Abstract
Intermittent and periodic fasting (IF and PF, respectively) are emerging as safe strategies to affect longevity and healthspan by acting on cellular aging and disease risk factors, while causing no or minor side effects. IF lasting from 12 to 48 hours and repeated every 1 to 7 days and PF lasting 2 to 7 days and repeated once per month or less have the potential to prevent and treat disease, but their effect on cellular aging and the molecular mechanisms involved are only beginning to be unraveled. Here, we describe the different fasting methods and their effect on longevity in organisms ranging from yeast to humans, linking them to the major nutrient-sensing signaling pathways and focusing on the benefits of the fasting and the refeeding periods. We also discuss both the therapeutic potential and side effects of IF and PF with a focus on cancer, autoimmunity, neurodegeneration and metabolic and cardiovascular disease.
Dietary restriction (DR) refers to regimens including the reduction of the intake of either calories or of specific components of the diet, such as protein or certain amino acids, and to intermittent and periodic fasting (IF and PF, respectively), which may or may not require an overall reduction in calorie intake. Instead, calorie restriction (CR), which in most cases involves a chronic reduction in calorie intake by 20–40% below the standard, extends lifespan and healthspan in yeast, invertebrates, laboratory rodents and non-human primates1–7. In humans, 15% CR reduces markers or risk factors for a range of age-related diseases, including diabetes, cancer and cardiovascular disease, but it also causes side effects, which include a low or very low body mass index (BMI) when applied chronically1,8. Moreover, a chronic CR of 20–60% in mice can have positive effects on aging and immune function9,10, but can increase susceptibility to certain pathogens, such as the influenza virus and intestinal parasites11,12.
Protein restriction (PR) independently of calorie intake13–15 also extends lifespan in mice16,17 and improves the health of young and middle-age mice and humans, but moderate to severe PR can lead to frailty and/or disease in old mice or individuals over the age of 65 (refs. 16,18). Severe PR, in which calories from proteins are below 5% of the total, could also have detrimental effects, including weight loss in younger organisms, as shown in mice18. Signaling pathways by which CR and PR extend lifespan include those activated by growth hormone, insulin-like growth factor-1 (IGF-1) and insulin, and involve downstream factors, including phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin complex 1 (mTORC1), protein-kinase A (PKA), AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), sirtuins and forkhead transcription factors (FOXOs), that are well established to regulate or affect aging and longevity19–23. Thus, both CR and PR can have strong effects on nutrient signaling pathways and aging, but there is a need to identify novel interventions that optimize healthspan while minimizing side effects and the burden imposed by chronic dietary interventions. These studies could allow the identification of specific dietary regimens effective in delaying, and even partially reversing, aging and age-related diseases.
In this Review, we discuss the role of dietary restriction in aging and disease, with a focus on IF and PF regimens (Table 1 and Box 1). The use of generic terms like IF, fasting and even dietary restriction, which include interventions lasting from a few hours to months and represent many dietary compositions and severities of caloric restriction, needs to be limited and replaced by specific terminology referring to a clearly defined intervention so that use can be standardized for the laboratory, the clinic and eventually the public.
Table 1 |.
Experimental strategies to promote healthspan in different organisms
| Type of fasting regimens | Macronutrients composition | Feeding pattern | Description | Refs. | |
|---|---|---|---|---|---|
| Intermittent fasting (IF) | ADF | Standard (15% protein, 30% fat, 55% carbohydrate) | 24-hour fast (water-only)/24-hour eating period | Complete fasting every other day | 12–15 |
| 5:2 | Standard (15% protein, 30% fat, 55% carbohydrate) | 2-day fast, or very low calorie consumption (500–700 kcal)/5-day eating period | Alternation of 2 days of 500–700 kcal with a 5-day ad libitum eating period. The 2 days of fasting can be consecutive or separated | 17,18 | |
| TRF | Standard (15% protein, 30% fat, 55% carbohydrate) Obesogenic (10% protein, 60% fat, 30% carbohydrate) | 12- to 18-hour fast/6- to 12-hour eating period | Food intake restricted to 6–12 hours per day | 19,20 | |
| Modified ADF | Standard (15% protein, 30% fat, 55% carbohydrate) | 24-hour restriction (75% calories)/24-hour eating period | Alternation of 24 hours of a very low calorie consumption with a 24-hour ad libitum eating period | 14,16 | |
| Periodic fasting (PF) | Prolonged fasting (water-only) | Standard (15% protein, 30% fat, 55% carbohydrate) | 3- to 21-day water-only fast/ 7 days or longer-day refeeding period | Up to 21 days of water-only fasting followed an ad libitum eating period of at least 7 days | 9,65,66,107 |
| FMD | FMD (9–10% protein, 50–60% fat, 30–40% carbohydrate) | 4- to 7-day FMD/10- to 25-day refeeding period | 30–50% of the normal caloric intake for 4–7 consecutive days, followed by a refeeding ad libitum period once per month | 7,8,10,78 |
Box 1 |. Glossary.
Autophagy. The digestion of cellular components by enzymes of the same cell to allow for their degradation and recycling.
Calorie restriction (CR). A dietary regimen with a 20–40% reduction of the normal daily calorie intake without malnutrition.
Chromatin regulators. Factors that allow dynamic chromatin remodeling by accessing condensed genomic DNA regions to control gene expression.
Dietary restriction (DR). A dietary regiment which accounts restriction of one or more macronutrients. Calorie intake can be normal or restricted.
Epigenetic drift. Epigenetic changes acquired with age that modify the DNA-methylation landscape of a cell.
Fasting-mimicking diet (FMD). A diet designed to achieve fasting-like effects on the serum levels of IGF-1, IGFBP-1, glucose and ketone bodies, while providing both macro- and micronutrients to minimize the burden and adverse effects of water-only fasting.
Forkhead transcription factor FOXO. A subgroup of the forkhead transcription factor family regulated by the insulin–PI3K–AKT pathway and involved in activation of stress response and survival genes.
Healthspan. The period of an organism’s life during which it is generally healthy and free from serious or chronic illness.
Insulin–IGF-1 signaling. A conserved nutrient-sensing pathway among different organisms that coordinates growth, differentiation, metabolism and longevity in response to changing environmental conditions and nutrient availability.
Intermittent fasting (IF). An eating pattern in which short periods of fasting (16–48 hours) and eating periods (8–120 hours) are alternated.
Ketone bodies. Intermediates of the lipid metabolism that are produced by the liver from fatty acids during periods of low food intake, such as fasting or calorie restriction.
Protein restriction (PR). A dietary regiment with a reduction of total protein intake or a reduction of specific amino acids.
p16. A tumor suppressor protein with an important role in cell-cycle arrest. Its increase is related to increased senescence and cellular aging.
Periodic fasting (PF). An extreme dietary restriction period characterized by a complete restriction of food (water-only) or a severely calorie-restricted diet (FMD) for a prolonged duration (more than two days), followed by an undertermined ad-libitum refeeding period. PF can be repeated but infrequently.
Pluripotency markers. Markers that identify pluripotent cells, or cells that are able to differentiate into any cell type.
Ribosomal biogenesis. A cellular process that results in the biosynthesis assembly and arrangement of constituent parts of ribosome subunits.
Sirtuins. Sirtuins are both histone deacetylases (HDAC, class III), able to remove the acetyl group from acetylated lysines inhistones) and ADP-ribosyltransferases. They regulate cellular metabolic signaling and protein post-translational modifications.
Stem-cell self-renewal. Stem cells’ ability to go through cycles of cell division while maintaining the undifferentiated state.
Starvation. A chronic nutritional restriction that, in contrast to IF and PF, results in detrimental effects, which can include a deficit in fat stores and the atrophy of muscles.
Time-restricted feeding (TRF). A daily eating pattern in which all nutrient intake occurs within a limited number of hours (usually ≤12 hours).
These intermittent or periodic dietary interventions can promote cell protection and repair as well as the clearance of damaged cells and intracellular components, in part through the modulation of conserved stress-response or nutrient-sensing pathways. Whereas the dietary-restriction field has mostly focused on the benefits of continuous caloric or macronutrient restriction, here we focus both on the importance of much shorter restriction periods and the refeeding and post-refeeding phases, which is accompanied by a regenerative process that is not observed or is much less active during chronic restrictions19,21,22,24. We summarize the metabolic and cellular responses triggered by these feeding regimens, their impact on nutrient signaling pathways and their link to age-related diseases.
Fasting methods and effects
Common fasting methods.
Different fasting regimens (Table 1) can affect metabolism, aging, disease and mortality in simple organisms and mammals. IF includes various eating patterns; water-only fasting or severely restricted (over 50%) calorie intake lasts between 12 and 48 hours, and in most cases is repeated in cycles occurring every day to once per week25. There are several types of IF diets commonly adopted in rodents and human clinical studies: complete water-only fasting that occurs every other day (also called alternate-day fasting (ADF))26–28; 70% energy restriction every other day27–29; the 5:2 diet, which provides between 500 and 700 calories for 2 days per week30,31; and time-restricted feeding (TRF), in which food intake is in most cases restricted to 6–12 hours per day32,33. Thus, IF regimens usually encompass a period in which only water is consumped or calorie intake is very low, which is followed by a normal feeding period that in most cases lasts between 12 and 48 hours.
In contrast, PF refers to a prolonged and severely calorie-restricted or water-only fasting period lasting in most cases between 48 hours and 1 week, although several studies have investigated longer fasting periods25. Unlike IF, it can occur at specific intervals or on an ‘as needed’ basis, but it is usually carried out less than once every 2 weeks and in most cases only a limited number of times per year for periods lasting 2 days or longer in mice or 4 days or longer in humans25,34. There are two major methods of PF: (1) water-only PF25 and (2) a fasting-mimicking diet (FMD), which is a plant-based caloric-restricted diet containing low proteins, low sugars and high unsaturated fats that are able to mimic the effects that water-only fasting has on IGF-1, IGF binding protein 1 (IGFBP-1), ketone bodies and glucose19,34.
