Abstract
Production of a metallo-β-lactamase activity was detected in a carbapenem-resistant Pseudomonas aeruginosa clinical isolate (isolate VR-143/97) from an Italian inpatient at the Verona University Hospital (northern Italy). The metallo-β-lactamase determinant was isolated from a genomic library of VR-143/97, constructed in an Escherichia coli plasmid vector, by screening for clones with reduced susceptibility to imipenem. Sequencing of the cloned gene revealed that it encoded a new class B β-lactamase that was named VIM-1. At the sequence level VIM-1 was rather divergent from the other class B enzymes (16.4 to 38.7% identity), overall being more similar to members of subclass B1 including the β-lactamase II of Bacillus cereus (Bc-II), the Bacteroides fragilis CcrA, the Chryseobacterium meningosepticum BlaB, and the cassette-encoded IMP-1 enzymes. Among these, VIM-1 showed the highest degree of similarity to Bc-II. Similarly to blaIMP, blaVIM was also found to be carried on a gene cassette inserted into a class 1 integron. The blaVIM-containing integron was located on the chromosome of P. aeruginosa VR-143/97, and the metallo-β-lactamase-encoding determinant was not transferable to E. coli by conjugation. Expression of the integron-borne blaVIM gene in E. coli resulted in a significant decrease in susceptibility to a broad array of β-lactams (ampicillin, carbenicillin, piperacillin, mezlocillin, cefotaxime, cefoxitin, ceftazidime, cefoperazone, cefepime, and carbapenems), revealing a very broad substrate specificity of the VIM-1 enzyme.
Metallo-β-lactamases are threatening resistance determinants from the clinical standpoint due to their usually broad substrate specificities, which always include carbapenems, and to their resistance to mechanism-based β-lactamase inactivators (4, 20, 26, 29). Thus far, the overall prevalence of these enzymes among clinical isolates has remained low since most metallo-β-lactamases are encoded by genes resident in species that are of minor clinical relevance and that are not transferable to major bacterial pathogens (4, 20, 29). Recently, however, the spread of a previously unknown metallo-β-lactamase gene, blaIMP, has been reported in Japan among isolates of various members of the family Enterobacteriaceae, Pseudomonas aeruginosa, and other nonfastidious, gram-negative nonfermenters (12, 15, 34, 35). This gene, whose original host remains unknown, was found to be carried on mobile gene cassettes inserted in plasmid- or chromosome-borne integrons (2, 16, 18, 25, 41). Its product, the IMP-1 enzyme, exhibits a very broad substrate profile (19, 25) and contributes to β-lactam resistance in bacterial hosts that have acquired the blaIMP gene (15, 17, 34). Nosocomial IMP-1-producing isolates of the family Enterobacteriaceae and Pseudomonas have been found in Japanese hospitals since 1991, and their clinical impact has been reviewed (12).
Although epidemic diffusion of blaIMP has not been reported elsewhere, production of a metallo-β-lactamase identical or very similar to IMP-1 was recently detected in an Italian isolate of Acinetobacter baumannii (8), while production of a metallo-β-lactamase apparently different from IMP-1 was reported in a P. aeruginosa isolated in the United Kingdom (40), suggesting that the spread of similar resistance determinants is also commencing in Europe.
In this paper we report on the characterization of a new integron-borne metallo-β-lactamase gene, named blaVIM, carried by a carbapenem-resistant P. aeruginosa clinical isolate that represents the index strain responsible for a nosocomial outbreak that recently occurred at the Verona University Hospital (northern Italy). This new determinant is different from either blaIMP or other known metallo-β-lactamase genes, and its acquisition can contribute to broad-spectrum β-lactam resistance in the bacterial host.
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
P. aeruginosa VR-143/97 was a clinical isolate from a surgical wound. It was isolated in February 1997 from an Italian patient admitted at the Intensive Care Unit Department of the Verona University Hospital (northern Italy). The patient had been transferred to the Intensive Care Unit from another department of the same hospital and did not report any recent history of travel or of other hospitalization. Escherichia coli DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1; Bethesda Research Laboratories, Bethesda, Md.] was used as the host in electrotransformation experiments and for all recombinant plasmids. E. coli MKD-135 (argH rpoB18 rpoB19 recA rpsL) was used as a recipient strain in conjugation experiments. Bacterial strains were always grown aerobically at 37°C. Plasmid pACYC184 (6) was used as the vector for construction of the P. aeruginosa genomic library. Plasmid pBC-SK (Stratagene, La Jolla, Calif.) was used for some subcloning steps.
