ABSTRACT
Pathogenic free-living amoebae affecting the central nervous system are known to cause granulomatous amoebic encephalitis (GAE) or primary amoebic meningoencephalitis (PAM). Although hosts with impaired immunity are generally at a higher risk of severe disease, amoebae such as Naegleria fowleri and Balamuthia mandrillaris can instigate disease in otherwise immunocompetent individuals, whereas Acanthamoeba species mostly infect immunocompromised people. Acanthamoeba also cause a sight-threatening eye infection, mostly in contact lens wearers. Although infections due to pathogenic amoebae are considered rare, recently, these deadly amoebae were detected in water supplies in the USA. This is of particular concern, especially with global warming further exacerbating the problem. Herein, we describe the epidemiology, presentation, diagnosis, and management of free-living amoeba infections.
KEYWORDS: Free-living amoebae, acanthamoeba, Balamuthia, Naegleria, keratitis, CNS infection, encephalitis, meningoencephalitis
Introduction
Pathogenic free-living amoebae, such as Acanthamoeba spp., Naegleria fowleri and Balamuthia mandrillaris, cause infection of the central nervous system (CNS) [1, 2]. The detection of brain-eating amoebae in drinking water supplies is of concern, which further indicates the severe threat posed by free-living amoebae to communities [3–6]. Furthermore, infection of the CNS with Acanthamoeba spp., Naegleria fowleri and Balamuthia mandrillaris almost always leads to mortality [7]. Moreover, cases of amoebic infection are under-reported worldwide, because of lack of awareness and diagnostic modalities, as well as misdiagnosis, due to similarity in symptoms, of amoebic infection of CNS to other common CNS infections such as bacterial meningitis, and thus, the true burden of cases due to these amoebae is unknown [8,9].
Acanthamoeba spp. infect the CNS, causing granulomatous amoebic encephalitis (GAE), and can also cause a sight-threatening eye infection known as Acanthamoeba keratitis (AK) [10, 11, 12]. B. mandrillaris is known to instigate Balamuthia amoebic encephalitis (BAE) in the CNS and infect other organs such as the lungs and skin, in both immunocompetent and immunocompromised individuals [11]. N. fowleri infects the CNS, causing primary amoebic meningoencephalitis (PAM), triggering a prompt onset of disease and leading to death within days [11, 13]. Treatment of CNS infection with amoebae is complicated and hampered by the selectivity of the blood-brain barrier (BBB) that affects drug permeability into the brain. The purpose of this review is to briefly describe the epidemiology, presentation, diagnosis and management of CNS complications due to free-living amoeba and keratitis caused by pathogenic Acanthamoeba.
Acanthamoeba spp
Acanthamoeba spp. are free-living protists that exist in a variety of environments, such as water, soil and air [14]. Acanthamoeba spp. can exist in two forms, namely, active trophozoites and dormant cysts, by transitioning under stressful conditions, such as starvation and desiccation, from trophozoite to cyst [15,16]. Acanthamoeba trophozoites, the metabolically and reproductively active form of the amoeba, is the form that the amoebae assume when under favorable conditions, such as nutrient-rich environment and appropriate pH, osmolarity and temperature [17]. Acanthamoeba trophozoites are irregular in shape and pseudopods are used for movement, while acanthopodia, spike-like protrusions are responsible for adhesion to inert and biological surfaces [10,18]. Based on 16S rDNA sequencing, genus Acanthamoeba consists of at least 22 genotypes (T1 – T22), while genotype T4 is proportionally over-represented in infections [19], only one species of Balamuthia genus, i.e. B. mandrillaris, infects humans and only one species of Naegleria, i.e. N. fowleri, is known to infect human [11]. Pathogenic Acanthamoeba spp. are more genetically diverse than both B. mandrillaris and N. fowleri, perhaps suggesting a more recent evolution of the latter two species (Figure 1). Whereas pathogenicity is limited to a single species in both Balamuthia and Naegleria, many genotypes, broadly equivalent to species, show human pathogenicity in Acanthamoeba. It has been proposed that N. fowleri evolved from N. lovaniensis and has spread throughout the world with the winds and minor differences detected in the ITS1 region allow the progression of the natural spread of Naegleria fowleri to be deduced [20]. This distribution indicates a relatively recent series of events since this contravenes the ‘everything is everywhere’ concept [21], which is generally found to be applicable to protists and seems to hold true for Acanthamoeba [22].
Figure 1.
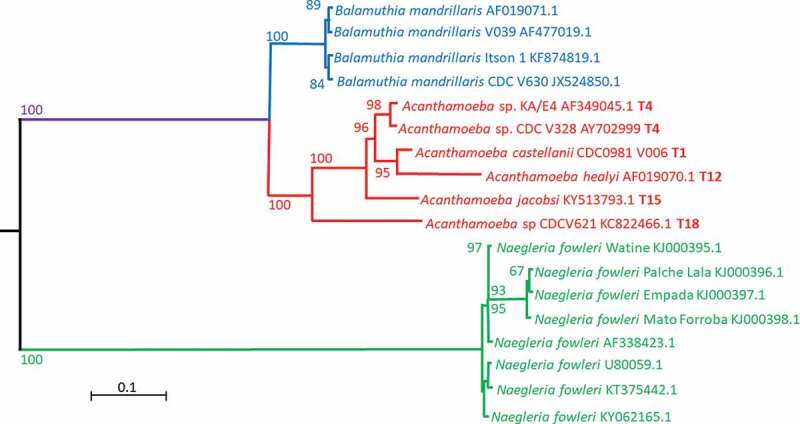
A PhyML phylogenetic tree (GTR model) based on the 18S ribosomal gene of the three pathogens discussed in this study. Sequences obtained from genbank (https://www.ncbi.nlm.nih.gov/genbank/) were aligned using ClustalW version 2 [179] and the trees calculated using seaview version 4 [189]. The tree was rooted with Giardia intestinalis (AF473852.1) and Giardia ardeae (Z17210.1). Bootstrap analysis was performed using 1000 pseudo-replicates. The scale bar shows the evolutionary distance for the nucleotide substitutions per site.
Intracellular components of Acanthamoeba trophozoites, enclosed within the plasma membrane, include mitochondria, nucleus, Golgi complex, endoplasmic reticulum and vacuoles [23]. Acanthamoeba trophozoites (15–45 μm) transform into a more compact dormant cyst stage (10–25 μm) when exposed to unfavorable conditions, including extreme temperatures or pH, high salination or osmolarity and lack of nutrients or drought [24]. Acanthamoeba cysts are round and are surrounded by a capsule, consisting of an inner endocyst and an outer ectocyst, made up primarily of cellulose and consolidated by binding of cellulose-binding lectins to glycopolymers [14,24–26]. Acanthamoeba cysts may survive in the environment for prolonged periods of more than 20 years without losing their virulence [27] and resist extreme physiological, chemical and radiological conditions [24]. Acanthamoeba spp. can infect the CNS, causing GAE, and the eyes, leading to a devastating infection known as AK, and may cause infections secondary to other (mainly bacterial) infections that have disrupted tissues, especially the skin in immunocompromised individuals [28, 29, 13]. It is widely recognized that the route of entry for Acanthamoeba involves the respiratory tract, leading to invasion of the alveolar blood vessels, followed by hematogenous spread and that the blood-brain barrier is where entry into the CNS most likely occurs [11].
Epidemiology
Due to increased awareness, AK, originally considered a rare infection, has been progressively considered as important in human health [14]. The incidence rate of AK varies worldwide, and some indicative rates are up to 3.3 per 1000 contact lens wearers in Hong Kong, up to 0.5 per 1000 in Holland, up to 0.1 per 1000 in the USA, up to 14.9 per 1000 in Scotland and up to 1.9 per 1000 in England [30–32]. A recent study revealed that out of 129 patients, 55 patients (38.4%) had infections in their left eyes, 60 (41.9%) had infections in their right eyes and 14 (9.7%) were suffering in both eyes [33]. Moreover, another study revealed that out of 245 patients, 243 patients suffered from infection in one eye and 2 suffered in both eyes [34]. A ten-year survey revealed that the most common age (75.5%) for patients suffering from AK was between 15 to 25 years [35]. Other studies found that out of 245 cases, 48.2% (118 cases) were between 40 amd 59 years old, 28.6% (70 cases) were under 40 years and 23.3% (57 cases) were over 60 years [34]. A study, comprising 194 cases, also revealed that ‘bad outcomes’, defined as having keratoplasty, corneal perforation, ocular surgeries, more than 10.5 months of antiamoebic treatments or degradation of vision from normal to 20/80, were more frequent (66%) in older, 35 to 76 years old, as compared to younger individuals, 15 to 34 years old, irrespective of gender [36]. While statistics are readily available for AK, it is difficult to ascertain the incidence of GAE due to lack of proper monitoring or healthcare systems, limited diagnostic expertise and low autopsy rates [37]. The disease was found to occur mostly in men and at ages between 20 years and 40 years [38]. Only 2–3% survival rates are observed in the cases reported in the literature and the approximate mortality rate was calculated to be 1.57 GAE deaths per 10,000 HIV/AIDS deaths in the USA, for HIV/AIDS patients [39].