Metabolic effects of fasting.
In most mammals, the liver serves as the main reservoir of glucose, which is stored in the form of glycogen. In humans, depending upon their level of physical activity, 12–24 hours of fasting typically results in a decrease in serum glucose and depletion of hepatic glycogen, accompanied by a switch to a metabolic mode in which glucose, fat-derived ketone bodies and free fatty acids are used as energy sources25. After hepatic glycogen depletion, lactate, pyruvate, fat-derived glycerol and amino acids account for the gluconeogenesis-dependent generation of approximately 80 g per day of glucose, which is mostly utilized by the brain25. Depending on the severity and length of the restriction, fatty acids are mobilized, leading to an increase in circulating ketone bodies and adiponectin and a lowering of circulating leptin29,35. In humans, the fed-state blood levels of ketone bodies are at or below the limit of detection and reach levels of 0.2–0.5 mM within 8–12 hours after the onset of fasting, but reach levels between 1 and 2 mM by 48 hours36,37. This metabolic switch occurs more rapidly in rodents, as plasma ketone levels are elevated within 4–8 hours after the onset of fasting and reach millimolar levels by 24 hours38.
Intermittent fasting, metabolism and aging
Intermittent fasting in simple organisms and rodents.
IF affects longevity in multiple organisms. In worms, IF activates mitochondrial-network plasticity as they are switched between fasted and fed states, which may contribute to longevity extension39. Moreover, recent studies in mice show that cycles of fasting lasting from 12 to 72 hours followed by a refeeding period have beneficial effects on longevity, markers for health, stress, metabolic response, age-related diseases and tissue regeneration; cycles of fasting lasting 48 hours or longer will be covered in more detail in the ‘Effects of periodic fasting and fasting-mimicking diet’ section21,25,40,41. In flies, chronic IF has failed to extend lifespan, suggesting that flies can be sensitive to a shorter starvation period42,43. In mice, however, IF can have both neutral or positive effects on lifespan (see following section) and the beneficial effects of DR on longevity appear to be due, at least in part, to the time-restricted access to food, and therefore to extended daily fasting periods16. New studies in flies investigating short-term IF regimens for only a part of the lifespan followed by a switch to ad libitum feeding at later stages indicate stronger effects on the aging process44. Again, more marked effects are observed when the flies are switched back to an ad libitum diet after 30 days of IF, in agreement with a series of studies in mice, indicating that the refeeding period is as important as the fasting or restricted stage19,20,22,23.
A number of studies have investigated the effect of IF on the health and lifespan of rodents. In one of the earliest studies, Goodrick et al. reported an increase of up to 80% in the average lifespan of rats maintained on a regimen of ADF, started at 5 weeks of age45. Later studies show much smaller effects of ADF, but several of them confirm an extension of median, as well as maximal, lifespan46,47. In addition, longevity in male C57BL/6J mice subjected to ADF is associated with a delayed onset of lethal neoplastic disorders48 that typically limit natural lifespan in many mouse strains49,50, but this was achieved without a delay of the aging process, in agreement with earlier studies48. Thus, the magnitude of the effects of ADF on longevity in rodents depends upon the species (mouse versus rat), strain and age at regimen initiation, and can range from a small negative effect to as much as an 80% lifespan extension47,51. Similarly, it has emerged from a genetic screening that CR can exert positive or even detrimental effects on longevity, depending on genetic variations in different mouse backgrounds52. For example, in two different mouse genetic backgrounds (A/J and C57BL/6J), IF did not extend mean lifespan, and even reduced lifespan, when initiated at 10 months47. When initiated at 1.5 months, IF either increased longevity or had no effect47. However, in rodents, IF enhances cognitive performance53, which may be caused in part by its stimulatory effect on synaptic plasticity54, improves insulin sensitivity and reduces blood pressure and heart rate55,56. Moreover, 2-month-old male C57BL/6J mice fed a time-restricted high-fat diet (16-hour daily fasting period), show protection against obesity, hyperinsulinemia, hepatic steatosis and inflammation with improved motor coordination, despite a caloric intake equivalent to that of the group with ad libitum access to a high-fat diet57. At the molecular level, TRF in mice affects the energy signaling mediated by c-AMP responsive element binding protein (CREB) and AMPK, the progrowth mTOR pathways and the expression of circadian clock genes57.
A deep understanding of the type and length of fasting and mechanisms that can maximize longevity effects, as well as of the detrimental effects that may be counterbalancing the positive ones, is required. Fasting may be consistently protective in young and middle-aged laboratory rodents that are affected by cellular damage and aging, which lead to insulin resistance, inflammation, genomic instability and so on, but may have at least some detrimental effects in old or very old animals after they begin to lose weight or become frail25.
Human studies on intermittent fasting, aging and disease risk factors.
A rapidly increasing number of studies have investigated the effects of different IF regimens on humans41. A recent study showed that 8-hour TRF without intentional calorie limitation produces mild caloric restriction and weight loss in obese adults58. A number of trials testing IF in humans show positive effects on metabolic markers but, together with epidemiological studies, also point to potential side effects, particularly after long-term use. Varady and colleagues showed that ADF in overweight or obese adults with insulin resistance produces a greater reduction in insulin levels and insulin resistance than CR does, despite achieving a similar decrease in body weight59. On the other hand, Madeo and colleagues found that either short-term (4 weeks) or long-term (6 months) ADF carried out in healthy middle-aged humans has beneficial effects on metabolic and cardiovascular markers alongside reduced levels of soluble intracellular adhesion molecule-1 (sICAM-1), an age-associated inflammatory marker, low-density lipoprotein (LDL) and the metabolic regulator triiodothyronine60. Panda and colleagues, who had shown the beneficial effects of TRF for preventing and treating obesity and metabolic disorders in mice32–34, showed similar cardiometabolic benefits in people with metabolic syndrome who consumed food for only 10 hours daily61,62. TRF prevents excessive body-weight gain, improves sleep, attenuates age- and diet-induced deterioration in cardiac performance and improves blood pressure and accumulation of atherogenic lipids62. Cienfuegos and colleagues also reported that 4- and 6-hour TRF reduces body weight, insulin resistance and oxidative stress compared with results from non-time-restricted controls, supporting further studies on TRF as a promising intervention for weight loss and cardiometabolic protection63.
The health effects of IF are accompanied by weight loss, but how much reduced adiposity affects disease risk factors remains poorly understood. In one trial, 16 healthy participants (aged between 23 and 53 years with a BMI between 20 and 30) assigned to a regimen of ADF for 22 days lost 2.5% of their initial weight and 4% of their fat mass, with a 57% decrease in fasting insulin levels64. In two other trials, overweight women (approximately 100 women in each trial) were assigned to either a 5:2 IF regimen or a 25% reduction in daily caloric intake. The women in the two groups lost the same amount of weight during the 6-month period, but those in the group assigned to the 5:2 IF had a greater increase in insulin sensitivity and a larger reduction in waist circumference30,65.
However, there are also a number of studies indicating that frequent fasting cycles may not only be difficult to carry out for long periods, but also increase side effects and even mortality. For example, the risk of gallstone disease nearly doubles between women who fast for 8 hours per day and those who fast for over 14 hours per day66. Furthermore, skipping breakfast, which is perhaps the most common method adopted to reach a daily 14- to 18-hour daily fasting period, is associated with an increased risk of mortality from cardiovascular and all-cause mortality in the US population67. Clearly, epidemiological data are not easy to interpret and the association between long daily fasting periods and increased incidence of disease or mortality could be explained by factors other than the fasting itself. However, until further studies, including randomized clinical studies and additional epidemiological studies, are conducted, the use of this type of daily fasting intervention should be limited to short-term periods and applied to only people with disease for which regular IF has been demonstrated to be effective. It is also important to gain an understanding of the effect of long daily fasting periods in which dinner is skipped instead of breakfast. In contrast, daily fasting/TRF periods of approximately 12 hours appear to be associated with benefits without known negative effects.
Effects of periodic fasting on aging
Periodic fasting and fasting-mimicking diet.
In contrast to the short and very frequent fasting periods of IF, PF or FMD last in most cases between 2 and 7 days (2–3 days in mice and 4–7 days in humans) and are followed by a high-nourishment refeeding period of at least 1 week25,68,69. Another major difference from IF is that PF can be periodic and does not have to be carried out at a specific interval, but can be applied for one or several cycles either as a preventive measure or to treat a specific disease or condition. PF was traditionally carried out in specialized clinics with water-only or very-low-calorie methods, but outside of a clinic, such a regimen can be difficult to maintain and unsafe because it can cause side effects, including malnourishment, rapid weight loss, reduced blood pressure and hypoglycemia70,71, as well as the exacerbation of existing micronutrient deficiencies. These safety concerns and the scarcity of preclinical and clinical data may explain why historically the potential benefits of PF have emerged multiple times within the medical community, but have eventually disappeared and have not been integrated into standard-of-care practices. Thus, FMDs were developed to promote the effects of fasting while standardizing dietary composition, providing nourishment and minimizing the burden and side effects associated with water-only fasting19. Notably, these steps are necessary for PF and possibly IF to move toward approval from the US Food & Drug Administration and standard-of-care applications.
