Abstract
Bempedoic acid is an ATP citrate lyase inhibitor approved for the treatment of hypercholesterolemia. The objective of this phase I study was to assess the pharmacokinetics (PKs) and safety of bempedoic acid in 24 subjects with normal renal function or mild, moderate, or severe renal impairment. All subjects received a single oral bempedoic acid 180‐mg dose and PK parameters were monitored for up to 23 days. Resulting estimates of area under the concentration‐time curve exposure following bempedoic acid treatment were 1.5‐fold, 2.2‐fold, and 2.2‐fold higher in subjects with mild, moderate, or severe renal impairment, respectively, compared with subjects with normal renal function. With decreases in renal function, plasma free fraction was increased up to 20.1%, whereas total and unbound clearances were decreased by 55.2% and 62.6%, respectively, in subjects with severe renal impairment relative to those with normal renal function. These observed decreases in total and unbound oral clearance in subjects with decreased renal function are not explained by the increases in free fraction and might therefore also be attributable to changes in bioavailability or intrinsic clearance. Bempedoic acid was generally well‐tolerated and the incidence and type of adverse events were not affected by the degree of renal impairment. In conclusion, bempedoic acid exposures in subjects with renal impairment were increased up to approximately two‐fold with no safety signals identified, consistent with findings in phase III patients with mild or moderate renal impairment. No dose adjustments are necessary for patients with mild or moderate renal impairment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
In the United States, bempedoic acid is approved as an adjunct to diet and maximally tolerated statin therapy for adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of low‐density lipoprotein cholesterol. The pharmacokinetics (PKs) and safety of bempedoic acid in patients with renal impairment have not yet been fully characterized.
WHAT QUESTION DID THIS STUDY ADDRESS?
This phase I study assessed the PKs and safety of bempedoic acid following a single oral 180‐mg dose in 24 subjects with normal renal function or mild, moderate, or severe renal impairment.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our results demonstrated that bempedoic acid area under the concentration‐time curve (AUC) exposure was increased by 1.5‐fold, 2.2‐fold, and 2.2‐fold in subjects with mild, moderate, and severe renal impairment, respectively, compared with subjects with normal renal function. [Correction added on 23 December 2021, after first online publication: The increase in bempedoic acid area under the concentration‐time curve (AUC) exposure was modified in this version to align with data presented in the manuscript.] Alterations in observed PK are consistent with a shift from renal to hepatobiliary elimination of bempedoic acid‐glucuronide, resulting in a greater fraction of glucuronide metabolite being subjected to intestinal deconjugation and enterohepatic recirculation. Bempedoic acid treatment was generally well‐tolerated, regardless of renal function category.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Although bempedoic acid exposure was increased in subjects with renal impairment compared with subjects with normal renal function, no safety concerns were identified, and based on safety results from randomized placebo‐controlled phase III studies, no bempedoic acid dosage adjustments are necessary for patients with mild or moderate renal impairment.
INTRODUCTION
Approximately 15% of the adult population in the United States is estimated to have chronic kidney disease. 1 The incidence of renal dysfunction is even greater for patients with comorbidities, such as cardiovascular disease or diabetes. 2 Thus, characterizing the influence of impaired renal function on drug pharmacokinetics (PKs) and/or pharmacodynamics is recommended by regulatory agencies. 3 , 4 For drugs whose primary clearance pathway is by renal elimination, circulating drug concentrations in patients with renal impairment may differ relative to individuals with normal kidney function treated at the same dose. However, for drugs eliminated primarily by nonrenal pathways, such as bempedoic acid, the impact of renal impairment on drug PKs can often be unpredictable.
Bempedoic acid (ETC‐1002) is a small‐molecule prodrug that is activated to a coenzyme A ester (ETC‐1002‐CoA) by very long‐chain acyl‐CoA synthetase 1 (ACSVL1) expressed in human liver. 5 The active ETC‐1002‐CoA metabolite is a potent and highly selective inhibitor of adenosine triphosphate citrate lyase in the cholesterol biosynthesis pathway, leading to significant decreases in serum low‐density lipoprotein cholesterol compared with placebo. 6 , 7 , 8 , 9
Bempedoic acid is a low extraction drug with terminal half‐life of 21 h and accumulation of ~ 2.3‐fold at steady‐state. Approximately 90% of an oral bempedoic acid dose is absorbed and the distribution of bempedoic acid is characterized by high (99.3%) in vitro plasma protein binding. 10 Bempedoic acid elimination occurs primarily by hepatic metabolism, with formation of an acyl glucuronide metabolite by uridine 5′ diphospho‐glucuronosyltransferase (UGT) 2B7–mediated conjugation. Bempedoic acid undergoes reversible metabolism to a keto metabolite ESP15228 by hepatic oxidoreductase activity. 10 , 11 ESP15228 circulates in plasma at ~ 20% of bempedoic acid concentrations and is activated by ACSVL1 to a pharmacologically active metabolite ESP15228‐CoA. 10 , 12 , 13 , 14 , 15
The extent to which renal impairment might impact bempedoic acid PKs is currently unknown, as renal excretion of unchanged bempedoic acid is considered a minor route of drug elimination, accounting for less than 2% of total clearance. 10 Although bempedoic acid metabolism by UGT2B7 could be altered by renal impairment, other drugs, such as morphine, which is converted to morphine 6‐O‐glucuronide by UGT2B7, 16 are not greatly impacted by the magnitude of renal impairment. 17 , 18 The current phase I study was undertaken to evaluate single dose oral PKs of bempedoic acid in subjects with mildly, moderately, and severely impaired renal function.
