Abstract
Moxidectin is a frontrunner drug candidate in the treatment of strongyloidiasis. A dose of 8 mg is recommended to treat this indication, which shows a reasonably good efficacy and tolerability profile. Yet, owing to the unique life cycle of Strongyloides stercoralis (S. stercoralis) that entails internal autoinfection, a curative treatment would be desirable. Population‐based pharmacometric modeling that would help to identify an ideal dosing strategy are yet lacking. The aims of this study were to (i) explore the exposure‐efficacy response relationship of moxidectin in treating S. stercoralis and (ii) evaluate whether moxidectin treatment outcomes in terms of cure rates at baseline as compared to post‐treatment could be optimized. Our pharmacodynamic model suggests high predictive power (area under the concentration time curve‐receiver operating characteristic [AUC‐ROC] 0.817) in the probability of being cured by linking an exposure metric (i.e., AUC0‐24 or maximum concentration [Cmax]) to baseline infection intensity. Pharmacometric simulations indicate that with a minimum dose of 4 mg a maximum cure rate of ~ 95% is established in the low infection intensity group (larvae per gram [LPG] ≥0.4–1), whereas in the moderate‐to‐high intensity group (LPG >1) the cure rate plateaus at ~ 87%, following an 8 mg dose. To enhance efficacy further, studies using repeated dosing based on the duration of the autoinfection cycle, for example a two‐dose regimen 3 weeks apart should be considered. Simulations revealed similar Cmax in both treatment courses of a two‐dose regimen; hence safety should not be a concern. Collectively, our results provide evidence‐based guidance for enhanced dosing strategies and should be considered when designing future treatment strategies.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Moxidectin is a promising candidate for addition to the depleted armamentarium of treatments for neglected tropical diseases, including strongyloidiasis. A recent dose‐finding trial showed moxidectin doses of 4–12 mg being efficacious in the treatment of strongyloidiasis. Because there is ample experience with a fixed dose of 8 mg in other indications, this dose was recommended for further evaluation.
WHAT QUESTION DID THIS STUDY ADDRESS?
To characterize exposure‐response relationship for moxidectin in S. stercoralis‐infected adults to explore whether treatment outcomes could be further improved by an optimized dosing strategy.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our results support the previously proposed 8 mg fixed dose for the treatment of S. stercoralis infections. Pharmacometric dose explorations, however, show that with a single dose regimen, the exposure‐response plateaus with increasing doses, with the plateau being dependent on infection intensity. Mean cure rates are not expected to surpass 97% or 90% in the low and moderate‐to‐high infection intensity groups, respectively.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our pharmacometric analyses pave the way for new strategies to improve and individualize moxidectin treatment for S. stercoralis infections. Single dose strategies achieve good but still suboptimal cure rates even at high dose. Alternative strategies, such as two‐dose regimens, may be necessary in patients, particularly those with a high burden of infection. Our work lays the groundwork for future phase II trials investigating dosing strategies for strongyloides‐infected adults to increase efficacy, as well as tailored strategies for other populations, such as pediatrics or obese adults.
INTRODUCTION
Strongyloidiasis is considered one of the most overlooked of the neglected tropical diseases (NTDs). 1 Yet, it is widely distributed and responsible for a significant global burden of disease with adverse impacts on human and economic development. 2 , 3 The disease is caused by infection with the soil‐transmitted helminth (STH) Strongyloides stercoralis (S. stercoralis) that presently parasitizes an estimated number of 300 million people worldwide. 2 , 4 The health consequences of strongyloidiasis are generally mild when infection intensity is low, but, if left untreated, infections can persist for life and potentially develop into life‐threatening conditions, such as hyperinfection syndromes. The persistence is a result of the unique ability of S. stercoralis to start new cycles of infection without the need of ever leaving the host. 1 , 5 These ongoing phases of autoinfection are responsible that S. stercoralis has a life‐threatening nature und ultimately implicate that interventions with very high effectiveness, close to 100%, are required for sustained treatment success. 6 , 7
To date, a worldwide consensus on the optimal public health strategy to control S. stercoralis has not been reached. The World Health Organization (WHO) recommends preventive chemotherapy along with targeted health education to control infections with STHs. Currently, the best treatment available against S. stercoralis is a single weight‐adjusted dose of ivermectin (200 µg/kg), which provides a cure rate between 55% and 95%, hence there is still room for improvement. 7 , 8 , 9 , 10 , 11
