Summary
Aneuploidy is a ubiquitous feature of human tumors, but the acquisition of aneuploidy typically antagonizes cellular fitness. To investigate how aneuploidy could contribute to tumor growth, we triggered periods of chromosomal instability (CIN) in human cells and then exposed them to different culture environments. We discovered that transient CIN reproducibly accelerates the acquisition of resistance to anti-cancer therapies. Single-cell sequencing revealed that these resistant populations develop recurrent aneuploidies, and independently deriving one chromosome-loss event that was frequently observed in paclitaxel-resistant cells was sufficient to decrease paclitaxel sensitivity. Finally, we demonstrated that intrinsic levels of CIN correlate with poor responses to numerous therapies in human tumors. Our results show that while CIN generally decreases cancer cell fitness, it also provides phenotypic plasticity to cancer cells that can allow them to adapt to diverse stressful environments. Moreover, our findings suggest that aneuploidy may function as an under-explored cause of therapy failure.
Introduction
One of the most common features of cancer cells is an aneuploid karyotype: approximately 90% of solid tumors and over 50% of hematopoietic cancers display some degree of aneuploidy (Vasudevan et al., 2021). Aneuploidy can result from chromosome mis-segregation events during mitosis where one or more chromosomes are not properly segregated to the resulting daughter cells (Bakhoum and Cantley, 2018). These events are usually rare in normal cells but occur more frequently during tumor development (McGranahan et al., 2012). The increased rate of mis-segregation events commonly found in cancer is called chromosomal instability, or CIN (Funk et al., 2016; Holland and Cleveland, 2012).
Despite the ubiquity of chromosomal alterations in human tumors, the induction of aneuploidy generally diminishes a cell’s proliferative capacity (Sheltzer and Amon, 2011; Sheltzer et al., 2017; Stingele et al., 2012; Williams et al., 2008). By altering the dosage of hundreds or thousands of genes simultaneously, aneuploidy increases a cell’s metabolic requirements and causes widespread problems in protein production, folding, and turnover (Donnelly et al., 2014; Ohashi et al., 2015; Oromendia et al., 2012; Santaguida et al., 2015). Aneuploidy also triggers a transcriptional program involving the upregulation of stress response genes and the down regulation of cell cycle genes, often leading to cell cycle arrest and senescence (Dürrbaum et al., 2014; Santaguida et al., 2017; Sheltzer, 2013; Sheltzer et al., 2012; Stingele et al., 2012). Additionally, abolition of the checkpoint responsible for ensuring proper chromosome segregation is lethal in mice and in cancer cells (Dobles et al., 2000; Kops et al., 2004; Michel et al., 2004). Nonetheless, in human tumors, high levels of aneuploidy are commonly associated with poor patient outcomes, suggesting that aneuploidy could contribute to certain aspects of tumor progression (Birkbak et al., 2011; van Dijk et al., 2021; McGranahan et al., 2012; Smith and Sheltzer, 2018, 2021; Turajlic and Swanton, 2016; Vasudevan et al., 2020).
Recent improvements in cancer therapies have significantly increased the survival and quality-of-life of patients with a diverse range of malignancies (Baudino, 2015; Johnson et al., 2017). However, prolonged patient survival is frequently thwarted by the development of genetic alterations that cause drug resistance, particularly in response to targeted therapies (Holohan et al., 2013). The standard model of how resistance arises is through the acquisition of mutations that either restore function to the therapeutic target in the presence of the drug or bypass the drug’s effects. For example, in BRAF-driven melanomas, BRAF inhibition commonly results in a therapeutic response, followed by the inevitable outgrowth of tumors harboring secondary mutations in BRAF, NRAS, MEK1, or other MAPK signaling proteins that allow continued proliferation (Sullivan and Flaherty, 2013). However, many causes of drug resistance remain unknown: in a recent study of melanoma patients who progressed on BRAF inhibitor treatment, 42% of patients lacked any genetic alteration known to cause BRAFi resistance (Rizos et al., 2014). Alternate routes to drug resistance that have been identified include cell-state transitions, epigenetic alterations, and the over-expression of drug-efflux pumps, but the relative importance of these pathways remains unknown (Housman et al., 2014). In general, our understanding of how cancers develop drug resistance remains incomplete, frustrating our ability to design strategies for long-lasting cancer control.
Studies in yeast have previously linked the acquisition of aneuploidy with decreased sensitivity to a variety of anti-fungal agents (Chen et al., 2012, 2015; Pavelka et al., 2010; Selmecki et al., 2009; Yang et al., 2019). For instance, in the pathogenic yeast Candida albicans, amplification of a single chromosome causes resistance to the drug fluconazole, and this resistance can be phenocopied by the over-expression of individual genes encoded on this chromosome (Selmecki et al., 2008). In humans, CIN has been reported to correlate with drug resistance, though a direct causative relationship has not been demonstrated (Kuznetsova et al., 2015; Lee et al., 2011; Swanton et al., 2009). We hypothesized that aneuploidy could represent a point mutation-independent driver of drug resistance in human tumors, potentially contributing to the observed association between aneuploidy and poor patient outcomes. To directly test whether CIN and aneuploidy can influence drug sensitivity, we assessed the evolution of drug resistance in cancer cells that have been induced to mis-segregate chromosomes.
Results
Inhibition of Mps1 accelerates the acquisition of resistance to a BRAF inhibitor
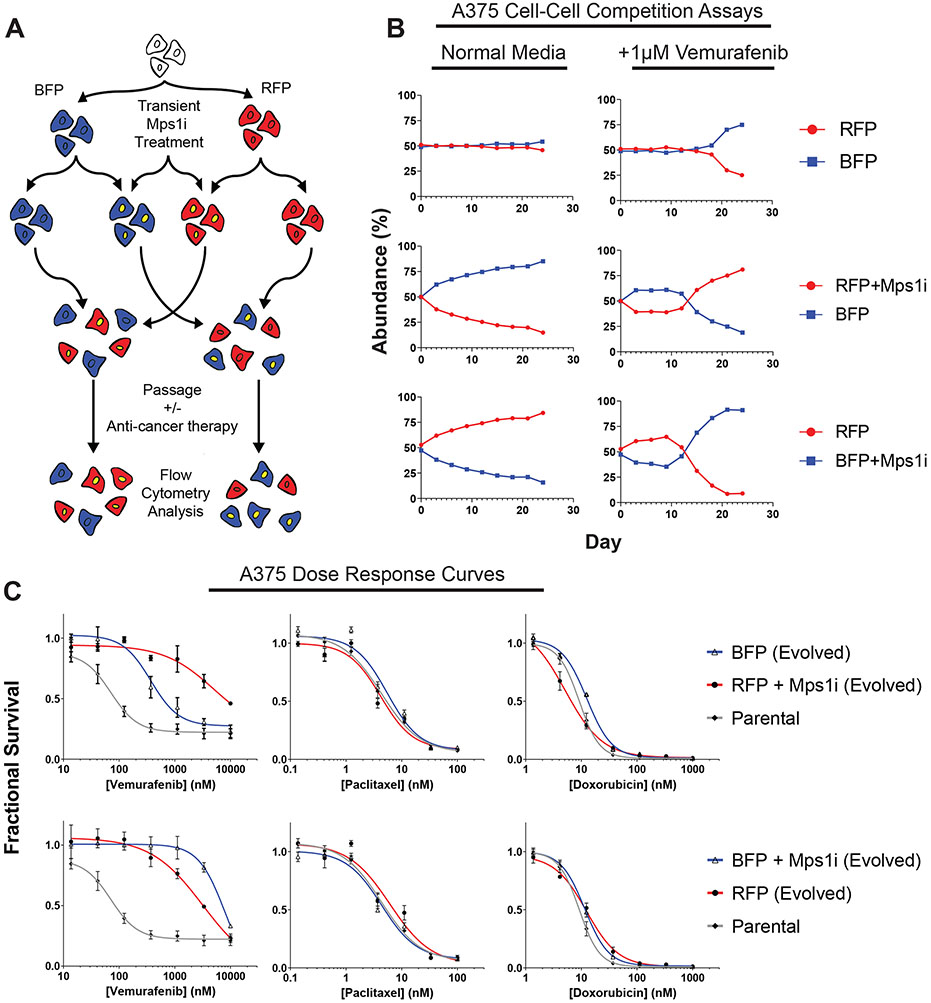
To determine the relationship between aneuploidy and therapeutic responses, we sought to transiently induce CIN in cancer cell lines. To achieve this, we treated cells with a small-molecule inhibitor of the spindle-checkpoint kinase Mps1, AZ3146. Treatment with 2μM AZ3146 causes a robust increase in the frequency of mitotic errors in cancer cell lines (Figure S1) (Santaguida et al., 2015). We have previously shown that mutations in the Mps1 kinase domain block the increase in errors caused by AZ3146, verifying that this represents an on-target effect of Mps1 inhibition (Vasudevan et al., 2020). We next devised a competition-based strategy to assess whether CIN is capable of driving the acquisition of resistance to an anti-cancer therapy (Figure 1A). A BRAF-mutant human melanoma cell line, A375, was transduced to express either of two fluorescent proteins, creating a pair of otherwise-identical cell populations. These fluorescently-labelled populations were then treated for 24-hr with Mps1 inhibitor (Mps1i) to induce a period of CIN. Next, the drug was washed out, and Mps1i-treated cell populations were co-cultured with an equal number of non-Mps1i-treated cells that had been labeled with a different fluorophore. These cell populations were then passaged for ~30 days in a variety of growth conditions. At each passage, the relative abundance of each cell population in the coculture was determined by flow cytometry, thus providing a temporal readout of the relative fitness of each population.