METHODS
Study design
This was a phase I, open‐label, single‐center, parallel‐group study to evaluate the safety, tolerability, and PKs of a single 180‐mg oral dose of bempedoic acid in subjects with mild, moderate, or severe renal impairment and compared with subjects with normal renal function. At screening, renal function was assessed by estimating creatinine clearance using the Cockcroft‐Gault equation (normal function group) and the estimated glomerular filtration rate (eGFR) calculated using the modification of diet in renal disease (MDRD) formula (renal impairment groups). 19 For analysis, subjects were uniformly allocated by absolute eGFR calculated using the MDRD formula, where normal renal function was defined as eGFR greater than or equal to 90 ml/min and renal impairment categories were defined as eGFR 60 less than 90 ml/min (mild), 30 less than 60 ml/min (moderate), or less than 30 ml/min and not requiring dialysis (severe). 3 , 4
The study enrolled male and female (not pregnant or lactating) subjects aged 18–75 years, with a body mass index between 18 and 40 kg/m2 and body weight of 50 kg or greater. Subjects had stable serum creatinine levels that did not change by more than 0.5 mg/dl from the time of screening to 1 day prior to treatment. Subjects who were judged to be in stable health based on their medical history, physical examinations, and routine laboratory tests were eligible for enrollment. Subjects with renal impairment and comorbid conditions (e.g., diabetes mellitus) were eligible to enroll in the study, but subjects with uncontrolled hypertension or clinically significant comorbid conditions, such as cardiovascular disease, liver disease or dysfunction, an active malignancy, recent history of drug or alcohol abuse, or abnormal laboratory test values, were not eligible to enroll.
Subjects were admitted to the clinical research unit and after a 10‐h overnight fast, they received a single bempedoic acid 180‐mg oral dose with water (240 ml) the following morning on day 1. Fasting continued for an additional 2 h after dosing. Serial blood samples for PK analyses were collected on day 1 immediately before dosing and at 1, 2, 3, 4, 6, 8, 12, and 24 h (day 2) after dosing, with additional samples drawn at 4 and 24 h after dosing for determination of plasma protein binding. Subjects were discharged from the research unit on day 2 and returned for additional PK blood collections on days 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 18, and 23 at approximately the same time each day to match the time of dosing. Blood was centrifuged at 4°C within 30 min of collection and separated plasma samples were stored frozen at −70°C until bioanalysis. The study was conducted at Prism Research LLC in accordance with the Good Clinical Practice Guideline as defined by the International Conference on Harmonization and The Declaration of Helsinki. Before the onset of the study, all protocols and procedures were approved by an institutional review board (Salis IRB) and all subjects provided written informed consent.
Assessments
Bempedoic acid and ESP15228 plasma concentrations were determined by liquid chromatography‐tandem mass spectrometry. 11 Plasma protein binding was determined using Centrifree Ultracentrifugation devices with 30,000 Da mass cutoff (EMD Millipore). Unbound fraction (funbound) was estimated as a ratio of unbound plasma concentration, determined in ultrafiltrate isolated by centrifugation at 2000 × g and 25°C for 60 min, relative to total plasma concentrations of bempedoic acid and ESP15228. PK analyses were performed by noncompartmental methods using Phoenix WinNonlin (version 6.3; Certara USA). PK parameters determined for bempedoic acid and ESP15228 included area under the concentration‐time curve (AUC) calculated from time 0 to the last measurable time point (AUClast) and AUC to infinity (AUCinf), maximum observed concentration (Cmax), time of maximum concentration (Tmax), elimination half‐life (t1/2), and ratio of ESP15228 AUC to bempedoic acid AUC (AUCm/AUCp). Apparent volume of distribution (Vz/F), oral clearance (CL/F), and unbound clearance (CLunbound/F), calculated using the average unbound fraction (funbound) in plasma at 4 and 24 h after dosing, were determined for bempedoic acid.
Summary statistics for bempedoic acid and ESP15228 PK parameters were calculated. Statistical comparisons of bempedoic acid PK exposure parameters were made using analysis of variance and 90% confidence intervals for the geometric mean ratios were calculated for comparisons of Cmax, AUClast, and AUCinf between study groups.