In vitro susceptibility testing.
MICs were determined by a broth macrodilution method (23) with cation-supplemented Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) and a bacterial inoculum of 5 × 105 CFU per tube. Results were recorded after incubation for 18 h at 37°C. MIC determinations were performed in triplicate. Results of susceptibility testing were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (23). Antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. Imipenem was from Merck Research Laboratories (Rahway, N.J.), meropenem was from Zeneca Pharmaceuticals (Cheshire, United Kingdom), ceftazidime was from Glaxo-Wellcome (Verona, Italy), cefepime was from Bristol-Myers Squibb (Wallingford, Conn.), and aztreonam was from the Squibb Institute for Medical Research (Princeton, N.J.). All antibiotic solutions were prepared immediately before use.
β-Lactamase assays.
β-Lactamase activity in crude cell extracts was assayed spectrophotometrically. Reactions were performed in 30 mM N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)–NaOH buffer (pH 7.0) (AB) at 25°C in a total volume of 0.75 ml. Imipenem hydrolysis was monitored at 299 nm (Δɛ = −9,000 M−1 cm−1) by using an initial substrate concentration of 150 μM. Inhibition of enzymatic activity by EDTA was assayed by measuring the residual carbapenemase activity after incubation of the crude extract for 20 min at 25°C in the presence of 2 mM EDTA (EDTA was added to the crude extract from a 100 mM stock solution in AB). A control without EDTA was always run in parallel. Reactivation by Zn2+ was assayed by measuring the carbapenemase activity after incubation of the EDTA-treated enzyme preparation for 20 min at 25°C in the presence of 2 mM Zn2+ (Zn2+ was directly added to the EDTA-treated extract as ZnCl2, which was from a 100 mM stock solution in 10 mM HCl). Controls for the effect of Zn2+ alone on the enzymatic activity and on the substrate stability were also included. Crude cell extracts were prepared as follows. Cells were grown in Mueller-Hinton broth aerobically at 37°C until the late exponential phase, collected by centrifugation, resuspended in AB (1/10 of the original culture volume), and disrupted by sonication (six times for 15 s each time at 50 W). The supernatant obtained after centrifugation at 10,000 × g for 10 min to remove the cell debris represented the crude extract. The protein concentration in the solution was determined with a commercial kit (Bio-Rad protein assay; Bio-Rad, Richmond, Calif.), with bovine serum albumin used as a standard.
Analytical IEF.
Analytical isoelectric focusing (IEF) of crude cell extracts was performed in precast 5% polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5) (Ampholine PAGplate; Amersham Pharmacia Biotech, Uppsala, Sweden) with a Multiphor II Apparatus (Pharmacia). Gels were focused at 0.1 W/cm2 for 2 h at 10°C. β-Lactamases were detected as purple bands after overlaying the gel with filter paper soaked with a 0.25 mM nitrocefin solution in AB supplemented with 2 mM ZnCl2.
Conjugation experiments.
Conjugation experiments were performed on solid medium as described previously (39). The initial donor/recipient ratio was 0.1. The plates used for mating were incubated at 30°C for 8 h. Transconjugants were selected on Mueller-Hinton agar containing ampicillin (50 μg/ml) plus rifampin (400 μg/ml). The detection sensitivity of the assay was of ≥5 × 10−7 transconjugants/recipient.
Recombinant DNA methodology.
Basic recombinant DNA procedures were performed as described by Sambrook et al. (33). Extraction of plasmid DNA from P. aeruginosa was performed by various methods (3, 14, 37). For construction of the genomic library of P. aeruginosa VR-143/97, genomic DNA was partially digested with Sau3AI and fragments in the 2- to 9-kb size range were purified by agarose gel electrophoresis with the Geneclean II kit (Bio 101, Inc., La Jolla, Calif.). The purified restriction fragments were ligated to the BamHI-linearized and dephosphorylated pACYC184 vector, and the ligation mixture was used to transform E. coli DH5α by electroporation. The ratio of recombinant clones to those carrying an empty religated vector was >20, as shown by replica plating of transformants, which were selected on Luria-Bertani (LB) agar plates containing chloramphenicol (70 μg/ml), onto plates containing both chloramphenicol and tetracycline (20 μg/ml). Electroporation of E. coli cells was performed with a Gene Pulser apparatus (Bio-Rad) according to the manufacturer’s instructions.
DNA sequencing and computer analysis of sequence data.
