Abstract
The aims of this study were to evaluate the effects of repeated chlorhexidine gluconate (CHG) pulsing on the viability and bacterial composition of microcosm dental plaques derived from human saliva. The biofilms were grown on bovine enamel discs in a constant-depth film fermentor fed with an artificial saliva which was supplemented thrice daily with sucrose. The microcosm plaques had total viable anaerobic counts of 5 × 108 CFU per mm2 and consisted of 12% Actinomyces spp., 85% streptococci, and 0.2% Veillonella spp. When pulsed twice daily with 0.2% CHG, there was an immediate 1.3-log10 reduction in the total viable (anaerobic) count. However, as pulsing continued, the viable counts recovered, and after 4 days, the anaerobic count reached its pre-CHG-pulsing level, although the bacterial composition of the biofilms had changed. The results of this study show that twice-daily pulsing with 0.2% CHG over a 4-day period was ineffective at reducing the total anaerobic viable count of the biofilms but did alter their bacterial composition.
Despite the complexity of the human diet, the only class of compounds found to greatly influence the ecology of the resident microflora is that of fermentable carbohydrates (14). Such carbohydrates can be broken down to acids by the microflora of the mouth. Thus, the frequent consumption of dietary carbohydrates is associated with a shift in the proportions of the constituents of the microflora of dental plaque towards a more cariogenic plaque (19). These plaques usually contain elevated numbers of mutans streptococci following sucrose rinsing (20) and may contain lower numbers of strict anaerobes, due possibly to the low pH and high Eh values resulting from acidogenic fermentation by the streptococci (15). These changes in composition of dental plaque are obviously important when determining its susceptibility to antimicrobial agents or the likely pathogenicity of the flora if left unchallenged. When developing new antibacterial or antiplaque agents for use in the oral cavity, it is important to consider such factors and to be able to use a model which can be adapted to mimic the nutrient source, substratum, and bacterial species which are present in vivo. The purpose of this investigation was to evaluate the effects of chlorhexidine gluconate (CHG) pulses on the viability and composition of mixed-species biofilms supplemented with sucrose by using a constant-depth film fermentor (CDFF) as a means of generating biofilms under conditions similar to those which would exist in vivo.
MATERIALS AND METHODS
Inoculum and media.
Saliva was used as an inoculum to provide a multispecies biofilm consisting of organisms found in the oral cavity. The saliva was collected from 10 healthy individuals, equal amounts from each person were pooled, and 1-ml aliquots were dispensed into cryovials and stored at −70°C for subsequent use. The nutrient source in all experiments was a mucin-containing artificial saliva, the composition of which has been described previously (16). In most of the experimental runs, this was supplemented with sucrose as described below.
Production of biofilms.
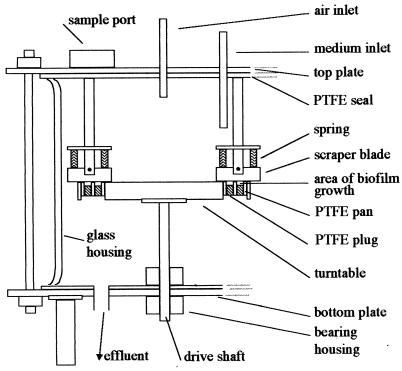
Biofilms were grown in a CDFF (University of Wales, Cardiff), shown schematically in Fig. 1 (16–18). The CDFF consists of a rotating turntable which holds 15 polytetrafluoroethylene (PTFE) pans located flush around its rim. Each pan contains five cylindrical holes containing PTFE plugs. The biofilms were grown on bovine enamel discs, 5 mm in diameter (Biomaterials Department, Eastman Dental Institute), which sit on PTFE plugs of the same diameter and are recessed to a depth of 300 μm.
FIG. 1.
Vertical section through the constant-depth film fermentor.
Inoculation of the CDFF.