Diagnosis
Due to its rarity and similarity of its symptoms, including fever, headache, hemiparesis, nausea, seizures, cranial nerve palsies, stiff neck, personality changes, depressed level of consciousness and coma [40], to other pathogens of the CNS such as fungi, virus and bacteria, GAE diagnosis is problematic and is linked to suspicion of amoebic infection, which, in turn, depends on expertise [41]. Following indication of brain defects such as lesions, detected through magnetic resonance imaging (MRI) or computed tomography (CT) scans, detection of pleocytosis with increased polymorphonuclear leukocytes, diminished glucose concentrations and enhanced protein concentrations in the cerebrospinal fluid (CSF) might indicate GAE [41]. Suspicion of GAE should be further strengthened by the absence of viral, fungal and bacterial pathogens and may be assessed by detecting elevated levels of Acanthamoeba-specific antibodies using indirect immunofluorescence (IIF) assays, by incubating patient’s serum on fixed amoeba-coated slides and fluorescein isothiocyanate (FITC)-labeled antibody [14]. Similarly, AK is difficult to diagnose and may be commonly misdiagnosed as adenovirus and Herpes Simplex virus infections [14]. To confirm amoebic infection, for both AK and GAE, materials from debridement, corneal scrap, corneal biopsy, corneal smear, contact lenses solutions, contact lens, CSF or brain biopsy can be observed using light or confocal microscopy [14, 34, 40]. Despite not being widely used in diagnosis, staining with Wright Giemsa (trophozoites appearing as purple bodies), Trichrome (trophozoites appear green and red, while cysts walls appear green) and Calcofluor white (trophozoites emit red, while cysts walls emits green fluorescence under ultraviolet illumination) are some example of stains that can be used to further improve the microscopic detection of Acanthamoeba spp [33,40,42]. Fluorescence microscopy can be used, by incubating the patient specimens with anti-Acanthamoeba spp. monoclonal antibodies followed by a secondary antibody with a fluorescent marker [40]. Moreover, transmission electron microscopy can be utilized to identify and differentiate Acanthamoeba spp. from host cells and other amoebae [40]. Beside microscopic techniques, molecular techniques, such as polymerase chain reaction (PCR), have been employed for the detection of Acanthamoeba spp [14,40,43]. The higher sensitivity (94%) of PCR as a diagnostic tool for Acanthamoeba spp. as compared to morphological detection (microscopic examination (33%) or culture (7%)) has been reported [44]. Specific rRNA genes or mitochondrial DNA used to detect Acanthamoeba spp. from patient samples using PCR and real-time PCR has been used to differentiate Acanthamoeba spp. from other amoebae [40,45–47]. Despite microscopic and molecular-based approaches, the most widely used technique is the cultivation of Acanthamoeba spp. from patient samples due to its simplicity, low cost and low number of cells required [14,48]. This is done by incubating the samples on 1.5% non-nutrient agar plates covered with Gram-negative bacteria at 30OC and observing the plates for the presence of Acanthamoeba spp [11,14,49–52].
Management
While a plethora of compounds have shown in vitro anti-Acanthamoeba spp. activity, a limited number of compounds have shown effects clinically in the treatment of GAE, mainly due to inability of compounds to cross the BBB [14]. Usually, using a cocktail of drugs, a hit and miss approach is used in the treatment of GAE and as such, ketoconazole (inhibits ergosterol synthesis) [53–55], amphotericin B (acts on ergosterol and generates disruption of membrane integrity) [56], fluconazole (inhibits ergosterol synthesis) [53,57], 5-fluorocytosine (inhibits synthesis of RNA and DNA) [55], trimethoprim-sulfamethoxazole (folate biosynthesis inhibitors) [53,54,57,58], voriconazole (inhibits ergosterol synthesis) [56, 180], rifampin (inhibits RNA transcription by acting on RNA polymerase) [53,54], miltefosine (inhibits cytochrome c oxidase, interacts with lipids and causes apoptosis-like cell death) [57, 58, 180], pentamidine (inhibits the synthesis of DNA, RNA, phospholipids and proteins) [58] and amikacin (inhibits mRNA binding) [180] are compounds that have demonstrated efficacy in reducing mortality associated with the disease [13]. The majority of drugs used in the treatment of Acanthamoeba spp. infections are highly toxic to human keratocytes and the required treatment duration is long and may last up to six months [176].
A 32-year-old male was diagnosed with GAE after hematopoietic stem-cell transplant [59]. He was treated with a regimen consisting of pentamidine isethionate (21 days), co-trimoxazole and azithromycin (28 days), metronidazole (60 days), fluconazole (120 days) and miltefosine (150 days) [59]. The treatment resulted in a successful outcome with continuous improvement in the patient condition [59]. Similarly, five months following an orthotopic heart transplantation, a 60-year-old woman was diagnosed with GAE [60]. Following consultation with CDC, she was treated with a regimen consisting of flucytosine, fluconazole and miltefosine for six months [60]. After six months of treatment, brain MRI demonstrated that the opacities have been cleared [60]. In another case, a 38-year-old male was diagnosed with AIDS and a ring-enhancing central nervous system lesion was revealed by brain imaging, which was later found to be caused by Acanthamoeba spp [61]. A regimen consisting of fluconazole, flucytosine, miltefosine and trimethoprim-sulfamethoxazole was administered for seven months [61]. The patient remained asymptomatic 5 months after discontinuation of antiamoebic treatment [61].
Despite advances in supportive care against infectious diseases, the mortality rate due to GAE remains alarmingly high and therefore, efforts toward developing repurposed, novel or improved drugs are ongoing [62]. Studies showed that food and drug administration (FDA)-approved drugs Amlodipine, a calcium channel blocker, prochlorperazine, a potassium channel blocker, loperamide, another calcium channel blocker, guanabenz, an adrenergic receptor blocker, and digoxin, inhibitor of the transport of potassium and sodium across cell membranes, possess anti-Acanthamoeba spp. activity [17,63,64]. Polyhexamethylene biguanide expressed both amoebicidal and cysticidal activities against Acanthamoeba spp. while exhibiting limited cytotoxicity against the eye surface [65,66], while chlorhexidine also showed both amoebicidal and cysticidal activities against Acanthamoeba spp., by interacting with the surface proteins inducing cellular damage [67]. A study in which the ability of chlorhexidine and polyhexamethylene biguanide to treat AK was compared concluded that the overall outcome was similar for both drugs [68]. Alexidine was shown to exhibit amoebicidal and cysticidal effects at 10 μg/mL and 100 μg/mL, respectively [69], acriflavine hydrochloride and proflavine were shown to exhibit amoebicidal activity against Acanthamoeba trophozoites at concentrations of 100 μg/mL and higher [70], while polymyxin B and E expressed an amoebicidal effect in vitro at concentrations of 50 μg/mL [70] and 62.5 μg/mL [71], respectively. Recently, staurosporine, isolated from a strain of Streptomyces sanyensis, was shown to be effective against both trophozoites and cysts of Acanthamoeba spp. by activating programmed cell death via the mitochondrial pathway [72].
Acanthamoeba spp. synthesizes ergosterol de novo from acetate, which is used in the membrane of the Acanthamoeba spp., while human cells possess cholesterol, which makes ergosterol a potential target against Acanthamoeba spp [73]. Various compounds that can target ergosterol, including ketoconazole, clotrimazole, miconazole, voriconazole, fluconazole and amphotericin B, have shown effectiveness against the amoeba [29]. Moreover, the cationic steroid antibiotic (CSA)-13, which is a ceraginin known to act by disrupting the cell membrane, was evaluated against Acanthamoeba spp. in vitro at a concentration of 25 mg/mL [74].
Also, propamidine isethionate was revealed to be effective in vitro against Acanthamoeba spp., showing amoebicidal activity at 1.95–10 μg/mL and cysticidal at 31.25–125 μg/mL [71,75]. Moreover, it was recently demonstrated that preserved propamidine (containing benzalkonium chloride) showed improved antiamoebic activities against both cysts and trophozoites of Acanthamoeba spp [76].
Bacterial and eukaryotic microbial cells synthesize folate, while mammalian cells do not, for the synthesis of nucleic acid, hence making the synthesis of folate a target. When the effect of folate inhibitors on Acanthamoeba spp. was studied in vitro, it was reported that 5‐fluorouracil and methotrexate exhibited amoebistatic activity at concentrations of 1.97 μg/mL and 2.45 μg/mL respectively, while pyrimethamine and metoprine exhibited amoebistatic activity at concentrations of 100 μg/mL and 50 μg/mL, respectively [77]. Rifampicin has been reported to possess an amoebicidal effect at a concentration higher than 32 μg/mL against Acanthamoeba spp. in vitro [78], while macrolide compounds, such as corifungin, rokitamycin and spiramycin [70,75,79,80], and aminoglycosides, including paromomycin, neomycin and N-chlorotaurine [70,71,81,82], have also shown anti-Acanthamoeba spp. activities. Trifluoperazine and chlorpromazine were shown to be more effective than ketoconazole, pentamidine, amphotericin B and miconazole when assessed against Acanthamoeba spp [78]. and when evaluated against Acanthamoeba spp. in vitro, artesunate was shown to exhibit amoebistatic activity that was dose dependent, increasing from 54% at 50 mg/mL to 93.2% at 100 mg/mL [83]. Recently, histone deacetylase inhibitors MPK472 and KSK64 showed both cysticidal and amoebicidal activities at 10 µM [84].
Recent studies have demonstrated that efficacy of compounds against Acanthamoeba spp. can be enhanced by synthesizing drug-conjugated nanoparticles through conjugation with metals, such as silver or gold [63]. Also, another recent study described the potent anti-Acanthamoeba spp. activity of a nanodrug consisting of iron oxide nanoparticles loaded with amphotericin B and conjugated with isoniazid [85]. Moreover, small interfering RNA molecules (siRNAs) have been shown to be able to improve the treatment of AK when used in combination with chlorhexidine [86]. Furthermore, aminoglycoside G418 was shown to cause programmed cell death in Acanthamoeba spp [87]. Another recent study depicted that ion transporters may be involved in the sensory perception of A. castellanii, suggesting their value as potential therapeutic targets to block cellular differentiation, which is one of the reasons that these amoebae infections are difficult to treat [88].
However, despite having shown promising results against Acanthamoeba trophozoites and cysts in vitro, the effects of these many of the compounds in vivo and in the clinical setting in cases of GAE are yet to be investigated. There are necessary to evaluate the actual effectiveness of the compounds in cases of GAE.