Moxidectin, a potent antiparasitic widely used in veterinary medicine, is approved for the treatment of onchocerciasis, and is presently being investigated as an alternative treatment option for strongyloidiasis. Like ivermectin, moxidectin is a macrocyclic lactone with cure rates of around 90% against S. stercoralis. 10 , 12 Both moxidectin and ivermectin exert their effect through glutamate‐gated chloride channels of the target nematode, leading to an increase in channel permeability and eventual paralysis and death of the parasite. 13 , 14 Despite their similarities, moxidectin and ivermectin exhibit important differences in their pharmacokinetic (PK) behavior. 15 , 16 , 17 In addition to six PK studies in healthy volunteers, the PKs of moxidectin was characterized in patients infected with Onchocerca volvulus as well as S. stercoralis‐infected adults, where this latter study provided an additional population PK model of moxidectin. 15 , 17 This study confirmed that moxidectin is characterized by a fast absorption, a large apparent volume of distribution, and presented a long mean terminal elimination half‐life of 11.5 days, while also showing a large unexplained variability. Simulations using this PK model are supportive in the use of a fixed dose regimen of moxidectin facilitating mass drug administration programs. 17 In addition, several further advantages of moxidectin over ivermectin, such as a slower elimination, and less neurotoxicity in nonclinical models, as well as efficacy in certain ivermectin‐resistant strains, indicate moxidectin as a promising candidate for future public health strategies. 16 , 18 , 19 , 20
To set moxidectin forth as a successful treatment option in strongyloidiasis, an investigation on the ideal dosing strategy to approach curative treatment is now needed. Our suggested single oral dose of 8 mg is based on a recent‐dose finding study that demonstrated that single moxidectin doses of 4–12 mg are highly efficacious and associated with few or no side effects, whereas a PK analysis indicated similar exposure for an 8‐mg fixed dose compared to weight‐based dosing. 12 , 17 However, a predicted cure rate of 87% in the 8 mg dose group still leaves room for improvement, given the persistent nature of strongyloidiasis. The aim of this study was to (i) develop an exposure‐efficacy response model for moxidectin in S. stercoralis‐infected adults that links drug exposure to the clinical outcome, and (ii) explore whether different dosing strategies could further enhance treatment success with moxidectin in strongyloidiasis.
METHODS
Clinical study design and procedure
Between November 27, 2019, and March 15, 2020, 223 S. stercoralis‐infected adults from four villages in Nambak, Laos, were enrolled and randomly assigned by use of computerized, stratified by infection intensity, block randomization into seven treatment groups to receive a single dose of 2–12 mg of moxidectin or placebo. Thereof, 209 participants completed the phase IIa dose‐finding study. Fourteen participants were lost to follow‐up. Out of the 180 participants receiving moxidectin, the first 96 volunteers were assigned for capillary blood sampling in a PK study. Capillary blood was taken before treatment as well as 2 h, 4 h, 6 h, 7 h, 24 h, 3 days, 7 days, and 28 days after treatment. The details and results of the phase IIa study and the population PK model were published elsewhere. 12 , 17 , 21 In brief, on the day of enrollment, an oily meat soup was provided before administration of a single oral dose of moxidectin (2 mg tablets, provided by Medicines Development for Global Health). Efficacy of the treatment was determined 28 days post‐treatment based on sextuplet fecal slides applying the quantitative Baermann method. Cure rates were calculated as the percentage of larvae‐positive subjects at baseline who became larvae‐negative after treatment. Stratification per baseline infection intensity was performed as follows: light greater than or equal to 0.4–1 larvae per gram (LPG) of stool, moderate greater than 1–10 LPG, and heavy greater than 10 LPG. 12 , 22
Ethical considerations
Ethical approval was obtained in Laos from the National Ethics Committee for Health Research, application No. 082‐NECHR on September 12, 2019, and in Switzerland from the Ethics Committee of Northwestern and Central Switzerland, application No. 2019–00558 on July 15, 2019. Informed written consent was obtained from participants after detailed written and oral information was provided during an information session. 12 The study is registered at ClinicalTrials.gov, identifier NCT04056325.
Software
Pharmacometric exposure‐response analyses were carried out using the Monolix 2019R2 software package (Lixoft SAS; Monolix version 2019R2). Data manipulation, statistical analysis, simulations, and visualization was performed using R (version 4.0.2 23 ) and Rstudio (version 1.3.959 24 ) using ggplot2, Hmisc, pROC, 25 and seperationplot 26 R packages. Model‐based simulations were performed in R using the simulx (part of mlxR) and Rsmlx packages.