One milliliter of pooled saliva was added to 500 ml of artificial saliva, mixed, and pumped into the CDFF for 8 h. After this time, the inoculum flask was disconnected and the CDFF was fed from a medium reservoir of sterile artificial saliva, with the waste being collected in an effluent bottle. The artificial saliva was delivered by a peristaltic pump (Watson-Marlow) at a rate of 0.72 liters per day, corresponding to the resting flow rate of saliva in man (1, 5, 12). In most experimental runs, 330 ml of a 10% (wt/vol) aqueous solution of sucrose was also pumped over the biofilms for 30 min via a second peristaltic pump. This was carried out thrice daily (9 a.m., 1 p.m., and 5 p.m.), thereby equating the total mean daily intake of sucrose of an adult in the United Kingdom (3).
CHG pulsing of biofilms.
Pulsing was carried out twice daily (9 a.m. and 5 p.m.) for 1 min with 10 ml of 0.2% (wt/vol) CHG (Sigma) delivered via a peristaltic pump.
Culture methods.
Pans were removed from the CDFF at various intervals, and the bovine enamel discs were aseptically removed and placed into neutralizing broth (Difco Laboratories, Detroit, Mich.) to prevent any further action by CHG before being vortexed for 1 min to disrupt the biofilm. Selective media were used to culture the following genera. Actinomyces spp. were isolated on cadmium fluoride-acriflavin-tellurite agar plates (25), Veillonella spp. were isolated on Veillonella agar (Difco), and streptococci were isolated on Mitis Salivarius agar (Difco). The total anaerobic count was performed on Wilkins-Chalgren agar (Oxoid) containing 8% horse blood. All the plates were incubated anaerobically for 4 days at 37°C. The total aerobic viable count was carried out on 8% blood agar (Oxoid) and incubated at 37°C aerobically.
Cryosectioning of biofilms.
The cryosectioning was carried out as described previously (17). Briefly, the biofilms were frozen and covered with OCT embedding compound (Raymond A. Lamb, London, United Kingdom) on a cryostat chuck, and 30-μm horizontal sections were cut at −19°C.
Determination of pH.
The pH of the biofilms was determined by using a pH meter (pH-boy; Camlab, Cambridge, United Kingdom). The meter consists of a flat electrode probe approximately 6 mm in diameter onto which an inverted disc containing the biofilm could be placed. The probe was recalibrated before each sample. The accuracy of the instrument was ±0.1 pH units.
RESULTS
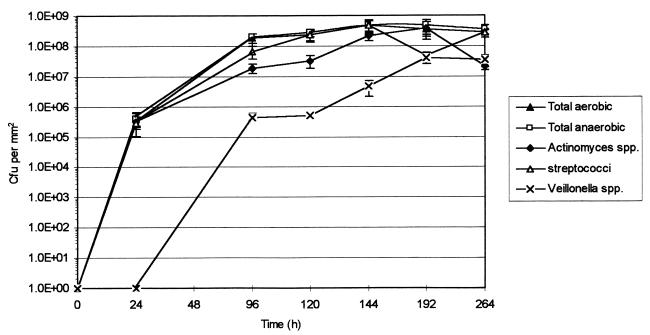
The microcosm plaques supplemented with sucrose produced total viable anaerobic counts in the region of 5 × 108 CFU per mm2 (Fig. 2). After 120 h, they consisted of 11.7% Actinomyces spp., 84.7% streptococci, and 0.2% Veillonella spp. The Veillonella spp. were undetectable until 24 h, but at 96 h, their numbers had reached 5 × 105 CFU per mm2 and continued to increase until 192 h, at which point the counts were 6 × 107 CFU per mm2.
FIG. 2.
Growth of various groups of bacteria comprising a microcosm plaque community pulsed thrice daily with sucrose. Error bars represent standard deviations; n = 4.