Balamuthia mandrillaris
B. mandrillaris are free-living protists that exist in an array of environments, such as water, soil and dust [89, 176; 90]. Also, similar to Acanthamoeba spp., B. mandrillaris exist in two stages, an active trophozoite and a dormant cyst stage. B. mandrillaris exist in the trophozoite form when exposed to favorable conditions, such as adequate pH, osmolarity and temperature and nutrient-rich environment. On the other hand, B. mandrillaris trophozoites, usually measuring around 15 µm to 60 µm in diameter, possess a distinctive irregular branching structure, a nucleus with varying numbers of nucleolus and other organelles including endoplasmic reticulum and mitochondria [91,92]. Binary fission, a form of mitosis whereby the amoebae and nucleus divide to form daughter cells, is the reproduction method employed by B. mandrillaris [91]. B. mandrillaris convert into a dormant and resistant cyst stage when exposed to harsh conditions, such as lack of food, excess of waste products, overcrowding of cells and extreme pH, temperatures and osmolarity [183]. The cysts of B. mandrillaris are approximately 10 µm to 30 μm, spherical and uninucleate and are surrounded by a cyst wall consisting of three layers, namely, an ectocyst, a thin irregular outer wall, a mesocyst, a fibrillar middle layer, and an endocyst, a thick inner wall [91,]. Cysts wall were found to contain mannose, glucose and trace amounts of galactose and linkage analysis revealed carbohydrates with linear and branching saccharides and the presence of cellulose [93]. B. mandrillaris cysts are resistant to temperatures of up to 70°C, repeated freeze–thawing (5 times), 0.5% SDS 200mJ ultraviolet irradiation cm2 and 25 ppm chlorine [94]. Under favorable conditions, such as neutral pH, moderate temperature (30 to 37°C) and availability of nutrients, cysts may differentiate into the active trophozoite stage [91]. B. mandrillaris may cause BAE and infect other organs such as lungs and skin, in both immunocompetent and immunocompromised individuals [11]. BAE is a chronic disease lasting between 3 and 24 months that almost always ends in fatality. It has been found in immunocompetent individuals, unlike GAE caused by Acanthamoeba spp [11,91,95]. Of note, reports have indicated the development of BAE in patients who have undertaken organ transplants, indicating that B. mandrillaris may be transmitted through donors [96]. While amoeba penetration of the olfactory neuroepithelium via the nasal route has been suggested, the more commonly recognized route is hematogenous dissemination, following entry of Balamuthia through the respiratory tract or skin and entry into the CNS achieved at the blood-brain barrier [91].
Epidemiology
The range of ages of patients suffering from BAE are large, such as 4 months to 91 years [97], 1 to 89 years [98] and 1.5 to 72 years [99], suggesting that BAE affects people of virtually all ages. Reports also show that BAE affects more males as compared to females [97–99]. Moreover, as opposed to GAE by Acanthamoeba spp., where the disease usually occurs in immunocompromised individuals, BAE mainly affects immunocompetent individuals [100].
Diagnosis
Symptoms such as abdominal pain, headache, hallucinations, fever, nausea, skin lesions, irritability, stiff neck, seizures, hemiparesis, photophobia, breathing difficulties, weight loss, sleep disturbance and slurred speech would indicate BAE [101]. Symptoms indicating BAE should lead to CT scans and MRI imaging that, in cases of BAE, would reveal ring-enhancing lesions [102–108]. Microscopic analysis of biopsies and CSF, usually extracted upon confirmation of CNS infection, may contain B. mandrillaris that can be seen more easily when using stains, including calcofluor white and Giemsa stain, but due to requirement of expert knowledge on morphology for diagnosis of B. mandrillaris, it is rarely used [91]. The method of choice for identification of B. mandrillaris is immunofluorescence assays [95,102–111]. Suspicion of BAE should be further strengthened by the absence of viral, fungal and bacterial pathogens and may be assessed by detecting elevated levels of B. mandrillaris-specific antibodies using indirect immunofluorescence (IIF) assays, by incubating patient’s serum with B. mandrillaris, followed by fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies and then quantified using flow cytometry or fluorescence microscope [112,113]. PCR is also used for identifying B. mandrillaris and sensitive primers specific to B. mandrillaris mitochondrial 16S rRNA gene have been established [109–111,114,115]. Matrix-assisted laser desorption-ionization time-of-flight MS (MALDI-TOF MS) can allow identification of B. mandrillaris within 15 min, by identifying the characteristic patterns of protein of B. mandrillaris [11]. Other techniques that can be used to detect the amoebae include metagenomics, the use of sequencing technology, such as next-generation sequencing (NGS), and bioinformatics [116].
Management
No effective drug currently exists for the treatment of BAE [117] and usually, a cocktail of drugs, using a hit and miss approach, is used in the treatment of BAE, similar to GAE; as such, artesunate (inhibits membrane glutathione S transferase), itraconazole (inhibits biosynthesis of ergosterol), metronidazole (inhibits synthesis of nucleic acid), 5-fluorocytosine, amphotericin B, fluconazole, pentamidine, trimethoprim-sulfamethoxazole, trifluoperazine (blocks central dopamine receptors), sulfadiazine (inhibits synthesis of folic acid), azithromycin, albendazole (inhibits polymerization of tubulin), clarithromycin (inhibits synthesis of proteins), ketoconazole, miltefosine, flucytosine (inhibits DNA and RNA synthesis) and thioridazine are the drugs that have been used in successful treatment of B. mandrillaris [95,105,110,118].
A 26-year-old Hispanic male was diagnosed with BAE and was treated with a regimen including miltefosine, azithromycin, trimethoprim-sulfamethoxazole, flucytosine, fluconazole, sulfadiazine, metronidazole, voriconazole, clarithromycin, pentamidine, albendazole, dexamethasone and amphotericin B [119]. Treatment with miltefosine continued for 3 weeks after stopping the regimen, while treatment with azithromycin and fluconazole continued for an additional 87 weeks and trimethoprim-sulfamethoxazole was continued for 39 weeks [119]. After 2 years, MRI imaging demonstrated that no signs of disease can be observed [119]. A 4-year-old girl was diagnosed with BAE and was treated with an initial regimen, consisting of flucytosine, fluconazole, azithromycin, pentamidine and sulfadiazine, to which miltefosine was later added and pentamidine was removed [181]. The condition of the patient gradually improved and was discharged but still given azithromycin, fluconazole and miltefosine after discharge [181]. MRI demonstrated a gradual reduction in the size of the lesions and no neurological signs or difficulties were reported following discharge [188]. Similarly, a 2-year-old boy was diagnosed with BAE and with MRI, showing mild ventricular enlargement, persistent parenchymal lesions and inflammation of the basilar cisterns [118]. He was treated with pentamidine, fluconazole, flucytosine, sulfadiazine, clarithromycin and thioridazine and gradually improved over 2 months of treatment [118]. The number and size of ring-enhancing lesions in the brain decreased as shown by subsequent brain MRI and no evidence of disease was observed in the patient after 22 months [118].
Despite being used in the treatment of BAE, drugs such as clarithromycin and pentamidine have been reported to have high toxicity [119]. The high mortality rate due to BAE warrants efforts toward developing repurposed, novel or improved drugs and as such, several compounds have been investigated against B. mandrillaris in vitro. Propamidine isethionate was shown to be able to exhibit antiamoebic activities against B. mandrillaris [120]. Cycloheximide can inhibit encystation and cytopathogenicity of B. mandrillaris [121]. Amlodipine showed antiamoebic activity against B. mandrillaris [122], while polymyxin B expressed antiamoebic activity against the amoebae at 1 μg/mL [120]. Apomorphine, demethoxycurcumin and haloperidol demonstrated antiamoebic activity against B. mandrillaris [122]. Gramicidin revealed antiamoebic activity against B. mandrillaris at 10 μg/mL [120], while resveratrol, loperamide, prochlorperazine and procyclidine were shown to cause irreversible damage to B. mandrillaris [122]. Diminazene aceturate has also been tested against both trophozoites and cysts of B. mandrillaris and showed better efficacy than amphotericin B, ciclopirox olamine, miltefosine, natamycin, paromomycin sulfate, pentamidine isethionate, protriptyline hydrochloride, spiramycin, sulconazole nitrate and telithromycin against both trophozoites and cysts [123]. Artemisinin and cytochalasin D inhibited encystation and inhibited cytopathogenicity of B. mandrillaris [121]. Nitroxoline was shown to be amoebicidal with an IC50 of 2.84 μM and cysticidal with an IC50 of 15.48 μM and at 35 μM, nitroxoline protected human brain tissue from the amoebae [124].
Recently, it has been revealed that quinazolinones have antiamoebic activities against B. mandrillaris while also reducing the cytopathogenicity of the amoebae [125]. Moreover, amoebicidal and amoebistatic activities of benzimidazoles, thiazoles, indazoles, indoles and tetrazoles against B. mandrillaris have also been reported [126,127]. Furthermore, curcumin was shown to have antiamoebic activities against B. mandrillaris [128]. Of interest, efficacy of compounds against B. mandrillaris can be enhanced by formation of drug-conjugated nanoparticles through conjugation with metals such as silver or gold [125–128].
Naegleria fowleri
N. fowleri, widely dispersed in nature, are free-living protists that exist in a variety of environments, such as water, soil and dust [129,130]. While Acanthamoeba spp. and B. mandrillaris exist in two stages, N. fowleri exist in three forms, active trophozoites, motile flagellates and dormant cysts. N. fowleri exist in the trophozoite stage, the infectious stage, under favorable conditions, such as adequate pH, osmolarity and temperature and nutrient-rich environment. N. fowleri trophozoites exhibit food cups, known as amoebastomes, that vary in size and numbers and are responsible for ingestion and attachment [131]. Some of the organelles found in the cytoplasm of the trophozoites include loosely organized endoplasmic reticulum, vesicular nucleus and mitochondria [132]. When in nutrient-poor aqueous environments, trophozoites convert into flagellates to allow long distance movement in search of nutrition. While the general ultrastructure of trophozoites and flagellates is similar, flagellates contain flagella that allow movement [132]. N. fowleri flagellates do not reproduce or form cysts [133]. When subjected to harsh conditions, such as excess of waste products, lack of food, overcrowding of cells and extreme pH, temperatures and osmolarity, N. fowleri trophozoites convert into a resistant, nonfeeding and nonreproductive cyst stage. A mature cyst wall consists of a thick inner and a thin outer component and some of the organelles found in cysts include endoplasmic reticulum, nucleus and mitochondria [134]. Little is known about the composition of cysts of N. fowleri, but it has been shown that enolase is one of the proteins expressed in N. fowleri cysts [135]. Following infection through exposure with contaminated water, N. fowleri infiltrate the cribriform plate and the nasal mucosa and then pass along the olfactory neuroepithelial route to gain entry to the brain [136].