Correlation matrixes between infection intensities and clinical or physical symptoms have been computed in Rstudio.
Pharmacometric exposure‐efficacy response analysis
A logistic regression model was fitted to the efficacy data with cure rates to estimate the probability of being cured at day 28 (Pcured) using the Stochastic Approximation Expectation – Maximization algorithm in Monolix. Equation 1 describes the logistic regression model:
| (1) |
where logit (P cured) is the logit expression of P cured. To analyze the exposure‐response relationship, individual post hoc PK parameters were generated for each individual in the efficacy dataset using the previously published population PK model with inclusion of interindividual variability by resampling of five different sets of PK parameters for each individual. 17 In the next step, several individual exposure metrics were simulated using the original dose that these individuals received, including area under the concentration time curve (AUC) values for different time intervals, such as AUC from start to infinity (AUC0–∞), over the first 24 h (AUC0–24h), or the second week (AUC7–14d). Other exposure metrics included capillary blood concentrations on days 7, 14, or 21 (C7d, C14d, and C21d) and the peak concentration (Cmax). These exposure metrics were tested for their ability to predict P cured using a linear model or standard maximum effect (Emax) model following Equations 2 and 3, respectively:
| (2) |
| (3) |
where BLlogit(Pcured) is the baseline logit probability of being cured at day 28, SLOPE is the effect parameter in the linear model, Emax is the maximum effect, EM is the exposure metric being used, and E50 is the value of the EM at which the effect is 50% of the maximum. In addition to exposure metrics, available covariates age, weight, gender, and infection intensity were tested in the model for their ability to predict the probability of being cured. Binary categorical covariates were tested in the model using Equation 4:
| (4) |
where BLlogit(Pcured) is the baseline logit probability of being cured at day 28, ZCOVi=1 is the effect of COV on parameter P when the covariate has value 1. Continuous covariates were tested using Equation 5:
| (5) |
where BLlogit( P cured) is the baseline logit probability of being cured at day 28, and ZCOV represents the change in logit probability with each unit of deviation of the individual covariate value COVi from the standard value COVstd. The COVstd typically is the population median value or, in case of body weight, a standardized weight of 70 kg.
Logit regression models with different predictors for P cured were compared using the corrected Bayesian information criterion (BICc) estimated by importance resampling and reported by Monolix, where the most predictive model is the one with the lowest BICc value. In addition, we assessed the performance using the AUC of the receiver operating characteristics curve (ROC‐AUC) and visual inspection of separation plots. 26 Performance of the model was assessed by visual predictive checks (VPC) comparing simulated probabilities of being cured versus the observed probability of being cured. Last, precision and robustness of the model parameters was assessed by bootstrap resampling (n = 500 replicates).
Pharmacometric simulations to evaluate dosing strategies
Based on the final moxidectin exposure‐efficacy response model, different single doses were explored using Monte Carlo simulations (n = 10,000). Each virtual subject was allocated an age, gender, body weight, and infection intensity with the same ranges (age and gender) and distribution (body weight and infection intensity) as in the original dataset. Each virtual subject was randomly allocated to receive a single moxidectin dose between 0 and 20 mg (in steps of 1 mg), followed by simulation of the AUC0–24h with inclusion of interindividual variability based on the previously published PK model. 17 For each subject, the probability of being cured at day 28 post‐treatment (P cured) was simulated using the exposure‐efficacy response model. Parameter uncertainty of the exposure‐efficacy response model was included by bootstrapping the original dataset (n = 100). The probability of being cured (mean ±95% confidence interval [CI]) was plotted versus the administered dose to assess the optimal dose for moxidectin where maximum efficacy is expected.