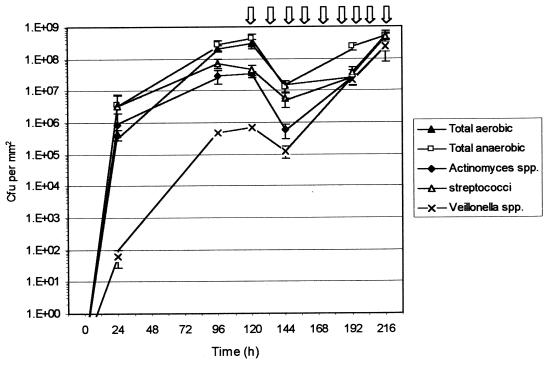
When these microcosm plaques were pulsed with 0.2% CHG at 120 h (Fig. 3), there was a reduction in the total aerobic and anaerobic counts of approximately 1.3 log10. The viable counts of the Streptococcus spp. and Veillonella spp. were reduced by less than 1 log10. However, because the initial viable counts of the Veillonella spp. were lower, this amounted to a much smaller number killed than in the case of the streptococci. The greatest reductions in counts were seen for the Actinomyces spp., from 4 × 107 to 8 × 105 CFU per mm2. When the CDFF was sampled at 192 h, all the viable counts had significantly increased (except for the total aerobic count), and by 216 h, all the counts had reached at least their pre-CHG-pulsing levels. In fact, the viable counts of the Actinomyces spp. and Veillonella spp. post-pulsing had increased compared to those found prior to pulsing. The relative proportions of species present post-pulsing were considerably different from those seen prior to pulsing. The biofilms now consisted of 34.4% Actinomyces spp., 47.2% streptococci, and 18.1% Veillonella spp.
FIG. 3.
Viable counts of sucrose-pulsed microcosm plaques additionally pulsed twice daily with 0.2% CHG after 120 h. Arrows represent the CHG pulsing twice daily from 120 to 216 h. Error bars represent standard deviations; n = 4.
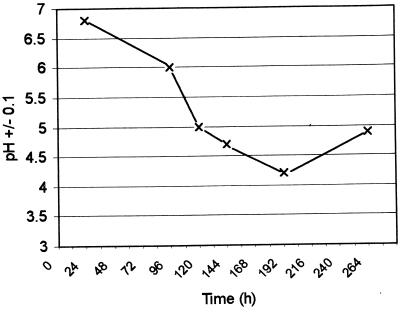
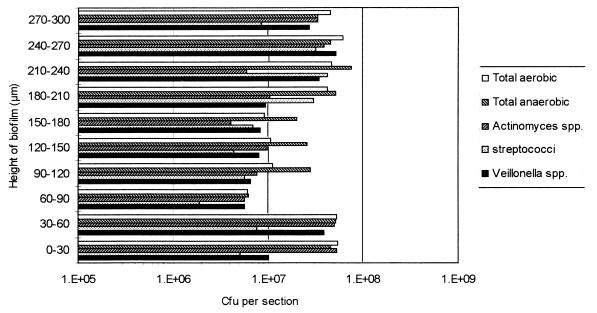
Figure 4 shows the pH of the biofilms from the run shown in Fig. 2. The pH of the microcosm plaques supplemented with sucrose dropped steadily from 6.8 at 24 h to 4.2 at 192 h; the pH then increased slightly to 4.8 when sampled at 264 h. Viable counts of sections of the microcosm plaques grown without sucrose supplementation revealed counts from each 30-μm section of approximately 5 × 107 CFU per section (Fig. 5). However, from 60 to 180 μm, the viable counts were approximately 107 CFU per section. The sections near the biofilm-air interface had high total aerobic counts, which would, of course, comprise both facultative anaerobes and obligate aerobes. Throughout the rest of the biofilm, the Actinomyces spp. and Streptococcus spp. tended to be numerically dominant.
FIG. 4.
The pH of microcosm plaques supplemented with sucrose.
FIG. 5.
Viable counts of 30-μm-thick sections through a 120-h-old microcosm plaque grown in the absence of sucrose. Bars represent means.
