both 1–2 × ULN, were reported post dosing. However, this subject also had an abnormal ALT measurement at screening (~ 1 × ULN). On follow‐up, the subject had reported excess alcohol consumption and the increase in ALT levels was not considered to be associated with any other clinical symptoms. This event was grade 1 in severity and assessed as not related to garadacimab. Both events resolved. No volunteers tested positive for ADAs to garadacimab during the study and no dose‐dependent elevations in cytokine plasma concentrations were observed.
Pharmacokinetics
Garadacimab PK parameters are summarized in Table 3. Garadacimab plasma concentration increased in a dose‐dependent manner when administered as an i.v. infusion or s.c. injection (Figure 1a and b). Maximum plasma concentration of garadacimab was achieved at the end of i.v. infusion (60 min), and ~ 7 days after s.c. injection. Mean garadacimab half‐life ranged between 14 and 20 days across the i.v. doses and between 18 and 20 days across the s.c. doses. The estimated plasma bioavailability of garadacimab s.c. versus i.v. administration was 63.3% for the 1 mg/kg dose, 54.3% for the 3 mg/kg dose, and 42.4% for the 10 mg/kg dose. Overall, absolute bioavailability of garadacimab after s.c. injection was 49.7%. Based on the Hummel power analysis for the i.v. cohort, the slope estimates for Cmax, AUC0–inf, and AUC0–t were 1.076, 0.981, and 1.031, respectively, and the 90% CIs were fully contained within the predefined critical interval of 0.85–1.15, inferring dose proportionality for the i.v. cohort based on available data (Table S3). For the s.c. cohort, slope estimates for Cmax, AUC0–inf, and AUC0–t were 0.800, 0.866, and 0.868, respectively. The 90% CIs were fully contained within the predefined critical interval of 0.7–1.3 for the AUCs, inferring dose proportionality for overall systemic exposure. However, the lower limit 90% CI for Cmax was slightly below the predefined critical interval, rendering dose proportionality inconclusive for peak systemic exposure.
TABLE 3.
Summary of garadacimab pharmacokinetic plasma parameters
| Parameters | I.V. garadacimab | S.C. garadacimab | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 mg/kg (n = 4) | 0.3 mg/kg (n = 4) | 1 mg/kg (n = 4) | 3 mg/kg (n = 4) | 10 mg/kg (n = 4) | 1 mg/kg (n = 4) | 3 mg/kg (n = 4) | 10 mg/kg (n = 4) | |
| AUC0–inf, h*µg/ml | ||||||||
| Mean (SD) | 967.1 (251.8) | 4348.4 (1263.3) | 9764.7 (1936.0) | 32,094.9 (7435.5) | 101,777.0 (24,163.9) | 6468.2 (2610.0) | 18,110.3 (7149.8) | 42,935.8 (7905.6) |