The DNA sequences on both strands were determined by the dideoxy-chain termination method with an automatic DNA sequencer (LICOR 4000; LI-COR Inc., Lincoln, Nebr.), the Thermosequenase DNA sequencing kit (Amersham), and IRD 800-labeled custom sequencing primers (MWG-Biotech, Munich, Germany). Similarity searches against sequence databases were performed with an updated version of the BLAST program (1). Computer analysis of the sequence data was performed with an updated version (version 8.1) of the University of Wisconsin Genetics Computer Group package (9). Codon usage tables were compared as described by Grantham et al. (11). Multiple sequence alignments, generated with the help of the PILEUP program, were manually refined by considering the information available on the three-dimensional structures of the Bc-II and CcrA enzymes (5, 7).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank/DDBJ sequence databases and has been assigned accession no. Y18050.
RESULTS
Production of metallo-carbapenemase activity by P. aeruginosa VR-143/97.
P. aeruginosa VR-143/97 was a clinical isolate resistant to carbenicillin, piperacillin, mezlocillin, cefoperazone, ceftazidime, cefepime, carbapenems, and aztreonam (Table 1).
TABLE 1.
β-Lactam susceptibilities of P. aeruginosa VR-143/97 and E. coli DH5α(pBCLL/39H) carrying the cloned integron-borne blaVIM genea
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| VR-143/97 | DH5α(pBCLL/39H) | DH5α(pBC-SK) | |
| Ampicillin | >128 | >128 | 1 |
| Carbenicillin | >128 | >128 | 4 |
| Piperacillin | >128 | 128 | 0.5 |
| Mezlocillin | >128 | >128 | 1 |
| Cefotaxime | >128 | 64 | ≤0.06 |
| Cefoxitin | >128 | 128 | 2 |
| Ceftazidime | >128 | 128 | 0.12 |
| Cefoperazone | >128 | >128 | 0.12 |
| Cefepime | >128 | 32 | ≤0.06 |
| Imipenem | >128 | 2 | 0.12 |
| Meropenem | 128 | 1 | ≤0.06 |
| Aztreonam | 32 | 0.25 | 0.25 |
The susceptibility of E. coli DH5α carrying the empty vector is also presented for comparison.
A crude extract prepared from this strain showed imipenem-hydrolyzing activity (Table 2). The carbapenemase activity was inhibited by treatment with EDTA and could be fully restored by subsequent addition of Zn2+ at the same molar concentration as EDTA, without significant dilution of the sample (Table 2). Enzyme reactivation under similar conditions was likely due to the initial presence of a certain amount of divalent cations in the crude extract.
TABLE 2.
Imipenem-hydrolyzing activities of crude extracts of P. aeruginosa VR-143/97 and of the two E. coli clones (DH5α[pAC2AL] and DH5α[pAC2IL]) able to grow on imipenem-containing mediuma
| Strain | Sp act (pmol/min/μg of protein)b
|
||
|---|---|---|---|
| Crude extract | Crude extract + EDTA | Crude extract + EDTA + Zn2+c | |
| VR-143/97 | 109 ± 6 | 15 ± 1 (14) | 112 ± 7 (103) |
| DH5α(pAC2AL) | 512 ± 20 | 40 ± 3 (8) | 493 ± 21 (96) |
| DH5α(pAC2IL) | 489 ± 20 | 38 ± 3 (8) | 481 ± 23 (98) |
| DH5α(pACYC184) | <2 | NAd | NA |
The basal activity measured with E. coli DH5α containing the empty vector is also reported for comparison. Specific activity data represent the mean ± standard deviations of three measurements.
Numbers in parentheses represent the percent activity measured after exposure to EDTA or subsequent Zn2+ addition, considering as 100% the activity of the crude extract. See Materials and Methods for experimental details.
The addition to crude extracts untreated with EDTA of Zn2+ at the same concentration used for the enzyme reactivation assay caused an increase (approximately 1.5-fold) in the carbapenemase activity. Zn2+ alone at the same concentration did not cause any detectable hydrolysis of the substrate.
NA, not assayed.
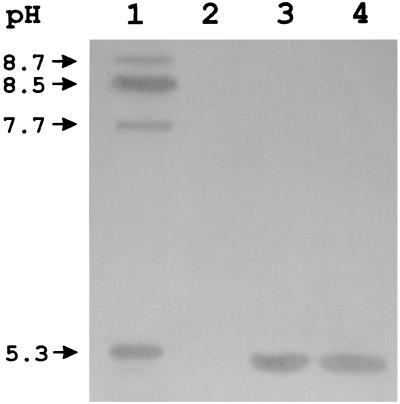
IEF analysis of the crude extract of VR-143/97 revealed the presence of three bands of β-lactamase activity that focused at alkaline pH values (a major band at pH 8.5 and two minor ones at pHs 8.7 and 7.7, respectively) and of one band that focused at pH 5.3 (Fig. 1). The bands with pIs of 8.7 to 8.5 are likely contributed by the resident AmpC enzyme of P. aeruginosa, the production of which appeared to be derepressed in VR-143/97.