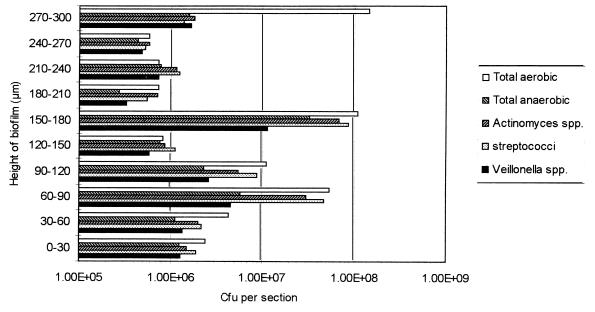
When the sucrose-supplemented biofilms were sectioned (Fig. 6), the counts from each section showed considerable variation. At the biofilm-air interface, the total aerobic count comprised 23% of the entire biofilm. In sections taken from depths of from 180 to 300 μm, the viable counts were lower, but the counts from the 150- to 180-μm sections showed that the numbers of Actinomyces spp., Veillonella spp., and Streptococcus spp. had significantly increased (P < 0.05) in this region. Towards the base of the biofilm, there were again generally low numbers of viable bacteria with significantly higher (P < 0.05) counts in the 60- to 90-μm section.
FIG. 6.
Viable counts of 30-μm-thick sections through a 120-h-old microcosm plaque grown in the presence of sucrose. Bars represent means.
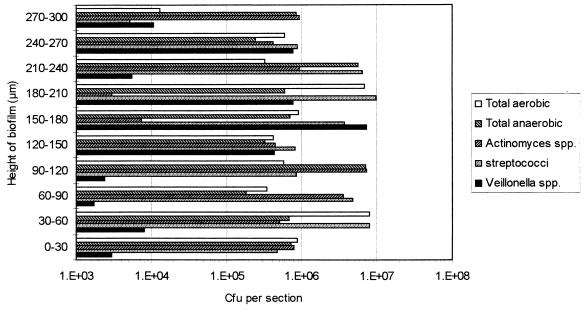
When these biofilms were pulsed with 0.2% CHG for 1 min and subsequently sectioned, there was an approximately 1.5-log10 reduction in the total viable counts of the entire biofilm (Fig. 7). The total aerobic count at the biofilm-air interface was greatly reduced (by approximately 4 log10). The total anaerobic count for each of the sections remained similar. However, the viable counts for the Veillonella spp. were reduced by between approximately 1.5 and 2.5 log10 towards the base of the biofilm (0 to 120 μm). The Actinomyces spp. in the center of the biofilm (150 to 210 μm) were also significantly reduced (P < 0.05) by the action of CHG.
FIG. 7.
Viable counts of 30-μm-thick sections through a 120-h-old microcosm plaque grown in the presence of sucrose following exposure to 0.2% CHG for 1 min. Bars represent means.
DISCUSSION
The purpose of this study was to determine the effect of twice-daily CHG pulsing on the viability and composition of microcosm plaques grown in artificial saliva and periodically supplied with sucrose. The addition of sucrose into the system had a profound effect on the bacterial composition of the biofilms. Microcosm plaques supplemented with sucrose had a much greater proportion of streptococci (85%) than nonsupplemented microcosm plaques (25%) observed in previous studies using an identical system (18). The presence of sucrose also resulted in very acidic biofilms, with the pH reaching levels as low as pH 4.2. This is similar to the pH of approximal plaque in vivo following a sucrose rinse (10). In vivo, pH fluctuations in plaque induced by dietary carbohydrate have a typical profile known as the Stephan curve (10, 19, 22). The results of such studies have indicated that low plaque pH levels in vivo are able to return to normal pH levels within 2 h. However, the inner regions of approximal plaques (i.e., those formed between the teeth) can become inaccessible to saliva exchanges due to their thickness and can therefore remain at low pH for long periods, thereby allowing enamel demineralization to take place (7). In our laboratory model, the thick (300-μm) plaques used would be similar to approximal plaques in that penetration of the artificial saliva (and hence its buffering ability) would be impaired, thus allowing a low pH to exist within the biofilms.