Epidemiology
PAM is a disease lasting an average of 4 days [137]. While N. fowleri is widespread in nature, the highest number of cases was reported in the United States (41%), Pakistan (11%) and Mexico (9%) [8,138]. Reports have demonstrated that patients of all ages were affected by PAM and that the mean and median age of patients suffering from PAM were around 29 years [137] and 14 years [138], respectively. Reports also show that PAM occurs mainly in males as compared to females [137,138]. The commonly reported exposure linked to PAM was swimming/diving (58%), bathing (16%), water sports (10%) and nasal irrigation (9%) while water sources were ponds/lakes/ reservoirs (45%), swimming pools (13%), ditches/canals/ puddles (12%) and tap water (12%) [138]. Interestingly, N. fowleri was recently detected in tap water in Texas USA [139] and, in August 2021, a seven-year-old boy died following N. fowleri infection in California [140].
Diagnosis
Initial symptoms such as headache, fever, nausea and fatigue and symptoms exhibited in the later stages, including altered nuchal rigidity, mental status, extremity weakness, seizures, coma, photophobia, drowsiness, blurred vision, abnormal gait, cranial nerve abnormalities and sensory abnormalities [138] together with a history of exposure to contaminated water, are indicative of PAM [13]. CT scan can indicate the involvement of the CNS and is usually followed extraction of CSF or brain biopsy [12]. Microscopic examination of samples may reveal N. fowleri due to its characteristic food cups and flagellate form [141,142]. Stains, such as Wright-Giemsa and Gram stain, are valuable in the microscopic identification of N. fowleri [143]. Inoculating samples onto bacteria-coated non-nutrient agar is a simple method with low cost and low cell number requirement for the detection of N. fowleri. Immunofluorescence staining, enzyme-linked immunosorbent assay, immune phosphate staining, flow cytometry and PCR assays can also be used in the detection of N. fowleri [136,141,144]. Due to its sensitivity and rapidity, PCR is the method of choice for detection and identification of N. fowleri [141]. NGS has also been utilized for the identification of N. fowleri [145].
While not tested in clinical setting, another method is the recognition through ‘untargeted metabolomics methods,’ which involves analyzing cell metabolites through liquid chromatography-mass spectrometry and then comparing results with available libraries and analyzing peaks through bioinformatics software for identification of proteins specific to N. fowleri [146,147].
Management
A cocktail of drugs using a hit and miss approach is used in the treatment of PAM, similar to GAE and BAE, and as such, sulfisoxazole (competitive inhibitor of dihydropteroate synthetase), ornidazole and miconazole (inhibit biosynthesis of ergosterol), chloramphenicol (inhibits protein synthesis), ceftriaxone and dexamethasone (prevent inflammation), azithromycin (interferes with their protein synthesis), amphotericin B, miltefosine, rifampin and fluconazole are some of the drugs that have been used in successful treatment of PAM [148–153].
A 12-year-old girl was diagnosed with PAM, a regimen consisting of azithromycin, amphotericin, fluconazole, rifampin and dexamethasone was initiated and miltefosine was then added to the regimen following suggestion from CDC [154]. Intraventricular shunt and controlled hypothermia was also used as treatment [154]. The patient recovered as demonstrated by brain imaging and negative PCR results [154]. Another 12-year-old girl was diagnosed with PAM and a regimen consisting of amphotericin B, fluconazole, rifampin, azithromycin, dexamethasone and miltefosine [150]. Induced hypothermia (32°C–34°C) was also used in the management of PAM [150]. The patient was discharged after 55 days of hospitalization and had normal levels of functioning and no deficits after 6 months [150]. A 73-year-old male was diagnosed with PAM and was treated with amphotericin B and rifampicin [155]. This treatment led to a positive outcome and after 4 weeks of treatment, CSF examination was normal and no neurological deficit was observed [155].
Amphotericin B, one of the primary drugs of choice in the treatment of PAM, has been associated with multiple side effects, including use-limiting renal toxicity [156]. Hence, development of treatments against N. fowleri is still ongoing and in this effort, several compounds have been investigated against the amoeba in vitro. Azole agents, such as voriconazole, itraconazole, clotrimazole and ketoconazole, are antifungal agents that are effective against N. fowleri and ketoconazole was reported to be as effective as amphotericin B against N. fowleri [157,158]. Chlorpromazine and rokitamycin were revealed to be active against N. fowleri, both in vitro and in vivo, since at 12.5 μg/mL and 6.25 μg/mL, respectively, they completely inhibited the growth of the parasite [157]. Corifungin gave rise to 100% survival rate in mouse models infected with N. fowleri, indicating its efficacy against the parasite, which is thought to be related to affecting the mitochondria [159]. Tritrpticin showed antiamoebic activities at 100 μg/mL [158]., while secondary metabolites of Larrea tridentata were also shown to be effective against N. fowleri [160]. Diamidines, having the capability to cross the BBB, were shown to possess antiamoebic activities against N. fowleri [161] and ebselen compounds, which can also cross the BBB, were shown to possess antiamoebic activities against N. fowleri [162]. Recently, HMG-CoA reductase inhibitors were revealed as drug leads against N. fowleri, since pitavastatin, an inhibitor of HMG Co-A reductase, was able to kill 80% of trophozoites within 16hrs [163] and farnesyltransferase inhibitor lonafarnib showed activity against N. fowleri with EC50 of 1.5 µM [164]. Also, fluvastatin and atorvastatin were shown to cause programmed cell death in N. fowleri by analyzing cell membrane damage, condensed chromatin, ROS generation and mitochondrial membrane potential [165]. Moreover, staurosporine, an indolocarbazole from Streptomyces sanyensis, was shown to induce programmed cell death in N. fowleri with a low IC50 of 0.08 µM [166]. Anisomycin, prodigiosin and obatoclax are also compounds that have been shown to possess activity against N. fowleri at low micromolar concentrations [167]. Importantly, the antiparasitic agent, miltefosine, has been shown to be effective in the successful treatment and it has been recommended by the Centers for Disease Control and Prevention against PAM [168].
Of note, it has been demonstrated that quinazolinones have antiamoebic activities against N. fowleri while also reducing the cytopathogenicity of the amoebae [125]. Moreover, amoebicidal and amoebistatic activities of benzimidazoles, thiazoles, indazoles, indoles and tetrazoles against N. fowleri have also been reported [126,127]. Furthermore, curcumin was shown to have amoebicidal activities against N. fowleri [128]. Also, trans-cinnamic acid and oleic acid were also shown to possess antiamoebic activities against N. fowleri [169,170]. Of interest, efficacy of drugs against N. fowleri can be enhanced by formation of drug-conjugated nanoparticles through conjugation with metals such as silver or gold [125–128,169,170]. In addition, anti-N. fowleri activity has shown hesperidin conjugated with silver nanoparticles [171]
Concluding remarks
Infections due to brain eating amoebae are on the rise and the discovery of brain-eating amoebae in drinking water supplies highlights the threat that these amoebae pose [3–6,172]. Infections due to pathogenic free-living amoebae are lethal, albeit they are rare, and thus, pharmaceutical companies are not eager to invest funding in developing novel therapies. Nevertheless, with the emergence of global warming and nature of these amoebae being thermophilic, it is logical to suggest that infectious diseases such as those caused by free-living amoebae will be escalating [8,173].
Several diagnostic techniques have been developed for Acanthamoeba spp., B. mandrillaris and N. fowleri, including CT scan, flow cytometry, immunohistochemistry, microscopic analysis, PCR assays, cell staining, immunofluorescence staining, enzyme-linked immunosorbent assay, NGS and liquid chromatography-mass spectrometry (Figure 2). However, less invasive, faster and more sensitive diagnostic techniques should be established. Infection of the CNS by free-living amoebae is a dangerous infection with extremely high mortality rates that warrant the development of novel treatment options.
Figure 2.
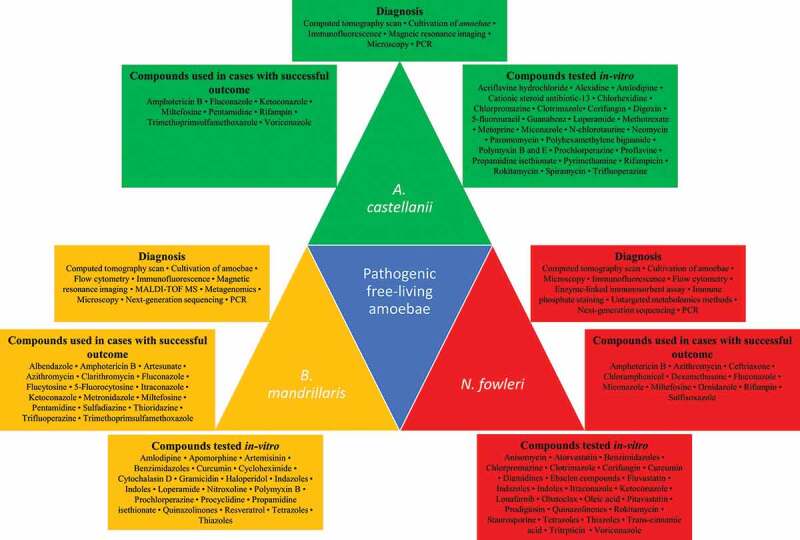
Diagnosis and management of brain-eating amoebae. Diagnostic tools and techniques used in the detection of A. castellanii, N. fowleri and B. mandrillaris are depicted. The compounds/drugs utilized in cases with successful outcomes and those tested in vitro against the three amoebae are also portrayed.