FIG. 1.
Results of IEF analysis of crude extracts of P. aeruginosa VR-143/97 (lane 1), E. coli DH5α(pACYC184) (lane 2), E. coli DH5α(pAC2AL) (lane 3), and E. coli DH5α(pAC2IL) (lane 4). Approximately 10 μg of total protein was loaded in each lane.
A blaIMP-specific probe containing the 0.5-kb HindIII fragment internal to the blaIMP gene (25) did not hybridize to VR-143/97 in a colony blot assay (data not shown), indicating that a metallo-β-lactamase other than IMP-1 was produced by this strain.
Cloning of the metallo-β-lactamase determinant of P. aeruginosa VR-143/97.
Since in P. aeruginosa acquired resistance determinants are often carried on plasmids, the presence of plasmid DNA in VR-143/97 was initially investigated by various extraction methods. No plasmid DNA was detectable by agarose gel electrophoresis in any plasmid preparation from that strain (data not shown). The same preparations did not yield any β-lactam-resistant transformant when they were used in electrotransformation experiments with E. coli DH5α as a recipient strain. Conjugation experiments also failed to demonstrate the occurrence of conjugative transfer of β-lactamase determinants from P. aeruginosa VR-143/97 to E. coli MKD-135.
The metallo-carbapenemase determinant was isolated from a genomic library of VR-143/97, which was constructed in the E. coli plasmid vector pACYC184 and transformed into the E. coli host DH5α, by screening the library for clones that had reduced susceptibility to imipenem. Two such clones (2AL and 2IL) were found among approximately 3,500 transformants after replica plating onto LB medium containing chloramphenicol (70 μg/ml) plus imipenem (5 μg/ml). Crude extracts prepared from these clones exhibited a strong carbapenemase activity that, similar to the one of VR-143/97, was susceptible to inhibition by EDTA and could be restored by subsequent addition of Zn2+ to the EDTA-treated samples (Table 2). IEF analysis of the crude extracts yielded, with both clones, a β-lactamase band at pH 5.3 that was not detectable in the parent E. coli strain and that apparently corresponded to one of those present in VR-143/97 (Fig. 1). The results presented above suggested that a cloned copy of the metallo-β-lactamase gene of VR-143/97 was carried and expressed in these clones and that the corresponding enzyme had an acidic pI.
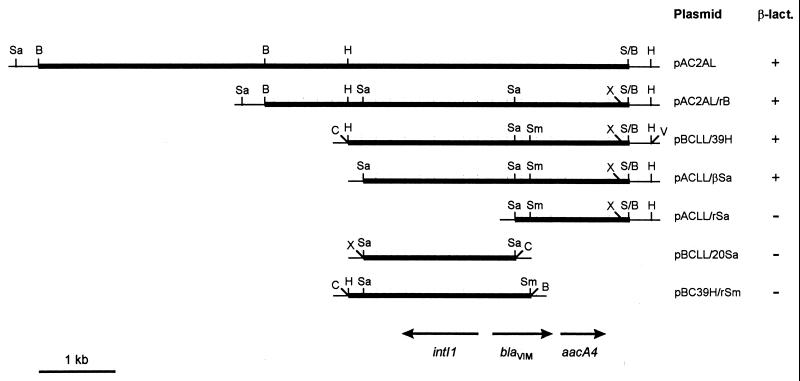
Restriction analysis showed that the recombinant plasmids harbored by these clones (named pAC2AL and pAC2IL, respectively) contained an apparently identical 7.8-kb insert (data not shown). The insert of plasmid pAC2AL (Fig. 2) was subjected to further characterization. Subcloning analysis showed that the β-lactamase-encoding determinant carried by pAC2AL was located within a 3.5-kb HindIII-XhoI fragment, being apparently interrupted by SalI and SmaI restriction sites located approximately 0.2 kb apart from each other (Fig. 2).
FIG. 2.