Figure 1. Transient inhibition of Mps1 accelerates the acquisition of resistance to a BRAF inhibitor.
(A) Schematic outline of the competition experiment.
(B) Relative abundance of each A375 cell population under the indicated growth conditions, as determined by flow cytometry.
(C) Dose-response curves of the parental A375 cell line or A375 cells purified by flow cytometry from day 24 of the competition experiments in (B), exposed to the indicated drug. N = 3 replicates.
To assess the effects of CIN on cell fitness, we first conducted competition experiments on melanoma cells grown in standard (drug-free) culture conditions. When BFP+ and RFP+ A375 cells were mixed together at a 50:50 ratio, each population remained at a ~50% abundance over 24 days in culture (Figure 1B). However, when either the BFP+ or RFP+ cell populations were pretreated with AZ3146 prior to the competition, the Mps1i-treated population showed a notable decrease in abundance compared to their untreated competitors. For instance, when A375 RFP+ cells were pretreated with AZ3146, they decreased from 50% to 15% abundance over 24 days in culture. These results suggest that periods of CIN are generally detrimental to cancer cell fitness under standard culture conditions, as has been previously reported (Kops et al., 2004; Santaguida et al., 2017; Sheltzer et al., 2017).
We next investigated the effects of CIN on cells exposed to the BRAF-inhibitor vemurafenib. Competitions conducted in medium containing 1μM vemurafenib initially resembled the competitions conducted under drug-free conditions, as both the RFP+ and BFP+ populations that were pretreated with Mps1i decreased in abundance. However, by day 12, both Mps1i-treated populations began to rise in abundance, eventually over-taking the untreated populations and dominating the competitions (Figure 1B). For instance, Mps1-treated RFP+ cells initially decreased from 50% abundance at the start of the competition to 39% abundance at day 9, then increased to 81% abundance by day 24. In the absence of Mps1i-pretreatment, both populations remained at a ~50:50 frequency for 18 days, and then a slight increase in BFP+ cell fitness was detected (discussed in greater detail below). In total, this experiment suggests that a transient period of CIN can accelerate the ability of melanoma cells to adapt to growth in a BRAF inhibitor.
To further characterize the populations that arose from these competition experiments, BFP+ and RFP+ cells with or without Mps1i-pretreatment were re-isolated by fluorescence activated cell sorting (FACS) and tested in 7-point drug sensitivity assays. All four cell populations that had been passaged in vemurafenib showed a decrease in vemurafenib sensitivity compared to their parental line (Figure 1C). However, consistent with the results of the competition assays, cells from the Mps1i-treated populations were more resistant to vemurafenib compared to the cells that were not exposed to Mps1i. These cells were not cross-resistant to two unrelated drugs, paclitaxel and doxorubicin (Figure 1C). This suggests that exposure to an Mps1 inhibitor did not cause a multi-drug resistance phenotype, and that the underlying mechanism(s) of resistance was likely specific to the conditions in which these cells were evolved.
To test whether the fitness benefits conferred by exposure to Mps1i were reproducible, we repeated the above competition experiment in four independent replicates (Figure S2A-D). While the relative abundance of each population varied across replicates, the overall dynamics of the experiment were maintained, and we found that Mps1i-treatment consistently accelerated the acquisition of resistance to BRAF-inhibition (Figure S2A-D). In 9 out of 10 competitions that we conducted, the Mps1i-treated cells eventually dominated the vemurafenib culture, while these same cells never outgrew their competitors in drug-free medium. In the absence of Mps1i-treatment, each population was typically maintained at a near-50:50 ratio for several passages, and then one population would eventually increase in abundance. This may reflect selection for underlying genetic or epigenetic alterations that can arise in a heterogeneous cancer cell line and can contribute to drug resistance. Overall, our results demonstrate that a transient period of CIN reproducibly accelerates the acquisition of resistance to vemurafenib, even in heterogeneous populations that harbor other alterations capable of influencing drug resistance.
Transient CIN can accelerate therapeutic resistance in multiple contexts
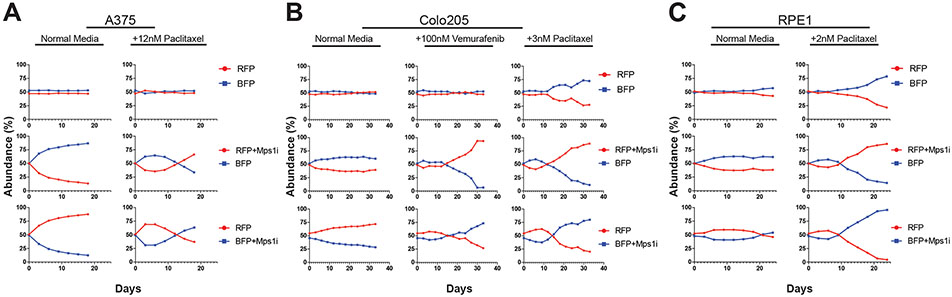
We next sought to investigate whether temporary Mps1 inhibition can accelerate the evolution of resistance to other drugs and in other genetic backgrounds. To test this, we first conducted competition experiments in A375 cells exposed to the microtubule poison paclitaxel. Consistent with our previous results, we found that Mps1i treatment before paclitaxel exposure resulted in a transient loss in cell fitness, followed by the outgrowth of the Mps1i-treated population (Figure 2A). Similar results were obtained in a set of replicate paclitaxel competitions (Figure S2E-F).
Figure 2. Transient inhibition of Mps1 accelerates the acquisition of drug resistance in multiple genetic backgrounds.
(A) Relative abundance of A375 cell populations +/− paclitaxel. See also Figures S2E-F.
(B) Relative abundance of Colo205 cell populations +/− vemurafenib, or +/− paclitaxel. See also Figure S2H.
(C) Relative abundance of RPE1 cell populations +/− paclitaxel. See also Figure S2G.
Next, we investigated whether this phenomenon was exclusive to A375 cells. To test this, we performed competitions in a BRAF-mutant colorectal cancer cell line, Colo205. Under drug-free growth conditions, Mps1i-treated cells decreased in abundance from ~50% to ~30% when competed for 30 days against untreated cells (Figure 2B). However, when competitions were performed in either vemurafenib or paclitaxel, we observed that the Mps1i-treated cells reproducibly dominated each culture. For instance, when Colo205 cells were competed in 100nM vemurafenib, Mps1i-treated populations initially dropped to ~40% abundance, then expanded to make up ~85% of their cultures between day 15 and day 33. Similar results were obtained in a set of replicate competitions in vemurafenib, though the magnitude of the benefit conferred by Mps1i treatment varied between replicates, underscoring the stochastic nature of these evolutionary adaptations (Figure S2H).
To explore whether the benefits conferred by transient CIN were restricted to malignant cells, we next examined the consequences of Mps1i exposure in immortalized retinal pigment epithelial (RPE1) cells. As we observed in A375 and Colo205 cancer cells, Mps1i pretreatment strikingly accelerated the growth of RPE1 cells cultured in paclitaxel in multiple independent competitions (Figure 2C and Figure S2G). Interestingly, the benefits of transient Mps1i treatment were not universal: exposure to Mps1i did not increase the fitness of EGFR-driven PC9 lung cells cultured in the EGFR inhibitor gefitinib or of BRAF-driven SK-MEL-5 cells cultured in vemurafenib (Figure S2J-K; discussed in greater detail below). Finally, we sought to examine whether Mps1-independent methods of producing CIN would also accelerate drug resistance. To accomplish this, we generated CIN by transiently exposing cells to the microtubule-destabilizing agent nocodazole (Thompson and Compton, 2008). We verified using H2B imaging that this treatment resulted in a significant increase in mitotic errors (Figure S1A). Then, we mixed nocodazole-treated and untreated A375 cells together and competed them in the presence of vemurafenib. We observed that pretreatment with nocodazole increased the fitness of cells grown in vemurafenib but not cells grown in normal media (Fig. S2I). In total, these results indicate that transient periods of CIN can accelerate the evolution of resistance to multiple targeted and cytotoxic agents in a variety of genetic backgrounds.