Currently, there are no standardized drugs to treat these infections and clinicians rely on a combination of therapies comprising antibiotic, anticancer, antifungal and anti-inflammatory drugs for therapy. Comprehensive research is essential over forthcoming years to determine the translation value of in vitro/early-stage drug leads and urgent collaborations between academia, the pharmaceutical industry and water companies are necessitated [174–178,182].
While several novel compounds have been investigated against the amoebae, conjugation of compounds with metal to form nanoparticles has been shown to be an effective strategy to improve efficacy of drugs against Acanthamoeba spp., B. mandrillaris and N. fowleri. However, the efficacy of drug-metal conjugated nanoparticles is yet to be investigated in vivo and their mechanism of actions is not completely understood. Recently, delivery of drugs via the intranasal route, leading to effective concentrations of drugs being delivered into the brain, has been suggested and may be a possible solution for successful treatment of brain infections, which is greatly hampered by the inability of existing drugs to cross the BBB [12, 133].
Acknowledgement
The authors acknowledge Department of Clinical Sciences, College of Medicine, University of Sharjah for support.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors contributions
NAK and RS conceived the idea. MRM reviewed the literature under the guidance of SM and RS. MRM prepared the first draft of the manuscript. NAK and RS corrected the manuscript. All authors read and approved the final manuscript.
References
- [1].Schuster FL, Visvesvara GS.. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat. 2004;7(1):41–51. [DOI] [PubMed] [Google Scholar]
- [2].Visvesvara GS. Infections with free-living amebae. Handb Clin Neurol. 2013;114:153–168. [DOI] [PubMed] [Google Scholar]
- [3].Edagawa A, Kimura A, Kawabuchi-Kurata T, et al. Isolation and genotyping of potentially pathogenic acanthamoeba and naegleria species from tap-water sources in Osaka, Japan. Parasitol Res. 2009;105(4):1109–1117. [DOI] [PubMed] [Google Scholar]
- [4].Gabriel S, Khan NA, Siddiqui R. Occurrence of free-living amoebae (Acanthamoeba, Balamuthia, Naegleria) in water samples in Peninsular Malaysia. J Water Health. 2019;17(1):160–171. [DOI] [PubMed] [Google Scholar]
- [5].Marciano-Cabral F, Jamerson M, Kaneshiro ES. Free-living amoebae, legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010;8(1):71–82. [DOI] [PubMed] [Google Scholar]
- [6].Ozçelik S, Coşkun KA, Yünlü O, et al. The prevalence, isolation and morphotyping of potentially pathogenic free-living amoebae from tap water and environmental water sources in sivas. Turkiye Parazitol Derg. 2012;36(4):198–203. [DOI] [PubMed] [Google Scholar]
- [7].Anwar A, Siddiqui R, Khan NA. Importance of theranostics in rare brain-eating amoebae infections. ACS Chem Neurosci. 2018;10(1):6–12. [DOI] [PubMed] [Google Scholar]
- [8].Maciver SK, Piñero JE, Lorenzo-Morales J. Is Naegleria fowleri an emerging parasite?. Trends Parasitol. 2020;36(1):19–28. [DOI] [PubMed] [Google Scholar]
- [9].Siddiqui R, Khan NA. Primary amoebic meningoencephalitis caused by Naegleria fowleri: an old enemy presenting new challenges. PLoS Negl Trop Dis. 2014;8(8):e3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Niederkorn JY, Alizadeh H, Leher H, et al. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999;1(6):437–443. [DOI] [PubMed] [Google Scholar]
- [11].Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: acanthamoeba spp, Balamuthia mandrillaris, Naegleria fowleri, and sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. [DOI] [PubMed] [Google Scholar]
- [12].Mungroo MR, Khan NA, Siddiqui R. Naegleria fowleri : diagnosis, treatment options and pathogenesis. Expert Opin Orphan Drugs. 2019;7(2):67–80. [Google Scholar]
- [13].Mungroo MR, Anwar A, Khan NA, et al. Brain-eating amoebae infection: challenges and opportunities in chemotherapy. Mini Rev Med Chem. 2019;19(12):980–987. [DOI] [PubMed] [Google Scholar]
- [14].Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. [DOI] [PubMed] [Google Scholar]
- [15].Kovacs CJ, Lynch SC, Rah MJ, et al. Acanthamoeba encystment: multifactorial effects of buffers, biocides, and demulcents present in contact lens care solutions. Clin Ophthalmol. 2015;9:1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Neff RJ, Neff RH. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51–81. [PubMed] [Google Scholar]
- [17].Elsheikha HM, Siddiqui R, Khan NA. Drug discovery against Acanthamoeba infections: present knowledge and unmet needs. Pathogens. 2020;9(5):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maciver SK, Asif M, Simmen MW, et al. A systematic analysis of Acanthamoeba genotype frequency correlated with source and pathogenicity: T4 is confirmed as a pathogen-rich genotype. Eur J Protistol. 2013;49(2):217–221. [DOI] [PubMed] [Google Scholar]
- [20].De Jonckheere JF. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol. 2011;11(7):1520–1528. [DOI] [PubMed] [Google Scholar]
- [21].O’Malley MA. ‘Everything is everywhere: but the environment selects’: ubiquitous distribution and ecological determinism in microbial biogeography. Stud History Philosophy Sci C. 2008;39(3):314–325. [DOI] [PubMed] [Google Scholar]
- [22].Corsaro D. Update on Acanthamoeba phylogeny. Parasitol Res. 2020;119(10):3327–3338. [DOI] [PubMed] [Google Scholar]
- [23].Bowers B, Korn ED. The fine structure of Acanthamoeba castellanii: i. the trophozoite. Int J Cell Biol. 1968;39(1):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bowers B, Korn ED. The fine structure of Acanthamoeba castellanii (neff strain) II. Encystment. Int J Cell Biol. 1969;41(3):786–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iovieno A, Gore DM, Carnt N, et al. Acanthamoeba sclerokeratitis: epidemiology, clinical features, and treatment outcomes. Ophthalmology. 2014;121(12):2340–2347. [DOI] [PubMed] [Google Scholar]
- [26].Magistrado-Coxen P, Aqeel Y, Lopez A, et al. The most abundant cyst wall proteins of Acanthamoeba castellanii are lectins that bind cellulose and localize to distinct structures in developing and mature cyst walls. PLoS Negl Trop Dis. 2019;13(5):e0007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mazur T, Hadaś E, Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Tropical Med Parasitol. 1995;46(2):106–108. [PubMed] [Google Scholar]
- [28].Grün AL, Stemplewitz B, Scheid P. First report of an Acanthamoeba genotype T13 isolate as etiological agent of a keratitis in humans. Parasitol Res. 2014;113(6):2395–2400. [DOI] [PubMed] [Google Scholar]
- [29].Khan NA.Acanthamoeba: biology and Pathogenesis. 2nd. Caister Academic Press; 2015. 424. Caister Academic Press; Norfolk, United Kingdom. ISBN: 978–1–908230–51–5 10.21775/9781908230508 [DOI] [Google Scholar]
- [30].Radford CF, Minassian DC, Dart JKG. Acanthamoeba keratitis in England and wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002;86(5):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seal DV, Kirkness CM, Bennett HGB, et al. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22(2):49–57. [DOI] [PubMed] [Google Scholar]
- [32].Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107(4):331–336. [DOI] [PubMed] [Google Scholar]
- [33].Haddad MHF, Shokri A, Habibpour H, et al. A review of Acanthamoeba keratitis in the Middle East and Iran. J Acute Dis. 2019;8(4):133–141. [Google Scholar]
- [34].Badawi AE, Moemen D, El-Tantawy NL. Epidemiological, clinical and laboratory findings of infectious keratitis at mansoura ophthalmic center, egypt. Int J Ophthalmol. 2017;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rezaeian M, Farnia S, Niati M, et al. Amoebic keratitis in Iran (1997–2007). Iran J Parasitol. 2007;3:1–6. [Google Scholar]
- [36].Carnt N, Robaei D, Minassian DC, et al. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complication. Br J Ophthalmol. 2018;102(10):1431–1435. [DOI] [PubMed] [Google Scholar]
- [37].Visvesvara GS, Maguire JH. Pathogenic and opportunistic free-living amebas: acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2006;2:1114–1125. [DOI] [PubMed] [Google Scholar]
- [38].Vyas S, Jain V, Goyal MK. Granulomatous amoebic meningoencephalitis. Neurol India. 2013;61(5):530–531. [DOI] [PubMed] [Google Scholar]
- [39].Duggal SD, Rongpharpi SR, Duggal AK, et al. Role of Acanthamoeba in granulomatous encephalitis: a review. J Infect Dis Immune Ther. 2017;1:2. [Google Scholar]
- [40].da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis. 2009;(2009):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moon EK, Hong Y, Chung DI, et al. Down-regulation of cellulose synthase inhibits the formation of endocysts in Acanthamoeba. Korean J Parasitol. 2014;52,131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].MacLean RC, Hafez N, Tripathi S, et al. Identification of Acanthamoeba sp. in paraffin-embedded CNS tissue from an HIV+ individual by PCR. Diagn Microbiol Infect Dis. 2007;57(3):289–294. [DOI] [PubMed] [Google Scholar]
- [44].Yera H, Zamfir O, Bourcier T, et al. Comparison of PCR, microscopic examination and culture for the early diagnosis and characterization of Acanthamoeba isolates from ocular infections. Eur J Clin Microbiol Infect Dis. 2007;26(3):221–224. [DOI] [PubMed] [Google Scholar]