RESULTS
Study population and samples
A total of 209 S. stercoralis‐infected adults concluded the dose‐escalating randomized placebo‐controlled phase IIa efficacy trial, out of which 180 patients received moxidectin with dose levels varying between 2 and 12 mg and the remaining participants obtained a placebo. These 209 individuals constitute the dataset for developing the exposure‐efficacy response model, with patient characteristics shown in Table S1. The observed cure rates were 14% in the placebo arm, and 73–97% in the treatment arms with 83% in the 8 mg arm (Table S2). Thereof, 96 individuals provided capillary blood samples for 762 moxidectin concentration measurements, which constituted the dataset of the PK analysis described and analyzed elsewhere. 17
Pharmacometric exposure‐response analysis
The exposure‐efficacy response analysis revealed that exposure metrics resulted in an improved prediction of the cure rate. Exposure metrics could be introduced with Emax models following Equation 3, as linear models (applying Equation 2) resulted in identifiability issues. The best results were obtained with the metrics Cmax and AUC0–24h, with BICc values of 857.7 and 857.5, corresponding to reductions of −310.6 and −310.8, respectively, compared to the base model without a drug effect. These models were significantly better than a model that includes the dose as predictor for Pcured, which resulted in a BICc of 863.1, corresponding to a drop of −305.2 compared to the model without a drug effect. AUC‐ROC curves were also highest for these exposure metrics (Cmax: 0.796 [95% CI 0.761–0.832], AUC0–24h: 0.794, [95% CI 0.758–0.831]) with separation plots improving substantially with a clear shift of observed individual being cured to the right side of the plots (Figure S1). Hence, both Cmax and AUC0–24h performed similar. Other AUC‐based exposure metrics improved the model as well but were inferior to AUC0–24 or Cmax (Table S3). In both models, we found a clear misspecification for individuals split for baseline infection intensity, which was resolved by inclusion of infection intensity as a binary categorical covariate (low versus moderate‐to‐high infection intensity) in the model. This resulted in a reduction in BICc (AUC0–24h: 841.9, Cmax: 843.2, corresponding to a reduction of −15.6 and −14.5 compared to the models without infection intensity, respectively) with an even higher predictive performance for predicting the probability of being cured (AUC‐ROC for AUC0–24h: 0.809 [95% CI 0.774–0.845], Cmax: 0.811 [95% CI 0.777–0.846]). In addition, the infection‐intensity optimized models clearly improved the VPC and the separation plot (Figure 1 and Figure S2). The VPC split for dose group also indicates a good performance across dose, although small variability in observed cure rates is not entirely captured (Supplementary Figure S2). A dose‐response model with infection intensity as a covariate resulted in a significantly worse BICc (847.2), while comparable predictive performance (AUC‐ROC 0.800 [95% CI 0.765–0.836] compared to exposure‐response models with AUC0‐24h or Cmax [Supplementary Table S3]). The bootstrap analysis (Table 1 and Table S4) confirms the robustness of the parameter estimates of the final exposure‐response model.
FIGURE 1.
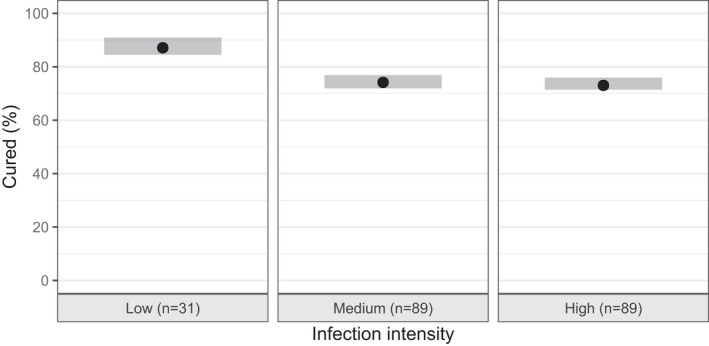
Visual predictive check (VPC) of the exposure‐efficacy response model, showing the percentage of moxidectin (2–12 mg) treated individuals being cured split for infection intensity. Shaded area’s indicate the simulation based 95% prediction interval of the percentage being cured (n = 500 datasets), where the black dots show the observed percentage of patients being cured
TABLE 1.
Parameters of the final exposure‐efficacy response model
| Pharmacodynamic model | Estimate (% RSE) | Bootstrap estimate b (95% CI) | |
|---|---|---|---|
|
|
|||
|
|
−0.69 (50) | −0.70 (−1.51–0.05) | |
| Emax | 4.60 (7.5) | 4.66 (3.95–5.49) | |
| E50 | 79.2 (29) | 77.8 (36.1–128.5) | |
| INT a | 1.42 (23) | 1.47 (0.80–2.27) |
Abbreviations: AUC0–24h, area under the concentration time curve from zero to 24 hours; BL, baseline; BLlogit(Pcured) , Logit transformed baseline probability of being cured; CI, confidence interval; E50, AUC0–24h where effect is half of Emax; Emax, maximum effect; INT, infection intensity factor (apply if infection intensity is moderate‐to‐high).
Include infection intensity factor only if infection intensity is moderate‐to‐high.
Based on n = 500 replicates.
