Abstract
The slender filefish is a master of adaptive camouflage and can change its appearance within 1–3 seconds. Videos and photographs of this animal’s cryptic body patterning and behavior were collected in situ under natural light on a Caribbean coral reef. We present an ethogram of body patterning components that includes large- and small-scale spots, stripes and bars that confer a variety of cryptic patterns amidst a range of complex backgrounds. Field images were analyzed to investigate two aspects of camouflage effectiveness: (i) the degree of color resemblance between animals and their nearby visual stimuli and (ii) the visibility of each fish’s actual body outline versus its illusory outline. Most animals more closely matched the color of nearby visual stimuli than that of the surrounding background. Three-dimensional dermal flaps complement the melanophore skin patterns by enhancing the complexity of the fish’s physical skin texture to disguise its actual body shape, and the morphology of these structures was studied. The results suggest that the body patterns, skin texture, postures and swimming orientations putatively hinder both the detection and recognition of the fish by potential visual predators. Overall, the rapid speed of change of multiple patterns, color blending with nearby backgrounds, and the physically complicated edge produced by dermal flaps effectively camouflage this animal among soft corals and macroalgae in the Caribbean Sea.
Keywords: color change, texture, papillae, cirrus, irregular marginal form, coral reef ecology, skin filaments, fronds, cutaneous appendages
INTRODUCTION
The modern study of animal camouflage originated about a century ago (Cott, 1940; Poulton, 1890; Thayer and Thayer, 1918), but there has only recently been empirical investigation of the principles of camouflage and pursuit of specific and consistent definitions (e.g., Fraser et al., 2007; Stevens, 2007; Stevens and Merilaita, 2009a; b; 2011; Troscianko et al., 2013). Briefly, visual camouflage is thought to work by retarding detection and/or recognition by a visual predator and includes strategies such as background matching, disruptive coloration and masquerade (Stevens and Merilaita, 2009a). Changeable camouflage is particularly interesting because it allows a prey animal to change its appearance according to its background and remain cryptic as it inhabits different microhabitats. Rapid adaptive camouflage (a change in appearance that takes less than 10 seconds) infers neuronal control of thousands (or millions) of pigmented or reflective skin elements (e.g., Fujii, 2000; Hanlon, 2007; Mäthger et al., 2003; Neill, 1940; Ramachandran et al., 1996). Determining the mechanisms and functions of changeable camouflage can enhance our understanding of visual perception: a prey animal must adjust its body pattern according to visual cues in its surroundings to avoid predation amidst a variety of visual backgrounds, while a visual predator must “break” that camouflage to find its next meal. In this study we used field images acquired under natural conditions to address several factors that contribute to these mechanisms.
One of the ways predators locate and identify their prey is through detection of edges, an essential task for object recognition (De Valois and De Valois, 1990; Marr, 1976; Tovée, 2008). Many of the mechanisms of camouflage include the modification of edges physically or visually through patterning. Here, we studied the slender filefish (Monacanthus tuckeri) to better understand the perception of two types of false edges: those created by color and pattern elements in the skin, and those created by three-dimensional skin projections called “dermal flaps.”
The slender filefish (Monacanthus tuckeri, Bean, 1906) is known to recreational SCUBA divers and photographers near shallow Caribbean reefs as a master of quick color and body pattern changes for camouflage and signaling (Bean, 1906; Clark, 1950), but has never been studied in the context of ethology and behavioral ecology. This small fish (usually 2–5cm standard length; 10cm maximum recorded standard length (Lieske and Meyers, 2002)) often hovers in a head-down position, moving inconspicuously among soft corals and macroalgae while foraging on tiny crustaceans and zooplankton (Ben-David and Kritzer, 2005; Randall, 1967; Randall and Randall, 1960). The slender filefish is prey to various marine teleost fishes, including groupers of the Family Serranidae (Randall, 1967), which are known for keen visual capabilities; in the present study, we observed successful predation on a slender filefish by a coney (Cephalopholis sp.).
The slender filefish is one of several cryptic animals whose three-dimensional shape is jagged or altered by the presence of projections variously referred to as skin flaps, dermal flaps, cirri, papillae, skin filaments, fronds, fleshy tabs, cutaneous appendages and leaf-like appendages (Curtis, 2006; Goffredo et al., 2004; Groves, 1998; Humann and DeLoach, 2002b; Lourie et al., 2004; Pietsch and Grobecker, 1987; Vasil’eva, 2007; Wallis, 2004). Various fish including seahorses, pipefishes, frogfishes, leafy seadragons, scorpionfish and wrasses have evolved a strong similarity to the structure of algae, coral or plants via these three-dimensional projections (Atz, 1951; Cott, 1940; Randall and Randall, 1960). Cott (1940) called these modifications in body shape a particular type of protective resemblance – “irregular marginal form” – and credited it with disguising an animal’s true body outline.
In this study, we examined the changeable camouflage body patterns of this species with field and laboratory data. From field imagery we determined the anatomical location of the changeable pattern components in this species and examined whether those components might contribute to camouflage by matching the color of nearby coral or macroalgae and/or emphasizing false edges. Next, we applied a Canny edge detector to visualize whether the animal’s true outline is readily discerned from its background and measured the contrast between animal and background at various points along its outline. Finally, we studied the internal structure of the dermal flaps and visualized their contribution to the slender filefish’s body outline and crypsis.
METHODS
Field research and image processing
SCUBA dives were completed in Bloody Bay, Little Cayman in April 2009 and June 2012 in clear water at depths ranging from 2–20m. Naturally behaving slender filefish were filmed with a Panasonic P2 high-definition video camera in a Gates underwater housing and photographed with a 24–70, 100 or 180mm lens on a Canon EOS 1-Ds Mark II digital SLR camera in a Subal underwater housing. No flash or video lights were used; all images were acquired under natural light. Behavioral data were collected from 21 animals in 2009 and 9 animals in 2012. Video and photograph data were collected via focal animal sampling, where the behavior of one individual was recorded for as long as feasible, limited by our air supply and keeping track of the animal. These data were used to create a camouflage body pattern ethogram (Fig. 1, Table 1) and to study the speed of color change and the diversity of camouflage body patterns (Figs. 2 and 3). Since it was not possible to use photographic calibration targets in each frame while working underwater with freely behaving wild animals, we carried out all intensity and color analyses in the camera’s device-dependent color space (i.e., not according to the visual perception capabilities of any animal) and avoided comparisons between different images. Images were viewed in Photoshop CS5 (Adobe Systems Inc.), drawings were created in Illustrator CS5 (Adobe Systems Inc.) and image processing was carried out using MATLAB R2013a (Mathworks, Inc) and Python (Python Software Foundation). When possible, backgrounds (predominantly macroalage, soft corals) were identified using Humann and DeLoach (2002a).
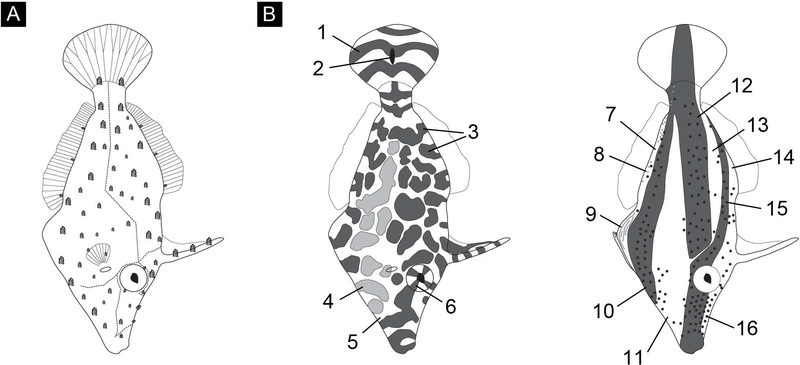
Figure 1:
(A) Drawing illustrating gross morphology including major lateral line (dashed line), fin rays and dermal flaps. Dermal flaps exist in several sizes and are roughly drawn to scale. (B) Drawing of the 16 body pattern components identified; numbers correspond to elements identified in the text and in Table 1. These drawings illustrate the location, size, placement, and value (i.e., expressed “light” or “dark”) of all possible components for this species, identified according to our field and laboratory data, not specific patterns.