- [45].Booton GC, Visvesvara GS, Byers TJ, et al. Identification and distribution of Acanthamoeba species genotypes associated with Nonkeratitis infections. J Clin Microbiol. 2005;43(4):1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yagi S, Schuster FL, Bloch K. Demonstration of presence of Acanthamoeb mitochondrial DNA in brain tissue and cerebrospinal fluid by PCR in samples from a patient who died of granulomatous amebic encephalitis. J Clin Microbiol. 2007;45(6):2090–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yagi S, Schuster FL, Visvesvara GS. Demonstration of Balamuthia and Acanthamoeba mitochondrial DNA in sectioned archival brain and other tissues by the polymerase chain reaction. Parasitol Res. 2008;102(2):211–217. [DOI] [PubMed] [Google Scholar]
- [48].Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487–499. [DOI] [PubMed] [Google Scholar]
- [49].Khan NA, Paget TA. Molecular tools for speciation and epidemiological studies of Acanthamoeba. Curr Microbiol. 2002;44(6):444–449. [DOI] [PubMed] [Google Scholar]
- [50].Martinez AJ. Free-living amebas: natural history, prevention, diagnosis, pathology, and treatment of disease. Crc Press; 2019. (cited 2020 Sep 2). Available from: ISBN-13: 978–1315893044. https://www.cabdirect.org/cabdirect/abstract/19860833524 [Google Scholar]
- [51].McKellar MS, Mehta LR, Greenlee JE, et al. Fatal granulomatous Acanthamoeba Encephalitis mimicking a stroke, diagnosed by correlation of results of sequential magnetic resonance imaging, biopsy, in vitro culture, immunofluorescence analysis, and molecular analysis. J Clin Microbiol. 2006;44(11):4265–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Visvesvara GS, Jones DB, Robinson NM. Isolation, identification, and biological characterization of Acanthamoeba polyphaga from a human eye. Am J Trop Med Hyg. 1975;24(5):784–790. [DOI] [PubMed] [Google Scholar]
- [53].Martinez MS, Gonzalez-Mediero G, Santiago P, et al. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group ii from brain tissue and successful treatment with sulfadiazine and fluconazole. J Clin Microbiol. 2000;38(10):3892–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Singhal T, Bajpai A, Kalra V, et al. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr Infect Dis J. 2001;20(6):623–627. [DOI] [PubMed] [Google Scholar]
- [55].Sison JP, Kemper CA, Loveless M, et al. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin Infect Dis. 1995;20(5):1207–1216. [DOI] [PubMed] [Google Scholar]
- [56].Walia R, Montoya JG, Visvesvera GS, et al. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl Infect Dis. 2007;9(1):51–54. [DOI] [PubMed] [Google Scholar]
- [57].Aichelburg AC, Walochnik J, Assadian O, et al. Successful treatment of disseminated Acanthamoeba sp. Infection with Miltefosine. Emerg Infect Dis. 2008;14(11):1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maritschnegg P, Sovinz P, Lackner H, et al. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treated with multimodal antimicrobial therapy and hyperbaric oxygen. J Clin Microbiol. 2011;49(1):446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Keane NA, Lane LM, Canniff E, et al. A surviving case of Acanthamoeba granulomatous amebic encephalitis in a hematopoietic stem cell transplant recipient. Am J Case Rep. 2020;21:e923219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brondfield MN, Reid MJ, Rutishauser RL, et al. Disseminated Acanthamoeba infection in a heart transplant recipient treated successfully with a miltefosine-containing regimen: case report and review of the literature. Transpl Infect Dis. 2017;19(2):e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].El Sahly H, Udayamurthy M, Parkerson G, et al. Survival of an AIDS patient after infection with Acanthamoeba sp. of the central nervous system. Infection. 2017;45(5):715–718. [DOI] [PubMed] [Google Scholar]
- [62].Martín-Navarro CM, López-Arencibia A, Sifaoui I, et al. Statins and voriconazole induce programmed cell death in Acanthamoeba castellanii. Antimicrob Agents Chemother. 2015;59(5):2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Anwar A, Mungroo MR, Anwar A, et al. Repositioning of guanabenz in conjugation with gold and silver Nanoparticles against pathogenic amoebae Acanthamoeba castellanii and Naegleria fowleri. ACS Infect Dis. 2019;5(12):2039–2046. [DOI] [PubMed] [Google Scholar]
- [64].Baig AM, Iqbal J, Khan NA. In vitro efficacies of clinically available drugs against growth and viability of an Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob Agents Chemother. 2013;57(8):3561–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Burger RM, Franco RJ, Drlica K. Killing acanthamoebae with polyaminopropyl biguanide: quantitation and kinetics. Antimicrob Agents Chemother. 1994;38(4):886–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Larkin DFP, Kilvington S, Dart JKG. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology. 1992;99(2):185–191. [DOI] [PubMed] [Google Scholar]
- [67].Perrine D, Chenu JP, Georges P, et al. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob Agents Chemother. 1995;39(2):339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lim N, Goh D, Bunce C, et al. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol. 2008;145(1):130–135. [DOI] [PubMed] [Google Scholar]
- [69].Alizadeh H, Neelam S, Cavanagh HD. Amoebicidal activities of alexidine against 3 pathogenic strains of Acanthamoeba. Eye Contact Lens. 2009;35(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nagington J, Richards JE. Chemotherapeutic compounds and Acanthamoebae from eye infections. J Clin Pathol. 1976;29(7):648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kilvington S, Larkin DF, White DG, et al. Laboratory investigation of Acanthamoeba keratitis. J Clin Microbiol. 1990;28(12):2722–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cartuche L, Sifaoui I, Cruz D, et al. Staurosporine from streptomyces sanyensis activates programmed cell death in Acanthamoeba via the mitochondrial pathway and presents low in vitro cytotoxicity levels in a macrophage cell line. Sci Rep. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Raederstorff D, Rohmer M. Sterol biosynthesis de nova via cycloartenol by the soil amoeba Acanthamoeba polyphaga. Biochem J. 1985;231(3):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Polat ZA, Savage PB, Genberg C. In vitro amoebicidal activity of a ceragenin, cationic steroid antibiotic-13, against Acanthamoeba castellanii and its cytotoxic potential. J Ocul Pharmacol Ther. 2011;27(1):1–5. [DOI] [PubMed] [Google Scholar]
- [75].Duma RJ, Finley R. In vitro susceptibility of Pathogenic Naegleria and Acanthamoeba species to a variety of therapeutic agents. Antimicrob Agents Chemother. 1976;10(2):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Heaselgrave W, Hamad A, Coles S, et al. In vitro evaluation of the inhibitory effect of topical Ophthalmic Agents on Acanthamoeba viability. Transl Vis Sci Technol. 2019;8(5):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mehlotra RK, Shukla OP. In vitro susceptibility of Acanthamoeba culbertsoni to inhibitors of folate biosynthesis 1. J Eukaryot Microbiol. 1993;40(1):14–17. [DOI] [PubMed] [Google Scholar]
- [78].Ondarza RN, Iturbe A, Hernández E. In vitro antiproliferative effects of neuroleptics, antimycotics and antibiotics on the human pathogens Acanthamoeba polyphaga and Naegleria fowleri. Arch Med Res. 2006;37(6):723–729. [DOI] [PubMed] [Google Scholar]
- [79].Debnath A, Tunac JB, Silva-Olivares A, et al. Vitro efficacy of corifungin against Acanthamoeba castellanii Trophozoites and cysts. Antimicrob Agents Chemother. 2014;58(3):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mattana A, Biancu G, Alberti L, et al. In vitro evaluation of the effectiveness of the macrolide rokitamycin and Chlorpromazine against Acanthamoeba castellanii. Antimicrob Agents Chemother. 2004;48(12):4520–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fürnkranz U, Nagl M, Gottardi W, et al. Cytotoxic activity of N-Chlorotaurine on Acanthamoeba spp. Antimicrob Agents Chemother. 2008;52(2):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Matoba AY, Pare PD, Le TD, et al. The effects of freezing and antibiotics on the viability of Acanthamoeba cysts. Arch Ophthalmol. 1989;107(3):439–440. [DOI] [PubMed] [Google Scholar]
- [83].Nacapunchai D, Phadungkul K, Kaewcharus S. In-vitro effect of artesunate against Acanthamoeba spp. Southeast Asian J Trop Med Public Health. 2003;33:49–52. [PubMed] [Google Scholar]
- [84].Lee HA, Park SM, Chu KB, et al. Application of histone deacetylase inhibitors MPK472 and KSK64 as a potential treatment option for Acanthamoeba keratitis. Antimicrob Agents Chemother. 2020;AAC.01506–20. DOI: 10.1128/AAC.01506-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Iqbal K, Abdalla SAO, Anwar A, et al. Isoniazid conjugated magnetic nanoparticles loaded with Amphotericin B as a potent Antiamoebic agent against Acanthamoeba castellanii. Antibiotics. 2020;9(5):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zorzi GK, Schuh RS, Maschio VJ, et al. Box Behnken design of siRNA-loaded liposomes for the treatment of a murine model of ocular keratitis caused by Acanthamoeba. Colloids Surf B Biointerfaces. 2019;173:725–732. [DOI] [PubMed] [Google Scholar]