Physical map of the insert of plasmid pAC2AL and subcloning strategy. Thick lines represent cloned DNA, and thin lines correspond to vector sequences. Plasmids whose names begins with pAC are pACYC184 derivatives, while those whose name begins with pBC are pBC-SK derivatives. Production of metallo-β-lactamase activity (β-lact.) was assayed with crude extracts as described in Materials and Methods. B, BamHI; C, ClaI; H, HindIII; Sa, SalI; Sm, SmaI; S/B, Sau3AI/BamHI junction; V, EcoRV; X, XhoI.
Sequence of the β-lactamase determinant.
The nucleotide sequence of the insert of plasmid pBCLL/39H around the restriction sites that apparently interrupted the β-lactamase determinant was determined. An open reading frame (ORF) which encoded a polypeptide showing high similarity scores with other class B β-lactamases was identified (Fig. 3) in a BLAST search. Results of the subcloning experiments were consistent with the identification of this ORF, named blaVIM, as the β-lactamase-encoding determinant (Fig. 2). A Southern blot analysis of undigested genomic DNA of P. aeruginosa VR-143/97, performed with the 0.24-kb SalI-SmaI fragment internal to the blaVIM ORF (Fig. 2) as a probe, yielded a strong hybridization signal that corresponded to the chromosomal DNA band (data not shown), confirming the origin and the chromosomal location of blaVIM.
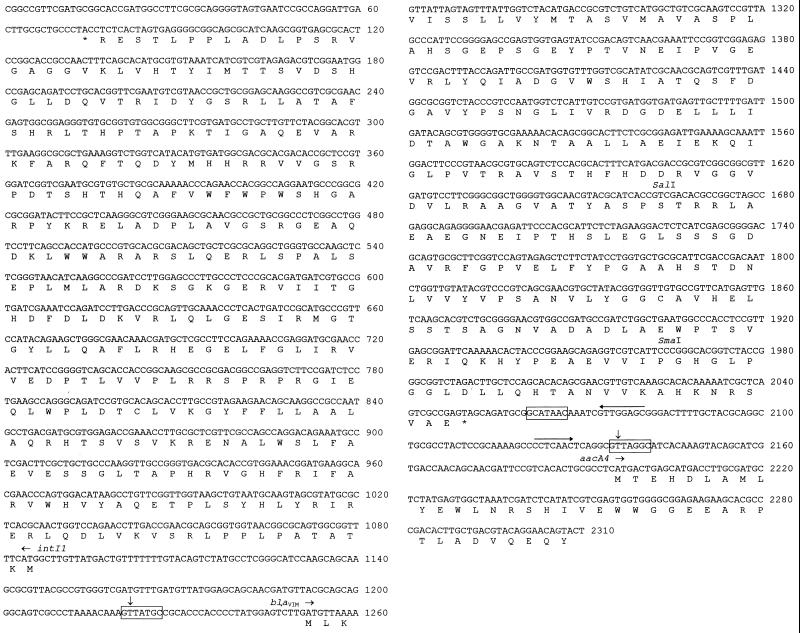
FIG. 3.
Nucleotide sequence of the blaVIM gene cassette and flanking regions. Initiation codons of the various ORFs are indicated, and protein translation is reported below the sequence. The blaVIM cassette boundaries are indicated by vertical arrows. The conserved recombination core sites located at the cassette boundaries and the inverse core site are boxed. The internal 2L and 2R core sites of the 59-base element (36) are overlined with arrows.
The blaVIM ORF encodes a 266-amino-acid polypeptide whose amino-terminal sequence exhibits features typical of those of bacterial signal peptides that target protein secretion into the periplasmic space via the general secretory pathway (Fig. 3). According to known patterns (28), the cleavage site could be located after either the Ala-20 or the Ser-26 residue: in the former case the calculated molecular mass and the pI value of the mature protein would be 25,915 Da and 4.97, respectively; in the latter case they would be 25,322 Da and 4.90, respectively. In either case the theoretical pI value of the VIM-1 enzyme is in good agreement with the IEF results (Fig. 1).
Sequence analysis of the blaVIM-flanking regions revealed that the gene was located on a mobile cassette inserted into a class 1 integron. The blaVIM cassette exhibited all the features typical of these mobile elements (31, 36), including two conserved core sites for recombination crossover at the cassette boundaries, an inverse core site located a few base pairs downstream of the blaVIM termination codon, and a 59-base element bounded by the inverse core site and the downstream conserved core site and containing putative IntI1-binding domains (Fig. 3). The blaVIM cassette was preceded by an intI1 allele that encoded the DNA integrase typical of class 1 integrons (31) but that was oriented in the opposite direction and was followed by a second gene cassette containing an aacA4 allele (Fig. 3) which, in the sequenced part, was identical to that carried by other integrons found in Pseudomonas and members of the family Enterobacteriaceae (22, 24, 27).