The viable counts from the 30-μm sections through the sucrose-supplemented microcosm plaques showed a pattern different from those of nonsupplemented plaques. The variability in viable counts in the 10 sections comprising the sucrose-supplemented plaques may indicate the presence of channels or pores (24). The differences between these plaques suggest that an overall change in the structure of the biofilm had taken place. Computer modelling of biofilm formation has shown that the availability of substrates may play a major role in the structure of biofilms (2, 24), and in vitro studies of oral bacterial biofilms have revealed that the availability of sucrose exerts a profound effect on biofilm structure due mainly to increased synthesis of exopolysaccharide (4).
The effects of pulsing with 0.2% CHG on sucrose-supplemented biofilms indicated that the total anaerobic count of the biofilm was able to recover to that seen prior to pulsing. The MICs of CHG for oral bacterial species likely to exist in the microcosm plaques range from 0.0008 to 0.0125% (21), indicating that the bacteria existing in the biofilm were considerably less susceptible to the action of CHG and that many managed to survive even when being pulsed with 16- to 250-fold-higher concentrations. However, the composition, and possibly the structure, of the biofilm was markedly affected by the CHG pulsing. Hence, the proportions of Actinomyces spp. and Veillonella spp. were considerably greater after CHG pulsing while the proportion of streptococci was greatly reduced. The higher proportion of Veillonella spp. may have implications for the cariogenicity of the microcosm plaque, as it has been documented that Veillonella spp. can stimulate the growth and glycolytic activity of streptococci by the continual removal of lactate (6, 23). Directly after the first CHG pulse, the large variations in the viable counts between sections seen prior to pulsing with CHG were no longer evident. This may be due to differing kills being achieved in the various sections or could reflect some structural change in the biofilms.
This investigation has demonstrated the versatility of the CDFF, in that it permits supplementation with dietary nutrients to mimic some of the external factors influencing plaque in vivo. A sucrose rinse lasting a few minutes, followed by clearance by artificial saliva, simulates the processes that arise in vivo during the intake of sucrose drinks (11, 13), and this rinse is commonly used in modelling cariogenic challenges (8, 9). It was also possible to monitor the pH of the biofilm, an important aspect of caries and its control, and to test the susceptibility of these biofilms to an antimicrobial agent.
REFERENCES
- 1.Bell G, Emslie-Smith D, Patterson C. Textbook of physiology. London, United Kingdom: Churchill Livingstone; 1980. [Google Scholar]
- 2.Brown M R, Allison D G, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988;22:777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 3.Burt B A. Relative consumption of sucrose and other sugars: has it been a factor in reduced caries experience? Caries Res. 1993;27:56–63. doi: 10.1159/000261604. [DOI] [PubMed] [Google Scholar]
- 4.Embleton J V, Newman H N, Wilson M. Influence of growth mode and sucrose on susceptibility of Streptococcus sanguis to amine fluorides and amine fluoride-inorganic fluoride combinations. Appl Environ Microbiol. 1998;64:3503–3506. doi: 10.1128/aem.64.9.3503-3506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyton A C. Human physiology and mechanisms of disease. W. B. Philadelphia, Pa: Saunders Co.; 1992. [Google Scholar]
- 6.Hamilton W A, Ng S K C. Stimulation of glycolysis through lactate consumption in a resting cell mixture of Streptococcus salivarius and Veillonella parvula. FEMS Microbiol Lett. 1983;20:61–65. [Google Scholar]