Table 1:
Ethogram of body patterning in the slender filefish. Numbers refer to elements indicated in Figure 1B.
| Component Number (Fig. 1B) | Component Name |
|---|---|
|
| |
| 1 | dark tail bar |
| 2 | black tail spot |
| 3 | dark patches |
| 4 | light patches |
| 5 | white scrawling |
| 6 | eye sectors |
| 7 | light ventral stripe |
| 8 | blue iridescent spots (approx. 16 per side) |
| 9 | dewlap stripes |
| 10 | major dark ventral stripe |
| 11 | light medial stripe |
| 12 | major dark medial stripe |
| 13 | light dorsal posterior stripe |
| 14 | light dorsal anterior stripe |
| 15 | minor dark medial stripe |
| 16 | dark spots (approx. 161 per side) |
Figure 2:
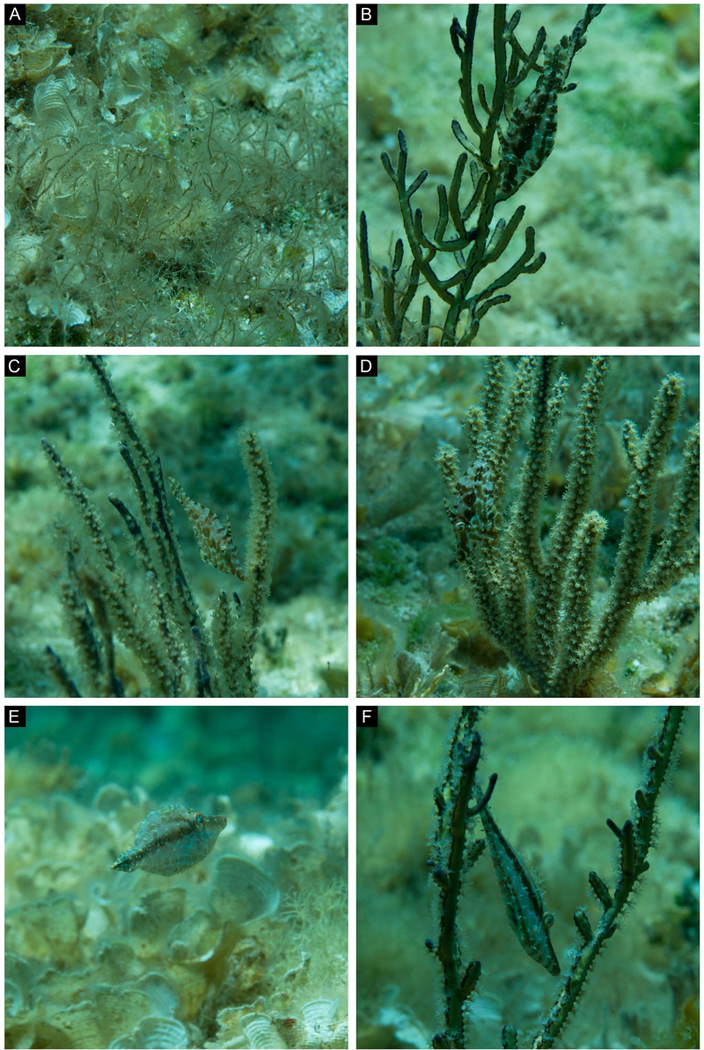
(A-B) Field images from an animal moving around a shelf-knob sea rod (Eunicea succinea); B was taken 11 seconds after A. (C-H) Field images from a different animal showing a variety of body patterns against different soft corals. (C-D) This filefish (white arrows) changed its body pattern when it moved from the base of a bent sea rod (Plexaura flexusa, polyps retracted) to nearby macroalgae (Sargassum sp.); D was taken 4 seconds after C. (E-G) A variety of patterns in the same animal on a single type of coral (bent sea rod, P. flexuosa); F was taken 33 seconds after E, G was taken 237 seconds after F. (H) The same animal changed its color and pattern according to a new visual background (a differently colored bent sea rod); H was taken 28 seconds after G.
Figure 3:
(A-F) A single animal adjusted its body pattern according to a variety of visual backgrounds. (A) Drifting from one soft coral to the next, the animal remains cryptic against benthic white scroll algae (Padina jamaicensis) and manatee grass (Syringodium filiforme). (B) Animal camouflaging near a yellow sea whip (Pterogorgia citrina). B was taken 89 seconds after A. (C-D) Same animal showing different cryptic body patterns near different gorgonians. D was taken 150 seconds after C. (E) Same animal showing a single stripe while swimming swiftly in a horizontal body orientation over white scroll algae (P. jamaicensis). E was taken 285 seconds after D. (F) Same animal showing a striped body pattern in front of a yellow sea whip (P. citrina). Note that different body patterns can be effective against the same visual background (compare B with F). F was taken 67 seconds after E.
Color resemblance between animal skin and backgrounds
Thirteen images of naturally behaving filefish in situ were selected for analysis, one image from each of 9 animals and two images of a tenth and eleventh animal. Eleven images of camouflaged animals were chosen to represent multiple individual animals and a diversity of patterns (Figs. 4–6 and S1-S8, image A; Figs. 5 and S8 are the same animal); two images of a pair of signaling fish (presumably courtship behavior) were included for comparison (Figs. S9-S10; the animal on the right in both images is the same animal camouflaging in Fig. S1). Using Photoshop CS5, a user created masks that identified the “animal” (filefish), the “stimulus” (the fish’s immediate visual background: adjacent coral and/or macroalgae), and the “background” (water column or sea floor: visual background presumably not influencing the animal’s camouflage body pattern) (Figs. 4–6, S1-S8, images B-C; S9-S10 images B-D).
Figure 4:
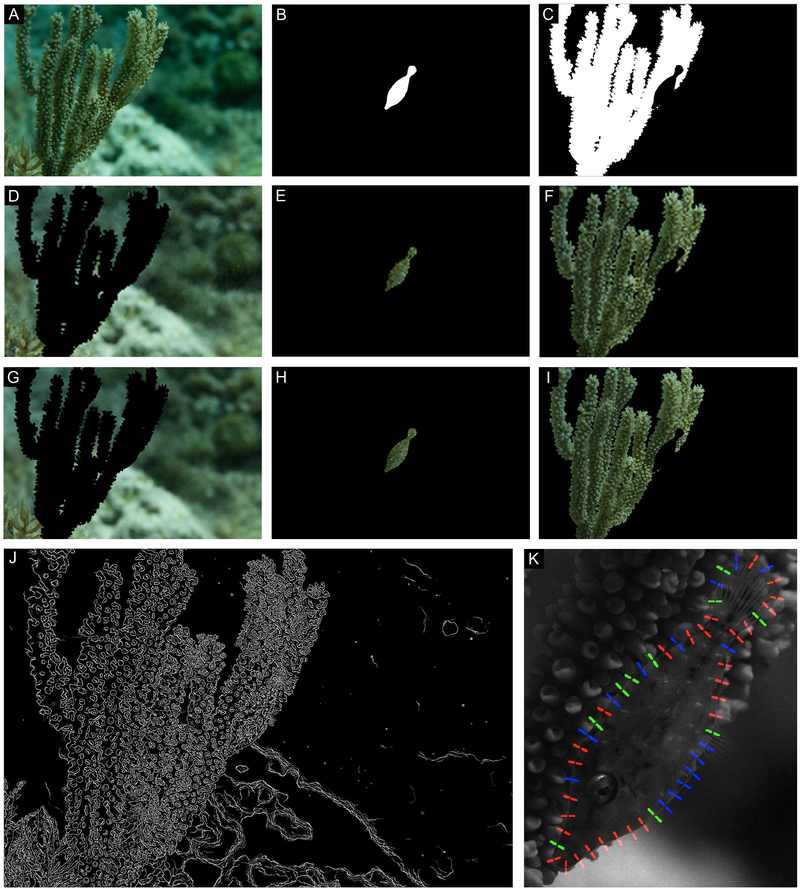
Same image as Fig. 3D. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Figure 6:
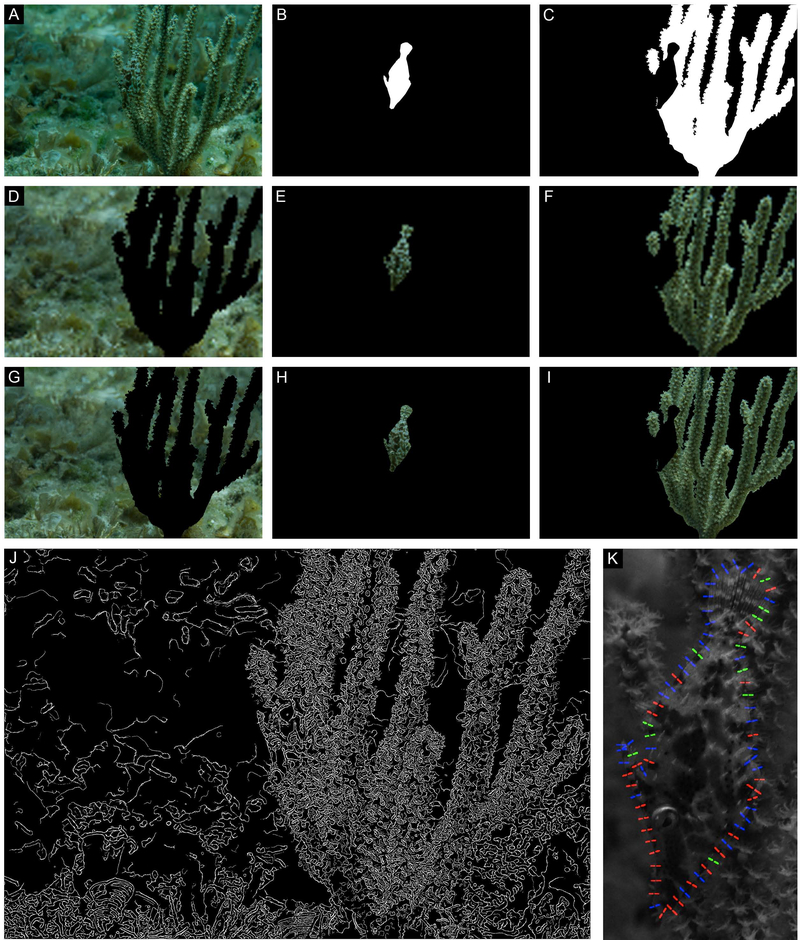
A) Filefish camouflaged in situ; animal was en route from one coral to another. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Figure 5:
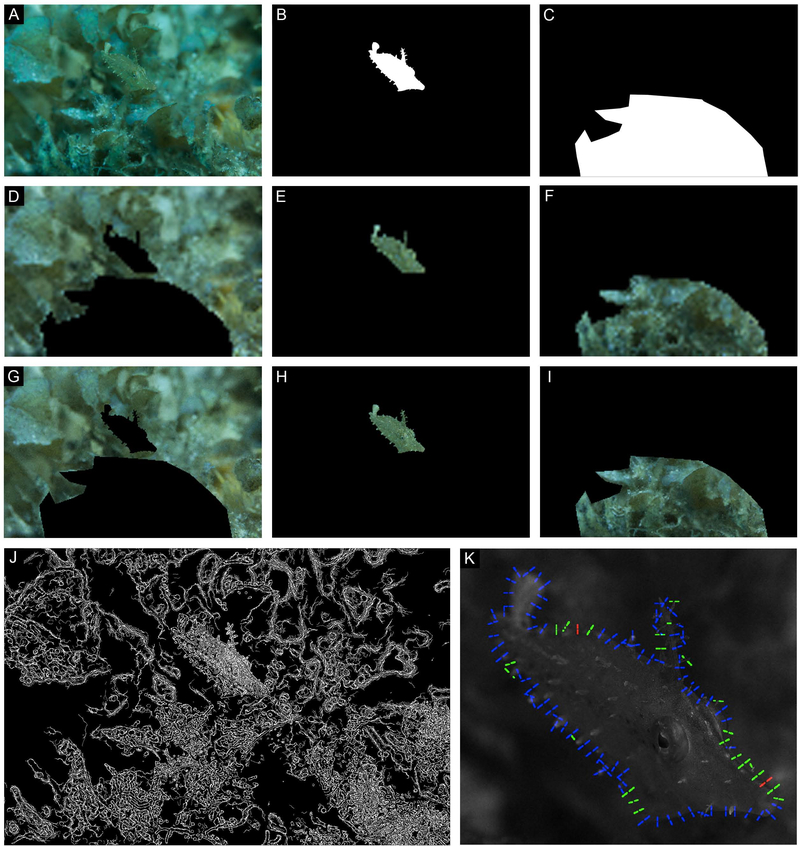
Same animal as Fig. S8. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
We used a permutation analysis to analyze the color match between the filefish’s skin and its “stimulus,” the coral or macroalgae in the fish’s immediate visual field, compared with the “background” (e.g., water column, distant reef). By design, permutation tests are insensitive to consistent measurement errors across groups, so they are ideal for identifying significant differences in uncalibrated photography (Good, 2004). Unlike statistical tests that assume a particular distribution of values, a permutation test only requires that it is equally possible that the fish matches the stimulus as that the fish matches the background.