| Median (range) | 974.1 (711.8–1215.3) | 4336.4 (3145.6–5574.5) | 9774.6 (7390.2–12,119.5) | 31,613.7 (24,676.1–4476.3) | 95,751.4 (79,801.1–135,804.1) | 6023.0 (3885.1–9941.7) | 17,178.8 (11,147.3–26,936.4) | 44,660.8 (31,959.2–50,462.4) |
| AUC0–t, h*µg/ml | ||||||||
| Mean (SD) | 761.7 (217.1) | 4062.9 (1027.5) | 9483.9 (1861.6) | 31,278.8 (7166.9) | 100,100.6 (24,598.4) | 6006.8 (2245.8) | 17,133.3 (6301.9) | 40,701.4 (8776.2) |
| Median (range) | 717.145 (570.8–1041.6) | 4121.6 (3032.5–4975.9) | 9437.7 (7252.3–11,807.7) | 30,838.1 (24,174.8–39,264.3) | 93,015.5 (78,909.8–135,461.8) | 5652.0 (3799.4–8923.9) | 16,544.3 (10,871.9–24,572.5) | 42,412.3 (28,746.6–49,234.4) |
| CL, ml/h | ||||||||
| Mean (SD) | 9.8 (3.2) | 5.6 (1.0) | 7.0 (1.5) | 7.1 (1.8) | 8.6 (2.0) | – | – | – |
| Median (range) | 8.0 (8.0–13.5) | 5.8 (4.5–6.5) | 6.4 (6.0–9.1) | 7.2 (5.2–8.6) | 8.6 (6.5–10.9) | – | – | – |
| CL/F, ml/h | ||||||||
| Mean (SD) | – | – | – | – | – | 13.3 (3.4) | 14.6 (5.0) | 18.5 (4.3) |
| Median (range) | – | – | – | – | – | 12.6 (10.1–18.0) | 14.3 (8.9–20.6) | 17.6 (14.3–24.4) |
| Cmax, µg/ml | ||||||||
| Mean (SD) | 2.7 (0.4) | 11.0 (1.7) | 32.5 (6.3) | 109.6 (27.1) | 410.1 (17.5) | 9.4 (2.2) | 24.7 (8.0) | 59.0 (20.6) |
| Median (range) | 2.6 (2.4–3.4) | 10.5 (9.6–13.5) | 31.6 (26.6–40.3) | 100.6 (88.3–149.1) | 414.4 (386.3–425.4) | 9.7 (6.8–11.4) | 25.3 (16.0–32.4) | 62.6 (33.7–77.1) |
| t 1/2, h | ||||||||
| Mean (SD) | 353.0 (64.2) | 489.7 (206.0) | 408.2 (47.5) | 393.4 (12.2) | 344.0 (116.4) | 439.7 (101.6) | 437.2 (94.7) | 470.2 (104.4) |
| Median (range) | 337.8 (297.0–439.4) | 526.7 (209.0–696.2) | 407.1 (352.6–466.1) | 390.0 (383.2–410.5) | 332.0 (214.9–497.1) | 422.6 (347.2–566.6) | 412.9 (351.0–572.1) | 468.9 (353.0–589.7) |
| Median (IQR) Tmax, h | 3.5 (8.0) | 1.0 (5.0) | 1.0 (2.5) | 1.0 (0.0) | 1.0 (2.5) | 168.1 (38.4) | 133.2 (95.1) | 168.8 (23.9) |
| V z, L | ||||||||
| Mean (SD) | 4.8 (1.1) | 3.8 (1.2) | 4.0 (0.4) | 4.0 (1.1) | 4.2 (1.3) | – | – | – |
| Median (range) | 5.1 (3.6–5.8) | 4.3 (2.0–4.5) | 3.9 (3.7–4.6) | 4.1 (2.9–5.1) | 4.6 (2.3–5.4) | – | – | – |
| V z/F, L | ||||||||
| Mean (SD) | – | – | – | – | – | 8.1 (1.2) | 8.7 (1.6) | 12.9 (5.7) |
| Median (range) | – | – | – | – | – | 8.1 (6.7–9.6) | 8.6 (7.4–10.4) | 11.8 (7.3–20.8) |
Abbreviations: AUC0–inf, area under the plasma concentration–time curve extrapolated to infinity; AUC0–t, area under the plasma concentration–time curve from the time of dosing up to collection time t; CL or CL/F, total systemic clearance; Cmax, maximum plasma concentration; IQR, interquartile range; t 1/2, terminal elimination half‐life; Tmax, time to reach maximum concentration in plasma; V z or V z/F, volume of distribution during the elimination phase.
FIGURE 1.