- 7.Hudson D E, Donoghue H D, Perrons C J. A laboratory microcosm (artificial mouth) for the culture and continuous pH measurement of oral bacteria on surfaces. J Appl Bacteriol. 1986;60:301–310. doi: 10.1111/j.1365-2672.1986.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi K, Lee I K, Schachtele C F. Comparison of in vivo human dental plaque pH changes within artificial fissures and at interproximal sites. Caries Res. 1989;23:417–422. doi: 10.1159/000261220. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi K, Lee I K, Schachtele C F. Effect of dental plaque age and bacterial composition on the pH of artificial fissures in human volunteers. Caries Res. 1990;24:52–58. doi: 10.1159/000261239. [DOI] [PubMed] [Google Scholar]
- 10.Kleinberg I, Jenkins G N. The pH of dental plaques in the different areas of the mouth before and after meals and their relationship to the pH and rate of flow of resting saliva. Arch Oral Biol. 1964;9:493–516. doi: 10.1016/0003-9969(64)90015-9. [DOI] [PubMed] [Google Scholar]
- 11.Lagerlof F, Dawes R, Dawes C. Salivary clearance of sugar and its effects on pH changes by Streptococcus mitior in an artificial mouth. J Dent Res. 1984;63:1266–1270. doi: 10.1177/00220345840630110201. [DOI] [PubMed] [Google Scholar]
- 12.Lamb J F. Essentials of physiology. Oxford, United Kingdom: Blackwell Scientific; 1991. [Google Scholar]
- 13.Lindfors B, Lagerlof F. Effect of sucrose concentration in saliva after a sucrose rinse on the hydronium ion concentration in dental plaque. Caries Res. 1988;22:7–10. doi: 10.1159/000261076. [DOI] [PubMed] [Google Scholar]
- 14.Marsh P D. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc Finnish Dent Soc. 1991;87:515–525. [PubMed] [Google Scholar]
- 15.Mühlemann H R, de Boever J. Radio-telemetry of the pH of inter dental areas exposed to various carbohydrates. In: McHugh W D, editor. Dental plaque. Edinburgh, Scotland: Livingstone; 1970. pp. 179–186. [Google Scholar]
- 16.Pratten J, Wills K, Barnett P, Wilson M. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J Appl Microbiol. 1998;84:1149–1155. doi: 10.1046/j.1365-2672.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 17.Pratten J, Barnett P, Wilson M. Composition and susceptibility to chlorhexidine of multi-species biofilms of oral bacteria. Appl Environ Microbiol. 1998;64:3515–3519. doi: 10.1128/aem.64.9.3515-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratten J, Smith A W, Wilson M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J Antimicrob Chemother. 1998;42:453–459. doi: 10.1093/jac/42.4.453. [DOI] [PubMed] [Google Scholar]
- 19.Sissons C H, Cutress T W, Faulds G, Wong L. pH responses to sucrose and the formation of pH gradients in thick ’artificial mouth’ microcosm plaques. Arch Oral Biol. 1992;37:913–922. doi: 10.1016/0003-9969(92)90062-d. [DOI] [PubMed] [Google Scholar]
- 20.Staat R H, Gawronski T H, Cressey D E, Harris R S, Folke L E. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J Dent Res. 1975;54:872–880. doi: 10.1177/00220345750540042801. [DOI] [PubMed] [Google Scholar]
- 21.Stanley A, Wilson M, Newman H N. The in vitro effects of chlorhexidine on subgingival plaque bacteria. J Clin Periodontol. 1989;16:259–264. doi: 10.1111/j.1600-051x.1989.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 22.Stephan R M. Intra-oral hydrogen-ion concentration associated with dental caries activity. J Dent Res. 1944;23:257–266. [Google Scholar]
- 23.van der Hoeven J S, Toorop A I, Mikx F H M. Symbiotic relationship of Veillonella alcalescens and Streptococcus mutans in dental plaque in gnotobiotic rats. Caries Res. 1978;12:142–147. doi: 10.1159/000260324. [DOI] [PubMed] [Google Scholar]
- 24.Wimpenny J W, Colasanti R. A unifying hypothesis for the structure of microbial biofilms based on cellular automaton models. FEMS Microbiol Lett. 1997;22:1–16. [Google Scholar]
- 25.Zylber L J, Jordan H V. Development of a selective medium for detection and enumeration of Actinomyces viscosus and Actinomyces naeslundii in dental plaque. J Clin Microbiol. 1982;15:253–259. doi: 10.1128/jcm.15.2.253-259.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]