The FMD composition, which includes low protein, low sugar and high unsaturated fat, achieves a reduction in IGF-1 and glucose, and an increase in ketone bodies, and IGFBP-1, similar to that caused by water-only fasting in mice34. Various versions of the rodent FMD have been utilized, but in most cases, they provide between 10% and 50% of the normal caloric intake for periods ranging from 2 to 5 days, with the most severe restrictions lasting from 2 to 3 days7. A longer regimen with less caloric restriction is also used, which consists of 5 days with a caloric intake ranging from 50% on the first day to 30% for the rest of the days19,72. Mice undergoing FMD cycles lose about 15–20% of their body weight, which is recovered upon refeeding. In fact, the severe caloric restriction is compensated by overeating during the refeeding period, resulting in the same or similar caloric intake in the FMD and control groups19.
Rodent studies on prolonged fasting or fasting-mimicking diet and longevity.
Sixteen-month-old female C57BL/6 mice placed on a periodic 4-day FMD twice per month, alternating with a normal diet, display an 11% increase in their median lifespan, in addition to significant weight and visceral-fat loss, without loss of muscle mass. Moreover, FMD cycles reduce tumor incidence by 45% and delay tumor development, with most being detected after 26 months of age. FMD cycles that are started at middle age reduce skin inflammation including dermatitis by 50% and improve motor coordination along with long- and short-term memory19. Notably, the FMD cycles also promote changes leading to an immune-system profile in 20.5-month-old mice more similar to that of younger mice (4 months old), in agreement with the effect of PF on hematopoietic stem cell (HSC)-dependent regeneration of immune cells21. In addition, FMD cycles selectively reduce visceral fat without an overall reduction in per-month calorie intake, indicating a potential acceleration in metabolism during the refeeding period. In summary, similarly to the well-established effects of CR73, FMD cycles delay the onset and reduce the incidence of age-related diseases19, but achieve this with minimal or no long-term reduction in calorie intake and with positive effects on immunity and a targeted reduction in visceral fat. Thus, PF/FMD but potentially also certain DR, including IF, may achieve many beneficial effects by mechanisms that are independent of reduced calorie intake. In fact, chronic protein restriction, without calorie restriction, is well established to extend longevity and healthspan16,74. Notably, IF and PF, similarly to chronic CR, delay disease incidence but also reduce the lifelong portion of animals that develop any type of disease, and particularly cancer19,25,34.
One limitation of studies with PF and IF is that they have been conducted on only a few mouse strains, and mostly on C57BL6 mice19,57,75,76, so we do not yet know whether the health benefits and lifespan increase is not dependent on rodent strain. On the other hand, several of these studies were conducted in both female19 and male mice, and some of them began when the mice were young75 and others when the mice were already middle aged (16 months)19, indicating that the health- and lifespan benefits of IF or PF can be achieved when started at middle age. A recent study has shown that there is memory of CR done during early mouse life, and that age-dependent mortality can depend on the nutrition earlier in life77. This is another effect of CR, IF and PF that is poorly understood and could help achieve the goal of maximizing healthspan and longevity with minimal burden.
Human studies on prolonged fasting/fasting-mimicking diet and longevity.
A study assessed longer periods of fasting in large cohorts that included non-obese participants78,79. In a 1-year observational study, 1,422 non-obese participants aged between 18 and 99 participated in a fasting program consisting of fasting periods of between 4 and 21 days in which they fasted with a daily caloric intake of 200–250 kcal accompanied by a moderate-intensity lifestyle program. Significant reductions in weight, abdominal circumference and blood pressure were observed, along with a reduction in blood glucose levels and increase in ketone bodies, proving the fasting-related metabolic switch79. However, these effects are detected during the fasting period. Also, a single period of extended fasting with concomitant weight reduction leads to significant, rapid improvement of fatty-liver index in people with or without type 2 diabetes (age ≥ 18 and BMI ≥ 19 kg per m2) by the end of the fasting cycle, but the long-term effect of this regimen on fatty liver after return to the normal diet is not known78. The major limitation of these very severe and prolonged fasting periods is that they must be carried out in a specialized clinic to avoid adverse events. Furthermore, we do not know the long-term effects of the long periods (weeks) of water-only or very-low-calorie fasting on health. In fact, long periods of CR have been associated with a reduction in metabolic rates80, which could actually promote, rather than reduce, fat accumulation after the end of the fasting or CR period, which would result in the regain of weight as established in studies involving severe restrictions or fasting lasting 10 days and longer. In addition, multiple cycles of weight loss (ranging between 7% and 10%) and regain (yo-yo diets) are associated with increased mortality81, indicating that long and severe fasting periods could have short-term benefits followed by long-term beneficial as well as detrimental effects.
The periodic use of FMD and refeeding cycles was studied to identify interventions to maximize effects against aging and age-related diseases, while minimizing side effects and the burden of frequent restrictions. In a randomized clinical trial with 100 relatively healthy volunteers, FMD cycles lasting 5 days, carried out once per month for 3 months, reduce multiple risk factors for age-related diseases, including diabetes, cancer and cardiovascular disease72. These effects include reduced body weight and trunk fat, lowered blood pressure and decreased IGF-1, along with decreased BMI, glucose, triglycerides, cholesterol and C-reactive protein 5 to 7 days after returning to a normal diet in people that displayed elevated levels of these markers at baseline72. Notably, the beneficial effects of the FMD on several of these markers/risk factors were maintained for months after subjects returned to a normal diet72. These studies underline the potential of PF, FMDs and other types of fasting for extension of not only healthspan but also ‘youthspan’ (the range of time in which an organism remains youthful, healthy and fully functional)82. Thus, PF and FMD cycles that alternate with normal refeeding periods appear to be safe in both rodents and humans and to have beneficial effects on disease or disease risk factors when started at middle-old age (16 months in rodents). Although FMDs appear to be safe, their use should be limited to three times per year in healthy people with normal levels of disease risk factors, until additional and long-term clinical studies demonstrating the safety of more frequent use are carried out. In contrast, the more severe and longer forms of fasting should only be done in a specialized clinic in the presence of medical personnel. How much of the benefit of PF is due to effects on cellular aging and dysfunction versus the effect of weight loss remains unclear and is addressed in the following sections.
Because there is a concern that all types of fasting methods may in the long term cause side effects, including those listed earlier, when done too frequently, the beneficial effects of IF and PF must be weighed against their potential side effects, particularly in healthy or relatively healthy individuals. Both basic and clinical research should focus more on interventions that maximize efficacy against aging and age-related diseases while minimizing side effects and the burden of the intervention, which is usually inversely correlated with long-term compliance.
Periodic fasting, intermittent fasting and nutrient signaling pathways
Reduced activity of the nutrient-sensing pathways that regulate aging in yeast (Fig. 1), worms and flies can also extend longevity in rodents (Fig. 2). Thus, inhibition of the mTOR pathway either pharmacologically with rapamycin83 or genetically by deletion of the ribosomal S6 kinase 1 (S6K1) extends longevity in mice84. In addition, S6K1-mutant mice show a delayed onset of age-related phenotypes, such as bone-matrix loss, immune and motor dysfunction and insulin resistance84.
Fig. 1 |. Fasting, nutrient signaling and longevity in yeast.
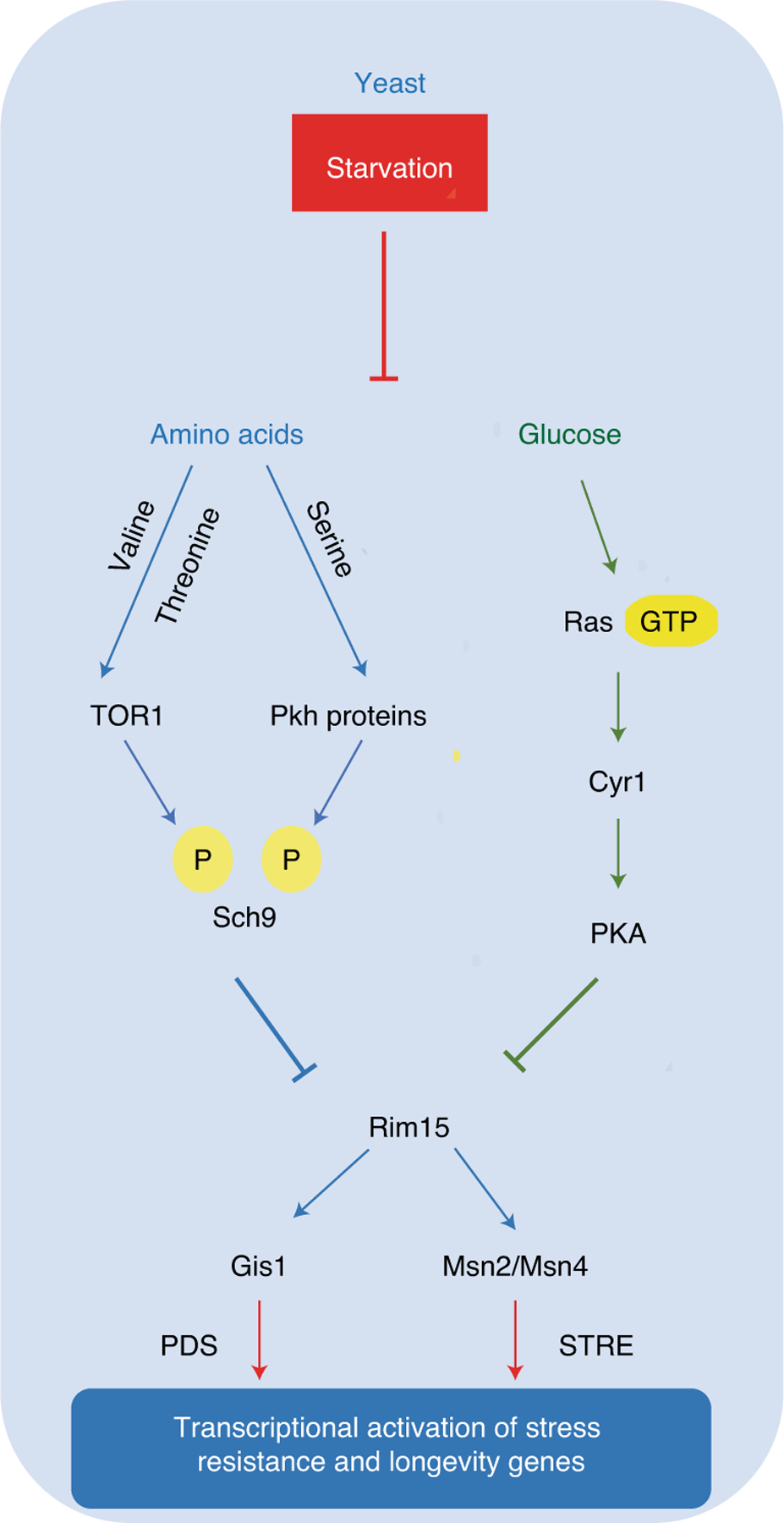
Starvation conditions in yeast cause a major lifespan extension mediated in large part by the lack of amino acids and sugars. On one hand, amino-acid restriction causes the inactivation of TOR–Pkh–S6k signaling; on the other hand, low glucose levels promote reduced activity of the Ras–adenylate cyclase (Cyr1)–PKA pathway. Both the amino-acid and the sugar pathways converge on and inactivate the serine threonine kinase Rim15. This, in turn, contributes to the activation of stress-resistance transcription factors Gis1, which binds to post diauxic shift (PDS) motif, and Msn2 and Msn4, orthologs of mammalian early growth response protein 1 (EGR1)1,19, which bind to stress-responsive element (STRE) motif.