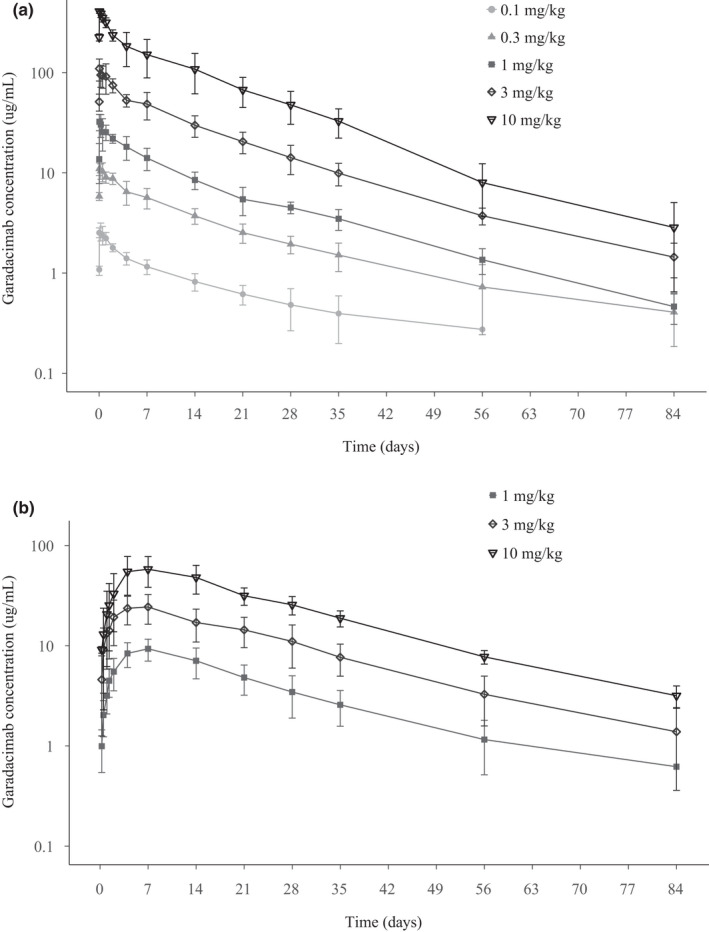
PK profiles in volunteers receiving i.v. (a) and s.c. (b) garadacimab (PK analysis set). Data are presented as a log linear scale. PK, pharmacokinetic
FXIIa‐mediated kallikrein activity and aPTT
A concentration‐dependent inhibition of FXIIa‐mediated kallikrein activity was observed after single increasing doses of i.v. and s.c. garadacimab (Figure 2a,b and Figure S3). Near‐complete inhibition was observed after the 3 mg/kg dose, and complete inhibition was observed after the 10 mg/kg dose; at these doses, inhibition was sustained beyond day 28. A dose‐dependent increase in aPTT was observed after both i.v. and s.c. administration of garadacimab and no change in prothrombin time was observed in any volunteers at any dose (Figure 3). The aPTT prolongation outside the normal range was observed at FXIIa‐mediated kallikrein activity inhibition of greater than or equal to 85% (Figure 4). The time at which maximum inhibition of FXIIa‐mediated kallikrein activity and aPTT prolongation was observed for all doses coincides with the time at which maximal PK concentration was achieved. The inhibition of FXIIa‐mediated kallikrein activity and aPTT prolongation starts returning to baseline as the concentration of the drug decreases. A quantitative analysis describing this relationship between PK and PD will be published separately. The aPTT prolongation was not associated with any bleeding outcomes during the study.
FIGURE 2.