- [87].Koutsogiannis Z, MacLeod ET, Maciver SK. G418 induces programmed cell death in Acanthamoeba through the elevation of intracellular calcium and cytochrome c translocation. Parasitol Res. 2019;118(2):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Siddiqui R, Roberts SK, Ong TYY, et al. Novel insights into the potential role of ion transport in sensory perception in Acanthamoeba. Parasit Vectors. 2019;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Baquero RA, Reyes-Batlle M, Nicola GG, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in guinea-bissau. Pathog Glob Health. 2014;108(4):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cabello-Vílchez AM, Reyes-Batlle M, Montalbán-Sandoval E, et al. The isolation of Balamuthia mandrillaris from environmental sources from Peru. Parasitol Res. 2014;113(7):2509–2513. [DOI] [PubMed] [Google Scholar]
- [91].Matin A, Siddiqui R, Jayasekera S, et al. Increasing Importance of Balamuthia mandrillaris. Clin Microbiol Rev. 2008;21(3):435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Visvesvara GS, Martinez AJ, Schuster FL, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28(12):2750–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Siddiqui R, Khan NA, Jarroll EL. The cyst wall carbohydrate composition of Balamuthia mandrillaris. Parasitol Res. 2009;104(6):1439–1443. [DOI] [PubMed] [Google Scholar]
- [94].Siddiqui R, Ortega-Rivas A, Khan NA. Balamuthia mandrillaris resistance to hostile conditions. J Med Microbiol. 2008;57(4):428–431. [DOI] [PubMed] [Google Scholar]
- [95].Deetz TR, Sawyer MH, Billman G, et al. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003;37(10):1304–1312. [DOI] [PubMed] [Google Scholar]
- [96].Farnon EC, Kokko KE, Budge PJ, et al. Transmission of Balamuthia mandrillaris by Organ Transplantation. Clin Infect Dis. 2016;63(7):878–888. [DOI] [PubMed] [Google Scholar]
- [97].Cope JR, Landa J, Nethercut H, et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. Clin Infect Dis. 2019;68(11):1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Diaz JH. The public health threat from Balamuthia mandrillaris in the southern United States. J La State Med Soc. 2011;163:197–204. [PubMed] [Google Scholar]
- [99].Centers for Disease Control and Prevention . Balamuthia amebic encephalitis–california, 1999–2007. MMWR Morb Mortal Wkly Rep. 2008;57:768. [PubMed] [Google Scholar]
- [100].Bravo FG, Seas C. Balamuthia mandrillaris amoebic encephalitis: an emerging parasitic infection. Curr Infect Dis Rep. 2012;14(4):391–396. [DOI] [PubMed] [Google Scholar]
- [101].Siddiqui R, Khan NA. Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog. 2008;44(2):89–97. [DOI] [PubMed] [Google Scholar]
- [102].Bakardjiev A, Azimi PH, Ashouri N, et al. Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases. Pediatr Infect Dis J. 2003;22(5):447–452. [DOI] [PubMed] [Google Scholar]
- [103].Denney CF, Iragui VJ, Zak LU, et al. Amebic Meningoencephalitis caused by Balamuthia mandrillaris: case report and review. Clin Infect Dis. 1997;25(6):1354–1358. [DOI] [PubMed] [Google Scholar]
- [104].Deol I, Robledo L, Meza A, et al. Encephalitis due to a free-living amoeba (Balamuthia mandrillaris): case report with literature review. Surg Neurol. 2000;53(6):611–616. [DOI] [PubMed] [Google Scholar]
- [105].Jung S, Schelper RL, Visvesvara GS, et al. Balamuthia mandrillaris Meningoencephalitis in an Immunocompetent Patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004;128(4):466–468. [DOI] [PubMed] [Google Scholar]
- [106].Katz JD, Ropper AH, Adelman L, et al. A case of Balamuthia mandrillaris meningoencephalitis. Arch Neurol. 2000;57(8):1210–1212. [DOI] [PubMed] [Google Scholar]
- [107].Kodet R, Nohýnková E, Tichý M, et al. Amebic encephalitis caused by Balamuthia mandrillaris in a Czech child: description of the first case from Europe. Pathol Res Pract. 1998;194(6):423–429. [DOI] [PubMed] [Google Scholar]
- [108].Pritzker AS, Kim BK, Agrawal D, et al. Fatal granulomatous amebic encephalitis caused by Balamuthia mandrillaris presenting as a skin lesion. J Am Acad Dermatol. 2004;50(2):38–41. [DOI] [PubMed] [Google Scholar]
- [109].Jayasekera S, Sissons J, Tucker J, et al. Post-mortem culture of Balamuthia mandrillaris from the brain and cerebrospinal fluid of a case of granulomatous amoebic meningoencephalitis, using human brain microvascular endothelial cells. J Med Microbiol. 2004;53(10):1007–1012. [DOI] [PubMed] [Google Scholar]
- [110].Martínez DY, Seas C, Bravo F, et al. Successful treatment of balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010;51(2):e7–e11. [DOI] [PubMed] [Google Scholar]
- [111].Tavares M, Da Costa JMC, Carpenter SS, et al. Diagnosis of first case of balamuthi Amoebic Encephalitis in Portugal by Immunofluorescence and PCR. J Clin Microbiol. 2006;44(7):2660–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Huang ZH, Ferrante A, Carter RF. Serum antibodies to balamuthia mandrillaris, a free-living amoeba recently Demonstrated to cause Granulomatous Amoebic Encephalitis. J Infect Dis. 1999;179(5):1305–1308. [DOI] [PubMed] [Google Scholar]
- [113].Siddiqui R, Kulsoom H, Lalani S, et al. Isolation of Balamuthia mandrillaris-specific antibody fragments from a bacteriophage antibody display library. Exp Parasitol. 2016a;166:94–96. [DOI] [PubMed] [Google Scholar]
- [114].Booton GC, Carmichael JR, Visvesvara GS, et al. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol. 2003;41(1):453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yagi S, Booton GC, Visvesvara GS, et al. Detection of Balamuthia Mitochondrial 16S rRNA Gene DNA in clinical specimens by PCR. J Clin Microbiol. 2005;43(7):3192–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Siddiqui R, Khan NA. Current treatment options of Balamuthia mandrillaris: a patent overview. Pharm Pat Anal. 2020;9(4):121–123. [DOI] [PubMed] [Google Scholar]
- [118].Cary LC, Maul E, Potter C, et al. Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics. 2010;125(3):e699–e703. [DOI] [PubMed] [Google Scholar]
- [119].Vollmer ME, Glaser C. A Balamuthia survivor. JMM Case Rep. 2016;3(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34(2):385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Siddiqui R, Matin A, Warhurst D, et al. Effect of Antimicrobial Compounds on Balamuthia mandrillaris Encystment and Human Brain Microvascular endothelial cell Cytopathogenicity. Antimicrob Agents Chemother. 2007;51(12):4471–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kalsoom H, Baig AM, Khan NA, et al. Laboratory testing of clinically approved drugs against Balamuthia mandrillaris. World J Microbiol Biotechnol. 2014;30(9):2337–2342. [DOI] [PubMed] [Google Scholar]
- [123].Ahmad AF, Heaselgrave W, Andrew PW, et al. The in vitro efficacy of antimicrobial agents against the pathogenic Free-Living Amoeba Balamuthia mandrillaris. J Eukaryot Microbiol. 2013;60(5):539–543. [DOI] [PubMed] [Google Scholar]
- [124].Laurie MT, White CV, Retallack H, et al. Functional Assessment of 2,177 U.S. and international drugs identifies the quinoline Nitroxoline as a potent amoebicidal agent against the pathogen Balamuthia mandrillaris. MBio. 2018;9(5):e02051–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mungroo MR, Shahbaz MS, Anwar A, et al. Aryl quinazolinone derivatives as novel therapeutic agents against brain-eating amoebae. ACS Chem Neurosci. 2020a;11(16):2438–2449. [DOI] [PubMed] [Google Scholar]
- [126].Anwar A, Mungroo MR, Khan S, et al. Novel Azoles as Antiparasitic remedies against brain-eating Amoebae. Antibiotics. 2020;9(4):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mungroo MR, Anwar A, Ali F, et al. Synthetic nanoparticle-conjugated bisindoles and hydrazinyl arylthiazole as novel antiamoebic agents against brain-eating amoebae. Exp Parasitol. 2020b;218:107979. [DOI] [PubMed] [Google Scholar]
- [128].Mungroo MR, Anwar A, Khan NA, et al. Gold-Conjugated Curcumin as a novel therapeutic agent against brain-eating Amoebae. ACS Omega. 2020c;5(21):12467–12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Maclean RC, Richardson DJ, LePardo R, et al. The identification of Naegleria fowleri from water and soil samples by nested PCR. Parasitol Res. 2004;93(3):211–217. [DOI] [PubMed] [Google Scholar]
- [130].Willaert E, Stevens AR. Experimental pneumonitis induced by Naegleria fowleri in mice. Trans R Soc Trop Med Hyg. 1980;74(6):779–783. [DOI] [PubMed] [Google Scholar]
- [131].Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988;52,114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Schuster F. An Electron microscope study of the Amoebo-flagellate, Naegleria gruberi (Schardinger). I. the Amoeboid and flagellate stages. J Protozool. 1963a;10(3):297–313. [DOI] [PubMed] [Google Scholar]
- [133].Siddiqui R, Ali IKM, Cope JR, et al. Biology and pathogenesis of Naegleria fowleri. Acta Trop. 2016b;164:375–394. [DOI] [PubMed] [Google Scholar]
- [134].Schuster F. An Electron Microscope Study of the Amoebo-flagellate, Naegleria gruberi (Schardinger). II. The cyst stage. J Protozool. 1963b;10(3):313–320. [DOI] [PubMed] [Google Scholar]
- [135].Chávez‐Munguía B, Segovia‐Gamboa N, Salazar‐Villatoro L, et al. Naegleria fowleri: enolase is expressed during cyst differentiation. J Eukaryot Microbiol. 2011;58(5):463–468. [DOI] [PubMed] [Google Scholar]
- [136].Siddiqui R, Ali IKM, Cope JR, et al.Brain-eating amoebae: biology and pathogenesis of Naegleria fowleri.Caister Academic Press; 2016;10:816-817. ISBN: 9781910190531. [Google Scholar]
- [137].Naqvi AA, Yazdani N, Ahmad R, et al. Epidemiology of primary amoebic meningoencephalitis-related deaths due to Naegleria fowleri infections from freshwater in Pakistan: an analysis of 8-year dataset. Arch Pharm Pract. 2016;7(4):1–12. [Google Scholar]