Fig. 2 |. Conserved nutrient-sensing response pathways in worms, flies and mammals.
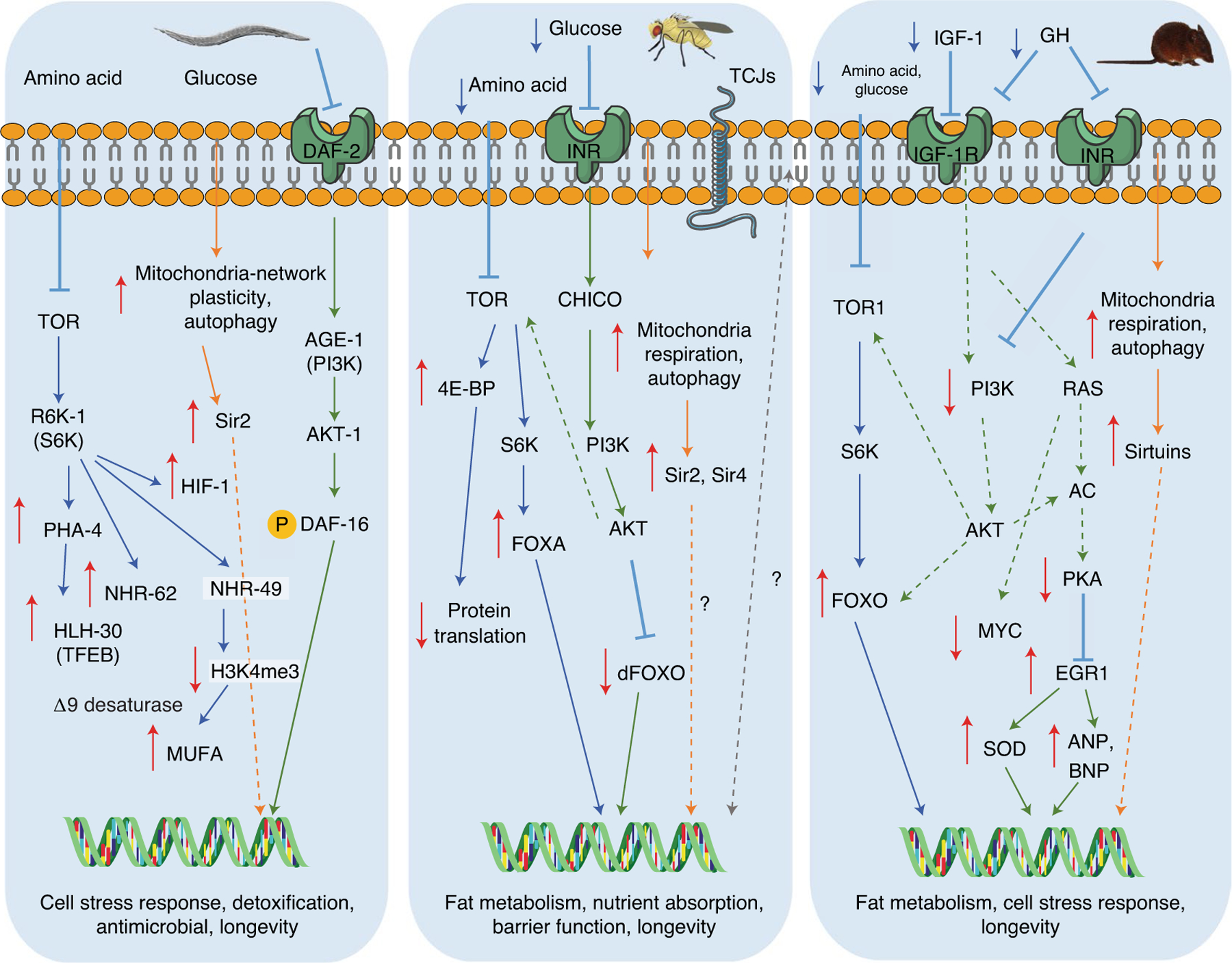
This model summarizes the conserved nutrient-sensing pathways that regulate longevity and stress-response mechanisms in different model organisms1. Fasting or calorie restriction reduces the activity of amino acids and glucose signaling pathways through membrane receptors by reducing circulating ligands such as growth factors like mammalian IGF-1. The fasting-inhibited TOR–S6K pathway (labeled in blue) promotes the expression of nuclear transcription factors such as the hypoxia-inducible factor-1 (HIF-1), the FOXA ortholog PHA-4, the nuclear hormone receptors NHR-62 and NHR-49 and the TFEB ortholog HLH-30 (worms), the FOXA nuclear factor (flies) and the increase of the FOXO nuclear factor (mammals). These transcription factors commonly activate antiaging systems and processes, including autophagy and ribosomal biogenesis, stress response and cellular-protection genes, including antioxidant SODs. The RAS–AC–PKA pathway (labeled in green) is also partially conserved between species. Similar to what was observed in yeast, glucose in certain mammalian cells can signal through the PKA pathway and the transcription factor EGR1, the mammalian ortholog of Msn2/Msn4 in yeast. In worms, flies and mice, downregulation of TOR–S6K signaling has conserved proaging effects1. The fasting-dependent effects on longevity in different organisms may also involve sirtuin pathway activation (labeled in orange), the increase in mitochondria respiration and the activation of autophagy39,40. Also, fasting in flies delays the disruption of tricellular junctions (TCJs), which is linked to improved intestinal barrier integrity and therefore longevity (labeled in gray)148.
The GH–IGF-1 axis, acting upstream of mTOR–PI3K–AKT-1 and PKA signaling, has been intensively studied in mammals because of its effects on the incidence of age-related diseases and lifespan. Indeed, mice lacking the GH receptor binding protein (GHR-BP) display a 40% longer average lifespan than that of controls, with reduced tumor incidence85.
A number of additional studies have shown similar extended lifespans for mice with defects leading to both GH and IGF-1 deficiencies86, which are also associated with downregulation of TOR signaling in multiple cell types. Whereas the mTORC1 inhibitor rapamycin increases longevity in wild-type mice83,87,88, in mice with knocked out growth hormone receptor that have constitutively suppressed mTORC1 and upregulated mTORC2 signaling, it leads to a drastic reduction in mTORC2 in liver, muscle and subcutaneous fat, which in turn causes an elevation of the inflammation marker interleukin-6, reduced numbers of functional immune cells and a shortened lifespan89. These results together, with a series of previous studies, indicate that mTORC2 is not as clearly linked to aging as mTORC1 and that its inhibition may even be deleterious90–92.
Because carbohydrates and proteins play a central role in growth, it is not surprising that the protein and sugar restriction associated with PF, IF and CR causes conserved changes in growth factors and nutrient signaling. It is well established that higher protein levels increase IGF-1 levels93 and that several amino acids are sufficient to promote an increase in the levels of this growth factor in the serum and also in TOR–S6K signaling94,95. For example, methionine regulates the growth hormone (GH)–IGF-1 axis, whereas amino acids including leucine and arginine can activate TOR–S6K signaling74,95,96. Moreover, the balance of macronutrients, not just their levels, and particularly of protein and carbohydrate can affect lifespan. In the mouse liver, when protein is replaced with carbohydrate, compensatory mechanisms are inhibited and protein uptake is suppressed, leading to mTOR inhibition16. Furthermore, the quality of macronutrient, for instance animal- versus plant-derived proteins, can influence aging and disease. In fact, high protein intake in adult life (up to age 65) is associated with increased risk of overall mortality and cancer-related death, however this association is attenuated or eliminated when the higher intake of proteins is from plant-derived sources18. It has also emerged that amino-acid quality can have an important effect on aging, and in fact an imbalance of branched-chain amino acids compared with other amino acids (high BCAA:non-BCAA ratio) leads to reduced longevity through a mechanism independent of mTOR activation and caused by hyperphagia97.