Images were downsampled (1 pixel/pupil and 4 pixels/pupil) to eliminate pixel noise and differences in focus due to the shallow depth-of-field with macrophotography. Downsampling also allowed us to simulate the spatial frequency information received by observers with different acuities or at different viewing distances. Using an algorithm written in Python, those pixels constituting the fish (74–242 pixels for the 1 pixel/pupil resolution; 1196–3876 pixels for the 4 pixels/pupil resolution) were compared to a random sample of 1000 pixels from the stimulus and the pixel matches within a threshold were counted (results reported for (R-R) + (G-G) + (B-B) < 10; a threshold of 5 gave similar results). Likewise, all pixels constituting the fish were compared to a random sample of 1000 pixels from the background. The ratio of matches between the fish and the stimulus to the matches between the fish and the background was computed. We next compiled 1000 random permutations of comparisons between the fish and “simulated stimulus” and “simulated background” pixels, both drawn from a pool of combined stimulus and background pixels. Finally, the real ratio of stimulus/background match was compared to all permuted stimulus/background matches to determine a p-value.
Edge design – Canny edge detector and local contrast analysis
We took two approaches to investigate the combined role of a filefish’s pattern and skin elements in visually disrupting its outline. With the first approach, we analyzed the overall animal pattern following the edge detection methodology described in Stevens and Cuthill (2006). We repeatedly applied a Canny edge detector (Canny, 1986) to the cropped grayscale images of filefish, varying the width (σ) of the Gaussian filter from 0.5 to 5.5, in increments of 0.5. We then thresholded each resulting image using Otsu’s method (Otsu, 1979) and added the adjacent σ images (e.g., σ = 1.5 and 2.0 were added together), and re-thresholded the resulting images. This step essentially eliminated pixels that might be incorrectly identified as edges; by summing adjacent values of σ, only edges that appear at two or more of the adjacent spatial frequency levels were kept (Marr and Hildreth, 1980; Stevens and Cuthill, 2006). Finally, all pairs of thresholded adjacent sigma images were averaged to yield the final edge image. This approach allowed us to process the entire filefish pattern, instead of just its perimeter, across multiple spatial frequencies.
With the second approach, we compared grayscale intensities of points along the perimeter of the animal’s body to the intensities of points directly in the background. This “local contrast analysis” method provides an objective quantification of local contrast from a grayscale image; points with abrupt changes in intensity suggest where an edge might be seen. Using a program written in MATLAB R2013a (Mathworks, Inc), a user cropped the animal and nearby surroundings from each grayscale image and traced the outline of the animal’s body, excluding the transparent dorsal and anal fins but including the tail (caudal fin) and any dermal flaps that were part of the animal’s outline. We then chose a random starting point on the perimeter of the filefish’s body and traced the entire outline by marking points 100 pixels apart from each other. Next, we drew a line perpendicular to the animal’s body at each of these points that was 100 pixels long; 50 pixels fell inside the animal’s body, and 50 pixels fell outside. We then calculated the average intensity of the pixels that fell on either side. The resulting “edge maps” showed the locations where the contrast between the animal and the background were measured. Areas where the animal was darker than the background were labeled with red, areas where the animal was lighter than the background were labeled with blue, and areas where the contrast between animal and background was low (within 10%) were labeled in green.
Microscopy
Three slender filefish collected from the wild (purchased from Dynasty Marine Associates, Inc. in Florida) were maintained in the Marine Resources Center at the Marine Biological Laboratory (MBL) in Woods Hole, Massachusetts, USA according to the Institution’s Animal Care and Use Committee’s protocol 12–38. Over the course of their care, two laboratory animals were discovered freshly dead of unknown causes and were fixed in Hollande’s derivative of Bouin’s solution or 10% formalin. Individual skin flaps were excised and prepared for brightfield or scanning electron microscopy (SEM).
For brightfield microscopy, 2 samples were first decalcified in Evans and Krajian fluid (10g sodium citrate crystals, 25mL 90% formic acid, 75mL distilled water) for 6.25h. All samples were washed in 2 changes of TRIS buffered natural seawater (pH 8.0) and 2 changes of 20mM glycine (15min each) before being dehydrated with a stepwise ethanol series (35%, 50%, 70%, 85%, 3 changes of 95%, 100%; 15min each). Dermal flaps were then infiltrated and embedded in polyester wax (Hallstar PEG 400 Distearate, melting point 36°C). Serial cross sections were cut with a rotary microtome (Leica; 6μm thickness) and mounted using 2% paraformaldehyde on glass slides pre-coated with Weaver’s subbing solution (Weaver, 1955). After drying, sections were hydrated, stained with Mallory’s triple connective tissue stain (Mallory 1: acid fuchsin; Mallory 2: orange G, aniline blue), Bielschowsky’s silver stain, Müller’s colloidal iron, Van Gieson’s stain or Weigert’s hematoxylin (Humason, 1967), dehydrated, cleared with toluene, and coverslipped with histomount. Stained slides were examined with a Zeiss AxioSkop and photographed with a Canon 5D-Mark II digital SLR camera.
For SEM, dermal flaps excised with a bit of surrounding tissue were washed with TRIS buffered natural seawater before being dehydrated in a stepwise ethanol series (35%, 50%, 70%. 85%, 95%, 3 changes of 100% ethanol; approximately 30min each), then 3 changes of hexamethyldisalizane (2 changes at 10 minutes each; third change allowed to evaporate in a hood overnight (Bray et al., 1993)). Samples were attached to metal mounts with double sided cellophane tape and a small amount of silver paint. Samples were then sputter coated with 7.5nm of platinum before being visualized with a Zeiss Supra 40VP SEM.
Gross morphology of the laboratory specimens
The larger (63mm, standard length, “SL”) of the two laboratory specimens had segmented fin rays: 12 caudal, 34 dorsal, 34 anal, 10 pectoral (Fig. 1A), 2 pairs of well-developed retrorse caudal spines (rostral pair was much larger) and a large dewlap (ventral flap) with a marginal stripe. Combined, this morphology suggests the specimen was male (Berry and Vogele, 1961; Clark, 1950). The smaller specimen was 54mm SL and had segmented fin rays: 12 caudal, 36 dorsal, 36 anal, 11 pectoral. Instead of caudal spines, this specimen had two small pairs of spines (approximately the size of surrounding hook-like spinules) pointing toward the tail. This specimen had a reduced dewlap compared to the other animal but it was difficult to determine whether it had a marginal stripe. This morphology suggests the smaller specimen was female (Berry and Vogele, 1961; Clark, 1950).
RESULTS
Ethogram of body patterns
Our fieldwork yielded 909 photographs (811 in 2009 and 98 in 2012) from 30 different fish and over 100 minutes of video footage (58min in 2009 and 42.4min in 2012). In conjunction with laboratory observations, the diverse body patterns captured in these photographic and video data allowed us to construct a body pattern ethogram for this species (Fig. 1B; Table 1). Sixteen body pattern components were identified for this species. Some components appeared to be the result of combinations of other components; for example, the major dark medial stripe (component 12) is created by the darkening of adjacent dark patches (component 3) when pigmented melanocytes disperse within melanophores that overlie and obscure the white scrawling (component 5) that would otherwise reveal separation among adjacent dark patches. The dermal flaps (Fig. 1A) are positioned along the white scrawling (component 5), in the space between the dark and light patches (components 3 and 4; Fig. 1B). Two dermal flaps occur on each eye (Fig. 1A).