Cells that acquire drug resistance after transient CIN display specific recurrent aneuploidies
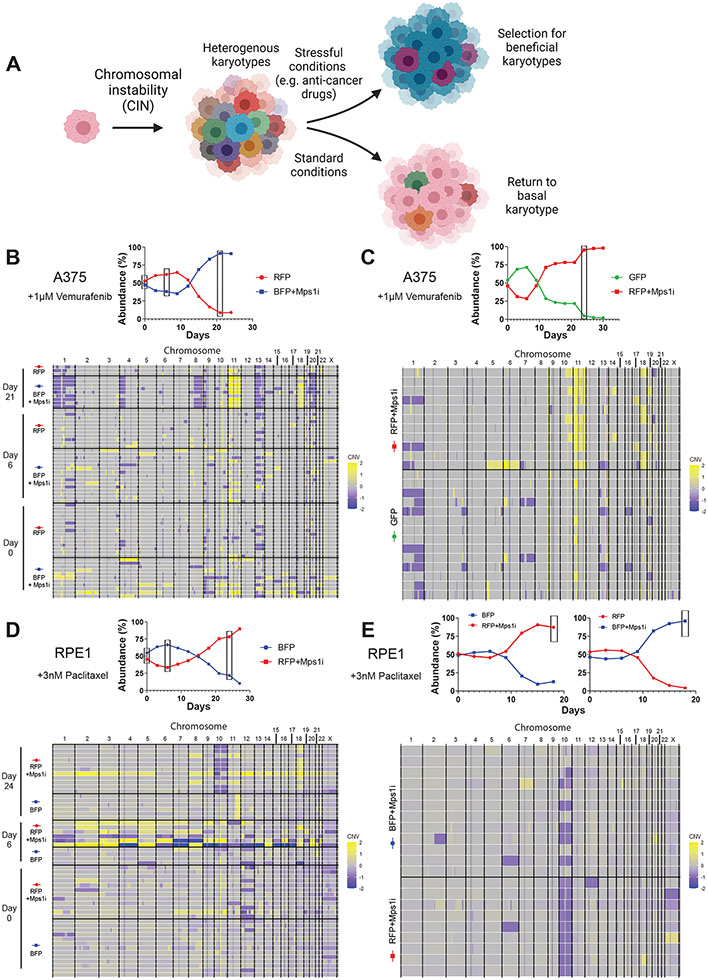
To explain the dynamics that we observed in our competition experiments, we hypothesized that treatment with an Mps1 inhibitor creates an initial population of cells with diverse karyotypes. As most of these aneuploidies are detrimental to cell fitness (Sheltzer et al., 2017; Williams et al., 2008), the Mps1i-treated cells are initially outcompeted by the untreated cells. However, certain rare aneuploidies could promote resistance to specific drugs, as has previously been observed in yeast cells treated with antimycotics (Pavelka et al., 2010; Selmecki et al., 2008; Yang et al., 2019). Over time, growth in the presence of anti-cancer drugs could select for cells harboring aneuploidies that are advantageous under those specific conditions, revealing a beneficial role for CIN in the evolution of drug resistance (Figure 3A).
Figure 3. Cells that develop drug resistance following Mps1i exposure display recurrent aneuploidies.
(A) Diagram modeling selection for beneficial aneuploidies under stressful conditions.
(B-C) (Top) Plot of A375 competition in vemurafenib from which cells were isolated for single cell sequencing. Boxes indicate time points when cells were isolated. (Bottom) Heatmap displaying the karyotypic alterations of single cells isolated from the above competitions. See also Figure S3B.
(D-E) (Top) Plot of RPE1 competition in paclitaxel from which cells were isolated for single cell sequencing. Boxes indicate time points when cells were isolated. (Bottom) Heatmap displaying the karyotypic alterations of single cells isolated from various time points of the above competition.
N.B. – Competition plots shown in (B), (C), and (E) were previously shown in Figures 1B, S2A, and S2G, respectively.
To investigate this hypothesis, we performed single-cell genomic sequencing on cells from different competition experiments. First, we isolated single A375 cells from either Mps1i-treated (BFP-expressing) or untreated (RFP-expressing) cells competed in vemurafenib for 0, 6, or 21 days. We performed read-depth analysis to identify copy number changes in each cell relative to the untreated parental cell population. As expected, the Mps1i pulse caused a significant increase in karyotype heterogeneity that was apparent at day 0 and day 6 (Figure 3B). Interestingly, at day 21, when the Mps1i-treated population had overtaken the untreated population, the heterogeneity among Mps1i-treated cells had declined, and a nearly-clonal aneuploid karyotype had emerged. These new aneuploidies included a gain of chromosome 11 (detected in 7/8 cells), a gain of chromosome 18 (detected in 7/8 cells), and several chromosome loss events. These karyotypic changes were either not detected or were recovered at a lower frequency in the untreated population. In contrast, single-cell sequencing of Mps1i-treated cells competed in normal growth medium revealed that by day 21 these cells had lost much of the karyotypic heterogeneity observed at day 0 and had nearly returned to their basal karyotype (Figure S3A).
If the aneuploidies that we recovered in the Mps1i-treated cells competed in vemurafenib conferred a fitness advantage in vemurafenib, then we would anticipate that they would independently recur in separate competitions. To test this, we performed single-cell sequencing from cells collected in three additional A375+vemurafenib competitions. In the first replicate assay, we again recovered amplifications of chromosome 11 (detected in 9/11 cells) and chromosome 18 (detected in 5/11 cells), but recurrent chromosome losses were not observed (Figure 3C). In the second replicate assay, only two Mps1i-treated cells passed our quality-control thresholds for single-cell sequencing, but both cells exhibited gains of chromosome 11 and chromosome 18 (Figure S3B). In the third assay, we generated karyotypic diversity using nocodazole washout in lieu of Mps1i treatment. Remarkably, the most common aneuploidy recovered from this competition was the gain of chromosome 11, which was found in 7/16 nocodazole-treated cells and 0/18 untreated cells (Figure S3C). Thus, in every A375-vemurafenib competition that we have analyzed, we observed recurrent amplifications of chromosome 11, while chromosome 18 gains were observed in three of four competitions. Other aneuploidies that were sporadically recovered could represent “passenger” alterations, particularly if they first arose in cells that also harbored gains of chromosome 11 and/or 18.
We next sought to discover whether these same karyotypic alterations would arise in cells treated with a different anti-cancer agent. To address this, we performed single-cell sequencing on two competitions of Mps1i-treated A375 cells cultured in paclitaxel. Interestingly the most common chromosomal alterations across both competitions were the loss of chromosomes 16, 19, and 20, while gain of chromosome 11 was rarely observed (Figure S3D-E). Thus, consistent with our drug-sensitivity experiments that demonstrated that vemurafenib-resistant cells were not cross-resistant to paclitaxel (Figure 1C), these sequencing results suggest that unique karyotypes can promote resistance to different therapeutic agents.
We next investigated whether the evolutionary pressure conferred by anti-cancer agents selects for clonal aneuploidies in other cell lines. To address this question, we performed single-cell sequencing on three independent competitions of Colo205 cells cultured in vemurafenib. We observed that Mps1i-treated Colo205 frequently acquired gains of chromosome 7 when grown in vemurafenib in independent competitions (Figure S3F-H). This suggests that the same anti-cancer drug can select for different aneuploidies in different genetic backgrounds, and no universal “vemurafenib-resistance” karyotype exists.
Finally, we analyzed karyotypes from Mps1i-treated and untreated RPE1 cells competed in paclitaxel. We isolated single cells from either Mps1i-treated (RFP-expressing) and untreated (BFP-expressing) cells competed in 3nM paclitaxel for 0, 6, and 24 days. At day 0 and 6, an increase in karyotype heterogeneity was apparent in the Mps1i-treated cells compared to the untreated counterparts (Figure 3D). By day 24, when the Mps1i-treated population dominated the competition, their karyotypic heterogeneity had declined and a near clonal karyotype emerged. The dominant Mps1i-treated cells displayed loss of chromosome 10 (detected in 8/11 cells) and gain of chromosome 18 (detected in 8/11 cells). Single-cell analysis from an independent experiment again revealed chromosome 10 loss, suggesting that this karyotypic alteration drove resistance to paclitaxel (Figure 3E). In total, we conclude that transient CIN can generate populations of karyotypically-heterogeneous cells, and certain aneuploidies reproducibly arise to near-clonal levels when cells are competed under the selective pressure of anti-cancer drugs (Table 1).
Table 1.
Summary of Single-Cell Karyotypic Analysis of Mps1i-Treated Cell Lines.
| Cell Line |
Drug | Mps1i- related Resistance |
Recurrent Aneuploidiesa |
Sporadic Aneuploidiesb |
|---|---|---|---|---|
| A375 | Vemurafenib | Yes | Gain: 11, 18 | Loss: 1, 4p, 8q, 13, 20 |
| A375 | Paclitaxel | Yes | Gain: 18 Loss: 16, 19, 20 |
Loss: 13 |
| A375 | No Drug | N/A | Return to basal karyotype | - |
| Colo205 | Vemurafenib | Yes | Gain: 7 | Gain: 8, 18 Loss: 1q, 13 |
| RPE1 | Paclitaxel | Yes | Loss: 10 | Gain: 18 |
| PC9 | Gefitinib | No | Whole-genome duplication | - |
Aneuploidies were considered recurrent if they were present in at least 50% of Mps1i-treated cells in two or more independent replicates
Aneuploidies were considered sporadic if they were present in at least 50% of Mps1i-treated cells in only one replicate
To better understand why Mps1i treatment failed to promote the acquisition of drug resistance in certain contexts, such as in PC9 cells competed in gefitinib, we performed single-cell sequencing on cells isolated from this experiment (Figure S4). Interestingly, we observed that many Mps1i-treated PC9 cells displayed whole-genome duplications, while such events were rare in other cell types. This observation is consistent with the results of our live-cell imaging analysis, which revealed that Mps1i treatment in PC9 cells frequently induces premature mitotic exit, in which cells progressed directly from prophase or prometaphase to interphase without anaphase or cytokinesis (Figure S1A). Thus, Mps1i exposure causes tetraploidization in PC9 cells. It is possible that the level of single-chromosome mis-segregation events generated by this treatment did not produce enough karyotypic diversity to confer drug resistance in PC9 cells, or that other, aneuploidy-independent resistance mechanisms are more common in this genetic background.