Sugars can also activate various pathways including Ras or PKA signaling either through insulin action or by an insulin-independent mechanism of insulin and resulting in reduced antioxidant protection and cellular stress sensitivity98. Thus, it is not surprising that GH- or GHR-deficient mice and those undergoing IF or PF, all of which reduce common proaging pathways, share an extended longevity and a major reduction in disease incidence99. For example, CR (from 10% to 50%) reduces IGF-1 and insulin, as well as Tor–S6K signaling, in rodent models. In agreement with mouse studies, in humans CR reduces IGF-1 levels only when protein intake is also restricted, underlining the need to focus both on calorie intake and dietary composition and their effects on nutrient signaling pathways1,100. IF, including ADF and TRF (8-week fast, 16 hours per day (16:8)), also decrease IGF-1, blood glucose and insulin levels while increasing insulin sensitivity and adiponectin levels101.
PF and FMDs also affect the levels TOR–S6K, IGF-1, insulin and glucose in mice, but these effects are reversed when animals return to the normal diet19,25,34. Thus, it is likely that a long-lasting reduction of factors including glucose, insulin and IGF-1 caused by CR is not necessary for at least part of the lifespan and healthspan effects1. However, in humans, FMD cycles can have long-lasting effects on IGF-1, insulin and glucose, raising the possibility that at least some of the effects of dietary restriction and FMDs on longevity may involve long-term effects on the levels of these factors72.
Another mechanism that may explain the role of the temporary reduction of these factors on the longevity extension caused by PF is the activation of HSC-based and other regenerative processes discussed later in this Review. Notably, these effects depend on the activation of regenerative processes that begin during fasting periods but are completed after mice return to a normal diet19–21. IF lasting between 12 and 24 hours can also have effects on IGF-1, IGFBP-1, glucose and ketone bodies, but most of the changes are smaller than those obtained by the longer PF or the chronic CR. For example, in mice, 4 weeks of every-other-day IF did not elevate ketone bodies but instead caused a reduction in β-hydroxybutyrate levels and β-hydroxybutyrate dehydrogenase activity in the mouse liver but not in the cerebral cortex, where levels remained unchanged or enhanced, supporting the importance of further investigating the mechanisms of ketone-body production, release and delivery102. However, circulating IGF-1, insulin and glucose were decreased after resistance-trained men (aged 29–33 and weighing 85–92 kg) were placed on a 16:8 fast, indicating that IF can have a positive effect on these factors101. Notably, because this IF period also caused loss of fat mass, it is difficult to establish whether the effects of IF on insulin and glucose and possibly IGF-1 are mediated by effects on adipose tissue. Importantly, these positive effects in men undergoing the 16:8 TRF were also accompanied by negative ones, including an over 20% decrease in total testosterone101. These results point to the importance of continuing to study both the short-term and long-term molecular changes caused by IF, PF and CR and to connect them to the positive as well as negative effects on metabolic markers, but also diseases and other conditions.
Fasting–refeeding and regeneration
Fasting and refeeding regimens are powerful promoters of stem-cell self-renewal mechanisms and activators of tissue regeneration, in part through inhibition and reactivation of the IGF-1, PKA and mTOR pathways19–21,103 (Fig. 3). The PF and FMD regimens can promote a rejuvenation process in tissues, organs and cells through the activation of cell death and autophagy followed by the activation of stem or progenitor cells19–21. Notably, the refeeding period appears to be responsible for a major component of the regeneration process leading to the replacement of senescent or damaged cells with new cells arising from tissue-specific stem cells19–22. Not surprisingly, the effects of short-term fasting and FMD on stem-cell function and regeneration depend on the content of the diet, its effects on different pathways and the timing and duration of the regimen. For example, lifelong CR does not prevent the age-related functional decline of HSCs in mice24, whereas short-term fasting periods, as well as FMD regimens followed by refeeding periods, do promote regenerative and rejuvenating effects in the hematopoietic and immune systems19. This is a fundamental distinction between cycles of fasting and refeeding compared with chronic dietary interventions, which may have smaller regenerative effects compared to fasting/refeeding by preventing or limiting the regenerative phase which requires high levels of macro- and micronutrients and possibly higher calories to support macromolecular synthesis, cellular division and growth and tissue and organ expansion18.
Fig. 3 |. Periodic fasting and tissue regeneration and rejuvenation in mice.
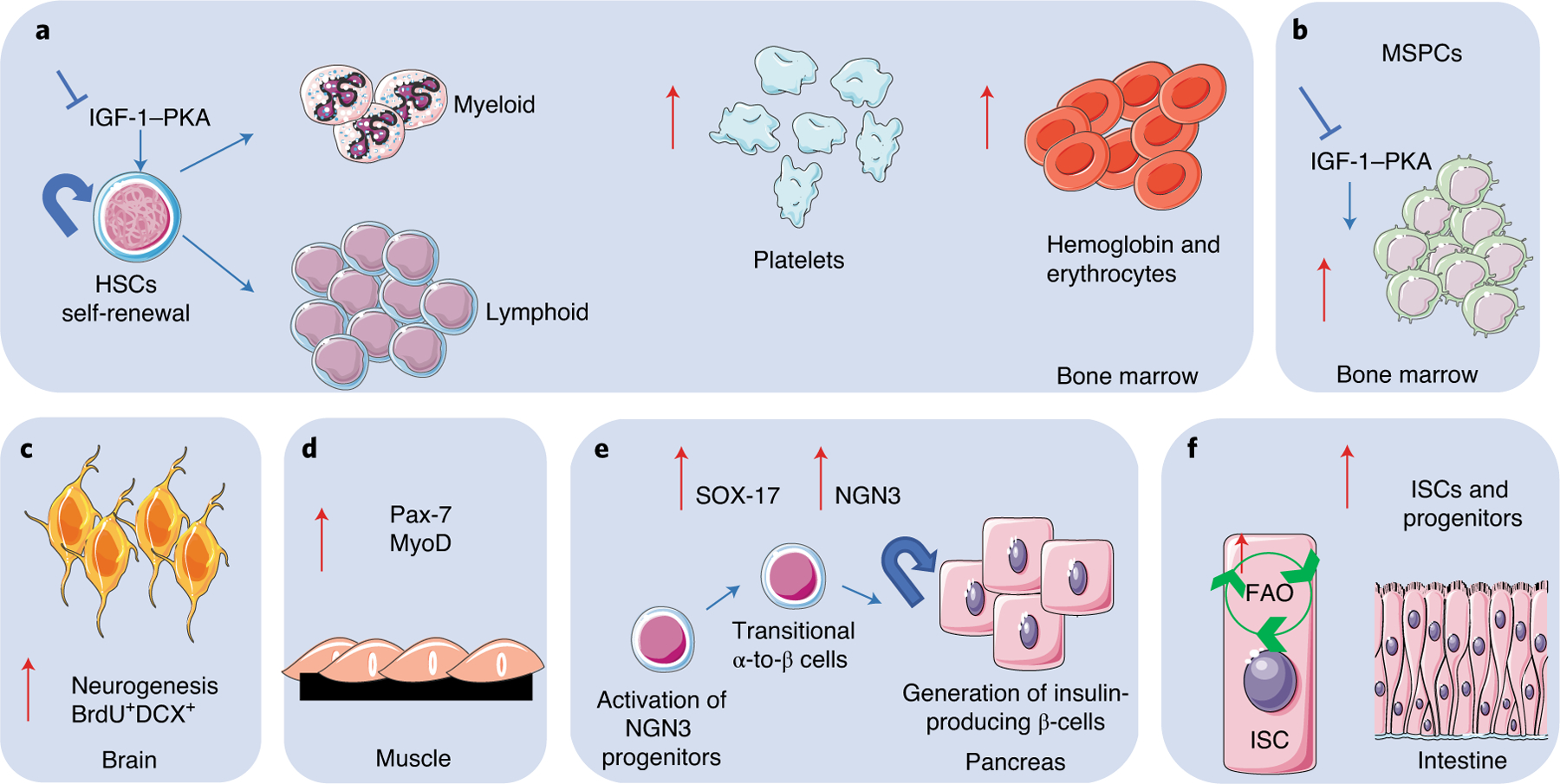
Periodic fasting or FMD can affect tissue regeneration in multiple systems and organs in mice. a, In bone marrow, PF or FMD drives self-renewal of HSCs and lineage-balance regeneration of the immune system, leading to a lymphoid-biased phenotype. b, PF and FMD increase mesenchymal stem and progenitor cells (MSPCs) in bone marrow. c, PF and FMD increase neurogenesis in brain tissue, represented by increase in doublecortin (DCX) levels in newly generated bromodeoxyuridine (BrdU+) neurons. d, In muscle tissue, PF or FMD modulates the expression of the pair box protein Pax-7, a stem-cell marker mainly expressed by muscle satellite stem cells, and MyoD, a marker of early muscle differentiation. e, In the pancreas, PF and FMD drive increased expression of early developmental markers, including SOX-17, and of the downstream NGN3 transcription factor, leading to the regeneration of insulin-producing β cells. f, In intestinal tissue acute one-day-only fasting, PF and FMD increase levels of ISCs and progenitors in part by inducing a fatty-acid oxidation (FAO) program or by modulating gut microbiota.
Stem-cell-based regeneration is stimulated by IF and PF through nutrient-sensing pathways19,21,103. The positive effects of fasting have been described in multiple stem-cell types, including muscle stem cells, HSCs, intestinal stem cells (ISCs) and neuronal stem cells (NSCs)19,21,103–105. The common mechanisms through which fasting affects stem-cell function involve modulation of the amino-acid- and glucose-sensing pathways, including IGF-1–TOR–PKA, also implicated in longevity regulation. However, the fasting–refeeding cycles also affect inflammation and can cause long-lasting epigenetic changes or changes in the stem-cell niche, which could contribute to the biological age of cells and organs40.