Examination of the location of the dark patches (component 3, Table 1) revealed that adjacent body elements had patches that appeared continuous. For example, the dark patch on the soft tissue between the dorsal edge of the animal and the prominent first dorsal spine was continuous with the dark patches on the first spine when the spine was held erect (as drawn; Fig. 1B, left). Likewise, when the first spine was held flat against the dorsal edge of the animal, dark patches on the dorsal edge of the animal aligned with the dark patches on the spine. Similarly, dark patches surrounding the eye aligned with those on the iris (eye sectors, component 6, Fig. 1B, left), according to the direction of the animal’s gaze (Fig 1B, left). In some body patterns, expression of the major dark medial stripe (component 12) extended continuously onto the caudal fin (Fig. 1B, right).
Speed of change
We recorded 46 body pattern changes on video and measured the speed of change to the nearest full second (mean = 3.04s, median = 2s, mode = 2s, standard deviation = +/− 2.31s; Figs. 2 and 3; Supp. videos 1 and 2). Changes were defined as a switch from one stable (i.e., held without a lightening or darkening of pattern components) body pattern to another. While some changes were as fast as 1s, the slowest recorded change took 13s. Changes included both subtle lightening or darkening within a body pattern component or dramatic, whole-body pattern changes, the latter typically when an animal changed visual background (as in Fig. 2G,H) or moved quickly between soft corals (as in Fig. 3E).
Crypsis via “background matching”
As indicated qualitatively in Figs. 2, 3, 4A, 5A, 6A, S1-S8, this fish species appears highly cryptic (at least to human vision) on a wide variety of backgrounds that include soft corals, algae, sponges and rocks. For example, in Fig. 3A, the pattern, brightness, color and contrast of the slender filefish are so similar to the surrounding algae that the fish is difficult to distinguish even in a close-up photograph. This species also has three-dimensional dermal flaps that are about the size of coral polyps, providing it with a physical texture that is similar to its background (e.g., Fig. 3D). At a distance of a meter, the fish was extremely difficult to distinguish with human vision. As divers, it was difficult to find fishes and we often lost them while filming, photographing or observing them during focal animal sampling. Figs. 3B-D also illustrate background matching amidst different types of soft corals. A head-down posture is a key component of camouflage in this species; such vertical posture enhances crypsis by orienting the longitudinal body shape of the fish to the vertical orientations of the soft corals, especially as it moves in rhythm with the coral branches. Body patterns such as those illustrated in Figs. 2F, 3F, S1, S4A and S5A include high-contrast large components of light and dark, which collectively are characteristic of some disruptive camouflage patterns in other animals.
Color resemblance between animal skin and backgrounds
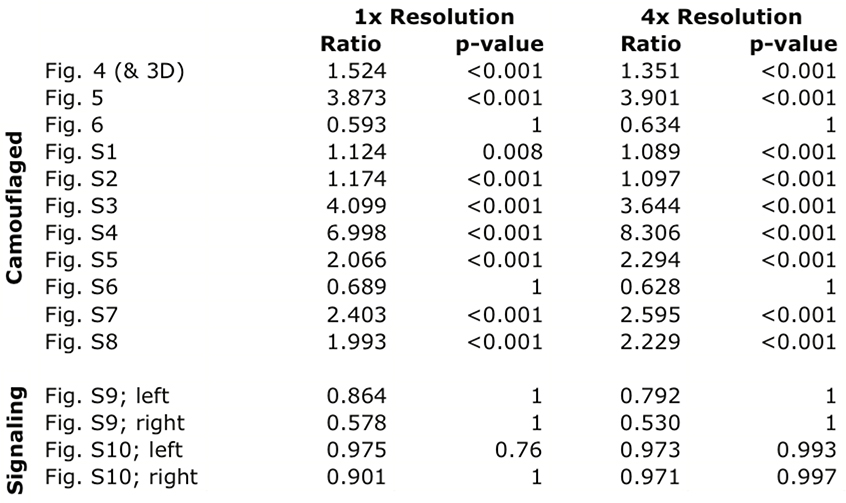
The color of camouflaged filefish more closely resembled the coral or algae stimulus than the surrounding background in 9 of the 11 images (Table 2). In one of the remaining images, the colors of the stimulus, background, and fish were all similar (Fig. 6). This result is not surprising because the sea floor in the background appeared to be mostly covered with similar algae. Furthermore, this photograph was taken while the animal was en route from one coral to another, a situation where a general match to the sea floor would be advantageous compared to a high-fidelity match to a particular nearby background element. In the other image (Fig. S6), it appears that the fish’s dark spots (component 16), white scrawling (component 5), and iridescence near the eye result in a difference in color when the animal is compared to the stimulus coral. In this case, the animal might have relied more on pattern scale, edge breaking and body orientation than a perfect color match. For the images of signaling animals, neither filefish significantly matched the stimulus better than the surrounding background (Table 2, Figs. S9-S10). There was no difference in statistical outcomes between the two image resolutions tested.
Table 2:
Results of the permutation analysis for color match. “Ratio” refers to the ratio of the number of matches between the fish and the stimulus to the number of matches between the fish and the background. The two resolutions tested were based on the number of pixels in the animal’s pupil; 1 pixel for 1x resolution and 4 pixels for 4x resolution.
|
Edge design – Canny edge detector and local contrast analysis
Internal pattern elements were more readily detected by the Canny edge detector than was the animal’s true body outline for an animal showing a large-scale mottled pattern against a coral with mostly extended polyps (Fig. 4J) and an animal showing a small-scale mottled pattern against a coral with locally retracted polyps (Fig. 5J). A filefish imaged at an oblique angle drifting along the sea floor above some leafy flat-blade algae (Stypopodium zonale) had prominent dermal flaps. Edge contrast and Canny edge detector analysis revealed that this animal’s edges – including the dermal flaps – were likely to be detected by their contrast with the background. However, the resulting irregular body outline was not immediately recognizable as fishlike (Fig. 6J). Results were similar in one of the signaling filefish (Fig. S10M, animal on left). Notably, the results of the Canny edge detector showed that patterns on the animals’ eyes – but not their stereotypical roundness – were often not readily identified by the edge detector (Figs. 4J, S2J, S5J, S7J). Even when the roundness of the eye socket was detected, it was similar in size and shape to elements on the nearby coral (Figs. 5J, S1J, S6J, S8J). Signaling animals appeared relatively conspicuous when analyzed with the Canny edge detector (Figs. S9M-S10M).
The same example images of camouflaged and signaling animals used to investigate color matching were processed to inspect the relative contrast between animal’s body edge and the nearby stimulus (Figs. 4–6; S1-8, image K). Images of animals showing mottled patterns with small- to medium-scale light and dark patches were often difficult to visually separate from their surroundings where their body edge intersected with a stimulus (e.g., coral) (e.g., Figs. 4A, 5A, S8A). Animal edges that intersected with the water column were more readily identified than edges adjacent to stimuli (e.g., Figs. 3B, ventral side; S3A, compare tail with dorsal edge). The results of the local contrast analysis parallel this subjective observation; regions where an animal’s body edge was directly adjacent to a background element often had an alternating contrast pattern (repeating light and dark pattern) along the obscured edge. In other words, a small part of the animal’s edge was darker than the adjacent surrounds (indicated in red), followed by a small part of the animal’s edge that was lighter than (indicated in blue) or roughly equal to (contrast less than 10%) the adjacent surrounds (indicated in green) (Figs. 4K, 5K). On the other hand, animal edges viewed against the water column usually resulted in continuous regions of the same contrast pattern (animal consistently darker or lighter than surrounds without alternating pattern; Figs. 4K, nose, 5K, ventral side, S5K). Animals showing uniform or striped patterns often had large portions of body edges with non-alternating contrast patterns (Figs. S3-5, image K). Like other cases of fish viewed against the far background, the signaling animals generally had long series of similar contrast along their edges (fish lighter than background; Figs. S9-S10).