Safety and tolerability were assessed during the study through a review of adverse events (AEs), severity of AEs, and AE relationship to study drug. Other safety assessments included vital sign measurements, changes found in laboratory tests (hematology, serum chemistry, coagulation, and urinalysis), electrocardiograms (ECGs), and physical examinations. The AE reporting period began at the time of bempedoic acid treatment and ended after the last study visit with serious AEs monitored up to 30 days after the last study visit.
RESULTS
Subjects
A total of 24 subjects were enrolled and completed the study. Subjects were categorized for analysis according to baseline eGFR calculated using the MDRD formula, resulting in a distribution of six subjects with normal renal function, and eight subjects with mild, five subjects with moderate, and five subjects with severe renal impairment. Subjects with normal kidney function were enrolled last and matched to subjects with severe renal impairment of similar age (±10 years), sex, and body weight (±10 kg). Of the subjects enrolled in the study, most were male and of White race, and none were Hispanic or Latino (Table 1).
TABLE 1.
Demographics and baseline characteristics of subjects according to renal function category
| Characteristic | Renal function a | |||
|---|---|---|---|---|
|
Normal function (n = 6) |
Mild Impairment (n = 8) |
Moderate impairment (n = 5) |
Severe impairment (n = 5) |
|
| Age, years, (mean ± SD) | 56.5 ± 6.4 | 59.5 ± 6.9 | 64.0 ± 8.1 | 59.8 ± 10.1 |
| Sex, n (%) | ||||
| Male | 5 (83.3%) | 4 (50.0%) | 3 (60.0%) | 3 (60.0%) |
| Female | 1 (16.7%) | 4 (50.0%) | 2 (40.0%) | 2 (40.0%) |
| Race, n | ||||
| White | 6 (100.0%) | 7 (87.5%) | 4 (80.0%) | 3 (60.0%) |
| Black | 0 | 1 (12.5%) | 1 (20.0%) | 2 (40.0%) |
| Weight, kg (mean ± SD) | 91.9 ± 25.8 | 81.9 ± 9.8 | 85.5 ± 8.7 | 77.0 ± 6.9 |
| BMI, kg/m2 (mean ± SD) | 28.6 ± 5.1 | 29.3 ± 1.9 | 30.9 ± 4.0 | 27.7 ± 2.9 |
| eGFR, ml/min (mean ± SD) | 107.8 ± 15.2 | 75.1 ± 8.8 | 41.0 ± 8.0 | 21.8 ± 4.1 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; n, number of subjects; SD, standard deviation.
Normal renal function = eGFR ≥ 90 ml/min, mild renal impairment = eGFR 60–<90 ml/min, moderate renal impairment = eGFR 30–<60 ml/min, and severe renal impairment = eGFR <30 ml/min.
Pharmacokinetics
Mean bempedoic acid and metabolite plasma concentrations were generally higher in subjects with decreased renal function (Figures 1 and 2). Comparisons of bempedoic acid PK profiles in subjects with renal impairment suggest a potential decrease in clearance relative to subjects with normal renal function. Concentration‐time profiles of individual subjects with severe renal impairment were also consistent with decreased bempedoic acid clearance and longer elimination half‐life (t1/2), with evidence of secondary peaks in two subjects, compared with subjects having normal renal function (Figure 3).
FIGURE 1.
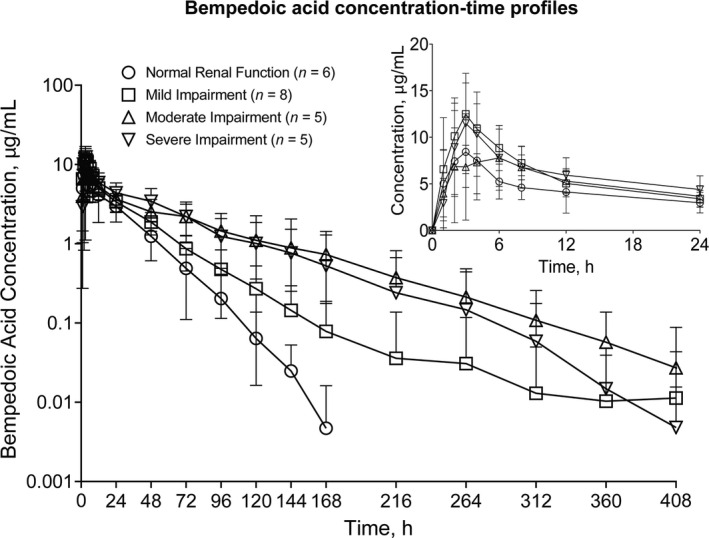
Arithmetic mean (±SD) bempedoic acid plasma concentration‐time profiles in subjects with normal renal function and mild, moderate, and severe renal impairment after single bempedoic acid 180‐mg dose administration. Insert depicts linear profiles on a 0–24 h time scale. Assay lower limit of quantitation, 20 ng/ml; inter‐run precision, 1.5 to 4.4% CV; accuracy, −3.3% to 1.6% mean bias
FIGURE 2.