Point mutations and slow growth are unable to explain the beneficial effects of CIN
Our results suggested that specific aneuploidies arising from periods of CIN cause drug resistance in a drug and cell type-specific manner. However, we also considered two alternate explanations for our findings. First, we investigated the possibility that Mps1-induced point mutations, rather than aneuploidy, accelerates the development of drug resistance. To assess this, we performed whole-exome sequencing on cell populations from two Colo205 competitions that had been evolved in the presence of vemurafenib with or without Mps1i treatment. We did not identify any recurrent point mutations in genes previously associated with BRAFi resistance, including NRAS, MEK1, MEK2, NF1, or PTEN, in any evolved population (Figure S5A). Cells treated with Mps1i did not acquire more indels or SNVs compared to untreated populations (Figure S5B). Furthermore, Mps1i treatment did not lead to the frequent amplification of the BRAFV600E allele that drives growth in this cell line (Figure S5C).
Secondly, we considered the possibility that, as CIN has been observed to slow proliferation and trigger a transient cell cycle arrest, this arrest could protect cells from drug toxicity (McClendon et al., 2012). To examine this possibility, we transduced A375 and RPE1 cells with a doxycycline-inducible vector encoding the cell cycle inhibitor p21. We then titrated the concentration of doxycycline to mimic via p21 overexpression the approximate ~50% decrease in cell proliferation observed after Mps1i washout (Figure S5D-E). However, we observed that p21-overexpressing cells lost in competitions in either vemurafenib or paclitaxel to cells overexpressing GFP, demonstrating that slow growth alone is insufficient to phenocopy the beneficial effects of Mps1 inhibition (Figure S5F). We consider it possible that CIN-induced slow growth could have a more-pronounced effect on resistance to DNA-damaging chemotherapies, as G1 arrests are particularly protective against these agents (Replogle et al., 2020). In total, these experiments provide evidence against two alternate explanations for the beneficial effects of Mps1 inhibition and support our theory that Mps1i-induced aneuploidy drives resistance.
A recurrent aneuploidy observed in competition experiments is sufficient to confer resistance to paclitaxel
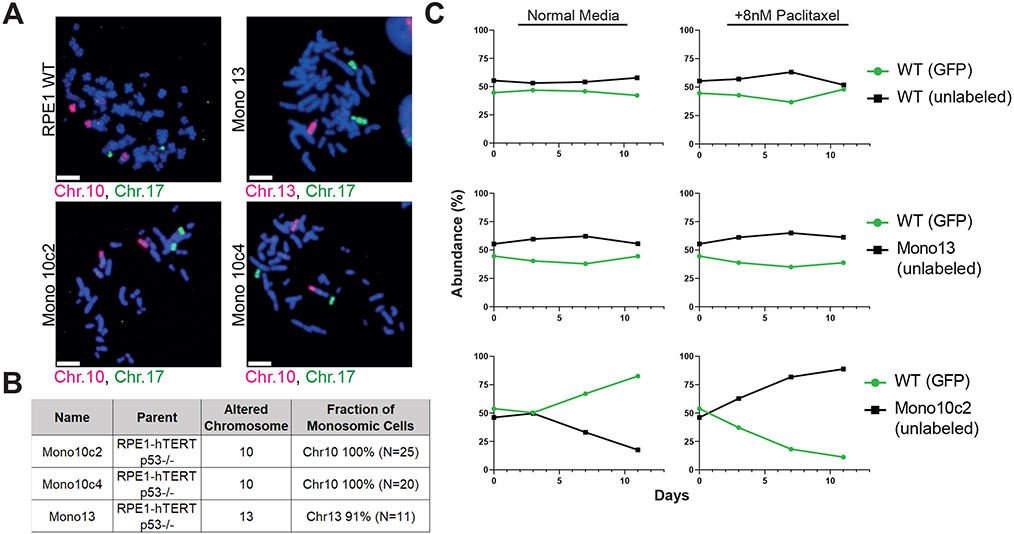
We hypothesized that our single-cell sequencing experiments recovered the same aneuploidies in independent competitions because these aneuploidies decreased a cell’s sensitivity to the anti-cancer agent that was being applied. Alternately, it is possible that these aneuploidies are “passenger” events, and their prevalence during cellular competitions occurs by chance. To differentiate between these two possibilities, we sought to discover whether an aneuploidy that we detected in our single-cell sequencing experiments could in fact confer drug resistance.
We isolated and karyotyped clones derived from single RPE1 clones without functional p53, which we deleted to facilitate proliferation of aneuploid cells (Chunduri et al., 2021). We successfully isolated two RPE1 p53−/− clones that had spontaneously lost a copy of chromosome 10, which was the most common aneuploidy that we observed in RPE1 cells cultured in paclitaxel (Figure 4A-B). (Note that the parental RPE1 cell line is trisomic for chromosome 10q, and thus these clones are monosomic for 10p and disomic for 10q). As a control, we also isolated an RPE1 clone that had lost a copy of chromosome 13, which we never observed in our RPE1 evolution experiments. We then competed these chromosome-loss clones against a parental population of RPE1 p53−/− cells. We found that losing chromosome 13 had minimal effect on cellular fitness in either normal growth medium or in medium that contained paclitaxel (Figure 4C). Losing chromosome 10 reduced the fitness of RPE1 cells in normal growth medium, as this clone decreased to ~12% abundance over 11 days in culture. Remarkably, this same aneuploidy increased in abundance from 46% to 89% when competed in medium containing paclitaxel (Figure 4C). Similar abundance changes were observed in replicates of these competition experiments (Figure S6). These results demonstrate that an aneuploidy that compromises proliferation under normal growth conditions can enhance cell fitness in the presence of a chemotherapy agent. Moreover, these findings indicate that the aneuploidies recovered in our cellular competition assays are sufficient to cause drug resistance.
Figure 4. A recurrent aneuploidy recovered in cellular competition experiments is sufficient to confer resistance to paclitaxel.
(A) Micrographs of chromosome paints for chromosomes 10, 13, and 17 in RPE1 clones to confirm monosomies. Scale bar – 10μm.
(B) Summary of the chromosome painting experiments.
(C) Relative abundance of GFP-expressing RPE1 cells competed against unlabeled monosomies +/− paclitaxel. See also Figure S6.
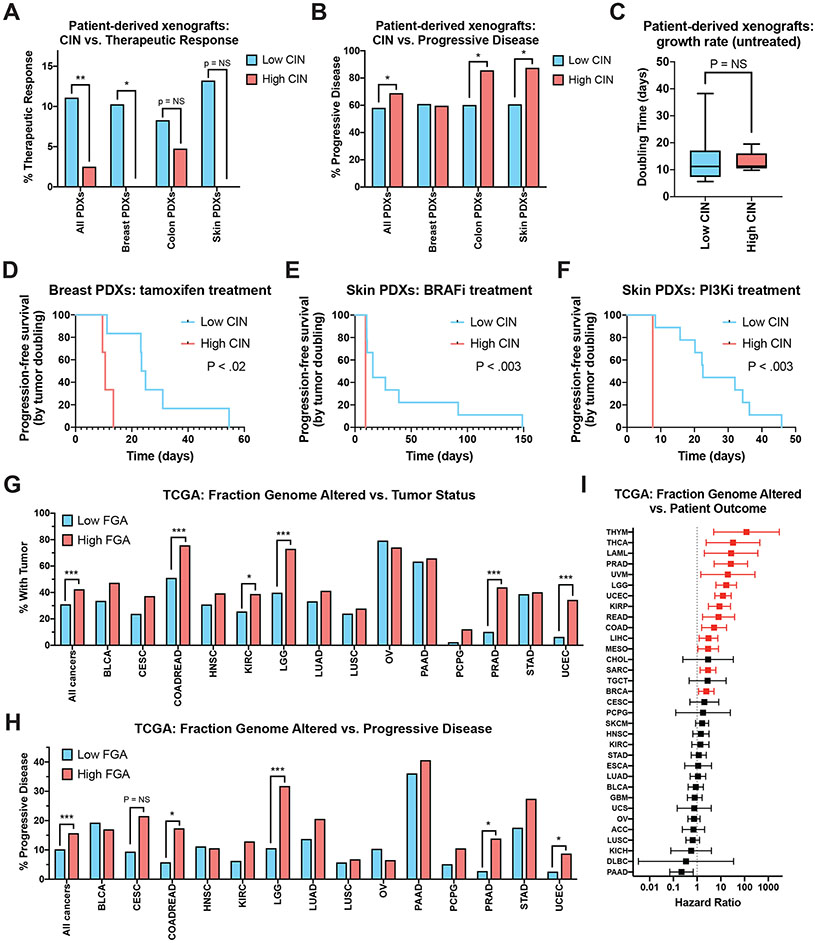
CIN and copy number alteration burden correlate with treatment resistance and disease progression in human tumors
We next sought to discover whether endogenous CIN, in the absence of an exogenous CIN-inducing agent, could also decrease cancer treatment efficacy. To investigate this question, we analyzed a large dataset of patient-derived xenografts (PDXs) that had been profiled over multiple passages in mice (Gao et al., 2015). To determine the level of endogenous CIN in each PDX, we calculated the average number of arm-length copy-number changes that occurred each time a PDX was passaged. We defined PDXs that exhibited fewer than one arm-length change per passage as “low CIN” cancers, while PDXs that exhibited four or more arm-length changes per passage were defined as “high CIN” cancers.