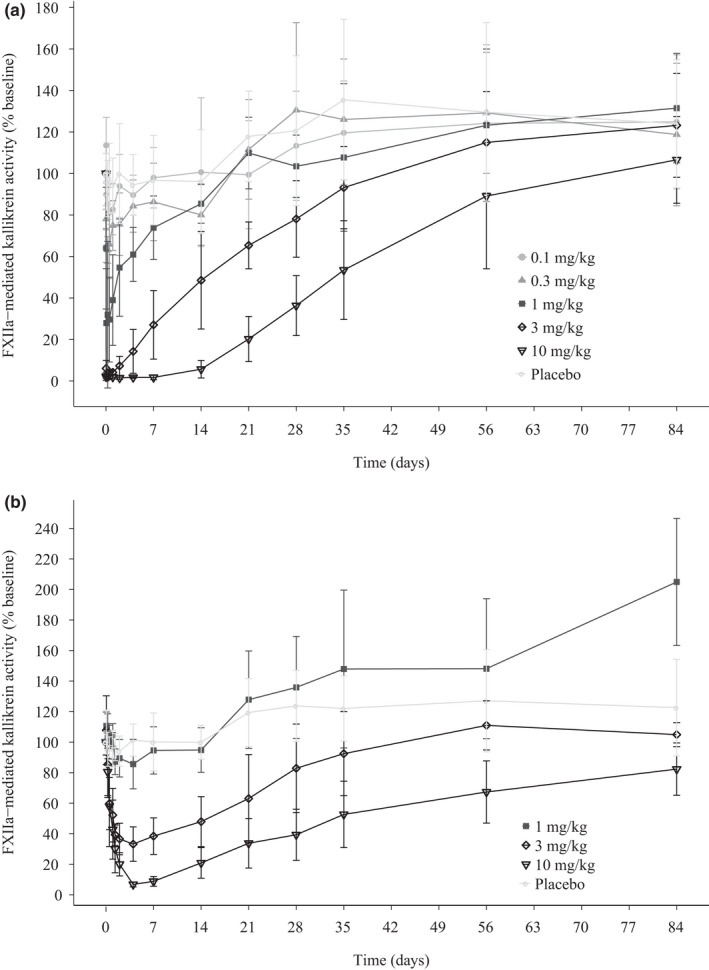
FXIIa‐mediated kallikrein activity inhibition in volunteers receiving i.v. (a) and s.c. (b) garadacimab at a range of doses (PD analysis set). FXII, Factor XII; PD, pharmacodynamic
FIGURE 3.
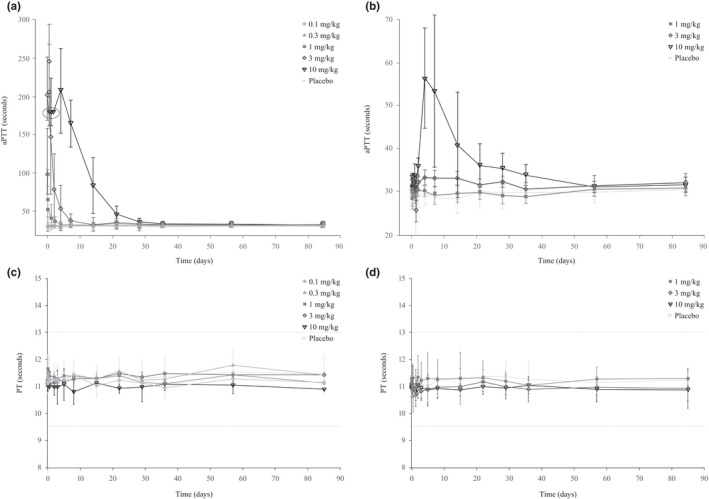
The aPTT and PT in volunteers receiving i.v. (a, c) and s.c. (b, d) garadacimab. Values circled for 10 mg/kg reported as greater than 180 s. Dotted lines on c and d represent the upper and lower limits of the reference range. aPTT, activated partial thromboplastin time; PT, prothrombin time
FIGURE 4.