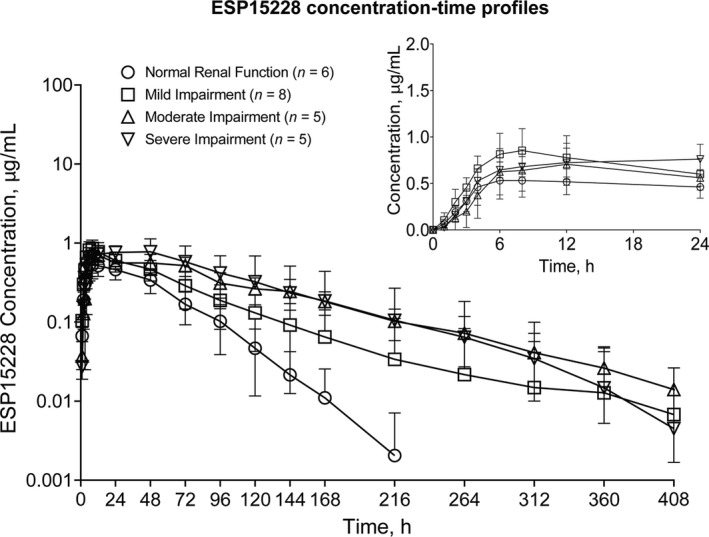
Arithmetic mean (±SD) ESP15228 plasma concentration‐time profiles in subjects with normal renal function and mild, moderate, and severe renal impairment after single bempedoic acid 180‐mg dose administration. Insert depicts linear profiles on a 0–24 h time scale. Assay lower limit of quantitation, 10 ng/ml; inter‐run precision, 2.6% to 5.7% CV; accuracy, −1.5% to 2.9% mean bias
FIGURE 3.
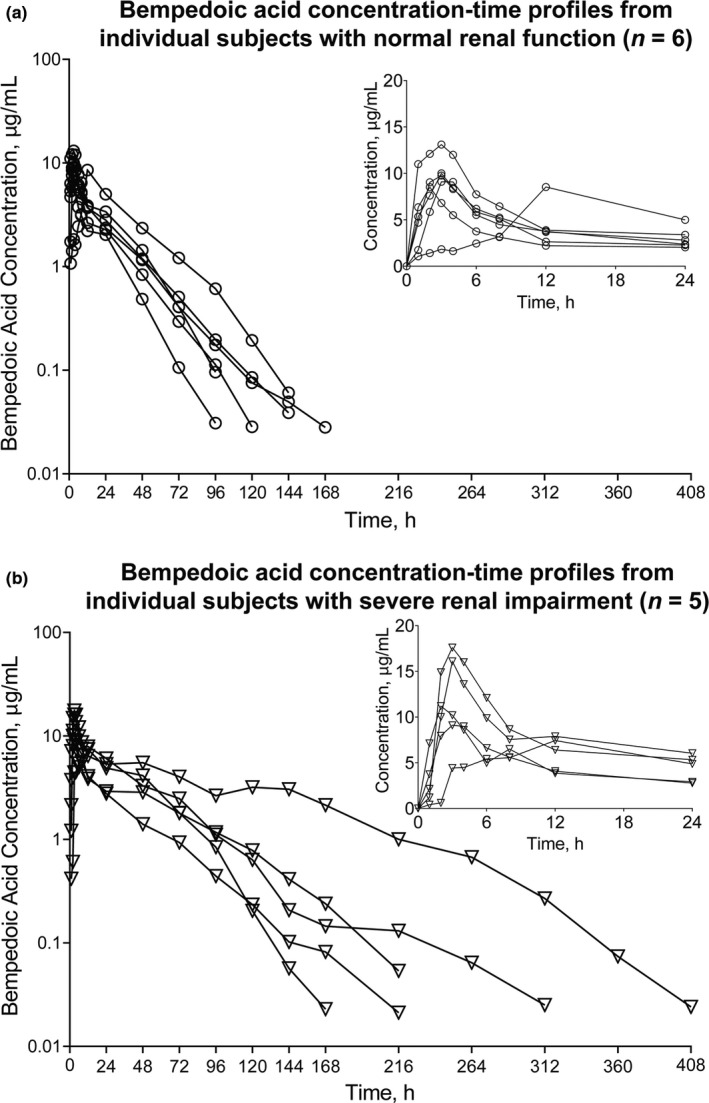
Bempedoic acid plasma concentration‐time profiles from individual subjects with (a) normal renal function (n = 6) and (b) severe renal impairment (n = 5) after single bempedoic acid 180‐mg dose administration. Insert depicts linear profiles of subjects with (a) normal renal function and (b) severe renal impairment on a 0–24 h time scale
Peak concentrations of bempedoic acid were comparable across groups and the effect of renal impairment on bempedoic acid Vz/F was variable (Table 2). In contrast to Vz/F, bempedoic acid CL/F and CLunbound/F were lower in subjects with reduced kidney function and estimates of bempedoic acid t1/2 were generally longer in subjects with decreased renal function. Bempedoic acid AUCinf exposure estimates were approximately 1.5‐fold, 2.2‐fold, and 2.2‐fold higher in subjects with mild, moderate, and severe renal impairment, respectively, compared with subjects with normal renal function (Table 3).