Whether changes in stem-cell function in response to nutrient availability are mediated by epigenetic changes is still unclear, but a few studies have indicated this possibility. For instance, the regulation of NSC proliferation in response to decreased glucose availability is governed by the nutrient sensors CREB and SIRT1, and the effects of CR are accompanied by an increase in histone H3 acetylated at Lys 9 (H3K9Ac)106. Changes in dietary intake are accompanied by massive changes in chromatin states in several species107,108. On the basis of these studies and on previous results of PF and FMD on tissue regeneration19–22,72, we speculate that FMD and/or refeeding periods may cause long-lasting epigenetic changes, although further studies are required to test this hypothesis and to understand its possible role in stem-cell maintenance during aging.
During aging, a skewing in the lineage of differentiation of HSCs occurs with relatively more myeloid cells being produced than lymphoid cells. This skewing contributes to an age-related impairment of adaptive immunity109. PF reduces IGF-1–PKA signaling and promotes HSC self-renewal and long-term repopulation capacity upon serial transplantations and lineage-balanced regeneration of the immune system21. Also, four periodic cycles of PF or FMD started at 16 months of age rejuvenate the hematopoietic system, leading to an increased number of white blood cells 1 week after refeeding, and to a partial reversion of the age-dependent lineage skewing19. Moreover, FMD cycles can partially revert the age-dependent decline of mesenchymal stem and progenitor cells, thus indicating that they promote proliferation of new stem and progenitor cells in various tissues19.
Quiescent satellite cells express the transcription factor paired box 7 (Pax-7), and when activated, coexpress Pax-7 and MyoD, the myoblast determination protein, which promotes transcription of muscle-specific target genes and plays a role in muscle differentiation. When they proliferate, they downregulate Pax-7 and differentiate110. Interestingly, mice undergoing FMD cycles show increases in Pax-7 and MyoD in muscle tissue, with associated reversal of markers of impaired autophagy19. Of note, the boost in protein expression of the regenerative markers was detected during the refeeding period, while no major changes were measured at the end of the fasting period. These results indicate that different systems may activate quiescent stem or progenitor cells at different stages during the fasting and refeeding periods. Notably, in old mice, short-term administration of spermidine, a known CR mimetic111, reverses the age-associated defect of autophagy in muscle cells, normally characterized by loss of proteostasis, increased mitochondrial dysfunction and oxidative stress. The molecular mechanism of spermidine action involves the increase of protein deacetylation and autophagy activation through induction of arginine and NO (nitric oxide) metabolism, as well as downregulation of proinflammatory cytokines in muscle stem cells (satellite cells)112.
In the mouse pancreas, PF and FMD promote a decrease in the numbers of differentiated or committed pancreatic cells, followed by the induction of transitional α-to-β or β-to-α cells that coexpress both α (that is, glucagon) and β (that is, PDX-1 or insulin) cell markers, and finally a major increase in the proliferation and number of insulin-generating β cells20. The metabolic reprogramming caused by the FMD also affects lineage determination in pancreatic islets with increased pluripotency and β cell reprogramming markers, especially one day after refeeding20. Results in human pancreatic islets from healthy donors and people with type 1 diabetes indicate that FMD induces the expression of SOX-2, NGN3 and insulin in part by reducing IGF-1 and inhibiting both mTOR and PKA signaling20. Recent work confirms the effect of FMD on pancreatic regeneration and diabetes in a genetic mouse model of type 2 diabetes20,113 and also confirms that a FMD promotes reduced blood glucose, increased insulin sensitivity, β cell proliferation and NGN3 expression113. The protein content of this specific FMD formulation was higher (17% versus 6%), whereas fat was lower (14% versus 65%), than the content of the Cheng et al. formulation20, but the diet was administered for nearly twice as long and was much more calorically restricted (7 days at 30% of daily calorie intake). Thus, in the latter study, changes in the key signaling pathways, similar to those caused by the shorter FMD, were likely to be achieved by a longer and more severely restricted period with a less-fasting-mimicking macronutrient ratio. Thus, the beneficial regenerative effects achieved are affected by dietary composition, severity of the calorie restriction and length of the PF or FMD period.
In another study, one day of fasting was sufficient to increase ISCs and progenitor activity both in young and old mice by inducing a fatty-acid oxidation program103. Interestingly, a recent study carried out in a mouse model of intestinal bowel disease shows that FMD cycles stimulate protective gut microbiota, reduce intestinal inflammation, increase stem-cell number and reverse intestinal pathology23. Notably, water-only fasting increases regenerative and reduces inflammatory markers without reversing pathology23, supporting the possibility that specific nutrients contained in the FMD, and possibly prebiotic ingredients contained in plant-based foods, can have a crucial impact on microbiota and consequently on the course of the disease. A recent study highlights that dietary macronutrients, in particular carbohydrate and protein, can also be major drivers of microbial response and can reshape microbiome by dictating nitrogen availability to bacteria114.
Previous studies also indicate that long-term dietary restriction can increase the regenerative capacity of ISCs via an extrinsic mechanism involving reduction of mTORC1 in the niche surrounding Paneth cells, suggesting that the refeeding period is not necessary for ISC activation104, although it may enhance or maximize regeneration as indicated in other studies19–22,72. Taken together, these studies suggest that the mechanisms of regeneration may vary depending on the timing, duration, composition and severity of both the fasting and refeeding diet. However, mechanisms involving IGF-1, TOR and PKA are likely to represent common denominators in the protective and regenerative effects of IF, PF and CR. Notably, both the inhibition of these signaling proteins and enzymes during the fasting and their activation during the refeeding are likely to be important for the regenerative process. In fact, their role in regeneration during the refeeding period is poorly understood and should be investigated further in multiple tissues.
Intermittent fasting, periodic fasting and aging-related diseases
Advancing age is the major risk factor for most major diseases, including cancer, diabetes and neurodegenerative115, cardiovascular and immunological diseases116–118. Because IF, PF and FMD cycles have been shown to slow down and partially reverse cellular aging in rodent models, a number of studies have investigated their potential application to the prevention and treatment of age-related diseases (Table 2).
Table 2 |.
Fasting and disease risk factors in humans
| Diet intervention | Disease risk factors decreased with fasting | References |
|---|---|---|
| ADF | Weight loss, total cholesterol, triglycerides, fat mass, LDL | 14,15,60,149 |
| 5:2 | Weight loss, insulin resistance | 17,61 |
| TRF | weight loss, fat mass, leptin, systolic blood pressure | 54,110,150 |
| FMD | Weight loss, systolic blood pressure, diastolic blood pressure, IGF-1, glucose, triglycerides, total cholesterol, LDL, C-reactive protein | 78 |
Neurodegeneration.
Fasting has been shown to protect neurons and ameliorate cognitive impairment in animal models119. A triple transgenic mouse model of Alzheimer’s disease (AD), which expresses familial AD mutations in the β-amyloid precursor protein (APP), presenilin 1 and Tau, fed either a 40% CR or ADF dietary regimen for 1 year starting at 5 months of age, develop reduced cognitive impairment compared with that in ad libitum-fed control mice; interestingly, 40% CR, but not ADF, reduces the levels of β amyloid (Aβ) and Tau accumulation in the brains of the AD mice120. The intermittent restriction of essential amino acids also protects mice from pathology and cognitive decline in triple transgenic AD mice, suggesting that the protein-restriction component of the fasting plays a key role in its protective effects121. The latter study did not detect reduced levels of Aβ after restriction of essential amino acids, although it did observe reduced accumulation of phosphorylated Tau in the hippocampus121. This suggests that fasting and protein restriction can protect the nervous system even in the presence of high levels of Aβ, although their effect on the fibrillar versus soluble forms of Aβ remain poorly understood. The mechanism by which fasting protects neurons from degeneration has been linked to increased expression of neurotrophic factors important for neuronal cell growth and stress resistance, including BDNF and FGF2 (refs. 122,123). Studies also suggest important roles for the ketone body β-hydroxybutyrate and the mitochondrial sirtuin SIRT3 in the neuroprotective mechanism of IF. Ketone bodies may be protective against GABAergic interneurons degeneration through a mechanism dependent on SIRT3, which was shown to reduce anxiety-like behavior and to improve hippocampus-dependent memory in mouse models of AD124,125.
A common genetic mouse model for Parkinson’s disease (PD) that overexpresses human α-synuclein exhibits progressive accumulation in neurons and shows motor dysfunction and premature death126. When these mice were treated with ADF, the autonomic nervous system deficit was reversed, while it was exacerbated even more in mice on a high-fat diet127. Bai et al. in a recent study showed that, in response to rapamycin treatment and the consequent inhibition of mTOR, these PD model mice displayed reduced oxidative stress and synaptic damage and an overall improvement of motor function128. Hence, ADF cycles have the potential to improve the overall pathology progression. Although it has not been tested with AD mouse models yet, PF or FMD has been shown to increase neural stem cells and increase cognitive performance in normal, old mice19. Major issues remaining to be addressed when considering human trials are the burden of fasting every other day and the side effects described earlier, particularly in people with PD or AD, who in most cases will be over age 70. The identification of the specific dietary compositions responsible for neuroprotective and regenerative effects and the description of the mechanisms involved should eventually allow the design and development of fasting-like interventions that are able to protect against neurodegeneration with minimal side effects.
Diseases of the immune system.