Figure 2 shows the AUC‐ROC for the final exposure‐efficacy response model based on AUC0–24h as exposure metric and infection intensity as covariate. The final model parameters, including the 95% CIs based on the bootstrap analysis are shown in Table 1. The AUC‐ROC curve and parameters of the alternative exposure‐efficacy response model with Cmax instead of AUC0–24h are shown in the Supplementary Figure S3 and Table S4.
FIGURE 2.
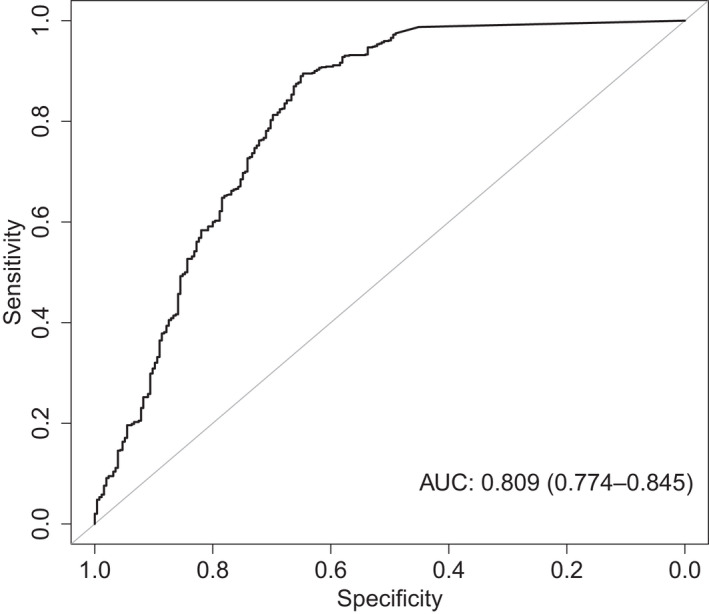
Area under the curve of the receiver operated characteristics (AUC‐ROC) curve for the final exposure‐efficacy response model incorporating AUC0–24h and infection intensity. The AUC is shown in the figure with 95% confidence interval between brackets
Pharmacometric simulations to evaluate dosing strategies
The probability of being cured for several dose levels, based on Monte Carlo simulations (n = 10,000 individuals) with the final exposure‐efficacy response model, including parameter uncertainty are shown in Figure 3 and Table S5. Figure 3a shows that for all moxidectin‐treated participants, the probability of being cured increases with the dose with an 88% (95% CI 81–98%) cure rate at an 8 mg dose. At 12 mg, the mean cure rate is 90% (95% CI 85–98%) and levelling off at even higher doses, indicating that there is only a minor increase expected with increasing the doses above 8 mg. Figure 3b shows that in addition to the dose, the cure rate is highly dependent upon the infection intensity. In the low infection intensity group, a mean cure rate of 95% (95% CI 90–98%) is reached with a 4 mg dose, levelling at 97% in the 8 mg dose group. In the moderate‐to‐high infection intensity group, the lower limit of the 95% CI does not surpass a cure rate of 90%. It is, however, visible that with doses above 8 mg, there is not much improvement of the probability of being cured; a mean cure rate of 87% (95% CI 81–91%) and 89% (95% CI 84–92%) in the 8 and 12 mg group, respectively, indicate that a “plateau” has been reached. Similar results are obtained when the same simulations are performed with the alternative model that includes an exposure‐response relationship based on Cmax values instead of AUC0–24h (Figure S4).
FIGURE 3.