Mice bearing these PDXs were treated with a variety of anti-cancer agents and subsequent changes in tumor volume were determined. RECIST criteria were used to identify tumors that exhibited a therapeutic response, stable disease, or progressive disease, during systemic treatment (Gao et al., 2015). We discovered that, across all cancer types and therapies, 11% of low CIN PDXs exhibited a partial or complete therapeutic response to drug treatment, compared to only 3% of high CIN cancers (Figure 5A; P < .002). Likewise, 69% of high CIN cancers exhibited progressive disease upon treatment, compared to 58% of low CIN cancers (Figure 5B; P < .03). Similar overall trends were observed in individual cancer types. For instance, 10% of low CIN breast cancer PDXs exhibited a therapeutic response to the given treatments, while 0% of high CIN breast cancers responded (Figure 5A; P < .007).
Figure 5. High levels of endogenous CIN are associated with poor drug responses in a set of patient-derived xenografts.
(A) The percent of PDXs that displayed a partial or complete therapeutic response to systemic therapy, sorted according to their degree of CIN.
(B) The percent of PDXs that displayed progressive disease in response to systemic therapy, sorted according to their degree of CIN.
(C) Box-plots showing the time required for an untreated PDX to double in volume, sorted according to their degree of CIN. Boxes indicate the 25th, 50th, and 75th percentiles, while the bars represent the 10th and 90th percentiles.
(D-F) Kaplan-Meier survival analysis of progression-free survival, defined as the time required for a treated PDX to double in volume, for (D) breast cancer PDXs treated with tamoxifen, (E) skin cancer PDXs treated with a BRAF inhibitor, (F) skin cancer PDXs treated with a PI3K inhibitor.
(G) The percent of patients from the TCGA who were classified as “not tumor free” at the end of the observation period, sorted according to their degree of FGA.
(H) The percent of patients from the TCGA who were classified as exhibiting disease progression following their frontline therapy, sorted according to their degree of FGA.
(I) A forest plot showing hazard ratios and 95% confidence intervals for Cox proportional hazards regression between FGA and patient outcome for each of the indicated cancer types. The hazard ratios plotted in red represent those that are significant at a P < .05 threshold.
Statistical comparisons in 5A, 5B, 5G, and 5H were performed using Fisher’s exact test. Statistical comparisons in 5C were performed using a two-sided t-test. Statistical comparisons between survival curves in 5D, 5E, and 5F were performed using a log-rank test. *, p < .05; **, p < .005; ***, p < .0005.
We considered the possibility that high CIN cancers grew more rapidly than low CIN cancers, thereby explaining this difference in therapeutic response rates. However, in mice that were not treated with any anti-cancer agents, both low CIN and high CIN PDXs exhibited a median tumor-volume doubling time of 11 days, suggesting that they exhibited similar proliferative capacities (Figure 5C). Finally, we sought to determine whether different response patterns could be detected in PDXs treated with specific therapeutic agents. We calculated a surrogate “progression-free survival” period for each PDX, based on the time required for the tumor volume to double during treatment. Consistent with the in vitro competition experiments that we conducted, we discovered that high CIN skin cancer PDXs treated with BRAF inhibitors exhibited a significantly shorter progression-free survival time compared to low CIN skin cancer PDXs (Figure 5D, < .003). Similar results were observed in several other treatment conditions, including breast cancer PDXs treated with tamoxifen and skin cancer PDXs treated with PI3K inhibitors (Figure 5E-F).
To investigate whether CIN correlates with therapy resistance in endogenous human tumors, we analyzed clinical and copy number data from The Cancer Genome Atlas (TCGA). As TCGA cancers were profiled at a single time point, we were unable to directly measure CIN in these samples. As a proxy for CIN, we assessed the Fraction Genome Altered (FGA), a measure of each cancer’s aneuploidy burden, which has previously been shown to correlate with CIN (Schonhoft et al., 2020; Xu et al., 2021). We divided each TCGA cohort into a low-FGA and high-FGA set, representing the bottom 25% and top 25% of FGA values, respectively. We found that, at the end of the clinical observation period, individuals with high-FGA cancers were significantly less likely to be tumor-free compared to individuals with low-FGA tumors (Figure 5G; P < .0001). For instance, among individuals diagnosed with prostate cancer, 90% of low-FGA patients were tumor-free at the end of the study, compared to only 56% of high-FGA patients. Similarly, while the patients that comprise the TCGA were not treated in a uniform manner, individuals with high-FGA cancers were significantly more likely to exhibit progressive disease following their frontline therapy compared to individuals with low-FGA cancers (Figure 5H; P < .0001). Among the cancer types in which copy number and therapy response data were available for >100 patients, high-FGA status was significantly associated with disease progression in colorectal cancer, low-grade glioma, prostate cancer, and endometrial cancer (Figure 5H).
Finally, we performed Cox proportional hazards regression to assess the link between FGA and patient outcome. We discovered that FGA was significantly associated with shorter survival times in 14 of 34 cancer types, including in breast cancer (hazard ratio: 2.4), kidney papillary carcinoma (hazard ratio: 8.8), and acute myeloid leukemia (hazard ratio: 27.1; Figure 5I). We note that this clinical association could reflect several distinct consequences of CIN, including the previously-reported association between CIN and metastasis (Bakhoum et al., 2018). In total, our analysis of patient-derived xenografts and human tumors supports the potential clinical relevance of our in vitro experiments. High levels of endogenous CIN, without supplemental treatment with an Mps1 inhibitor, are correlated with an increased likelihood of progressive disease and therapy resistance across a wide variety of cancer types.
Discussion
Here we demonstrated that inducing exogenous CIN can accelerate the acquisition of resistance to both targeted and cytotoxic therapies. Single cell karyotypic analysis of the drug-resistant cells across multiple replicates revealed consistent but unique karyotypic alterations for each drug-cell line combination. Contemporaneously, Ippolito et al. employed a similar experimental approach to study the role of CIN in promoting drug resistance (Ippolito et al., this issue of Developmental Cell). Using different CIN-induction approaches, different cell lines, and different anti-cancer therapies, they also report that CIN can promote drug resistance. These findings highlight the generalizability of this phenomenon as a cause of anti-cancer therapy failures.
While CIN and the aneuploidies that result from CIN cause numerous cellular stresses, chromosomal errors are ubiquitous during tumorigenesis, and high levels of aneuploidy are associated with poor patient outcomes (Bakhoum and Cantley, 2018; Sheltzer and Amon, 2011; Sheltzer et al., 2017; Shukla et al., 2020; Vasudevan et al., 2020, 2021). It remains unclear to what extent the aneuploidy that arises during tumorigenesis functions as a driver of cancer progression, and to what extent aneuploidy arises as a byproduct of the loss of checkpoint control that often occurs in advanced malignancies (Davoli et al., 2013; Zimonjic et al., 2001). Previous studies have shown that CIN can accelerate the adaptation to oncogene withdrawal in mouse models of KRAS- and BRAF-driven cancers (Kwong et al., 2017; Salgueiro et al., 2020). Similarly, our work shows that CIN can drive the evolution of cells challenged with different anti-cancer therapies, and we demonstrate that one such aneuploidy which arises in these populations is sufficient to confer drug resistance. We believe that our findings coupled with those of Ippolito et al. may help explain the large fraction of tumors for which no known resistance mechanism can be identified (Rizos et al., 2014). CIN allows cancer cells to sample the fitness landscape by acquiring random aneuploidies, most of which will be deleterious, and a few of which may provide a fitness benefit under selective conditions. Over the course of tumor progression, this selection for beneficial aneuploidies arising from CIN would result in higher degrees of aneuploidy, causing the observed clinical association between CIN, aneuploidy, and aggressive malignancies.
It has previously been reported that cancer cell lines with CIN show a reduced sensitivity to a broad range of cytotoxic and targeted therapies compared to their euploid counterparts (Lee et al., 2011). However, we observed that the immediate consequence of exogenously-induced CIN is reduced fitness under both normal and stressful growth conditions. We only detected an increase in the fitness of the karyotypically heterogenous cells after several passages, and single-cell sequencing revealed that this increase co-occurred along with the rise of certain clonal aneuploidies. This may suggest that CIN does not provide “intrinsic” resistance to anti-cancer therapies, and instead endows a genomic plasticity to cancer cells that allows them to acquire beneficial aneuploidies. In support of this model, it has previously been observed that certain karyotypic alterations are associated with improved proliferation in serum-starved or hypoxic conditions, underscoring aneuploidy’s ability to promote growth in stressful environments (Rutledge et al., 2016).
In our experiments, we recovered the same aneuploidies in independent competitions, suggesting that these chromosomal alterations drove the drug-resistance phenotype. Moreover, by reconstituting one aneuploidy in drug-naïve cells, we were able to demonstrate that this karyotypic change was sufficient to decrease paclitaxel sensitivity. Aneuploidy has been proposed to promote tumor development by increasing the dosage of growth promoting oncogenes and decreasing the dosage of growth inhibitory tumor suppressors (Beroukhim et al., 2010; Davoli et al., 2013; Gordon et al., 2012; Schukken and Sheltzer, 2021). Similarly, we hypothesize that the altered expression of certain dosage-sensitive genes encoded on the chromosomes we recovered drives selection for these specific aneuploidies. However, the identity of these gene(s) is at present unknown. Chromosome-specific and drug-specific interactions may complement the multi-drug resistance phenotype that has been observed in near-tetraploid cells that have undergone whole-genome duplications (Kuznetsova et al., 2015; Shukla et al., 2020; Viganó et al., 2018). However, Mps1i treatment did not accelerate evolution in every context tested. We speculate that different classes of mitotic errors may exhibit different effects on cellular evolvability, and in certain contexts whole-genome duplications, as observed in PC9 lung cancer cells, may promote senescence rather than drug resistance.