Dermal flap morphology
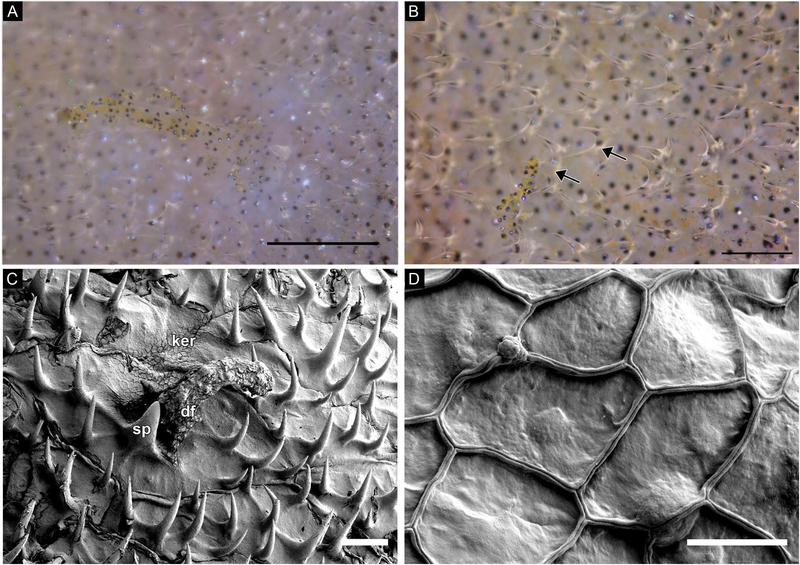
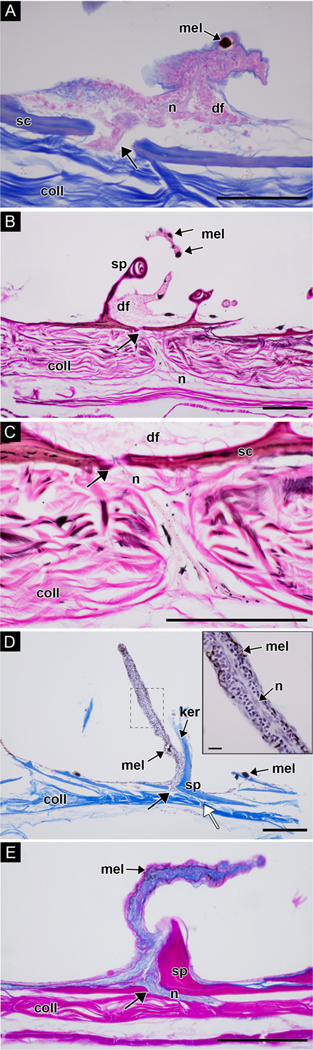
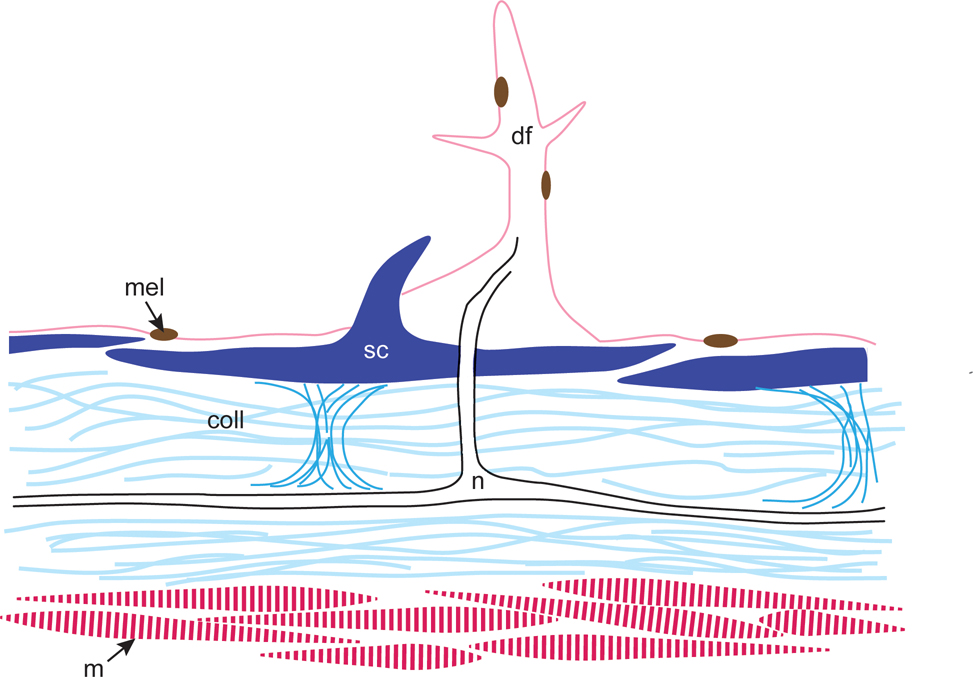
Analysis of field images and laboratory specimens showed that this species has approximately 63 dermal flaps of varying size on both lateral surfaces of the body, along the edge of the eye, and on both sides of the prominent dorsal spine (Fig. 1A, 7A-B). Examination revealed that the dermal flaps were typically branched and covered with melanophores; in general, the dermal flaps on the larger (presumably male) specimen were larger and had more branches than the dermal flaps on the smaller (presumably female) specimen (Figs. 7A-B, 8). Among the dermal flaps were much smaller and more numerous hook-like spinules curved towards the posterior (Fig. 7A-B). Near the rostral end of the larger specimen, spinules were small and simple, while around the pectoral fin and more caudally, spinules were often longer and bifurcated or trifurcated. These results contradict Berry and Vogele’s (1961) suggestion that “the scale spines of Monacanthus never branch – each spine arises individually from the scale base” (Fig. 7B). Examination of whole mounts of skin patches using SEM and serial cross sections of dermal flaps using brightfield microscopy revealed that the dermal flaps overlie scales containing multiple spinules (Fig. 7C). Squamous keratinocytes occur along the surface of the scales, dermal flaps, and spinules (Figs. 7C,D; 8D). When analyzed with brightfield microscopy, the dermal flaps appeared similar to the surrounding epidermis in staining affinities and cell composition (Fig. 8) and appeared to be predominantly composed of stratified cuboidal epithelial cells enveloped in mucopolysaccharide-rich connective tissue (Fig. 8E). An opening in the underlying scale was found in sections through the center of a dermal flap (Fig. 8A-E). Nerve fibers extended from the dermal flap to an underlying nerve network that coursed through two layers of collagen (Figs. 8, 9). Collagen held the scales in place (Figs. 8D, white arrow; 9) and a layer of muscles was found below the second collagen layer (Fig. 9). All dermal flaps examined contained melanophores (Figs. 7A-B, 8).
Figure 7:
(A-B) Freshly dead tissue; photographs of a branched dermal flaps covered with brown and yellow melanocytes and surrounded by smaller, transparent, forked spinules. (C) SEM image of a dermal flap surrounded by hook-like spinules. Squamous keratinocytes overlie the surface of the skin. Note the outlines of scales. (D) SEM image of the squamous keratinocytes. Abbreviations: dermal flap (df), keratinocytes (ker), spinule (sp). Scale bars: A-B, approx. 100μm; C, 100μm; D, 10μm.
Figure 8:
Light micrographs of dermal flaps cut in cross section. (A) Mallory’s triple connective tissue stain. The dermal flap is composed of stratified cuboidal epithelial cells; note the nerve bundle passing through the scale (arrow). Epithelial cells and nerve bundle, pink; scales, blue and orange; collagen, blue; melanophores, dark brown. (B-C) Bielschowsky’s silver and Van Gieson’s stain. C is a high magnification view of the nerve bundle passing through the scale in B (arrows). Nerve bundle connects to fibers beneath the collagen layer. Epithelial cells and nerve bundle, light pink; scales, red and purple; nerves, pink and black; melanophores, dark brown/black; collagen, bright pink. (D-E) Two samples decalcified in Evans and Krajian fluid. (D) Weigert’s hematoxylin and Mallory’s 2 (orange G and aniline blue) stains. A nerve runs through the middle of the dermal flap (see inset). Nuclei of epithelial cells, dark purple; nerve bundle, light purple; scale and spinule, bright blue; collagen, bright blue. (E) Müller’s colloidal iron and Van Gieson’s stains. Epithelial cells are wrapped in mucopolysaccharide-rich connective tissue (blue); spinule, scale and collagen, bright pink. White arrow in D indicates attachment point between collagen and scale. Abbreviations: collagen (coll); dermal flap (df); keratinocyte (ker); melanophore (mel); nerve (n); scale (sc); spinule, (sp). Scale bars: 100μm; inset in D, 10μm.
Figure 9:
Schematic diagram of a dermal flap in cross section showing skin elements. Scales are held in place with collagen; nerve fibers between collagen layers extend through an overlying scale to the core of the dermal flap. Light and medium blue, collagen (coll); pink, dermal flap (df), dark pink, muscle (m); brown, melanophore (mel); black, nerve tract (n); dark blue, scales (sc), center scale with spinule.
DISCUSSION
The slender filefish resides on a coral reef, a complex habitat that is occupied by a variety of teleost fish predators known to have diverse and powerful visual capabilities (Losey et al., 2003). The ability to quickly and dramatically adjust its body pattern according to its surroundings allows the slender filefish to forage stealthily among a diversity of three-dimensional reef structures such as soft corals, sponges, macroalgae, and rocks. The speed of pattern change (approx. 2s) is comparable to some other teleost reef fishes (e.g., Mathger et al., 2003; Ramachandran et al., 1996; Tyrie et al. 2015; Watson et al., 2014), including other filefish (Okaichi et al., 1958), and is driven putatively via neural, rather than hormonal, mechanisms (e.g., Fujii, 2000; Fujii and Novales, 1969; Sköld et al., 2013). This species may be a suitable comparative model for the study of the mechanisms and functions of camouflage because it exhibits a variety of cryptic patterns and behaviors that can be classified as “background matching,” “disruptive coloration,” and possibly even “masquerade.” The 16 camouflage pattern components described for this species rivals the repertoire of some cephalopods (e.g., Hanlon and Messenger, 1988; Packard and Sanders, 1971; Roper and Hochberg, 1988) and flatfish (e.g., Kelman et al., 2006; Ramachandran et al., 1996; Tyrie et al., 2015). Each of these 16 components is presumably (as in cephalopods) a neurophysiological unit guided by visual input; these neural mechanisms have barely been studied in fishes (cf. Bagnara and Matsumoto, 1996; Demski, 1992; Fujii, 2000).
Color resemblance between animal and background
The results of our analysis of the colors of camouflaged or signaling filefish were intuitive; camouflaged animals usually closely resembled a nearby visual stimulus (coral or macroalgae) better than the surrounding background while animals engaged in visual signaling (presumably courtship behavior) did not. These results were not dependent on image resolution, suggesting that the color similarity between the animals’ skin and their stimuli may be effective against predators with a range of visual acuities or at a range of viewing distances. Although color match is only one of a suite of camouflage components, these results suggest that the slender filefish might achieve some color resemblance to nearby backgrounds that could hinder its detection or recognition by some predators that are sensitive to color.