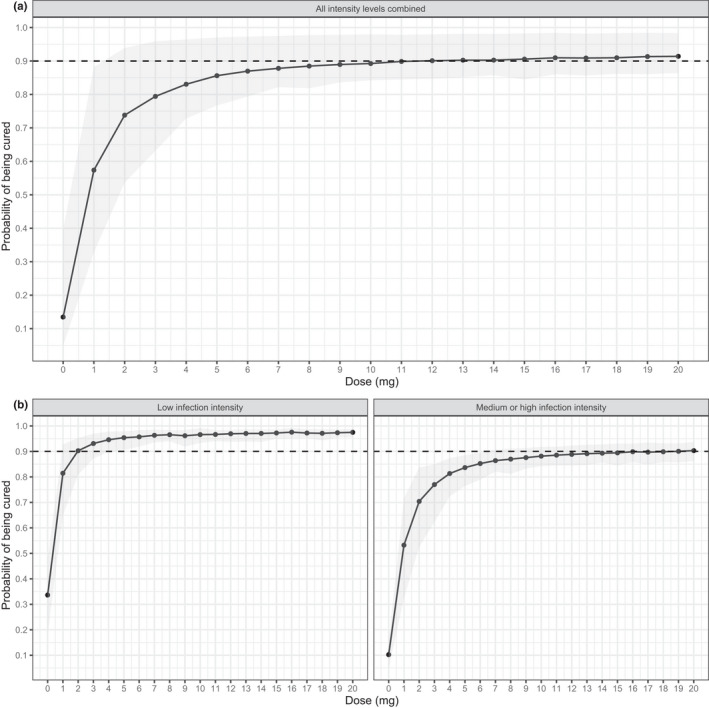
Predicted probability of being cured for dose levels between 0 and 20 mg, based on Monte Carlo simulations (n = 10,000) using the final exposure‐efficacy response model with AUC0–24h as exposure metric (Table 1) where parameter uncertainty was included for the exposure‐efficacy response model using bootstrap resampling (n = 100). Results are shown for (a) all individuals combined or (b) split for individuals with a low infection intensity (left panel) or moderate‐to‐high infection intensity (right panel). The solid line and black dots depict the probability of being cured for each dose level, where the grey area indicate the 95% confidence interval. The horizontal dashed line shows the 0.9 (90%) level. Raw data for this figure is shown in Table S5
The pharmacometric simulations fail to propose drivers for complete cure. To investigate whether a different treatment strategy, a two‐dose regimen, on the basis of the duration of the autoinfection cycle would reveal no safety issues simulations based on the earlier published PK model with two 8 mg doses given 3 weeks apart to several typical individuals were performed. 17 These resulted in similar peak concentrations and exposure following both courses (shown in Supplementary Table S6).
DISCUSSION
Maximizing treatment success is key in management of infections with S. stercoralis, as only cure can eliminate the risk of ongoing complications in light of recurrent autoinfection. To date, a single dose of 8 mg of moxidectin is advocated for the treatment of S. stercoralis infections, resulting in an observed cure rate of 83% in a dose‐finding study. 12 An exposure‐efficacy response characterization, linking dose, exposure, and response, is a necessary next step in the development of moxidectin for S. stercoralis, which might help to further improve the treatment outcome. Here, we report for the first time such an analysis to allow us to explore dosing strategies for moxidectin against strongyloidiasis.
Our analysis revealed that increased moxidectin exposure corresponds to increased probability of being cured levelling off at or above 6–8 mg. The early exposure metrics (AUC0–24h and Cmax) appear more predictive than late exposure metrics, such as total AUC or serum concentrations at days 7, 14, or 21. In addition, we found that the baseline infection intensity is a significant driver of treatment success. Our exposure‐efficacy response model results in a high predictive performance for curation with an AUC‐ROC of 0.81. Although this exposure‐response model was statistically significantly better than a dose‐response model, predictive performance was similar for both approaches. These results help to better understand the dose‐response relationship of moxidectin.
When pursuing a single‐dose strategy for moxidectin, the results presented here support a fixed 8 mg dose as a reasonable dose for treating strongyloidiasis. Doses lower than 8 mg result in suboptimal efficacy, particularly in the moderate‐to‐high infected individuals. Pharmacometric simulations show that in individuals with less than one LPG, cure rates of 95% are achieved with a low 4 mg dose of moxidectin, and slightly increase to 97% with a dose of 8 mg. In moderate‐to‐high infected patients, a dose of 8 mg just achieves a cure rate of close to 90%. Increasing the dose further would further improve efficacy, albeit only with a small increase with uncertain clinical importance. Moreover, increasing dosage on a large scale is always accompanied by an increase in treatment costs. Thus, cost‐effectiveness studies would have to be conducted to provide justification for a higher dose in mass drug administration programs. In addition, issues with toxicity may arise as an increase in dose inevitably leads to increased risk of adverse events. Hence, a single fixed 8 mg dose as approved for onchocerciasis and proposed earlier remains a reasonable choice, especially where the level of infection is unknown. In individualized clinical situations, a lower dose could theoretically be considered for lightly infected diagnosed patients to minimize the use of moxidectin with little loss of efficacy.