Aging is associated with progressive immunosenescence, which is caused in part by the age-dependent impairment of HSC function129. This results in a higher ratio of myeloid cells relative to lymphoid cells, accompanied by a decline in common lymphoid progenitors, and ultimately reduced T- and B-cell lymphogenesis as well as stem-cell exhaustion and reduced regenerative capacity130. Dysfunctional lymphocytes can consequently give rise to immunosuppression or immunosenescence, but may also contribute to autoimmune disorders such as asthma, systemic lupus erythematosus, multiple sclerosis (MS) and rheumatoid arthritis131. Certain dietary restriction regimens have the potential to prevent and/or reverse age-dependent immune dysfunction by killing or altering autoimmune cells and activating HSC-dependent regeneration21,132,133. Notably, severe CR (66% CR) and 8 weeks of ADF regimen prevent autoimmune encephalomyelitis (EAE) in mice134,135.
Although the molecular mechanisms of autoimmune disease suppression are not yet known, these diet regimens have been shown to decrease the amount of circulating T cell and inflammatory cytokines and chemokines132. In contrast to the prevention of autoimmunity described above, for the treatment of already-established MS symptoms and pathology, FMD–refeeding cycles were shown to attenuate EAE by modulating immune cells and promoting regeneration of oligodendrocyte precursor cells132. The FMD cycle increased apoptosis in autoreactive T cells, which are replaced in part by newly generated naive T cells during the refeeding period132. Notably, several studies show that fasting and CR can cause both the death and relocalization of different immune-cell populations136–138. In agreement with the rodent study, a clinical trial in humans also reported a reduction in lymphocytes upon FMD intervention and an improvement in quality of life in people with MS132. However, larger studies are necessary to determine whether the FMD can reduce multiple sclerosis pathology and progression in people.
Cancer.
Recently, a series of studies in animal models has shown that PF and FMD lasting two or more days can be as effective as chemotherapy at delaying the progression of a wide range of cancers but, more importantly, can protect normal cells from the toxic effects of chemotherapy while sensitizing cancer cells to the treatment68,69,139,140. PF and FMD cycles appear to increase the killing of cancer cells by causing system-wide changes that affect the ability of malignant cells to survive or adapt, which includes a reduction in IGF-1, insulin, glucose, leptin and cytokines, but likely also changes in hundreds of enzymes or pathways72,141. Notably, fasting and FMD are most effective against cancer cells when combined with chemotherapy, radiotherapy, kinase inhibitors, metabolic drugs or hormone therapy142–146. Another important mode of action of cycles of PF or FMDs and refeeding is the activation of the immunosurveillance to promote T-cell-dependent killing of cancer cells143,147. These effects of the fasting/FMD on immunity-dependent attack of cancer cells was confirmed by a recent study from Collins et al. showing that mice that underwent severe 50% CR for few weeks accumulated memory T cells in the bone marrow, and that caused enhanced protection against infections and tumors136. The broad effect of fasting and FMD in decreasing circulating levels of glucose, and IGF-1 and increasing ketone bodies, IGFBP-1 and so on together with the targeted toxicities of standard cancer therapies have the potential to promote major improvements in therapeutic index and cancer- or progression-free survival.
Diabetes and cardiovascular disease.
Both IF and PF or FMDs can be effective in reducing not only weight, abdominal circumference and body fat, but also risk factors for metabolic syndrome, diabetes and cardiovascular disease.
ADF and chronic CR caused similar effects on weight loss, reducing body and abdominal fat and lipids and improving insulin sensitivity in humans27. Notably, ADF had stronger effects on high-density lipoprotein (HDL) and LDL than did CR27. Another study on people with one or more risk factors for metabolic syndrome compared a 6-month ADF against chronic CR. Both groups experienced a similar decrease in body weight (8–9 kg), as well as improvements in blood pressure, triglycerides and HDL (Table 2).
In another study, 8 weeks of a 10-hour time-restricted eating decreased body weight (4 kg), waist circumference (4 cm), systolic BP (13 mmHg) and glucose in people with metabolic syndrome. No significant changes were found in LDL cholesterol, HDL and insulin or insulin resistance62 (Table 2).
For the PF interventions, three monthly cycles of a FMD were effective at reducing risk factors for diabetes and CVD in higher risk people. Independently of initial weight, people underwent a reduction in BMI, although individuals with BMI > 30 displayed a greater decrease (Table 2). Significant improvements in total cholesterol, LDL, fasting glucose, C-reactive protein and triglycerides were also observed in people with high levels of these risk factors at baseline72 (Table 2). In summary, both IF and PF or FMDs can cause a wide range of improvements in cardiometabolic risk factors, which are likely to lead to a reduction in diabetes and CVDs. Additional and larger studies will be important to determine which of these interventions can become standard of care in disease prevention and treatment.
Conclusion and future perspectives
IF and PF or FMD activate ancient programs that promote entry into alternative metabolic modes focused on conserving energy and on protecting the organism while enduring extended periods of food deprivation to optimize survival and reproduction once food becomes available. In fact, it is the refeeding period that has more recently emerged as an equally important process involved in the regeneration, and possibly rejuvenation, of systems, including organs, cells and organelles. In humans, the alternation of fasting and refeeding periods is accompanied by positive effects on risk factors for aging, diabetes, autoimmunity, cardiovascular disease, neurodegeneration and cancer. But not all fasting interventions are equal, and some are associated with smaller beneficial effects as well as side effects, including, in some cases, reduced longevity.
The IF and PF field should expand the investigation of the effects of dietary macronutrient composition, ratio and quality on healthspan. In fact, lifespan and healthspan extension can be reached by a specific macronutrient balance, independently of caloric intake. It is also important to identify the mediators of the effects of IF and PF not only on different types of mammalian cells and organs and on disease, but also on the microorganisms of the digestive system, with a focus on the molecular mechanisms mediating key effects on cellular protection, aging and regeneration.
It will not only be important to separate the positive effects of IF and PF from the adverse effects, but also to match the type and length of IF and PF with goals including healthspan extension, the prevention and treatment of specific diseases and weight management.
Acknowledgements
We would like to thank for their support the Associazione Italiana per la Ricerca sul Cancro (AIRC, IG nos. 17605 and 21820 to V.D.L.), the BC161452 grant of the Breast Cancer Research Program (US Department of Defense; to V.D.L.), and the NIA/NIH grants AG034906 and AG20642 to V.D.L.
Footnotes
Competing interests
V.D.L. declares the following competing interests: V.D.L. has equity interest in L-Nutra, a company that develops medical food. The University of Southern California has licensed intellectual property to L-Nutra. As part of this license agreement, the University has the potential to receive royalty payments from L-Nutra.
Additional information
Peer review information Nature Aging thanks Rozalyn Anderson, Stephen Simpson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Fontana L, Partridge L & Longo VD Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCay CM, Crowell MF & Maynard LA The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 5, 155–172 (1989). [PubMed] [Google Scholar]
- 3.Lin SJ, Ford E, Haigis M, Liszt G & Guarente L Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev 18, 12–16 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosono R, Nishimoto S & Kuno S Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp. Gerontol 24, 251–264 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Bross TG, Rogina B & Helfand SL Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell 4, 309–317 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Weindruch R & Walford RL Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215, 1415–1418 (1982). [DOI] [PubMed] [Google Scholar]
- 7.Colman RJ et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana L & Klein S Aging, adiposity, and calorie restriction. J. Am. Med. Assoc 297, 986–994 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Abe T et al. Suppression of experimental autoimmune uveoretinitis by dietary calorie restriction. Jpn. J. Ophthalmol 45, 46–52 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Jolly CA & Fernandes G Diet modulates TH1 and TH2 cytokine production in the peripheral blood of lupus-prone mice. J. Clin. Immunol 19, 172–178 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Kristan DM Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 6, 817–825 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Gardner EM Caloric restriction decreases survival of aged mice in response to primary influenza infection. J. Gerontol. A Biol. Sci. Med. Sci 60, 688–694 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Mair W, Piper MD & Partridge L Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 3, e223 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross MH Length of life and nutrition in the rat. J. Nutr 75, 197–210 (1961). [DOI] [PubMed] [Google Scholar]
- 15.McCay CM, Dilley WE & Crowell MF Growth rates of brook troutreared upon purified rations, upon dry skim milk diets, and upon feed combinations of cereal grains. J. Nutr 1, 233–246 (1929). [Google Scholar]
- 16.Solon-Biet SM et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solon-Biet SM et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep 11, 1529–1534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine ME et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandhorst S et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng C-W et al. Fasting-mimicking diet promotes Ngn3-driven β-cell regeneration to reverse diabetes. Cell 168, 775–788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng C-W et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi IY et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 15, 2136–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangan P et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep 26, 2704–2719 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazare S et al. Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp. Hematol 53, 26–30 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Longo VD & Mattson MP Fasting: molecular mechanisms and clinical applications. Cell Metab 19, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anson RM et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl Acad. Sci. USA 100, 6216–6220 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trepanowski JF et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med 177, 930–938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varady KA et al. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci. Rep 5, 7561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JB et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med 42, 665–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvie MN et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. (Lond.) 35, 714–727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattson MP, Longo VD & Harvie M Impact of intermittent fasting on health and disease processes. Ageing Res. Rev 39, 46–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaix A et al. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29, 303–319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaix A, Zarrinpar A, Miu P & Panda S Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo VD & Panda S Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23, 1048–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan R et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem 21, 413–417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cahill GF Starvation in man. N. Engl. J. Med 282, 668–675 (1970). [DOI] [PubMed] [Google Scholar]
- 37.Browning JD, Baxter J, Satapati S & Burgess SC The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J. Lipid Res 53, 577–586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster DW Studies in the ketosis of fasting. J. Clin. Invest 46, 1283–1296 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weir HJ et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab 26, 884–896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longo VD & Cortellino S Fasting, dietary restriction, and Immunosenescence. J. Allergy Clin. Immunol 146, 1002–1004 (2020). [DOI] [PubMed] [Google Scholar]
- 41.de Cabo R & Mattson MP Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med 381, 2541–2551 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Grandison RC, Wong R, Bass TM, Partridge L & Piper MDW Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS ONE 4, e4067 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Bourg E & Minois N Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J. Gerontol. A. Biol. Sci. Med. Sci 51, B280–B283 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Catterson JH et al. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol 28, 1714–1724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR & Cider NL Effects of intermittent feeding upon growth and life span in rats. Gerontology 28, 233–241 (1982). [DOI] [PubMed] [Google Scholar]
- 46.Talan MI & Ingram DK Effect of intermittent feeding on thermoregulatory abilities of young and aged C57BL/6J mice. Arch. Gerontol. Geriatr 4, 251–259 (1985). [DOI] [PubMed] [Google Scholar]
- 47.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR & Cider N Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech. Ageing Dev 55, 69–87 (1990). [DOI] [PubMed] [Google Scholar]