TABLE 2.
Bempedoic acid pharmacokinetic parameters following administration of a single dose of bempedoic acid 180 mg by baseline renal impairment category
| Parameter | Unit |
Normal function (n = 6) |
Mild impairment (n = 8) |
Moderate impairment (n = 5) |
Severe impairment (n = 5) |
|---|---|---|---|---|---|
| AUCinf, μg•h/ml | GM (% CV) | 179 (33.2) | 269 (26.9) | 392 (52.2) | 399 (53.0) |
| % Change | — | 50.3 | 119 | 123 | |
| Cmax, μg/ml | GM (% CV) | 9.72 (16.1) | 13.6 (20.0) | 9.25 (48.0) | 11.6 (38.0) |
| % Change | — | 39.9 | −4.8 | 19.3 | |
| Tmax, h | Median (range) | 3.0 (2.0–12.1) | 3.0 (1.0–6.0) | 4.0 (2.0–12.0) | 3.0 (2.0–12.0) |
| % Change | — | 0.0 | 33.3 | 0.0 | |
| t½, h | GM (% CV) | 15.1 (23.6) | 23.5 (54.3) | 48.3 (20.2) | 25.0 (32.4) |
| % Change | — | 55.6 | 220 | 65.6 | |
| CL/F, ml/min | GM (% CV) | 16.8 (33.2) | 11.2 (26.9) | 7.66 (52.2) | 7.52 (52.9) |
| % Change | — | −33.3 | −54.4 | −55.2 | |
| CLunbound/F, L/h | Mean (% CV) | 41.2 (28.3) | 26.8 (32.2) | 16.1 (47.3) | 15.4 (52.9) |
| % Change | — | −35.0 | −60.9 | −62.6 | |
| Vz/F, L | GM (% CV) | 21.8 (36.6) | 22.7 (53.4) | 32.0 (52.5) | 16.3 (56.1) |
| % Change | — | 4.1 | 46.8 | −25.2 | |
| funbound | GM (% CV) | 0.0244 (14.1) | 0.0250 (12.2) | 0.0286 (5.3) | 0.0293 (22.4) |
| % Change | — | 2.5 | 17.2 | 20.1 | |
| AUCm/AUCp | GM (% CV) | 0.179 (20.6) | 0.200 (33.1) | 0.212 (30.4) | 0.211 (23.2) |
| % Change | — | 13.6 | 20.5 | 19.9 |
Abbreviations: AUC, area under the concentration‐time curve; AUCinf, area under the concentration‐time curve calculated from time 0 to infinity; AUCm/AUCp, ratio of ESP15228 AUC to bempedoic acid AUC; CL/F, oral clearance; Cmax, maximum observed concentration; CV, coefficient of variation; funbound, bempedoic acid unbound fraction; GM, geometric mean; % Change, percent change in geometric mean or median from subjects with normal renal function; t½, elimination half‐life; Tmax, time of maximum concentration; Vz/F, apparent volume of distribution.
TABLE 3.
Ratio of geometric least squares mean and 90% CI estimates of bempedoic acid and ESP15228 pharmacokinetic parameters
| Parameter | Treatment comparison a | Bempedoic acid | ESP15228 | ||
|---|---|---|---|---|---|
| Geometric LS mean ratio | 90% CI | Geometric LS mean ratio | 90% CI | ||
| Cmax, μg/ml | B/A | 1.398 | 1.059–1.845 | 1.377 | 1.095–1.732 |
| C/A | 0.952 | 0.697–1.299 | 1.363 | 1.054–1.762 | |
| D/A | 1.198 | 0.878–1.635 | 1.458 | 1.127–1.885 | |
| AUClast, μg•h/ml | B/A | 1.495 | 1.045–2.141 | 1.667 | 1.121–2.479 |
| C/A | 2.184 | 1.461–3.265 | 2.620 | 1.679–4.089 | |
| D/A | 2.233 | 1.493–3.339 | 2.669 | 1.710–4.166 | |
| AUCinf, μg•h/ml | B/A | 1.501 | 1.049–2.149 | 1.673 | 1.126–2.486 |
| C/A | 2.187 | 1.463–3.270 | 2.588 | 1.660–4.034 | |
| D/A | 2.228 | 1.490–3.331 | 2.623 | 1.683–4.089 | |
Abbreviations: AUCinf, area under the concentration‐time curve calculated from time 0 to infinity; AUClast, area under the concentration‐time curve from time 0 to time of last measurable concentration; CI, confidence interval; Cmax, maximum observed concentration; eGFR, estimated glomerular filtration rate; LS, least squares.