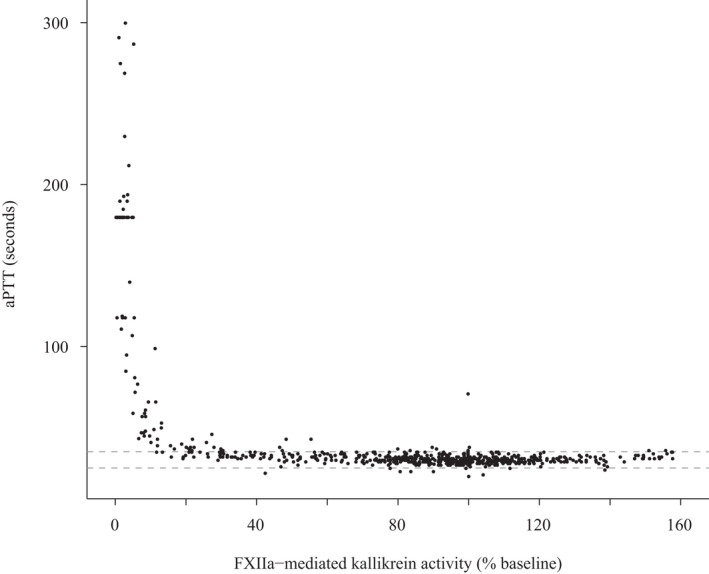
The aPTT by FXIIa‐mediated kallikrein activity in volunteers receiving garadacimab. Dotted line represents the normal biological range of aPTT. aPTT, activated partial thromboplastin time; FXII, Factor XII
DISCUSSION
This study shows that s.c. and i.v. administration of garadacimab in healthy male volunteers is well‐tolerated. No serious TEAEs were reported during the administration period or 85‐day safety follow‐up, and the majority of recorded TEAEs were mild in severity. No volunteers discontinued the study drug due to TEAEs. An increase in injection‐site reactions was apparent in the s.c. garadacimab cohorts compared with placebo. However, these events were not dose dependent, were of mild severity, and resolved without the need for treatment. Immunogenicity to garadacimab was not identified in this study as no volunteers tested positive for ADAs. No significant elevations in cytokines were observed.
Plasma concentrations of garadacimab increased in a dose‐dependent manner for all escalating i.v. and s.c. doses. The observed dose proportionality for peak systemic exposure was inconclusive for the s.c. doses. This may have arisen because of the small sample size or dose‐limiting absorption. Multiple hypotheses have been put forward to explain dose‐limiting absorption for monoclonal antibodies, 21 but further evaluation of each needs to be performed. In addition, the i.v. and s.c. doses were administered to different individuals, which may have also contributed to this inconclusive observation. The slow absorption after s.c. administration (Cmax ~ 7 days) along with the long mean terminal t 1/2 of garadacimab after s.c. injection (18 days) is typical for monoclonal antibodies. 22 The mean terminal t 1/2 observed in this study may have implications for future dosing schedules and may suggest the potential for a monthly dosing regimen pending further indication‐specific clinical studies. Decreasing the burden of daily or weekly injections may have a beneficial effect on patient quality of life in HAE.
Two PD biomarkers, FXIIa‐mediated kallikrein activity and aPTT were assessed in this study to explore the potential mechanism of action of garadacimab. Assessing FXIIa‐mediated kallikrein activity provides an insight into the effect of garadacimab in inhibiting the downstream generation of kallikrein that directly leads to kininogen cleavage in plasma. Measuring aPTT indicates the effect of garadacimab in inhibiting downstream thrombin generation. In addition, the PD biomarkers provide data to inform the selection of doses for later phase studies in the development program.
FXIIa‐mediated kallikrein activity was inhibited by garadacimab (i.v. and s.c.) in a dose‐dependent manner, with complete inhibition observed at 10 mg/kg. This effective target inhibition suggests that garadacimab has the potential to block bradykinin production and thus prevent attacks of edema in HAE. Antibody‐mediated FXIIa inhibition has been previously shown to inhibit edema in a mouse model to a greater extent than that reported for the HAE drugs icatibant and ecallantide. 18 It was suggested that this resulted from serum clearance being slower for a monoclonal antibody than the other drugs. 18 In our study, garadacimab inhibition of FXIIa‐mediated kallikrein activity at 3 mg/kg and 10 mg/kg was sustained beyond day 28.