Finally, in this work, we applied pharmacological inhibitors of Mps1 to generate periods of CIN. Mps1 inhibitors are also being investigated as potential cancer therapies, and at least five different Mps1-targeting compounds have entered clinical trials (Wang et al., 2019). However, our results underscore how CIN can drive genomic plasticity in cancer and promote the development of drug resistance in a variety of contexts. These findings suggest that Mps1 inhibitors should be used cautiously in the clinic, so as not to inadvertently accelerate the evolution of drug resistance in cancer patients.
Limitations of the Study
These experiments were performed on cell lines grown in culture, which are an imperfect model for autochthonous human cancers. The spectrum of both cell-autonomous and non-cell autonomous resistance mechanisms for tumors growing in vivo may be different than we observed in vitro. Aneuploidies such as chromosome 10 loss in RPE1 cells may preexist within these populations or may arise de novo. Additionally, the mechanism(s) linking aneuploidy with drug resistance are still under investigation.
STAR Methods
Resource Availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Jason Sheltzer (sheltzer@cshl.edu).
Materials availability
The plasmids used in this study are available through Addgene. The cell lines used in this study are available through ATCC and other cell line repositories (see the Key Resource Table).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Digoxigenin-Fluorescein, Fab fragments | Roche | Cat. No. 11207741910 |
| Bacterial and virus strains | ||
| Stbl3 E. coli | Thermo Fisher | Cat. No. C737303 |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| AZ3146 | Selleck Chemicals | Cat. No. S2731; CAS: 1124329-14-1 |
| Paclitaxel | Selleck Chemicals | Cat. No. S1150; CAS: 33069-62-4 |
| Vemurafenib | Selleck Chemicals | Cat. No. S1267; CAS: 918504-65-1 |
| Doxorubicin | Selleck Chemicals | Cat. No. S1208; CAS: 25316-40-9 |
| Gefitinib | Selleck Chemicals | Cat. No. S1025; CAS: 184475-35-2 |
| Nocodazole | Selleck Chemicals | Cat. No. S2775; CAS: 31430-18-9 |
| Doxycycline | Sigma-Aldrich | Cat. No. D3072; CAS: 10592-13-9 |
| Critical commercial assays | ||
| FISH probe, chromosome 10 | Chrombios | Cat. No. PH10OR |
| FISH probe, chromosome 13 | Chrombios | Cat. No. PH13OR |
| FISH probe, chromosome 17 | Chrombios | Cat. No. PH17DIG |
| Deposited data | ||
| Short Read Sequencing Data | NCBI BioProject | PRJNA725851 |
| Experimental models: Cell lines | ||
| Human: A375 | ATCC | Cat. No. CRL-1619; RRID:CVCL_0132 |
| Human: RPE1 | ATCC | Cat. No. CRL-4000; RRID:CVCL_4388 |
| Human: RPE1 monosomic cell lines | Dr Zuzana Storchova, TU Kaiserslautern | Chunduri et al. 2021 |
| Human: SK-MEL 5 | ATCC | Cat. No. HTB-70; RRID:CVCL_0527 |
| Human: SK-MEL 28 | ATCC | Cat. No. HTB-72; RRID:CVCL_0526 |
| Human: Colo205 | ATCC | Cat. No. CCL-222; RRID:CVCL_0218 |
| Human: PC-9 | Millipore Sigma | Cat. No. 90071810; RRID:CVCL_B260 |
| Experimental models: Organisms/strains | ||
| Oligonucleotides | ||
| Recombinant DNA | ||
| pMMLV-EGFP-P2A-Puro | Addgene | Cat. No. 160226; RRID:Addgene_160226 |
| pMMLV-EBFP-P2A-Puro | Addgene | Cat. No. 160227; RRID:Addgene_160227 |
| pMMLV-mRFP1-P2A-Puro | Addgene | Cat. No. 160228; RRID:Addgene_160228 |
| pLVX-TetOne-Puro-hAXL | Addgene | Cat. No. 124797; RRID:Addgene_124797 |
| Lenti-Cas9-gRNA-GFP | Addgene | Cat. No. 124770; RRID:Addgene_124770 |
| pcDNA3-HA p21 | Addgene | Cat. No. 78782; RRID:Addgene_78782 |
| pLVX-TetOne-Puro-GFP | Addgene | Cat. No. 171123; RRID:Addgene_171123 |
| pLVX-TetOne-Puro-p21 | Addgene | Cat. No. 171122; RRID:Addgene_171122 |
| pBABE-H2BGFP | Addgene | Cat. No. 26790; RRID:Addgene_26790 |
| pX330-U6-Chimeric_BB-CBh-hSpCas9 | Addgene | Cat. No. 42230; RRID:Addgene_42230 |
| Software and algorithms | ||
| Biomarker-survival (version 0.23) | Joan Smith | github.com/joan-smith/biomarker-survival |
| FGA Analysis | Joan Smith | github.com/joan-smith/survival-analysis-scripts/tree/master/fga |
| Volocity | Quorum Technologies | http://quorumtechnologies.com/volocity |
| Other | ||
Data and code availability
Raw short read sequencing data generated for this study have been deposited in the NCBI Short Read Archive and are available under BioProject accession number PRJNA725851.
Clinical data for TCGA patients were acquired from (Liu et al. 2018). FGA data for TCGA samples were acquired from cBioportal (Gao et al. 2013). Patient-derived xenograft drug response data were acquired from (Gao et al. 2015).
All original code is available at https://github.com/joan-smith and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper will be made available from the lead contact upon request.
Experimental Model and Subject Details
Cell Lines
The identity of each cell line was verified by STR profiling (University of Arizona Genetics Core, Tucson, AZ). A375 (sex: female), RPE1 (sex: female), SK-MEL-5 (sex: female), and SK-MEL-28 (sex: male) cell lines were cultured in DMEM (Gibco) with 10% FBS, 2mM glutamine, and 100 U/mL penicillin and streptomycin. Colo205 (sex: male) and PC9 (sex: male) cell lines were cultured in RPMI-1640 (Gibco) with 10% FBS, 2mM glutamine, and 100 U/mL penicillin and streptomycin. All cell lines were grown in a humidified environment at 37°C and 5% CO2 and passaged every 3 days.
Method Details
Construction of inducible GFP and inducible p21 expression plasmids
The pLVX-TetOne backbone was amplified from pLVX-TetOne-Puro-hAXL (Addgene #124797). The p21 coding sequence was amplified from pcDNA3-HA p21 (Addgene #78782) and the GFP coding sequence amplified from Lenti-Cas9-gRNA-GFP (Addgene #124770). The fragments were PCR purified and assembled together using NEB HiFi Assembly Master Mix (NEB, cat no E2621). Colonies were screened for proper assembly, and the resulting pLVX-TetOne-Puro-p21 (Addgene #171122) and TetOne-Puro-GFP (Addgene #171123) plasmids were sequence verified.
Virus generation and transduction
Virus for constructs expressing EGFP (Addgene #160226), EBFP (Addgene #160227), mRFP1 (Addgene #160228), inducible GFP (Addgene #171123), and inducible p21 (Addgene #171122) were generated using calcium phosphate transfection previously described (Chang et al. 2013; Giuliano et al. 2019). Virus was collected 48-hours and 72-hours post transfection and filtered through a 0.45μm syringe. Collected virus was either immediately applied to target cells with 4μg/mL polybrene for transduction or stored at −80°C for later use.
Live cell imaging
A375 and PC9 cells harboring a GFP-H2B construct (Addgene #26790) were plated on 8-well μ-slides (Ibidi; Cat. No. 80826) and half were treated with 2μM AZ-3146 (Selleck Chemicals; Cat. No. S2731) 3 hours prior to imaging. A375 cells harboring a GFP-H2B construct were treated with 100ng/mL nocodazole (Selleck Chemicals; Cat. No. S2775) for 8-hours followed by washout with fresh media. Mitotic cells were collected by shake-off an hour after washout and plated on 8-well μ-slides 3-hours prior to imaging. Live-cell imaging was performed in a humidified environment at 37°C using a spinning-disc confocal microscopy system (UltraVIEW Vox; PerkinElmer) and a charge-coupled device camera (ORCA-R2; Hamamatsu Photonics) fitted to an inverted microscope (DMI6000 B; Leica) equipped with a motorized piezo-electric stage (Applied Scientific Instrumentation). Overnight imaging was performed using a Plan Apochromat 20X 0.7 NA air objective with camera binning set to 2x2. Image acquisition and analysis were performed using Volocity version 6.3 (PerkinElmer).
Cells undergoing mitosis were tracked from nuclear envelope breakdown to anaphase exit, during which time errors such as lagging chromosomes, polar chromosomes, multipolar mitoses, anaphase bridges, and mitotic exit were observed and scored. Criteria for scoring errors are as follows: the presence of a chromosome between the migrating chromosomal masses during anaphase is scored as a lagging chromosome. The presence of a chromosome proximal to the spindle pole at the onset of anaphase is scored as a polar chromosome. The presence of chromosome spanning between migrating chromosomal masses during anaphase is scored as an anaphase bridge. Mitotic cells proceeding directly from prophase or prometaphase into anaphase without forming a metaphase plate is scored as metaphase skip. An anaphase where chromosomes migrate to more than two poles is scored as a multipolar mitosis. A cell that begins the process of mitosis but does not proceed through anaphase before chromosomal decondensing is scored as mitotic exit. A minimum of 40 mitoses were analyzed for each condition.