The G+C content of the blaVIM cassette was 56%, being considerably higher than that of the blaIMP cassette (40%). The codon usage of the blaVIM ORF was significantly different from that of blaIMP (D2 = 6.33).
Comparison of the VIM-1 enzyme with other class B β-lactamases.
The BLAST search performed with the VIM-1 protein as the query returned the highest similarity scores with other class B β-lactamases of subclass B1 including the Bacillus cereus β-lactamase II (Bc-II) (13), the Bacteroides fragilis CcrA (30), the cassette-encoded IMP-1 (2, 18, 25, 41), and the Chryseobacterium meningosepticum BlaB (32) enzymes.
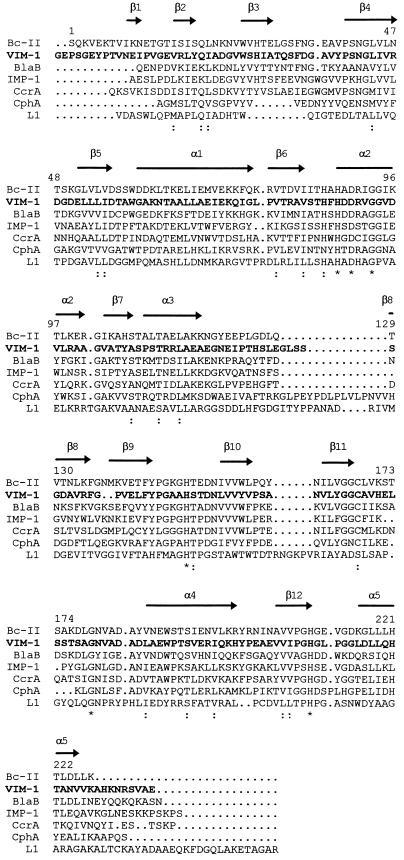
A multiple sequence alignment analysis confirmed the similarity of VIM-1 with other class B β-lactamases and the closer structural relationship of this enzyme with the other members of subclass B1 (Table 3; Fig. 4). In fact, VIM-1 could be aligned with the latter proteins without introducing major gaps except for a short insertion and a short deletion that, referring to the known three-dimensional structure of Bc-II (5), would be located in the loop between the α3 helix and the β8 strand and at the beginning of the β9 strand, respectively (Fig. 4). Among enzymes of subclass B1, VIM-1 exhibited the highest degree of similarity (38.7% identical residues) with Bc-II and was more distantly related to the others (Table 3). Analysis of the phylogenetic relationships among VIM-1 and the other class B β-lactamases confirmed that the former enzyme shares the same phylogeny as the other proteins of subclass B1 and has apparently diverged from the same ancestor as Bc-II during the evolutionary history of this lineage.
TABLE 3.
Percent identity between the amino acid sequences of class B β-lactamasesa
| Enzyme
|
% Identity
|
|||||
|---|---|---|---|---|---|---|
| BlaB | VIM-1 | IMP-1 | CcrA | CphA | L1 | |
| Bc-II | 36.4 | 38.7 | 36.6 | 33.5 | 29.9 | 18.8 |
| BlaB | —b | 26.3 | 30.6 | 28.1 | 28.2 | 11.6 |
| VIM-1 | — | 31.4 | 32.0 | 26.1 | 16.4 | |
| IMP-1 | — | 36.8 | 22.7 | 15.3 | ||
| CcrA | — | 27.3 | 14.7 | |||
| CphA | — | 16.6 | ||||
FIG. 4.
Comparison of the VIM-1 sequence (VIM-1; boldfaced) with those of other molecular class B β-lactamases. Bc-II, β-lactamase II of B. cereus 569/H (13); BlaB, BlaB enzyme of C. meningosepticum CCUG4310 (32); IMP-1, IMP-1 enzyme encoded by the blaIMP gene cassette found in various gram-negative bacteria (2, 18, 25, 41); CcrA, CcrA enzyme of B. fragilis TAL3636 (30); CphA, CphA enzyme of A. hydrophila AE036 (21); L1, L1 enzyme of S. maltophilia IID1275 (38). Identical residues are indicated by an asterisk. Conserved amino acid substitutions are indicated by a colon. Secondary structure elements of Bc-II (5) are also indicated above the sequences. The numbering scheme refers to the Bc-II enzyme.