A = normal renal function (eGFR ≥ 90 ml/min); B = mild renal impairment (eGFR 60–<90 ml/min); C = moderate renal impairment (eGFR 30–<60 ml/min); D = severe renal impairment (eGFR <30 ml/min).
Similar to bempedoic acid, ESP15228 peak concentrations were comparable across groups. Metabolite concentration‐time profiles suggest ESP15228 formation was delayed in subjects with severe renal impairment and t1/2 was longer among subjects with moderate and severe renal impairment (Figure 2). The ratio of circulating levels of ESP15228 to bempedoic acid was generally unchanged, with estimates of AUCm/AUCp ratios slightly greater in subjects with renal impairment compared with subjects with normal renal function. ESP15228 AUCinf exposure estimates were ~ 1.7‐fold, 2.6‐fold, and 2.6‐fold higher in subjects with mild, moderate, and severe renal impairment, respectively, compared with subjects with normal renal function (Table 3).
The extent of bempedoic acid and ESP15228 plasma protein binding was decreased with decreasing renal function, as mean estimates of unbound fraction ranged from 2.44% in subjects with normal renal function to 2.93% in subjects with severe renal impairment for bempedoic acid and 1.17% to 1.43% for ESP15228 in subjects with normal renal function and severe renal impairment, respectively.
Safety
Bempedoic acid treatment was generally well‐tolerated, regardless of renal function category. All AEs were mild or moderate in severity, with treatment‐emergent AEs reported for one subject (16.7%) with normal renal function, five (62.5%) with mild, two (40.0%) with moderate, and one (20.0%) with severe renal impairment (Table 4). There were no severe or serious AEs, deaths, or discontinuations due to AEs. The most frequent AE was blood vessel puncture site hemorrhage, which was reported for three subjects (37.5%) with mild renal impairment, one (20.0%) with severe renal impairment, and no subjects with normal renal function or moderate renal impairment. No relationship between the level of renal impairment and number of AEs was observed, and no new safety signals were identified in subjects with impaired renal function compared with subjects with normal renal function.
TABLE 4.
Treatment‐emergent adverse events
| Preferred term | Renal impairment category a , b | AE severity |
|---|---|---|
| Upper respiratory tract infection | Mild (n = 1); severe (n = 1) | Moderate |
| Vertigo | Mild (n = 1) | Moderate |
| Hypertension | Moderate (n = 1) | Moderate |
| Vessel puncture site hemorrhage | Mild (n = 3); severe (n = 1) | Mild |
| Headache | Mild (n = 1); moderate (n = 1) | Mild |
| Vessel puncture site hematoma | Mild (n = 2) | Mild |
| Leukocytosis | Normal (n = 1) | Mild |
| Constipation | Normal (n = 1) | Mild |
| Administration site extravasation | Normal (n = 1) | Mild |
| Oropharyngeal pain | Mild (n = 1) | Mild |
| Vessel puncture site erythema | Mild (n = 1) | Mild |
| Vessel puncture site paresthesia | Mild (n = 1) | Mild |
| Diarrhea | Moderate (n = 1) | Mild |
| Dizziness | Moderate (n = 1) | Mild |
| Confusional state | Moderate (n = 1) | Mild |
| Skin abrasion | Severe (n = 1) | Mild |
Abbreviations: AE, adverse event; eGFR, estimated glomerular filtration rate.
Normal renal function = eGFR ≥ 90 ml/min, mild renal impairment = eGFR 60–<90 ml/min, moderate renal impairment = eGFR 30–<60 ml/min, and severe renal impairment = eGFR <30 ml/min.
n refers to the number of events; some subjects reported multiple events.
DISCUSSION
In this phase I study, the PKs of bempedoic acid and ESP15228 were assessed following single bempedoic acid 180‐mg dose administration to subjects with normal or impaired kidney function of varying degrees. Unexpectedly, geometric mean AUCinf exposures of bempedoic acid were 1.5‐fold, 2.2‐fold, and 2.2‐fold higher in subjects with mild, moderate, and severe renal impairment, respectively, compared with subjects having normal renal function, whereas peak concentrations were similar across groups.