Although we did not explicitly compare the size scale of the light and dark components of filefish body patterns to the size scale of the light and dark elements of their background elements, the various patches that make up many of this species’ cryptic body patterns appeared to be similar in scale to those of their surroundings, thus aiding background matching (e.g., Figs. 4A, 5A, S6A, S8A). Collectively, this fish appears to be hindering detection by a combination of factors: (i) color resemblance, (ii) scale resemblance, (iii) expressing cryptic pattern elements and complicating the body’s edge with three-dimensional dermal flaps, (iv) moving in rhythm with the coral branches (Supp. videos 1, 2), and (v) maintaining a head-down body orientation that is similar to the main axes of the background elements.
Disruptive coloration: Sub-principles and pattern design
Disruptive coloration has been defined as “a set of markings that creates the appearance of false edges and boundaries, and hinders the detection or recognition of an object’s, or part of an object’s, true outline and shape” (Stevens and Merilaita, 2009a). We have no behavioral or experimental proof of disruptive coloration, but we do consider the components of cryptic patterns in this species in the context of the 5 sub-principles of disruptive coloration: “differential blending,” “disruptive marginal patterns,” “maximum disruptive contrast,” “disruption of surface through false edges,” and “coincident disruptive coloration” (Stevens and Merilaita, 2009b). Although not included as part of disruptive coloration by Stevens and Merilaita (2009b), the sub-principle “regularity avoidance,” the tendency of pattern elements to have irregular shapes, may also be important to camouflage patterns (Cott, 1940) and many of the slender filefish’s internal markings (e.g., dark patches, element 3) are irregularly shaped (Fig. 1B).
Differential blending occurs when some elements of an animal’s camouflage pattern visually blend into the background while other elements do not and may work best where pattern markings intersect the body’s true outline (Cuthill et al., 2005; Fraser et al., 2007; Merilaita, 1998; Stevens et al., 2006). In the slender filefish, all of the dark patches (component 3, Fig. 1B, left) and dark tail bars (component 1, Fig. 1B, left) intersected the slender filefish’s true edges when the animal was viewed from the side. The dark patches wrap around the dorsal (not shown, but see Supp. Video 1) and ventral (Fig. 3F) sides of the animal; this results in the same effect of the pattern elements intersecting with the animal’s true edge when the animal is viewed from above or below.
Disruptive marginal patterns break up an animal’s outline with repeating light and dark markings along a true edge (Cott, 1940); evidence of differential blending of disruptive marginal patterns is particularly evident in Figs. 2A, 3D, and 4A. We used a Canny edge detector and local contrast analysis to objectively investigate the appearance of the camouflaged filefishes’ edges against their immediate background. The discernable fishlike outline was very difficult to detect in many of the edge maps produced by the Canny analysis (e.g., Figs. 4J, 5J). Likewise, in cases of especially good edge disguise against a heterogeneous stimulus (Figs. 4K, 5K), parts of the animal’s outline were marked with regions of alternating contrast. In other words, the animal’s body was alternately darker (indicated by red) or lighter (indicated by blue) than the adjacent surroundings, breaking up the continuity of the true edge and resulting in “good” camouflage. Together, these results suggest that markings along the animal’s edge (“disruptive marginal patterns”) might function by “differential blending” to help the animal visually hide its body edge when viewed against its surroundings (Cott, 1940; Stevens et al., 2006; 2009).
In several cases (camouflaged animals in Figs. 6K, S1K, S3K, S4K; signaling animals in Figs. S9N, S10N), our local contrast analysis showed that relatively long, continuous portions of the animal’s edge had unequal contrast with the immediate background. In the images of camouflaged animals, the filefish probably rely on color resemblance (at least in Figs. S1, S3 and S4), body postures that were similar to the orientation of the coral branches (Figs. S1A, S3A, S4A), or appearing to be a piece of drifting detritus when moving over the sea floor form one coral to the next (Fig. 6K). This result was unsurprising for the signaling animals because they did not appear to be actively adjusting their patterns according to background stimuli.
Maximum disruptive contrast suggests that adjacent pattern elements should strongly contrast for the most camouflage benefit and there is some empirical evidence that this is the case, at least for internal (not edge) pattern elements (Stevens et al., 2009; Troscianko et al., 2013). Physiological changeable patterns allow the slender filefish to quickly modify the contrast of its internal (and edge) pattern components as necessary for crypsis in its surrounds; the slender filefish was observed expressing a cryptic pattern with high-contrast edge markings in combination with low-contrast internal markings (Fig. 3C). In this species, the light patches (component 4) and dark patches (component 3) can be expressed so that they weakly contrast each other (e.g., Fig. 2E) and/or strongly contrast with the white scrawling (component 5; e.g., Fig. 3D). Likewise, the major dark medial stripe (component 12) is an internal pattern component that can strongly (Figs. 2F, 3F, S9A) or weakly (Fig. 2G) contrast with the light medial stripe (component 11). In disguising true edges, patterns can contribute to camouflage by creating the illusion of edges where there are none through the “disruption of surface through false edges.” The slender filefish can create long, solid lines (false edges) by expressing the major dark medial stripe (component 12), for example (Figs. 2F, 3E-F, S8). It is important to remember that patterning and contrast must be considered in the context and the characteristics of the background rather than as rules for crypsis: the same components (light medial stripe, component 11 and major dark medial stripe, component 12) that create high-contrast internal markings and false edges for camouflage (Fig. S5) can be conspicuous when animals are engaged in signaling behavior (Figs. S9-S10).
Finally, coincident disruptive coloration concerns the extension of pattern elements across adjacent body sections such as eyes, wings, limbs, and fins to obscure true form by visually joining recognizable forms (Cott, 1940; Cuthill and Székely, 2009; Stevens and Merilaita, 2009b). Although this camouflage pattern characteristic appears to be widespread among animals, especially to conceal the eye (Cott, 1940, discussed examples of reptiles, insects, fish, amphibians, mammals and birds), it has rarely been studied in modern camouflage experiments. One study devoted to coincident disruptive coloration found that artificial patterns that employ this technique were more effective in avoiding detection by wild birds and human “predators” (Cuthill and Székely, 2009). Pattern components (e.g., dark patches, component 3; eye sectors, component 6; major dark medial stripe, component 12) on the predominant first spine, eye and caudal fin of the slender filefish aligned with patches on the surrounding regions of the fish, suggesting that this animal also takes advantage of coincident disruptive coloration to disguise its true form.
Canny edge detector, pattern elements and dermal flaps
In many images of camouflaged fish, the results of the Canny edge analysis showed that the animal was difficult to resolve from its background (Figs. 4–5, S5, S8). Close inspection revealed that many of the pattern elements – particularly those that intersected with the animal’s true edge – were grouped with the surroundings instead of along the true edge of the fish. This suggests that those pattern elements may have been functioning for crypsis through differential blending and disruptive marginal form. On the other hand, the dermal flaps were readily identified by the Canny edge detector (e.g., Fig. 6; Fig. S10, animal on left), which suggests that the irregular marginal form created by the three-dimensional projections may function to hide the animal’s true edge. In some cases, the dermal flaps closely resembled elements of the background such as polyps on the gorgonians (Fig. 3D) or small bits of sand and other debris on benthic algae (Figs. 6, S10). In those situations, the filefish might achieve crypsis through masquerade, a strategy “where recognition is prevented by resembling an uninteresting object, such as a leaf or stick” (Stevens and Merilaita, 2009a), especially when the dermal flaps are enhanced by chromatic elements. Moreover, masquerade can hinder both recognition and detection (Skelhorn et al., 2010a,b), and M. tuckeri seems to hinder the former when it is swimming head-down a short distance from gorgonians and the latter when it is immediately adjacent to gorgonians. Masquerade is not unique to M. tuckeri; another small filefish, the harlequin filefish (Oxymonacanthus longirostris), is known to masquerade amidst coral branches for overnight protection (Brooker et al., 2011).
Edge detectors in computer vision are used to identify locations of intensity changes in images (e.g., Canny, 1986). Intensity changes in images can be due to a variety of physical factors including, but not limited to, shadows, color differences, surface markings, texture, and occluding boundaries (e.g., Elder and Sachs, 2004; Marr and Hildreth, 1980; Troscianko et al., 2009). Through visual inspection, we can verify for our visual system that the outline of camouflaged filefish was difficult to detect against nearby coral and algae and was easier to identify against the water column or far background, especially in the signaling fish (Figs. 4J, 5J, S8J, S9M, S10M).
Dermal flaps – 3D skin appendages that aid crypsis
Although the number and location of dermal flaps is variable in frogfish (Pietsch and Grobecker, 1987) and some seahorses (Curtis, 2006), they appear to be fixed and consistent in location among individual slender filefish (Fig. 1A), although the size and complexity of individual flaps may vary. This anatomical feature might assist taxonomic identification. Although we examined the morphology of only one putative female and one putative male specimen, we cautiously suggest that the dermal flaps are more prominent and complex in males than in females. Stain affinities and tissue morphology suggested that the dermal flaps are constructed of stratified cuboidal epithelial cells with an overlying layer of squamous keratinocytes. Although there was no evidence that the fish can actively control the expression or appearance of the flaps, their epidermal composition suggests they are somewhat flexible and might bend according to microcurrents along the fish’s body as it swims. Internally, nerve fibers connected the center of each dermal flap to an underlying nerve tract (Fig. 8B-C). Although the function of these nerve fibers could not be determined with our methods, it is likely that they are motor neurons that control the expression of melanophores on the branches of the dermal flap (Figs. 7, 8). Alternatively, many fish have dermal sensory receptors (e.g., Kotrschal et al., 1993; Lane and Whitear, 1982; Meyer-Rochow, 1978; von Bartheld and Meyer, 1985), so it is possible that these neurons serve a sensory function.