- 48.Xie K et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun 8, 155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettan-Brewer C & Treuting PM Practical pathology of aging mice. Pathobiol. Aging Age Relat. Dis 10.3402/pba.v1i0.7202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackwell BN, Bucci TJ, Hart RW & Turturro A Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol 23, 570–582 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Arum O, Bonkowski MS, Rocha JS & Bartke A The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell 8, 756–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao CY, Rikke BA, Johnson TE, Diaz V & Nelson JF Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh R et al. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 34, 917–933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee GD et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin. Cancer Res 12, 198–205 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Mager DE et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J 20, 631–637 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Wan R, Camandola S & Mattson MP Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J 17, 1133–1134 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Hatori M et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabel K et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging 4, 345–353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabel K et al. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity (Silver Spring) 27, 1443–1450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stekovic S et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab 30, 462–476 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Melkani GC & Panda S Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol 595, 3691–3700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkinson MJ et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31, 92–104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cienfuegos S, Gabel K & Kalam F et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab 32, 366–378 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heilbronn LK, Smith SR, Martin CK, Anton SD & Ravussin E Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr 81, 69–73 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Harvie M et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr 110, 1534–1547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sichieri R, Everhart JE & Roth H A prospective study of hospitalization with gallstone disease among women: role of dietary factors, fasting period, and dieting. Am. J. Public Health 81, 880–884 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rong S et al. Association of skipping breakfast with cardiovascular and all-cause mortality. J. Am. Coll. Cardiol 73, 2025–2032 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Safdie FM et al. Fasting and cancer treatment in humans: a case series report. Aging 1, 988–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raffaghello L et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl Acad. Sci. USA 105, 8215–8220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldhamer A, Lisle D, Parpia B, Anderson SV & Campbell TC Medically supervised water-only fasting in the treatment of hypertension. J. Manipulative Physiol. Ther 24, 335–339 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Goldhamer AC et al. Medically supervised water-only fasting in the treatment of borderline hypertension. J. Altern. Complement. Med 8, 643–650 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Wei M et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med 9, eaai8700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell SJ et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23, 1093–1112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mirzaei H, Raynes R & Longo VD The conserved role of protein restriction in aging and disease. Curr. Opin. Clin. Nutr. Metab. Care 19, 74–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell SJ et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29, 221–228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varady KA, Roohk DJ, Bruss M & Hellerstein MK Alternate-day fasting reduces global cell proliferation rates independently of dietary fat content in mice. Nutr. Burbank Los Angel. Cty. Calif 25, 486–491 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Hahn O, Drews LF & Nguyen A et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab 1, 1059–1073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drinda S et al. Effects of periodic fasting on fatty liver index—a prospective observational study. Nutrients 11, 2601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S & Michalsen A Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 14, e0209353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Redman LM et al. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab 27, 805–815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oh TJ et al. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease and mortality: a 16-year prospective cohort study. J. Clin. Endocrinol. Metab 104, 639–646 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Longo VD Programmed longevity, youthspan, and juventology. Aging Cell 18, e12843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selman C et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeno Y et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci 64, 522–529 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Junnila RK, List EO, Berryman DE, Murrey JW & Kopchick JJ The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol 9, 366–376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bitto A et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C, Liu Y, Liu Y & Zheng P mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal 2, ra75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang Y et al. Effects of rapamycin on growth hormone receptor knockout mice. Proc. Natl Acad. Sci. USA 115, E1495–E1503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamming DW et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lamming DW et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13, 911–917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu D et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep 29, 236–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campbell RG, Johnson RJ, King RH, Taverner MR & Meisinger DJ Interaction of dietary protein content and exogenous porcine growth hormone administration on protein and lipid accretion rates in growing pigs. J. Anim. Sci 68, 3217–3225 (1990). [DOI] [PubMed] [Google Scholar]
- 94.Pedrosa RG, Donato J, Pires IS & Tirapegui J Leucine supplementation increases serum insulin-like growth factor 1 concentration and liver protein/RNA ratio in rats after a period of nutritional recovery. Appl. Physiol. Nutr. Metab 38, 694–697 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Wolfson RL et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chantranupong L et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solon-Biet SM et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab 1, 532–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Biase S et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol 15, e2001951 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartke A, Sun LY & Longo V Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev 93, 571–598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fontana L, Weiss EP, Villareal DT, Klein S & Holloszy JO Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moro T et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med 14, 290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sorochynska OM et al. Every-other-day feeding decreases glycolytic and mitochondrial energy-producing potentials in the brain and liver of young mice. Front. Physiol 10, 1432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mihaylova MM et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yilmaz ÖH et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J, Duan W, Long JM, Ingram DK & Mattson MP Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci 15, 99–108 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Fusco S et al. A CREB–Sirt1–Hes1 circuitry mediates neural stem cell response to glucose availability. Cell Rep 14, 1195–1205 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Horvath S et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang N et al. Dietary and genetic effects on age-related loss of gene silencing reveal epigenetic plasticity of chromatin repression during aging. Aging 5, 813–824 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schultz MB & Sinclair DA When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zammit PS et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci 119, 1824–1832 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Madeo F, Eisenberg T, Pietrocola F & Kroemer G Spermidine in health and disease. Science 359, eaan2788 (2018). [DOI] [PubMed] [Google Scholar]
- 112.García-Prat L et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Wei S et al. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr. Metab 15, 80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holmes AJ, Chew YV & Colakoglu F et al. Diet–microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab 25, 140–151 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Camandola S & Mattson MP Brain metabolism in health, aging, and neurodegeneration. EMBO J 36, 1474–1492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cignarella F et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab 27, 1222–1235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fulop T, Witkowski JM, Olivieri F & Larbi A The integration of inflammaging in age-related diseases. Semin. Immunol 40, 17–35 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Mirzaei H, Di Biase S & Longo VD Dietary interventions, cardiovascular aging, and disease: animal models and human studies. Circ. Res 118, 1612–1625 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Mattson MP Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 16, 706–722 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halagappa VKM et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis 26, 212–220 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Parrella E et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell 12, 257–268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arumugam TV et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol 67, 41–52 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng A et al. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun 3, 1250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun 10, 1886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng A et al. SIRT3 haploinsufficiency aggravates loss of GABAergic interneurons and neuronal network hyperexcitability in an Alzheimer’s disease model. J. Neurosci 40, 694–709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Crabtree DM & Zhang J Genetically engineered mouse models of Parkinson’s disease. Brain Res. Bull 88, 13–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Griffioen KJ et al. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol. Aging 34, 928–935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bai X et al. Rapamycin improves motor function, reduces 4-hydroxynonenal adducted protein in brain, and attenuates synaptic injury in a mouse model of synucleinopathy. Pathobiol. Aging Age Relat. Dis 5, 28743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denkinger MD, Leins H, Schirmbeck R, Florian MC & Geiger H HSC aging and senescent immune remodeling. Trends Immunol 36, 815–824 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Haan G & Lazare SS Aging of hematopoietic stem cells. Blood 131, 479–487 (2018). [DOI] [PubMed] [Google Scholar]
- 131.Ostroukhova M et al. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol 302, L300–307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi IY, Lee C & Longo VD Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell Endocrinol 455, 4–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang D et al. Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J. Exp. Med 213, 535–553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Piccio L, Stark JL & Cross AH Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol 84, 940–948 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kafami L et al. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicienna J. Med. Biotechnol 2, 47–52 (2010). [PMC free article] [PubMed] [Google Scholar]
- 136.Collins N et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jordan S et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nagai M et al. Fasting–refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178, 1072–1087 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Lee C et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med 4, 124ra27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buono R & Longo VD Starvation, stress resistance, and cancer. Trends Endocrinol. Metab 29, 271–280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei T, Ye P, Peng X, Wu L-L & Yu G-Y Circulating adiponectin levels in various malignancies: an updated meta-analysis of 107 studies. Oncotarget 7, 48671–48691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elgendy M et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A–GSK3β–MCL-1 axis. Cancer Cell 35, 798–815 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Di Biase S et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brandhorst S & Longo VD Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res 207, 241–266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Di Tano M et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun 11, 2332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caffa I et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pietrocola F et al. Caloric restriction mimetics enhance anticancer Immunosurveillance. Cancer Cell 30, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salazar AM et al. Intestinal snakeskin limits microbial dysbiosis during aging and promotes longevity. iScience 9, 229–243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Varady KA et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J 12, 146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tinsley GM et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci 17, 200–207 (2017). [DOI] [PubMed] [Google Scholar]