Plausible mechanisms to explain the observed increases in AUC without changes in Cmax could involve alterations in factors commonly observed in subjects with renal impairment, such as changes in plasma protein binding, clearance pathways, or bioavailability. Among subjects with severe renal impairment, bempedoic acid CL/F and CLunbound/F were decreased by 55.2% and 62.6%, respectively, and plasma free fraction was increased by 20.1% relative to subjects with normal renal function. Increases in the free fraction of low extraction drugs, such as bempedoic acid, would be expected to result in decreased total drug concentrations and would not necessarily account for changes observed in either CL/F or CLunbound/F. Given that the major pathway of elimination involves UGT2B7, a UGT expressed in both the kidneys and liver, with subsequent urinary excretion of bempedoic acid‐glucuronide, impaired renal function might be expected to decrease the elimination of bempedoic acid‐glucuronide but not bempedoic acid per se. Indeed, this is observed with other drugs such as morphine, which is converted to morphine‐6‐O‐glucuronide by UGT2B7. 17 , 18 , 20 Although renal impairment does not greatly impact morphine clearance, impaired renal function was associated with a reduction in urinary excretion and accumulation of morphine‐6‐O‐glucuronide. 17 , 18 , 20 However, as bempedoic acid glucuronide was not directly measured, this mechanism remains speculative. A reduction in clearance of another major metabolic pathway, reversible biotransformation to ESP15228, also does not support such a hypothesis as estimates of AUCm/AUCp ratio for ESP15228 to bempedoic acid were similar across groups. Finally, a change in apparent bioavailability might be responsible for alterations in observed PKs, where a shift from renal to hepatobiliary elimination of bempedoic acid‐glucuronide could result in a greater fraction of glucuronide metabolite being subjected to intestinal deconjugation and enterohepatic recirculation. As the estimated fraction of oral dose absorbed in the gut is high (90%) and bempedoic acid is a low extraction drug, an increase in absolute bioavailability is unlikely to explain the increases in bempedoic acid AUC. The hypothesized change in apparent bioavailability is supported by (1) the observed bempedoic acid PK parameter changes in CL/F and CLunbound/F, where both were substantially decreased, and (2) the observed differences in PK profiles, where secondary peaks were most prominently observed in subjects with severe renal impairment.
These PK findings indicate that subjects with severe renal impairment who received bempedoic acid treatment experienced a 2.2‐fold increase in bempedoic acid AUC exposure on average. Despite increases in PK exposure, a single dose of bempedoic acid was generally well‐tolerated by all subjects, regardless of the degree of renal impairment, and no safety concerns were identified relative to subjects with normal renal function. These findings from the current study were consistent with the safety and tolerability of bempedoic acid among patients with hypercholesterolemia having mild or moderate renal impairment in four phase III randomized, placebo‐controlled studies involving over 3600 patients, where no notable differences in AE incidence were observed between treated and placebo patients. 21 Although bempedoic acid treatment in the phase III studies was associated with small but reversible increases in serum blood urea nitrogen, creatinine, and uric acid in some subjects, these changes were not associated with baseline renal function. 21 For example, the mean percent increases from baseline in uric acid after 52 weeks of bempedoic acid treatment ranged from 13.1% to 13.9% in subjects with mild (n = 1168) and moderate (n = 268) renal impairment and normal renal function (n = 388). 21 In terms of clinical implications, no adjustments in bempedoic acid dosage are currently recommended for subjects with mild or moderate renal impairment based on the small number of subjects in the current study and safety analyses of patients with hypercholesterolemia who received steady‐state bempedoic acid dosing in four phase III studies. 13 Because the population in the current study included a small number of subjects with severe renal impairment (n = 5) and none were included in phase III studies; further investigation is needed to determine whether any dose adjustments may be required for individuals with eGFR less than 30 ml/min.
The limitations of this study include a small subject population (n = 24), which may not accurately reflect exposure with the larger population of patients receiving bempedoic acid therapy. The study population did not include any subjects with end‐stage renal disease and/or dialysis and therefore the disposition of bempedoic acid in these patients is currently unknown. Additionally, the data reported herein characterize bempedoic acid after single‐dose administration and the effects of impaired renal function may differ after prolonged treatment. Furthermore, plasma concentrations of bempedoic acid‐glucuronide were not measured. Future studies will focus on these questions to determine the mechanism by which bempedoic acid PK exposure is increased due to impaired renal function.
In conclusion, bempedoic acid AUC exposures were increased by approximately twofold in subjects with renal impairment following a single oral 180‐mg dose of bempedoic acid without a corresponding increase in Cmax compared with subjects with normal renal function. A switch from renal to biliary excretion of bempedoic acid glucuronide, the major clearance pathway for bempedoic acid, with subsequent enterohepatic recirculation of bempedoic acid seems to be the most plausible explanation. Bempedoic acid was generally well‐tolerated by all subjects, regardless of the degree of renal impairment. Based on the safety findings to date, no dose adjustments are currently required for patients with mild or moderate renal impairment.
CONFLICT OF INTEREST
B.M.A., M.G.E., W.J.S., and P.T. are current (B.M.A.) or former (M.G.E., W.J.S., and P.T.) employees of Esperion Therapeutics, Inc., and may hold stock and/or stock options. W.J.S. is currently the Principal of Cardiometabolic Consulting, LLC. D.K.R. is a current employee of Nucleus Network, Pty, Ltd., which had a corporate agreement with Esperion Therapeutics, Inc., during this study.
AUTHOR CONTRIBUTIONS
P.T. designed the research. D.K.R. performed the research. M.G.E. analyzed the data. B.M.A., W.J.S., P.T., and M.G.E. wrote the manuscript.