- [138].Gharpure R, Bliton J, Goodman A, et al. Epidemiology and clinical characteristics of primary amebic meningoencephalitis Caused by Naegleria fowleri: a global review. Clin Infect Dis. 2020;ciaa520:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].BBC NEWS . Brain-eating microbe: US city warned over water supply. 2020. [cited 2020 Sep 29]. Available from: https://www.bbc.com/news/world-us-canada-54313110?at_custom4=A0E1B0F6-0093-11EB-A52E-C4323A982C1E&at_campaign=64&at_medium=custom7&at_custom3=BBC+News&at_custom2=facebook_page&at_custom1=%5Bpost+type%5D&fbclid=IwAR36wNf0BOQLBHwE
- [140].Forbes . Brain-Eating Amoeba Kills 7-Year-Old Boy In California. 2021. [cited 2021 Sep 15]. Available from: https://www.forbes.com/sites/brucelee/2021/08/15/brain-eating-amoeba-kills-7-year-old-boy-in-california/?sh=7218ed1c4801
- [141].Bellini NK, Santos TM, Da Silva MTA, et al. The therapeutic strategies against Naegleria fowleri. Exp Parasitol. 2018;187:1–11. [DOI] [PubMed] [Google Scholar]
- [142].Jamerson M, Schmoyer JA, Park J, et al. Identification of Naegleria fowleri proteins linked to primary amoebic meningoencephalitis. Microbiology. 2017;163(3):322–332. [DOI] [PubMed] [Google Scholar]
- [143].Chávez‐Munguía B, Omaña‐Molina M, Castanon G, et al. ultrastructural study of the encystation and excystation processes in Naegleria sp. J Eukaryot Microbiol. 2009;56(1):66–72. [DOI] [PubMed] [Google Scholar]
- [144].Capewell LG, Harris AM, Yoder JS, et al. Diagnosis, clinical course, and treatment of primary amoebic meningoencephalitis in the United States, 1937–2013. J Pediatr Infect Dis Soc. 2015;4(4):e68–e75. [DOI] [PubMed] [Google Scholar]
- [145].Wang Q, Li J, Ji J, et al. A case of Naegleria fowleri related primary amoebic meningoencephalitis in China diagnosed by next-generation sequencing. BMC Infect Dis. 2018;18(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pediatr Infect Dis Moura H, Izquierdo F, Woolfitt AR, et al. Detection of Biomarkers of Pathogenic Naegleria fowleri through mass spectrometry and proteomics. J Eukaryot Microbiol. 2015;62(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yu Z, Miller HC, Puzon GJ, et al. Development of Untargeted Metabolomics Methods for the Rapid Detection of Pathogenic Naegleria fowleri. Environ Sci. 2017;51(8):4210–4219. [DOI] [PubMed] [Google Scholar]
- [148].Brown RL. Successful treatment of primary amebic meningoencephalitis. Arch Intern Med. 1991;151(6):1201–1202. [PubMed] [Google Scholar]
- [149].Jain R, Prabhakar S, Modi M, et al. Naegleria meningitis: a rare survival. Neurol India. 2002;50:470. [PubMed] [Google Scholar]
- [150].Linam WM, Ahmed M, Cope JR, et al. Successful treatment of an adolescent with Naegleria fowleri primary amebic meningoencephalitis. Pediatrics. 2015;135(3):e744–e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Seidel JS, Harmatz P, Visvesvara GS, et al. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306(6):346–348. [DOI] [PubMed] [Google Scholar]
- [152].Vargas-Zepeda J, Gómez-Alcalá AV, Vázquez-Morales JA, et al. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36(1):83–86. [DOI] [PubMed] [Google Scholar]
- [153].Wang A, Kay R, Poon WS, et al. Successful treatment of amoebic meningoencephalitis in a Chinese living in hong kong. Clin Neurol Neurosurg. 1993;95(3):249–252. [DOI] [PubMed] [Google Scholar]
- [154].Dunn AL, Reed T, Stewart C, et al. Naegleria fowleri that induces primary amoebic meningoencephalitis: rapid diagnosis and rare case of survival in a 12-year-old caucasian girl. Lab Med. 2016;47(2):149–154. [DOI] [PubMed] [Google Scholar]
- [155].Gautam PL, Kumar R, Puri S, et al. A rare case of survival from primary amebic meningoencephalitis. Indian J Crit Care Med. 2012;16(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother. 2015;59(11):6677–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kim JH, Jung SY, Lee YJ, et al. Effect of therapeutic Chemical Agents In vitro and on experimental Meningoencephalitis due to Naegleria fowleri. Antimicrob Agents Chemother. 2008;52(11):4010–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Tiewcharoen S, Junnu V, Chinabut P. In vitro effect of antifungal drugs on pathogenic Naegleria spp. Southeast Asian J Trop Med Public Health. 2002;33:38–41. [PubMed] [Google Scholar]
- [159].Debnath A, Tunac JB, Galindo-Gómez S, et al. Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob Agents Chemother. 2012;56(11):5450–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Debnath A, Nelson AT, Silva-Olivares A, et al. In vitro efficacy of ebselen and BAY 11–7082 against Naegleria fowleri. Front Microbiol. 2018;9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Bashyal B, Li L, Bains T, et al. Larrea tridentata: a novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PLoS Negl Trop Dis. 2017;11(8):e0005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Rice CA, Colon BL, Alp M, et al. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob Agents Chemother. 2015;59(4):2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hahn HJ, Abagyan R, Podust LM, et al. HMG-CoA reductase inhibitors as drug leads against Naegleria fowleri. ACS Chem Neurosci. 2020;11(19):3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hahn HJ, Debnath A. In vitro evaluation of farnesyltransferase inhibitor and its effect in combination with 3-hydroxy-3-methyl-glutaryl-coa reductase inhibitor against Naegleria fowleri. Pathogens. 2020;9(9):.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rizo-Liendo A, Sifaoui I, Arberas-Jiménez I, et al. Fluvastatin and atorvastatin induce programmed cell death in the brain eating amoeba Naegleria fowleri. Biomed Pharmacother. 2020a;130:110583. [DOI] [PubMed] [Google Scholar]
- [166].Rizo-Liendo A, Sifaoui I, Cartuche L, et al. Evaluation of indolocarbazoles from streptomyces sanyensis as a novel source of therapeutic Agents against the brain-eating Amoeba Naegleria fowleri. Microorganisms. 2020b;8(5):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ehrenkaufer G, Li P, Stebbins EE, et al. Identification of anisomycin, prodigiosin and obatoclax as compounds with broad-spectrum anti-parasitic activity. PLoS Negl Trop Dis. 2020;14(3):e0008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Cope JR, Conrad DA, Cohen N, et al. Use of the novel therapeutic agent miltefosine for the treatment of primary Amebic Meningoencephalitis: report of 1 fatal and 1 surviving case. Clin Infect Dis. 2016;62(6):774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Rajendran K, Anwar A, Khan NA, et al. Oleic acid coated silver nanoparticles showed better in vitro amoebicidal effects against Naegleria fowleri than Amphotericin B. ACS Chem Neurosci. 2019b;11(16):2431–2437. [DOI] [PubMed] [Google Scholar]
- [170].Rajendran K, Anwar A, Khan NA, et al. trans-cinnamic acid conjugated gold nanoparticles as potent therapeutics against brain-eating Amoeba Naegleria fowleri. ACS Chem Neurosci. 2019a;10(6):2692–2696. [DOI] [PubMed] [Google Scholar]
- [171].Siddiqui R, Rajendran K, Abdella B, et al. Naegleria fowleri: differential genetic expression following treatment with Hesperidin conjugated with silver nanoparticles using RNA-Seq. Parasitol Res. 2020;119(7):2351–2358. [DOI] [PubMed] [Google Scholar]
- [172].Sohail H, Iftikhar A, Hassan SM, et al. Brain eating Amoeba: alarming Rise of Naegleriasis in Pakistan. Ann Med Health Sci Res. 2020;10:816-817. [Google Scholar]
- [173].Maciver SK, McLaughlin PJ, Apps DK, et al. Opinion: iron, climate change and the ‘brain eating amoeba’ Naegleria fowleri. Protist. 2021;172(1):1. [Google Scholar]
- [174].Anwar A, Siddiqui R, Shah MR, et al. Gold nanoparticle-conjugated cinnamic acid exhibits antiacanthamoebic and antibacterial properties. Antimicrob Agents Chemother. 2018;62(9):e00630–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Milanez GD, Masangkay FR, Scheid P, et al. Acanthamoeba species isolated from Philippine freshwater systems: epidemiological and molecular aspects. Parasitol Res. 2020;1–7. [DOI] [PubMed] [Google Scholar]
- [176].Niyyati M, Dodangeh S, Lorenzo-Morales J. A review of the current research trends in the application of medicinal plants as a source for novel therapeutic agents against Acanthamoeba infections. Iran J Pharm Sci. 2016;15:893. [PMC free article] [PubMed] [Google Scholar]
- [177].Niyyati M, Karamati SA, Morales JL, et al. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res. 2016;115(2):541–545. [DOI] [PubMed] [Google Scholar]
- [178].Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba. Parasit Vectors. 2012;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. [DOI] [PubMed] [Google Scholar]
- [180].Webster D, Umar I, Kolyvas G, et al. Treatment of granulomatous amoebic encephalitis with voriconazole and miltefosine in an immunocompetent soldier. Am J Trop Med Hyg. 2012;87:715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Moriarty P, Burke C, McCrossin D, et al. Balamuthia mandrillaris encephalitis: survival of a child with severe meningoencephalitis and review of the literature. J Pediatric Infect Dis Soc. 2014;3(1):e4–9 [DOI] [PubMed] [Google Scholar]
- [182].Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27(2):221–224. [DOI] [PubMed] [Google Scholar]
- [183].Siddiqui R, Khan NA. 2012. Biology and pathogenesis of Acanthamoeba. Parasit Vectors.;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