Competition experiments
Cell lines transduced with different fluorophores were cultured in normal growth conditions, pulse-treated with 2μM AZ-3146 for the duration of approximately two cell doubling times (24 hours for A375 and RPE1; 48 hours for Colo205, PC-9, SKMEL-5). A375 cells were also pulse-treated with nocodazole for 8-hours followed by mitotic shake-off to collect dividing cells (Thompson and Compton 2008). Pulse-treated cells were then given a three-day recovery passage in normal growth conditions. Pulse-treated cells and non-treated cells harboring a different fluorophore were then mixed in a 50:50 ratio confirmed by flow cytometry (MACSQuant; Miltenyi Biotec). Gates were established using non-fluorescent parental cell lines as a negative control. Cell mixtures were then cultured in normal growth medium or medium supplemented with anti-cancer therapeutics. Cell mixtures were passaged every three days and the relative abundance measured by flow cytometry.
Single-cell sequencing and SMASH sequencing
The single-cell sequencing protocol was adapted from (Baslan et al. 2012). Cells from competition experiments were single-cell sorted into 96-well PCR plates (ThermoFisher Scientific; Cat. No. AB0731) containing lysis buffer (0.1% SDS, 2% Triton) and incubated at 65°C for 1 hour. Genomic DNA was enzymatically digested with NlaIII (NEB; Cat. No. R0125). Both ends of genomic fragments were tagged using oligonucleotides containing a cell-barcode, a universal primer, and several random nucleotides (varietal tags) through ligation and extension reactions. Cell barcodes allow for multiplexing of the samples and the varietal tags allow for counting of unique initial DNA fragments. After amplification with a universal primer, fragment size selection was performed with Agencourt AMPure XP beads (Beckman Coulter; A63881) to obtain fragments appropriate for sequencing. Barcoded sequencing adaptors were ligated to the ends of the fragments to allow for further multiplexing and to prepare the fragments for next generation sequencing. Next generation sequencing was performed on an Illumina MiSeq. Reads were demultiplexed and used to identify karyotypes as described in (Andrews et al. 2016). Heatmaps of single-cell karyotypes compared to parental basal karyotypes were generated in R.
Basal karyotypes of each cell line were identified through SMASH sequencing (Wang et al., 2016). Cells were trypsinized, resuspended in PBS, and pelleted. Total Genomic DNA was then extracted from the cell pellets via Qiagen QIAamp kit (Cat. No. 51036). The genomic DNA was then fragmented with dsDNA Fragmentase (NEB, Cat. No. M0348L) to a mean size of ~40bp followed by random ligation to form chimeric fragments approximately 400-700bp in length. Size selection of suitably sized fragments for NGS library preparation was performed with Agencourt AMPure XP beads (Beckman Coulter, Cat. No. A63881). Illumina compatible NEBNext Multiplex Dual Index Primer pairs and Adaptors (New England Biolabs, Cat. No. E6440S) were then ligated to the ends of the size-selected chimeric fragments. These fragments were then sequenced on an Illumina MiSeq. NGS-generated reads were demultiplexed and mapped to generate karyotypes as described in (Andrews et al. 2016).
Whole exome sequencing
Genomic DNA was extracted from Colo205 cells isolated from endpoints of two competition experiments conducted in 100nM vemurafenib. Additionally, genomic DNA was extracted from two samples of parental Colo205 cells and parental Colo205 cells treated for 48-hours with 2μM AZ-3146. These genomic DNA samples were sent to Novogene Corporation Inc. for whole exome sequencing and analysis. In short, genomic DNA was fragmented by sonication, adaptors ligated, and fragments amplified by PCR. Fragment libraries were then hybridized with biotinylated probes (Agilent SureSelect Human All Exome V6) and pulled down with streptomycin-coated magnetic beads to enrich for exonic fragments. Captured fragment libraries were then amplified and index tagged in a PCR reaction followed by purification by AMPure XP beads. Libraries were sequenced on the Illumina NovaSeq 6000 platform. Sequences were aligned using BWA, Samtools, and Picard. SNP/InDel detection performed with GATK, MuTect, Strelka.
Generation of monosomies and chromosome paints
The monosomic cell lines arose spontaneously following depletion of TP53 gene from human retinal pigment epithelium cell line RPE1 immortalized with hTERT overexpression. Briefly, the gRNA against TP53 was cloned in pX330 vector (Addgene: 42230) according to a modified protocol from (Shalem et al. 2014) and used to transfect RPE WT cells. Single cell derived clones were verified for the loss of p53 expression by sensitivity to Nutlin and immunoblotting for p53 and p21. The copy number status of the single cell derived clones were verified by low-pass whole genome sequencing. All the cell lines were cultured in DMEM + GlutaMAX™-I medium (Gibco) supplemented with 10% FBS and penicillin and streptomycin at 37°C in a humidified 5% CO2 incubator. To minimize the occurrence of secondary genomic changes, original stocks were thawed for every experiment and maintained for maximum of 4 to 5 passages.
Additional validation of monosomies was performed with chromosome paints. Cells were treated with 400 ng/mL colchicine for 5-6 h, trypsinized, and pelleted. Cell pellets were resuspended in 75 mM KCl and incubated for 10–15 min at 37°C. Cells were pelleted at 1000 rpm for 10 min and suspended in 3:1 methanol/acetic acid to fix the cells, then washed several times in 3:1 methanol/acetic acid. Fixed cells were dropped on a glass slide and dried at room temperature for 15 min. Each sample was labeled with chromosome FISH probes (Chrombios) specific for a monosomic chromosome and a control chromosome as per manufacturer’s instructions. Briefly, chromosome spreads were incubated with probe mixture (1 μL of each probe, adjusted to 10 μL with HybMix buffer). After denaturation at 72°C for 6 min, slides were kept at 37°C in a humid chamber overnight. Slides were washed for 5 min in 2x saline sodium citrate (SSC) solution and then for 1 min in prewarmed 70°C 0.4X SSC, 0.1% Tween solution, and, finally, in 4x SSC, 0.1% Tween solution for 5 min at room temperature. Then slides were incubated for 30 min at 37°C with 100 μL fluorescein isothiocyanate (FITC) sheep anti-digoxigenin (Roche) solution (1:300 in 4X SSC/0.1% Tween) and washed twice in 45°C pre-warmed 4x SSC/0.1% Tween solution for 5–10 min. Finally, cells were stained with DAPI and microscopic analysis was carried out using 3i software and spinning disc confocal microscopy (see below). For each sample, at least 25 metaphases were captured and analyzed (Chunduri et al. 2021).
Analysis of PDX models
Data on patient-derived xenograft drug responses was acquired from Gao et al (Gao et al. 2015). The degree of chromosomal instability in each model was determined as described in Ben-David et al (Ben-David et al. 2017). Low CIN PDXs were defined as PDXs that displayed an average of less than one arm-length copy number change per passage. High CIN PDXs were defined as PDXs that displayed an average of greater than four arm-length copy number changes per passage. Therapeutic responses were determined according to RECIST criteria, as described in Gao (Gao et al. 2015). Progression-free survival times were defined as the time required for a treated tumor to double in volume.
Analysis of TCGA data
FGA data from The Cancer Genome Atlas were acquired from cBioportal (Gao et al. 2013). Clinical data from The Cancer Genome Atlas were acquired from (Liu et al. 2018). Within the clinical dataset provided with this paper, we analyzed the response to “treatment_outcome_first_course” and “tumor_status” that was recorded for each patient. For the analysis of tumor status, we included patients who were classified as either “WITH TUMOR” or “TUMOR FREE”. For the analysis of treatment responses, we included patients whose response was classified as “Complete Remission/Response”, “Partial Remission/Response”, “Stable Disease”, and “Progressive Disease”. Patients with missing or disputed data were excluded from this analysis. We included the 14 cancer types that had >100 patients with both copy number data and response data and at least one patient with progressive disease. We divided each of these cancer types into a low-FGA bin (≤25% FGA) and a high-FGA bin (≥75% FGA). We then compared the number of patients classified as “with tumor” at the end of the observation period and the number of patients with progressive disease following their first treatment using Fisher’s Exact Test.
Patient survival analysis was performed as described in (Smith and Sheltzer 2018). In short, github.com/joan-smith/biomarker-survival (version 0.23) was used to prep clinical data and perform univariate cox proportional hazards analysis. tcga_cdr_utilities.py was used to prep the clinical data and select the appropriate endpoints. Proportional hazards were performed on each cancer type independently, with the FGA data acquired from The Cancer Genome Atlas. Code for this analysis is available at github.com/joan-smith/survival-analysis-scripts/tree/master/fga.
Quantification and Statistical Analysis
GraphPad Prism was used for statistical analysis in Figure 5. Details on the statistical tests performed can be found in the figure legends and Method Details section of STAR Methods. Statistical comparisons in 5A, 5B, 5G, and 5H were performed using Fisher’s exact test. Statistical comparisons in 5C were performed using a two-sided t-test. Statistical comparisons between survival curves in 5D, 5E, and 5F were performed using a log-rank test. Experiments depicted in figures represent single replicates unless otherwise noted. Independent replicates are displayed in separate figures (e.g., Figure 1B and S2A-D).