A dose‐dependent increase in aPTT with no change in prothrombin time was observed in this study after i.v. and s.c. administration of garadacimab; however, aPTT prolongation was not associated with any bleeding outcomes. These results are consistent with preclinical studies in which an anti‐FXIIa antibody effectively inhibited FXIIa‐mediated coagulation and thrombus formation in mouse and rabbit thrombosis models without impairing hemostatic capacity and increasing wound bleeding. 23 Results also agree with previously published in vitro data using FXII‐deficient human plasma in aPTT and prothrombin time assays. 24 Patients who have congenital deficiency of FXII do not exhibit a bleeding phenotype and can undergo surgery without bleeding complications, despite having a prolonged aPTT. 4 , 25 Blockade of FXIIa using garadacimab does not appear to confer an increased risk of bleeding events.
Limitations of this study include the small number of volunteers, single dosing administration of garadacimab, and the challenge in extrapolating these results to patients with disease states of interest; however, these limitations are inherent to phase I studies in healthy volunteers. An additional limitation is that only male subjects were recruited, as reproductive toxicology studies in animals at the time of the study initiation were limited. FXII levels are similar in men and women and we expect the results of this study to be applicable to women. 26 Phase II and III studies with garadacimab will include both men and women.
In conclusion, i.v. and s.c. administration of single‐dose garadacimab was well‐tolerated by healthy male volunteers, with no serious TEAEs reported at any of the escalating doses. The PKs reported in this study were dose dependent and the t1/2 suggests the potential for a monthly dosing regimen. The effects reported on the exploratory biomarkers, FXIIa‐mediated kallikrein activity and aPTT, are reflective of preclinical data and support the investigation of garadacimab in disease states, such as HAE, COVID‐19 associated with SARS‐CoV‐2 infection, and IPF. Additional ongoing studies with garadacimab include a phase II study of patients with HAE and a phase II study of patients with COVID‐19.
CONFLICTS OF INTEREST
A.M., S.M., D.P., and C.P. are employees and shareholders of CSL Limited. A.R. is an employee of CSL Limited. H.F. is an employee of CSL Behring Innovation GmbH and shareholder of CSL Limited.
AUTHOR CONTRIBUTIONS
A.M., A.R., S.M., H.F., C.P., and D.P. wrote the manuscript. A.M., A.R., S.M., H.F., C.P., and D.P. designed the research. A.M., A.R., S.M., H.F., C.P., and D.P. performed the research. A.M., A.R., S.M., H.F., C.P., and D.P. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the healthy volunteers for participating in this study. Writing support was provided by Jenny Feehan, BSc, of OPEN Health (London, UK) and was funded by CSL Behring. Additional support in the preparation of figures was provided by Fiona Glassman of CSL Behring.
McKenzie A, Roberts A, Malandkar S, Feuersenger H, Panousis C, Pawaskar D. A phase I, first‐in‐human, randomized dose‐escalation study of anti‐activated factor XII monoclonal antibody garadacimab. Clin Transl Sci. 2022;15:626–637. doi: 10.1111/cts.13180
Clinical trial registration: This trial was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR, https://www.anzctr.org.au/) with the registration number ACTRN 12616001438448.