All the six invariant residues shared by the other class B enzymes (His-88, Asp-90, Gly-93, His-149, Gly-179, and His-210, with the numbering for the Bc-II enzyme of B. cereus 569/H [5]) were also retained in the VIM-1 sequence. At positions 86 and 168, VIM-1 contained His and Cys residues, respectively, similar to most other enzymes of this family. Of the additional residues known to be in or close to the active site of Bc-II (5), five (Asn-42, Trp-59, Leu-114, Asn-180, and Asp-183) were found to be conserved in the VIM-1 protein, whereas two (Thr-150 and Lys-171) were conservatively substituted, and eight (Leu-110, Lys-117, Asn-118, Lys-147, Glu-151, Asp-177, Tyr-185, and Glu-214) were nonconservatively substituted (Fig. 4).
Patterns of β-lactam susceptibility of E. coli producing the VIM-1 enzyme.
The substrate specificity of VIM-1 and its contribution to resistance were investigated by testing the susceptibility to several β-lactams of E. coli DH5α(pBCLL/39H), which carries a recombinant plasmid containing the integron-borne blaVIM cassette and which produces the VIM-1 enzyme (Fig. 2), in comparison with that of DH5α, which carries an empty vector.
VIM-1 production was associated with a significant decrease in the in vitro susceptibility of the E. coli host to ampicillin, carbenicillin, piperacillin, mezlocillin, cefotaxime, cefoxitin, ceftazidime, cefoperazone, cefepime, imipenem, and meropenem but not to aztreonam (Table 1), indicating that the enzyme has a very broad substrate specificity and can contribute to broad-spectrum β-lactam resistance in the microbial host.
DISCUSSION
The appearance of metallo-β-lactamase genes capable of spreading among major bacterial pathogens is a matter of major concern for the future of antimicrobial chemotherapy. A similar occurrence was delayed until the early 1990s, when the integron-borne blaIMP metallo-β-lactamase gene made its appearance in Japan among members of the family Enterobacteriaceae, Pseudomonas, and other nonfastidious, gram-negative nonfermenters isolated in several hospitals (12, 15, 34, 35). Our findings demonstrate that additional integron-borne metallo-β-lactamase genes can be found in P. aeruginosa clinical isolates in different geographic areas and suggest that the spread of similar resistance determinants could become a more widespread problem in the near future. The metallo-β-lactamase gene which is carried by a P. aeruginosa strain recently isolated in the United Kingdom but which is different from blaIMP (40) also appears to be different from blaVIM by consideration of the very different pIs of their products and could represent yet another determinant of this type.
Although blaIMP and blaVIM are both carried on mobile gene cassettes inserted into integrons, their highly divergent sequences along with differences in the G+C content and codon usage indicate an independent phylogeny of these two determinants and of their associated cassette frameworks. In VR-143/97, the blaVIM-containing integron is located on the chromosome and is apparently not transferable by conjugation, at least to E. coli. However, the finding of blaVIM also on conjugative plasmids, similarly to what happens with blaIMP (15), should not be unexpected considering the mobile nature of gene cassettes (31). We are investigating additional VIM-1-producing P. aeruginosa isolates, collected during the nosocomial outbreak for which VR-143/97 represents the index strain, to verify their clonal relationships with VR-143/97 and between each other and to determine the genetic location of blaVIM alleles in these strains.
The VIM-1 enzyme is structurally rather divergent from other class B β-lactamases and represents a new member of this family. Its closest neighbors are the other proteins of subclass B1 and, among these, the B. cereus Bc-II enzyme, with which VIM-1 apparently shares the closest ancestry. The overall degree of similarity between VIM-1 and either Bc-II (38% identical residues) or CcrA (32% identical residues) suggests that the VIM-1 molecule overall retains the same three-dimensional fold as the latter proteins (5, 7). Also, the residues involved in Zn2+ binding are perfectly conserved in VIM-1 compared to those of Bc-II and CcrA, suggesting similar structures of their zinc centers. However, several differences were detected in residues that are known to be in or close to the active site in Bc-II (5). These differences might be relevant for the functional properties of the VIM-1 enzyme. Purification and biochemical characterization of VIM-1 are in progress to determine and compare its kinetic parameters with those of other class B β-lactamases.