Another important implication of our results is that in all cases, but especially in highly infected individuals, cure of all patients cannot be expected with a single dose strategy. Moreover, moxidectin, like most antiparasitic agents, including ivermectin, acts only on adult worms. 15 This implies a possible relapse by migrating larvae or hatching eggs at later time points. Prolongation of the treatment period and multiple treatment dosages in line with the duration of the autoinfection cycle could thus ensure enhanced treatment outcomes. 6 , 7 This hypothesis is substantiated by the finding that high larvae‐reduction rates (around 97%) are reached for any infection intensity and cure rates. 12 In addition, we have found that early exposure metrics (reflecting the exposure in the first days after treatment) are more predictive of moxidectin’s efficacy compared to late exposure metrics. These findings imply that a second treatment dose could further drive the efficacy. Repeating the application regimen in accordance to the life cycle, namely 14–21 days postdosing, could thus ensure higher efficacies along with higher effectiveness, as previously unaffected larvae and eggs have now developed into adults making them sensitive to a second treatment with moxidectin. 6 Such a treatment regimen has previously been proposed for ivermectin. However, a benefit of the two‐dose over a single‐dose could not be observed for ivermectin, yet it should be noted that no quantitative assessment of larval load was performed in this study. 27 , 28
Safety and tolerability of a second dose should be carefully evaluated in clinical studies. Of note, our simulations do not indicate any risk of accumulation with a further dose based on the PK of moxidectin. 28 Additionally, future studies should additionally address the route of re‐infection.
Some limitations of our study should be acknowledged. First, in this study, patients received a maximum of a 12 mg dose. Hence, the results of our simulations beyond 12 mg should be interpreted with some caution. However, given the large variability in the underlying PK model, the actual exposures, driving the exposure‐efficacy response model, are expected to overlap in doses between 12 and 16 mg. Therefore, we argue that our model is at least applicable to dosages up to 16 mg. Second, we found similar performance of Cmax and AUC0–24h as drivers for the cure rates. The difference might not be very relevant in the majority of the treated population, because these exposure metrics are expected to be highly correlated. However, some exceptions might occur in special populations, such as obese adults or in pediatrics, where volume of distribution (driving Cmax) or clearance (driving AUC0–24h) can change in different ways. Therefore, future research should be undertaken in these specific populations to ensure the correct dosing strategy for these groups.
CONCLUSION
This first characterization of the exposure‐response relationship of moxidectin in strongyloidiasis shows that moxidectin’s cure rate is related to early exposure metrics, such as Cmax or AUC0–24h. Both exposure‐response plateau with increasing dose and baseline infection intensity have a strong impact on clinical success. For single fixed dose strategies, our results support a dose of 8 mg moxidectin to treat S. stercoralis‐infected adults. Because a complete response is not expected, even at high doses, further studies should focus on treatment regimens with multiple doses to ensure cure and resilient treatment outcomes. In addition, extension of the developed exposure‐efficacy response model to other populations, such as children or obese adults is warranted.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
D.H., C.S., M.P., and J.K. wrote the manuscript. D.H. and J.K. designed the research. D.H. collected the data. D.H., C.S., and M.P. analyzed the data. D.H., C.S., M.P., S.S., and J.K. performed the research.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the study participants as well as the field team in Laos for their contribution in the clinical trial.
Hofmann D, Smit C, Sayasone S, Pfister M, Keiser J. Optimizing moxidectin dosing for Strongyloides stercoralis infections: Insights from pharmacometric modeling. Clin Transl Sci. 2022;15:700–708. doi: 10.1111/cts.13189
Hofmann and Smit shared first author.
Funding information
European Research Council (ERC) and Adiuvare Foundation supported the clinical trial as well as J.K and D.H. C.S. and M.P are financially supported by the Eckenstein‐Geigy Foundation in Basel, Switzerland. The funding source has no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Contributor Information
Marc Pfister, Email: marc.pfister@certara.com.
Jennifer Keiser, Email: jennifer.keiser@swisstph.ch.