Funding information
This study was funded by CSL Limited (CSL), 45 Poplar Road, Parkville, Victoria 3052, Australia
REFERENCES
- 1. Kenne E, Nickel KF, Long AT, et al. Factor XII: a novel target for safe prevention of thrombosis and inflammation. J Intern Med. 2015;278:571‐585. [DOI] [PubMed] [Google Scholar]
- 2. Renné T, Schmaier AH, Nickel KF, Blombäck M, Maas C. In vivo roles of factor XII. Blood. 2012;120:4296‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio‐oedema. Lancet. 1998;351(9117):1693‐1697. [DOI] [PubMed] [Google Scholar]
- 4. Lämmle B, Wuillemin WA, Huber I, et al. Thromboembolism and bleeding tendency in congenital factor XII deficiency – a study on 74 subjects from 14 Swiss families. Am J Clin Pathol. 1993;100:94‐98. [PubMed] [Google Scholar]
- 5. Ivanov I, Matafonov A, Gailani D. Single‐chain factor XII – a new form of activated factor XII. Curr Opin Hematol. 2017;24:411‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Björkqvist J, Sala‐Cunill A, Renné T. Hereditary angioedema: a bradykinin‐mediated swelling disorder. Thromb Haemost. 2013;109:368‐374. [DOI] [PubMed] [Google Scholar]
- 7. Cichon S, Martin L, Hennies HC, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79:1098‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382:1136‐1148. [DOI] [PubMed] [Google Scholar]
- 9. Björkqvist J, de Maat S, Lewandrowski U, et al. Defective glycosylation of coagulation factor XII underlies hereditary angioedema type III. J Clin Invest. 2015;125:3132‐3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Maat S, Björkqvist J, Suffritti C, et al. Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J Allergy Clin Immunol. 2016;138:1414‐1423. [DOI] [PubMed] [Google Scholar]
- 11. Bork K. Pasteurized C1 inhibitor concentrate in hereditary angioedema: pharmacology, safety, efficacy and future directions. Expert Rev Clin Immunol. 2008;4:13‐20. [DOI] [PubMed] [Google Scholar]
- 12. Roche JA, Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID‐19 respiratory complications. FASEB J. 2020;34:7265‐7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID‐19: mechanisms underlying disease severity and progression. Physiology. 2020;35:288‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shatzel JJ, DeLoughery EP, Lorentz CU, et al. The contact activation system as a potential therapeutic target in patients with COVID‐19. Res Pract Thromb Haemost. 2020;4:500‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hess R, Wujak L, Hesse C, et al. Coagulation factor XII regulates inflammatory responses in human lungs. Thromb Haemost. 2017;117:1896‐1907. [DOI] [PubMed] [Google Scholar]
- 16. Wygrecka M, Jablonska E, Henneke I, et al. Coagulation factor XII mediates fibrotic response to lung injury. Pneumologie. 2015;69:P05. [Google Scholar]
- 17. Wong M, Jaffar J, McMillan L, et al. CSL312, a novel anti‐FXII antibody, blocks FXII‐induced IL‐6 production from primary non‐diseased and idiopathic pulmonary fibrosis fibroblasts. Am J Respir Crit Care Med. 2020;201:A6363. [Google Scholar]
- 18. Cao H, Biondo M, Lioe H, et al. Antibody‐mediated inhibition of FXIIa blocks downstream bradykinin generation. J Allergy Clin Immunol. 2018;142:1355‐1358. [DOI] [PubMed] [Google Scholar]
- 19. Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267‐1281. [DOI] [PubMed] [Google Scholar]
- 20. Hummel J, McKendrick S, Brindley C, French R. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat. 2009;8:38‐49. [DOI] [PubMed] [Google Scholar]
- 21. Sánchez‐Félix M, Burke M, Chen HH, Patterson C, Mittal S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: open innovation challenge. Adv Drug Deliv Rev. 2020;167:66‐77. [DOI] [PubMed] [Google Scholar]
- 22. Mould DR, Sweeney KRD. The pharmacokinetics and pharmacodynamics of monoclonal antibodies: mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10:84‐96. [PubMed] [Google Scholar]
- 23. Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6(222):222ra17. [DOI] [PubMed] [Google Scholar]
- 24. Burns ER, Goldberg SN, Wenz B. Paradoxic effect of multiple mild coagulation factor deficiencies on the prothrombin time and activated partial thromboplastin time. Am J Clin Pathol. 1993;100:94‐98. [DOI] [PubMed] [Google Scholar]
- 25. Girolami A, Ruzzon E, Lombardi AM, Cabrio L, Randi ML. Thrombosis‐free surgical procedures in severe (homozygote) factor XII deficiency: report of four additional cases and literature review. Clin Appl Thromb Hemost. 2004;10:351‐355. [DOI] [PubMed] [Google Scholar]
- 26. Gallimore MJ, Harris SL, Jones DW, Winter M. Plasma levels of factor XII, prekallikrein and high molecular weight kininogen in normal blood donors and patients having suffered venous thrombosis. Thromb Res. 2004;114:91‐96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