Concluding remarks
This study examined changeable body patterning for camouflage in the slender filefish (Monacanthus tuckeri) through the quantification of in situ imagery and behavioral data as well as histological examination of the dermal flaps of preserved specimens. This marine fish quickly and effectively changes its body patterns according to its immediate surroundings and is an example of a species that implements many of the defined principles of camouflage (Cott, 1940; Stevens & Merilaita, 2009a; b; 2011). Many other marine teleost fishes change their body patterning appearance quickly for crypsis (e.g., Humann & DeLoach, 2002b; Marshall & Johnsen, 2011; Mäthger et al., 2003; Ramachandran et al., 1996; Stevens et al., 2014; Tyrie et al., 2015; Watson et al., 2014), but there are few systematic studies of this behavior. Marine fishes represent a sizeable repository of visual predators and cryptic prey that will yield new insights into visual perception and the many tactics of animal visual camouflage.
Supplementary Material
Supplementary Video 1: Video clip of a slender filefish in situ. Notice the appearance and disappearance of dark patches on the dorsal side of the body above the longitudinal dark stripe.
Supplementary Video 2: Video clip of a slender filefish in situ. Notice the overall lightening of the pattern and the disappearance of dark patches on the lateral side of the animal.
Supplementary Figure 1: Same animal as the filefish on the right in Figs. S9 and S10. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 2: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 3: Same image as Fig. 2H. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 4: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 5: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 6: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 7: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 8: Same animal as Fig. 5. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 9: (A) Filefish signaling in situ (presumably courtship behavior). Animal on the right is the same animal camouflaged in Fig. S1. The same pair of animals is presented in Fig. S10. (B-D) Masks created to isolate the animals (B-C) from their stimulus and background (C). (E-H) Low resolution (pupil is 1 pixel) images of background (E), animals (F-G), and stimulus (H) analyzed for color match. (I-L) Higher resolution (pupil is 4 pixels) images of background (I), animals (J-K), and stimulus (L) analyzed for color match. (M) Results of the Canny edge detection analysis. (N) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background are red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 10: (A) Filefish signaling in situ (presumably courtship behavior). Animal on the right is the same animal camouflaged in Fig. S1. The same pair of animals is presented in Fig. S9. (B-D) Masks created to isolate the animals (B-C) from their stimulus and background (C). (E-H) Low resolution (pupil is 1 pixel) images of background (E), animals (F-G), and stimulus (H) analyzed for color match. (I-L) Higher resolution (pupil is 4 pixels) images of background (I), animals (J-K), and stimulus (L) analyzed for color match. (M) Results of the Canny edge detection analysis. (N) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background are red, and regions where the animal is lighter than the background are blue.
ACKNOWLEDGEMENTS
A. C. (Watson) Hanson was a valuable member of our dive team. The staff of the Central Caribbean Marine Institute’s Little Cayman Research Centre were gracious hosts. M. Dahle, E. Ferguson, L. Ferretti, and K. Laurel kindly provided additional photographs taken during Digital Shootout 2012. John “Bucky” Wile and Forrest Young at Dynasty Marine Associates, Inc. collected and supplied the laboratory specimens. Many thanks to A. M. Kuzirian, R. M. Smolowitz and G. R. R. Bell for histology advice, to K. M. Ulmer, G. R. R. Bell, L. A. Siemann and E. Green-Beach for help with animal care, to R. J. Crook, R. Rosenholtz and L. A. Siemann for helpful discussions, and to three anonymous reviewers for constructive feedback. JJA appreciates support from a National Defense Science and Engineering Graduate Fellowship and a National Science Foundation Graduate Research Fellowship. DA is grateful for support from grant NIH-NEI EY021473 to R. Rosenholtz. AUS is thankful for support from a National Research Service Award (5F31NS083247–02). RTH acknowledges partial funding from ONR grants N0001406–1-0202 and N00014–10-1–0989; AFOSR grant FA9950090346; the Sholley Foundation; and the Digital Shootout Underwater Imaging Workshop at Little Cayman. Animal care and research protocols were approved by the Institutional Animal Care and Use Committee at the Marine Biological Laboratory, protocol 12–38.
REFERENCES
- Akkaynak D, Treibitz T, Xiao B, Gurkan UA, Allen JJ, Demirci U, Hanlon RT. 2014. Use of commercial off-the-shelf digital cameras for scientific data acquisition and scene-specific color calibration. Journal of the Optical Society of America A 31: 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atz JW. 1951. Fishes that look like plants. Animal Kingdom 54:130–136. [Google Scholar]
- Bagnara JT, Matsumoto J. 2006. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA and Ortonne J-P, eds. Pigmentary System. Oxford, UK: Oxford University Press. 11–59. [Google Scholar]
- Bean TH. 1906. A Catalogue of the Fishes of Bermuda, With Notes on a Collection Made in 1905 for the Field Mueseum. Chicago: Field Columbian Museum. [Google Scholar]
- Ben-David J, Kritzer JP. 2005. Early life history and settlement of the slender filefish, Monacanthus tuckeri (Monacanthidae), at Calabash Caye, Turneffe Atoll, Belize. Environmental Biology of Fishes 73:275–282. [Google Scholar]
- Berry FH, Vogele LE. 1961. Filefishes (Monacanthidae) of the Western North Atlantic. Fishery Bulletin of the Fish and Wildlife Service 61:61–109. [Google Scholar]
- Bray DF, Bagu J, Koegler P. 1993. Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microscopy Research and Technique 26:489–495. [DOI] [PubMed] [Google Scholar]
- Brooker RM, Munday PL, Jones GP. 2011. Coral obligate filefish masquerades as branching coral. Coral Reefs 30:803. [Google Scholar]
- Canny J 1986. A computational approach to edge detection. IEEE Transactions on Pattern Analysis and Machine Intelligence 8:679–698. [PubMed] [Google Scholar]
- Clark E 1950. Notes on the behavior and morphology of some West Indian Plectognath fishes. Zoologica 35:159–170. [Google Scholar]
- Cott HB. 1940. Adaptive Coloration in Animals. London: Methuen & Co. Ltd. [Google Scholar]
- Curtis JMR. 2006. A case of mistaken identity: skin filaments are unreliable for identifying Hippocampus guttulatus and Hippocampus hippocampus. Journal of Fish Biology 69:1855–1859. [Google Scholar]
- Cuthill IC, Stevens M, Sheppard J, Maddocks T, Párraga CA, Troscianko TS. 2005. Disruptive coloration and background pattern matching. Nature 434:72–74. [DOI] [PubMed] [Google Scholar]
- Cuthill IC, Székely A. 2009. Coincident disruptive coloration. Philosophical Transactions of the Royal Society B 364:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demski LS. 1992. Chromatophore systems in teleosts and cephalopods - a levels oriented analysis of convergent systems. Brain, Behavior and Evolution 40: 141–156. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. 1990. Spatial Vision. New York, NY: Oxford University Press. [Google Scholar]
- Elder JH, Sachs AJ. 2004. Psychophysical receptive fields of edge detection mechanisms. Vision Research 44:795–813. [DOI] [PubMed] [Google Scholar]
- Fraser S, Callahan A, Klassen D, Sherratt TN. 2007. Empirical tests of the role of disruptive coloration in reducing detectability. Proceedings of the Royal Society B 274:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Research 13:300–319. [DOI] [PubMed] [Google Scholar]
- Fujii R, Novales RR. 1969. Cellular aspects of the control of physiological color changes in fish. American Zoologist 9:453–463. [DOI] [PubMed] [Google Scholar]
- Goffredo S, Piccinetti C, Zaccanti F. 2004. Volunteers in marine conservation monitoring: a study of the distribution of seahorses carried out in collaboration with recreational scuba divers. Conservation Biology 18:1492–1503. [Google Scholar]
- Good PI. 2004. Permutation, Parametric, and Boostrap Tests of Hypotheses (Springer Series in Statistics). New York, NY: Springer Science + Business Media, Inc. [Google Scholar]
- Groves P 1998. Leafy sea dragons. Scientific American 279:84–89.9648300 [Google Scholar]
- Hanlon RT. 2007. Cephalopod dynamic camouflage. Current Biology 17: R400–R404. [DOI] [PubMed] [Google Scholar]
- Hanlon RT, Messenger JB. 1988. Adaptive coloration in young cuttlefish (Sepia officinalis L.): The morphology and development of body patterns and their relation to behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences 320:437–487. [Google Scholar]
- Hildreth EC. 1985. Edge Detection. Cambridge, MA: Massachusetts Institute of Technology. [Google Scholar]
- Humann P, DeLoach N. 2002a. Reef Coral Identification: Florida, Caribbean, Bahamas. Jacksonville, FL: New World Publications, Inc. [Google Scholar]
- Humann P, DeLoach N. 2002b. Reef Fish Identification: Florida, Caribbean, Bahamas. Jacksonville, FL: New World Publications, Inc. [Google Scholar]
- Humason GL. 1967. Animal Tissue Techniques. San Francisco and London: W. H. Freeman and Company. [Google Scholar]
- Kelman EJ, Tiptus P, Osorio D. 2006. Juvenile plaice (Pleuronectes platessa) produce camouflage by flexibly combining two separate patterns. Journal of Experimental Biology 209:3288–3292. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Whitear M, Finger TE. 1993. Spinal and facial innervation of the skin in the gadid fish Ciliata mustela (Teleostei). Journal of Comparative Neurology 331:407–417. [DOI] [PubMed] [Google Scholar]