REFERENCES
- 1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis ‐ the most neglected of the neglected tropical diseases? T Roy Soc Trop Med H. 2009;103(10):967‐972. [DOI] [PubMed] [Google Scholar]
- 2. Krolewiecki AJ, Lammie P, Jacobson J, et al. A public health response against Strongyloides stercoralis: time to look at soil‐transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7(5):e2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beknazarova M, Whiley H, Ross K. Strongyloidiasis: A disease of socioeconomic disadvantage. Int J Environ Res Public Health. 2016;13(5):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102(4):314‐318. [DOI] [PubMed] [Google Scholar]
- 6. Satoh M, Kokaze A. Treatment strategies in controlling strongyloidiasis. Expert Opin Pharmacother. 2004;5(11):2293‐2301. [DOI] [PubMed] [Google Scholar]
- 7. Luvira V, Watthanakulpanich D, Pittisuttithum P. Management of Strongyloides stercoralis: a puzzling parasite. Int Health. 2014;6(4):273‐281. [DOI] [PubMed] [Google Scholar]
- 8. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panic G, Duthaler U, Speich B, Keiser J. Repurposing drugs for the treatment and control of helminth infections. Int J Parasitol Drugs Drug Resist. 2014;4(3):185‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barda B, Sayasone S, Phongluxa K, et al. Efficacy of moxidectin versus ivermectin against Strongyloides stercoralis infections: A randomized, controlled noninferiority trial. Clin Infect Dis. 2017;65(2):276‐281. [DOI] [PubMed] [Google Scholar]
- 11. Buonfrate D, Salas‐Coronas J, Muñoz J, et al. Multiple‐dose versus single‐dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open‐label, phase 3, randomised controlled superiority trial. Lancet Infect Dis. 2019;19(11):1181‐1190. [DOI] [PubMed] [Google Scholar]
- 12. Hofmann D, Sayasone S, Sengngam K, Chongvilay B, Hattendorf J, Keiser J. Efficacy and safety of ascending doses of moxidectin against Strongyloides stercoralis infections in adults: a randomised, parallel‐group, single‐blinded, placebo‐controlled, dose‐ranging, phase 2a trial. Lancet Infect Dis. 2021;21(8):1151–1160. [DOI] [PubMed] [Google Scholar]
- 13. Laing R, Gillan V, Devaney E. Ivermectin ‐ old drug, new tricks? Trends Parasitol. 2017;33(6):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59(2):139‐156. [DOI] [PubMed] [Google Scholar]
- 15. Cotreau MM, Warren S, Ryan JL, et al. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43(10):1108‐1115. [DOI] [PubMed] [Google Scholar]
- 16. Brussee JM, Schulz JD, Coulibaly JT, Keiser J, Pfister M. Ivermectin dosing strategy to achieve equivalent exposure coverage in children and adults. Clin Pharmacol Therapeutics. 2019;106(3):661‐667. [DOI] [PubMed] [Google Scholar]
- 17. Smit C, Hofmann D, Sayasone S, Keiser J, Pfister M. Characterization of the population pharmacokinetics of moxidectin in adults infected with Strongyloides stercoralis: support for a fixed‐dose treatment regimen. Clin Pharmacokinet. 2021 [ahead of print]. 10.1007/s40262-021-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janko C, Geyer J. Moxidectin has a lower neurotoxic potential but comparable brain penetration in P‐glycoprotein‐deficient CF‐1 mice compared to ivermectin. J Vet Pharmacol Ther. 2013;36(3):275‐284. [DOI] [PubMed] [Google Scholar]
- 19. Prichard RK, Geary TG. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int J Parasitol Drugs Drug Resist. 2019;10:69‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pankavich JA, Berger H, Simkins KL. Efficacy of moxidectin, nemadectin and ivermectin against an ivermectin‐resistant strain of Haemonchus contortus in sheep. Vet Rec. 1992;130(12):241‐242, 3. [DOI] [PubMed] [Google Scholar]
- 21. Hofmann D, Sayasone S, Keiser J. Development and validation of an LC‐MS/MS method for the quantification of the anthelmintic drug moxidectin in a volumetric absorptive microsample, blood, and plasma: Application to a pharmacokinetic study of adults infected with Strongyloides stercoralis in Laos. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1166:122556. [DOI] [PubMed] [Google Scholar]
- 22. Schär F, Hattendorf J, Khieu V, et al. Strongyloides stercoralis larvae excretion patterns before and after treatment. Parasitology. 2014;141(7):892‐897. [DOI] [PubMed] [Google Scholar]
- 23. RCoreTeam . A language and environment for statistical computing. 2020.
- 24. RStudioTeam . RStudio: Integrated Development for R. 2020.
- 25. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenhill B, Ward MD, Sacks A. The separation plot: a new visual method for evaluating the fit of binary models. Am J Polit Sci. 2011;55(4):990‐1002. [Google Scholar]
- 27. Suputtamongkol Y, Premasathian N, Bhumimuang K, et al. Efficacy and safety of single and double doses of ivermectin versus 7‐day high dose albendazole for chronic strongyloidiasis. PLoS Negl Trop Dis. 2011;5(5):e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaha O, Hirata T, Kinjo F, Saito A, Fukuhara H. Efficacy of ivermectin for chronic strongyloidiasis: two single doses given 2 weeks apart. J Infect Chemother. 2002;8(1):94‐98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