Expression of the integron-borne blaVIM gene in E. coli caused a significant decrease in the in vitro susceptibility of the host to a broad array of β-lactams, including penicillins, cephalosporins, and carbapenems. Only aztreonam was apparently unaffected, in agreement with the properties of all other metallo-β-lactamases (4, 10, 19, 32, 42). VIM-1 therefore appears to be a class B enzyme of very broad substrate specificity, similarly to the IMP-1 (19, 25), CcrA (42), BlaB (32), and L1 (10) metallo-β-lactamases. Interestingly, although the in vitro susceptibility was significantly decreased, the VIM-1-producing E. coli host could not be classified as resistant to carbapenems by conventional susceptibility testing (23). A similar phenomenon, which was also noticed with blaIMP when the gene was expressed in E. coli and other members of the family Enterobacteriaceae (17, 18), should be kept in mind for the correct interpretation of the susceptibility results provided by the clinical microbiology laboratory for isolates with acquired metallo-carbapenemases. The much higher carbapenem MICs observed for P. aeruginosa VR-143/97 compared to those for VIM-1-producing E. coli are likely due to a contribution of additional resistance mechanisms in the former strain, including a derepressed high-level production of the AmpC enzyme and, possibly, a loss of OprD.
ACKNOWLEDGMENTS
This work was supported by the European research network on metallo-β-lactamases within the TMR program (contract FMRX-CT98-0232), by grants 97.04260.CT04 and 98.00510.CT04 from the Italian National Research Council (C.N.R.), and by a grant from MURST ex-40% project “structural biology.”
We acknowledge the excellent technical support of Tiziana di Maggio and Michela Cappelli and thank Gennadi Kholodii and S. Mindlin (Institute of Molecular Genetics, The Russian Academy of Sciences, Moscow) for kindly providing us with E. coli MKD-135.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K. Metallo-β-lactamases: a class apart. Clin Infect Dis. 1998;27(Suppl. 1):S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 5.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J-M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concha N, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 8.Cornaglia G, Riccio M L, Mazzariol A, Piccoli P, Lauretti L, Fontana R, Rossolini G M. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet. 1999;353:899–900. doi: 10.1016/s0140-6736(98)05954-6. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 9.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuel L, Frère J-M. An overview of the kinetic parameters of class B β-lactamases. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantham R, Gautier C, Gouy M, Jacobzone M, Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981;9:43–74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirakata Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, Matsuda J, Nakano M, Tomono K, Maesaki S, Kaku M, Yamada Y, Kamihira S, Kohno S. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–2011. doi: 10.1128/aac.42.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain M, Carlino A, Madonna M J, Lampen J O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyobe S, Yamada H, Minami S. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 17.Iyobe S, Tsunoda M, Mitsuhashi S. Cloning and expression in Enterobacteriaceae of the extended-spectrum β-lactamase gene from a Pseudomonas aeruginosa plasmid. FEMS Microbiol Lett. 1994;121:175–180. doi: 10.1111/j.1574-6968.1994.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 18.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frère J-M, Rossolini G M. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:890–901. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, de Pauw E, Amicosante G, Frère J-M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:902–906. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore D M. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massidda O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugnier P, Podglajen I, Goldstein F W, Collatz E. Carbapenems as inhibitors of OXA-13, a novel, integron-encoded β-lactamase in Pseudomonas aeruginosa. Microbiology. 1998;144:1021–1031. doi: 10.1099/00221287-144-4-1021. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.Nobuta K, Tolmasky M E, Crosa L M, Crosa J H. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne D J. Metallo-β-lactamases—a new therapeutic challenge. J Med Microbiol. 1993;39:93–99. doi: 10.1099/00222615-39-2-93. [DOI] [PubMed] [Google Scholar]
- 27.Preston K E, Kacica M A, Limberger R J, Archinal W A, Venezia R A. The resistance and integrase genes of pACM1, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid. 1997;37:105–118. doi: 10.1006/plas.1997.1284. [DOI] [PubMed] [Google Scholar]
- 28.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 32.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M, Galleni M, Frère J-M, Amicosante G. Characterization and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stokes H W, O’Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984;20:608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh T R, Hall L, Assinder S J, Nichols W W, Cartwright S J, MacGowan A P, Bennet P M. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 39.Willets N. Conjugation. Methods Microbiol. 1984;17:33–59. [Google Scholar]
- 40.Woodford N, Palepou M-F I, Babini G S, Bates J, Livermore D M. Carbapenemase-producing Pseudomonas aeruginosa in UK. Lancet. 1998;352:546–547. doi: 10.1016/s0140-6736(05)79255-2. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi H, Nukaya M, Sawai T. EMBL/GenBank database entry no. D29636. Hinxton, United Kingdom: EMBL-European Bioinformatics Institute; 1994. [Google Scholar]
- 42.Yang Y, Rasmussen B A, Bush K. Biochemical characterization of the metallo-β-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992;36:1155–1157. doi: 10.1128/aac.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]