- Lane EB, Whitear M. 1982. Sensory structures at the surface of fish skin I. Putative chemoreceptors. Zoological Journal of the Linnean Society 75:141–151. [Google Scholar]
- Lieske E, Myers R 2002. Coral Reef Fishes: Indo-Pacific and Caribbean. 3rd Ed. Princeton University Press, Princeton, NJ. [Google Scholar]
- Losey GS, McFarland WN, Loew ER, Zamzow JP, Nelson PA, Marshall NJ. 2003. Visual biology of Hawaiian coral reef fishes. I. Ocular transmission and visual pigments. Copeia, 3:433–454. [Google Scholar]
- Lourie SA, Foster SJ, Cooper EWT, Vincent ACJ. 2004. A Guide to the Identification of Seahorses. Washington, D. C.: University of British Columbia and World Wildlife Fund. [Google Scholar]
- Marr D 1976. Early processing of visual information. Philosophical Transactions of the Royal Society B 275:483–519. [DOI] [PubMed] [Google Scholar]
- Marr D, Hildreth E. 1980. Theory of edge detection. Proceedings of the Royal Society of London, B 207:187–217. [DOI] [PubMed] [Google Scholar]
- Marshall J, Johnsen S. 2011. Camouflage in marine fish. In: Stevens M, Merilaita S, eds. Animal Camouflage: Mechanisms and Functions. Cambridge, UK: Cambridge University Press:186–211. [Google Scholar]
- Mäthger LM, Land MF, Siebeck UE, Marshall NJ. 2003. Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus. Journal of Experimental Biology 206: 3607–3613. [DOI] [PubMed] [Google Scholar]
- Merilaita S 1998. Crypsis through disruptive coloration in an isopod. Proceedings of the Royal Society B 265:1059–1064. [Google Scholar]
- Meyer-Rochow VB. 1978. Skin papillae as possible electroreceptors in the deep-sea eel Cyema atrum (Cyemidae: Anguilloidei). Marine Biology 46:277–282. [Google Scholar]
- Neill RM. 1940. On the existence of two types of chromatic behaviour in teleostean fishes. Journal of Experimental Biology 17:74–78. [Google Scholar]
- Okaichi T, Kai H, Hashimoto Y. 1958. A rapid color change in the filefish. Bulletin of the Japanese Society of Scientific Fisheries 24:389–393. [Google Scholar]
- Otsu N 1979. A threshold seleciton method from gray-level histograms. IEEE Transactions on Systems, Man, and Cybernetics 9:62–66. [Google Scholar]
- Packard A, Sanders GD. 1971. Body patterns of Octopus vulgaris and maturation of the response to disturbance. Animal Behaviour 19:780–790. [Google Scholar]
- Pietsch TW, Grobecker DB. 1987. Frogfishes of the World: Systematics, Zoogeography, and Behavioral Ecology. Stanford, CA: Stanford University Press. [DOI] [PubMed] [Google Scholar]
- Poulton EB. 1890. The Colours of Animals: Their Meaning and Use, Especially Considered in the case of Insects. London: Kessinger Publishing, LLC. [Google Scholar]
- Ramachandran VS, Tyler CW, Gregory RL, Rogers-Ramachandran D, Duensing S, Pillsbury C, Ramachandran C. 1996. Rapid adaptive camouflage in tropical flounders. Nature, 379:815–818. [DOI] [PubMed] [Google Scholar]
- Randall JE. 1967. Food Habits of Reef Fishes of the West Indies. Miami, FL: University of Miami Institute of Marine Science. [Google Scholar]
- Randall JE, Randall HA. 1960. Examples of mimicry and protective resemblance in tropical marine fishes. Bulletin of Marine Science 10:444–480. [Google Scholar]
- Roper CFE, Hochberg FG. 1988. Behavior and systematics of cephalopods from Lizard Island, Australia, based on color and body patterns. Malacologia 29:153–193. [Google Scholar]
- Skelhorn J, Rowland HM, Ruxton GD. 2010a. The evolution and ecology of masquerade. Biological Journal of the Linnean Society 99: 1–8. [Google Scholar]
- Skelhorn J, Rowland HM, Speed MP, Ruxton GD. 2010. Masquerade: camouflage without crypsis. Science 327: 51. [DOI] [PubMed] [Google Scholar]
- Sköld HN, Aspengren S, Wallin M. 2013. Rapid color change in fish and amphibians - function, regulation, and emerging applications. Pigment Cell & Melanoma Research 26: 29–38. [DOI] [PubMed] [Google Scholar]
- Stevens M 2007. Predator perception and the interrelation between different forms of protective coloration. Proceedings of the Royal Society B 274:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill IC, Párraga CA, Troscianko T. 2006. The effectiveness of disruptive coloration as a concelament strategy. Progress in Brain Research 155:49–64. [DOI] [PubMed] [Google Scholar]
- Stevens M, Lown AE, Denton AM. 2014. Rockpool gobies change colour for camouflage. PLoS ONE 9:e110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Merilaita S. 2009a. Animal camouflage: current issues and new perspectives. Philosophical Transactions of the Royal Society of London B 364:423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Merilaita S. 2009b. Defining disruptive coloration and distinguishing its functions. Philosophical Transactions of the Royal Society of London B 364:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Merilaita S. 2011. Animal Camouflage: Mechanisms and Function. Cambridge University Press. [Google Scholar]
- Stevens M, Winney IS, Cantor A, Graham J. 2009. Outline and surface disruption in animal camouflage. Proceedings of the Royal Society B 276:781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer GH, Thayer AH. 1918. Concealing-Coloration in the Animal Kingdom. New York: The MacMillan Company. [Google Scholar]
- Tovée MJ. 2008. An Introduction to the Visual System. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Troscianko J, Lown AE, Hughes AE, Stevens M. 2013. Defeating crypsis: detection and learning of camouflage strategies. PLoS ONE 8:e73733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troscianko T, Benton CP, Lovell PG, Tolhurst DJ, Pizlo Z. 2009. Camouflage and visual perception. Philosophical Transactions of the Royal Society B 364:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrie EK, Hanlon RT, Siemann LA, Uyarra MC. 2015. Coral reef flounders, Bothus lunatus, choose substrates on which they can achieve camouflage with their limited body pattern repertoire. Biological Journal of the Linnean Society 114: 629–638. [Google Scholar]
- Vasil’eva ED. 2007. Seahorse species (genus Hippocampus, Pisces) described by C. Linné. Folia Zoologica 56:319–327. [Google Scholar]
- von Bartheld CS, Meyer DL. 1985. Trigeminal and facial innervation of cirri in three teleost species. Cell and Tissue Research 241:615–622. [Google Scholar]
- Wallis C 2004. Seahorses: Mysteries of the Oceans. Charlestown, MA: Bunker Hill Publishing. [Google Scholar]
- Watson AC, Siemann LA, Hanlon RT. 2014. Dynamic camouflage by Nassau groupers Epinephelus striatus on a Caribbean coral reef. Journal of Fish Biology 85:1634–1649. [DOI] [PubMed] [Google Scholar]
- Weaver HL. 1955. An improved gelatine adhesive for paraffin sections. Stain Technology 30:63–64. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. 2000. Color Science: Concepts and Methods, Quantitative Data and Formulae. New York: Wiley-Interscience. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1: Video clip of a slender filefish in situ. Notice the appearance and disappearance of dark patches on the dorsal side of the body above the longitudinal dark stripe.
Supplementary Video 2: Video clip of a slender filefish in situ. Notice the overall lightening of the pattern and the disappearance of dark patches on the lateral side of the animal.
Supplementary Figure 1: Same animal as the filefish on the right in Figs. S9 and S10. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 2: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 3: Same image as Fig. 2H. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 4: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 5: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 6: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 7: (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 8: Same animal as Fig. 5. (A) Filefish camouflaged in situ. (B-C) Masks created to isolate the animal (B) from its stimulus and background (C). (D-F) Low resolution (pupil is 1 pixel) images of background (D), animal (E), and stimulus (F) analyzed for color match. (G-I) Higher resolution (pupil is 4 pixels) images of background (G), animal (H), and stimulus (I) analyzed for color match. (J) Results of the Canny edge detection analysis. (K) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background is red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 9: (A) Filefish signaling in situ (presumably courtship behavior). Animal on the right is the same animal camouflaged in Fig. S1. The same pair of animals is presented in Fig. S10. (B-D) Masks created to isolate the animals (B-C) from their stimulus and background (C). (E-H) Low resolution (pupil is 1 pixel) images of background (E), animals (F-G), and stimulus (H) analyzed for color match. (I-L) Higher resolution (pupil is 4 pixels) images of background (I), animals (J-K), and stimulus (L) analyzed for color match. (M) Results of the Canny edge detection analysis. (N) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background are red, and regions where the animal is lighter than the background are blue.
Supplementary Figure 10: (A) Filefish signaling in situ (presumably courtship behavior). Animal on the right is the same animal camouflaged in Fig. S1. The same pair of animals is presented in Fig. S9. (B-D) Masks created to isolate the animals (B-C) from their stimulus and background (C). (E-H) Low resolution (pupil is 1 pixel) images of background (E), animals (F-G), and stimulus (H) analyzed for color match. (I-L) Higher resolution (pupil is 4 pixels) images of background (I), animals (J-K), and stimulus (L) analyzed for color match. (M) Results of the Canny edge detection analysis. (N) Results of the local contrast analysis; regions where contrast is similar (<10% different) are color coded green, regions where the animal is darker than background are red, and regions where the animal is lighter than the background are blue.