ACKNOWLEDGEMENTS
Medical writing support (funded by Esperion Therapeutics, Inc.) was provided by Callie A. S. Corsa, PhD, and Lamara D. Shrode, PhD, CMPP, of JB Ashtin, who developed the first draft based on an author‐approved outline and assisted in implementing author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations.
Amore BM, Sasiela WJ, Ries DK, Tresh P, Emery MG. Pharmacokinetics of bempedoic acid in patients with renal impairment. Clin Transl Sci. 2022;15:789‐798. doi: 10.1111/cts.13202
Funding information
This study was supported by Esperion Therapeutics, Inc.
REFERENCES
- 1. Centers for Disease Control and Prevention, Chronic kidney disease in the United States (2021). https://www.cdc.gov/kidneydisease/publications‐resources/ckd‐national‐facts.html. Accessed April 6, 2021.
- 2. Centers for Disease Control and Prevention, Chronic kidney disease risk factors and prevention (2021). https://www.cdc.gov/kidneydisease/publications‐resources/annual‐report/ckd‐risk‐prevention.html. Accessed April 6, 2021.
- 3. US Food and Drug Administration, Draft Guidance: Pharmacokinetics in patients with impaired renal function — study design, data analysis, and impact on dosing and labeling (2020). https://www.fda.gov/media/78573/download. Accessed April 6, 2021.
- 4. European Medicines Agency, Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function (2016). https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐evaluation‐pharmacokinetics‐medicinal‐products‐patients‐decreased‐renal‐function_en.pdf. Accessed April 6, 2021.
- 5. Pinkosky SL, Newton RS, Day EA, et al. Liver‐specific ATP‐citrate lyase inhibition by bempedoic acid decreases LDL‐C and attenuates atherosclerosis. Nat Commun. 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin‐intolerant patients with hypercholesterolemia: a randomized, placebo‐controlled study. Atherosclerosis. 2018;277:195‐203. [DOI] [PubMed] [Google Scholar]
- 7. Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022‐1032. [DOI] [PubMed] [Google Scholar]
- 8. Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low‐density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322:1780‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laufs U, Maciej Banach GB, Mancini J, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Food and Drug Administration, 211616Orig1s000 Integrated Review − Nexletol (bempedoic acid) Tablets (2020). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/211616Orig1s000IntegratedR.pdf. Accessed September 28, 2021.
- 11. Engel BJ, Preusch K, Brown C, Cramer CT, Shoup R. Measurement of bempedoic acid and its keto metabolite in human plasma and urine using solid phase extraction and electrospray LC‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1154:122291. [DOI] [PubMed] [Google Scholar]
- 12. Lalwani ND, Hanselman JC, MacDougall DE, Sterling LR, Cramer CT. Complementary low‐density lipoprotein‐cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC‐1002) to high‐dose atorvastatin background therapy in hypercholesterolemic patients: a randomized placebo‐controlled trial. J Clin Lipidol. 2019;13:568‐579. [DOI] [PubMed] [Google Scholar]
- 13. NEXLETOL (bempedoic acid) tablets for oral use . Prescribing Information. Esperion Therapeutics; 2020. [Google Scholar]
- 14. Hanselman JC, MacDougall DE, Emery MG, Sasiela WJ, Amore BM. A phase 1 drug‐drug interaction study assessing the effects of steady‐state (SS) probenecid (PB) on single dose bempedoic acid (BA) pharmacokinetics (PK) in healthy subjects. Clin Pharmacol Ther. 2020;107:S43. [Google Scholar]
- 15. Amore BM, Sasiela WJ, Emery MG. The effects of impaired hepatic function on pharmacokinetics of bempedoic acid, a first‐in‐class adenosine triphosphate (ATP) citrate lyase inhibitor, evaluated in an open‐label, single‐dose, parallel‐group study. Clin Pharmacol Ther. 2021;109:S53. [Google Scholar]
- 16. Oda S, Fukami T, Yokoi T, Nakajima M. A comprehensive review of UDP‐glucuronosyltransferase and esterases for drug development. Drug Metab Pharmacokinet. 2015;30:30‐51. [DOI] [PubMed] [Google Scholar]
- 17. Milne RW, Nation RL, Somogyi AA, Bochner F, Griggs WM. The influence of renal function on the renal clearance of morphine and its glucuronide metabolites in intensive‐care patients. Br J Clin Pharmacol. 1992;34:53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28:497‐504. [DOI] [PubMed] [Google Scholar]
- 19. Nyman HA, Dowling TC, Hudson JQ, Peter WLS, Joy MS, Nolin TD. Comparative evaluation of the Cockcroft‐Gault Equation and the Modification of Diet in Renal Disease (MDRD) study equation for drug dosing: an opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2011;31:1130‐1144. [DOI] [PubMed] [Google Scholar]
- 20. Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4:1065‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bays HE, Banach M, Catapano AL, et al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol. 2020;14:649‐659.e646. [DOI] [PubMed] [Google Scholar]