Supplementary Material
Acknowledgments
Research in the Sheltzer Lab is supported by an NIH Early Independence award (1DP5OD021385), NIH grant R01CA237652-01, Department of Defense grant W81XWH-20-1-068, a Damon Runyon-Rachleff Innovation award, an American Cancer Society Research Scholar Grant, and a grant from the New York Community Trust. This work was also supported by grants to M. Wigler from the Simons Foundation, Life Sciences Founders Directed Giving-Research (award numbers 519054) and the Breast Cancer Research Foundation (award number 19-174), and by a grant to Z. Storchova from the German Research Foundation (award number STO918-5). We thank Uri Ben-David (Tel Aviv University) for assistance with PDX analysis. Graphical abstract and Figure 3A were created with BioRender.com.
This work was performed with assistance from CSHL Shared Resources, including the CSHL Flow Cytometry Shared Resource, which are supported by the Cancer Center Support Grant 5P30CA045508.
Footnotes
Declaration of Interests
J.C.S. is a co-founder of Meliora Therapeutics, a member of the advisory board of RTP Ventures, and an employee of Google, Inc. This work was performed outside of her affiliation with Google and used no proprietary knowledge or materials from Google. J.M.S. has received consulting fees from Ono Pharmaceuticals and Merck, is a member of the advisory board of Tyra Biosciences, and is a co-founder of Meliora Therapeutics.
References
- Andrews PA, Iossifov I, Kendall J, Marks S, Muthuswamy L, Wang Z, Levy D, and Wigler M (2016). MUMdex: MUM-based structural variation detection. BioRxiv 078261. [Google Scholar]
- Bakhoum SF, and Cantley LC (2018). The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 174, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, et al. (2018). Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslan T, Kendall J, Rodgers L, Cox H, Riggs M, Stepansky A, Troge J, Ravi K, Esposito D, Lakshmi B, et al. (2012). Genome wide copy number analysis of single cells. Nat Protoc. 7, 1024–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino TA (2015). Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol 12, 3–20. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R, et al. (2017). Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet 49, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Rychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Lander ES, et al. (2010). The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkbak NJ, Eklund AC, Li Q, Mcclelland SE, Endesfelder D, Tan P, Tan IB, Richardson AL, Szallasi Z, and Swanton C (2011). Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res 71, 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Marran K, Valentine A, and Hannon GJ (2013). Packaging shRNA retroviruses. Cold Spring Harb. Protoc 2013, 734–737. [DOI] [PubMed] [Google Scholar]
- Chen G, Bradford WD, Seidel CW, and Li R (2012). Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 482, 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Mulla WA, Kucharavy A, Tsai H-J, Rubinstein B, Conkright J, McCroskey S, Bradford WD, Weems L, Haug JS, et al. (2015). Targeting the adaptability of heterogeneous aneuploids. Cell 160, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunduri NK, Menges P, Gotsmann VL, Zhang X, Mardin BR, Buccitelli C, Korbel JO, Willmund F, Kschischo M, Raeschle M, et al. (2021). Systems approaches identify the consequences of monosomy in somatic human cells. BioRxiv 2021.02.22.432226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, Xu AW, Mengwasser KE, Sack LM, Yoon JC, Park PJ, and Elledge SJ (2013). Cumulative Haploinsufficiency and Triplosensitivity Drive Aneuploidy Patterns to Shape the Cancer Genome. Cell 155, 948–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E, van den Bosch T, Lenos KJ, El Makrini K, Nijman LE, van Essen HFB, Lansu N, Boekhout M, Hageman JH, Fitzgerald RC, et al. (2021). Chromosomal copy number heterogeneity predicts survival rates across cancers. Nat. Commun 12, 3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobles M, Liberal V, Scott ML, Benezra R, and Sorger PK (2000). Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645. [DOI] [PubMed] [Google Scholar]
- Donnelly N, Passerini V, Dürrbaum M, Stingele S, and Storchová Z (2014). HSF1 deficiency and impaired HSP90-dependent protein folding are hallmarks of aneuploid human cells. EMBO J. 33, 2374–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrbaum M, Kuznetsova AY, Passerini V, Stingele S, Stoehr G, and Storchová Z (2014). Unique features of the transcriptional response to model aneuploidy in human cells. BMC Genomics 15, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk LC, Zasadil LM, and Weaver BA (2016). Living in CIN: Mitotic Infidelity and Its Consequences for Tumor Promotion and Suppression. Dev. Cell 39, 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, et al. (2015). High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med 21, 1318–1325. [DOI] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. (2013). Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano CJ, Lin A, Girish V, and Sheltzer JM (2019). Generating Single Cell–Derived Knockout Clones in Mammalian Cells with CRISPR/Cas9. Curr. Protoc. Mol. Biol 128, e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, and Pellman D (2012). Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet 13, 189–203. [DOI] [PubMed] [Google Scholar]
- Holland AJ, and Cleveland DW (2012). Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 13, 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohan C, Schaeybroeck SV, Longley DB, and Johnston PG (2013). Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726. [DOI] [PubMed] [Google Scholar]
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, and Sarkar S (2014). Drug Resistance in Cancer: An Overview. Cancers 6, 1769–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito MR, Martis V, Hong C, Wardenaar R, Zerbib J, Spierings DCJ, Ben-David U, Foijer F, and Santaguida S (2021). Aneuploidy-driven genome instability triggers resistance to chemotherapy. This issue of Developmental Cell. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Gross CP, Park HS, and Yu JB (2017). Use of alternative medicine for cancer and its impact on survival. J. Clin. Oncol 35, e18175–e18175. [DOI] [PubMed] [Google Scholar]
- Kops GJPL, Foltz DR, and Cleveland DW (2004). Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl. Acad. Sci. U. S. A 101, 8699–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova AY, Seget K, Moeller GK, de Pagter MS, de Roos JADM, Dürrbaum M, Kuffer C, Müller S, Zaman GJR, Kloosterman WP, et al. (2015). Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells. Cell Cycle Georget. Tex 14, 2810–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LN, Zou L, Chagani S, Sekhar Pedamallu C, Liu M, Jiang S, Protopopov A, Zhang J, Getz G, and Chin L (2017). Modeling Genomic Instability and Selection Pressure in a Mouse Model of Melanoma. 19, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJX, Endesfelder D, Rowan AJ, Walther A, Birkbak NJ, Futreal A, Downward J, Szallasi Z, Tomlinson IPM, Kschischo M, et al. (2011). Chromosomal Instability Confers Intrinsic Multi-Drug Resistance Europe PMC Funders Group. Cancer Res 71, 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, et al. (2018). An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 173, 400–416.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, Farber JL, Force T, Koch WJ, and Knudsen ES (2012). CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle 11, 2747–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Burrell RA, Endesfelder D, Novelli MR, and Swanton C (2012). Cancer chromosomal instability: therapeutic and diagnostic challenges. EMBO Rep. 13, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L, Diaz-Rodriguez E, Narayan G, Hernando E, Murty VVVS, and Benezra R (2004). Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc. Natl. Acad. Sci. U. S. A 101, 4459–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi A, Ohori M, Iwai K, Nakayama Y, Nambu T, Morishita D, Kawamoto T, Miyamoto M, Hirayama T, Okaniwa M, et al. (2015). Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat. Commun 6, 7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oromendia AB, Dodgson SE, and Amon A (2012). Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 26, 2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka N, Rancati G, Zhu J, Bradford WD, Saraf A, Florens L, Sanderson BW, Hattem GL, and Li R (2010). Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 468, 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replogle JM, Zhou W, Amaro AE, McFarland JM, Villalobos-Ortiz M, Ryan J, Letai A, Yilmaz O, Sheltzer J, Lippard SJ, et al. (2020). Aneuploidy increases resistance to chemotherapeutics by antagonizing cell division. Proc. Natl. Acad. Sci 117, 30566–30576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, et al. (2014). Predictive Biomarkers and Personalized Medicine BRAF Inhibitor Resistance Mechanisms in Metastatic Melanoma: Spectrum and Clinical Impact. Clin Cancer Res 20, 1965–1977. [DOI] [PubMed] [Google Scholar]
- Rutledge SD, Douglas TA, Nicholson JM, Vila-Casadesús M, Kantzler CL, Wangsa D, Barroso-Vilares M, Kale SD, Logarinho E, and Cimini D (2016). Selective advantage of trisomic human cells cultured in non-standard conditions. Sci. Rep 6, 22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgueiro L, Buccitelli C, Rowald K, Somogyi K, Kandala S, Korbel JO, and Sotillo R (2020). Acquisition of chromosome instability is a mechanism to evade oncogene addiction. EMBO Mol. Med 12, e10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Vasile E, White E, and Amon A (2015). Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 29, 2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Richardson A, Iyer DR, M’Saad O, Zasadil L, Knouse KA, Wong YL, Rhind N, Desai A, and Amon A (2017). Chromosome Mis-segregation Generates Cell-Cycle-Arrested Cells with Complex Karyotypes that Are Eliminated by the Immune System. Dev. Cell 41, 638–651.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoft JD, Zhao JL, Jendrisak A, Carbone EA, Barnett ES, Hullings MA, Gill A, Sutton R, Lee J, Dago AE, et al. (2020). Morphology-Predicted Large-Scale Transition Number in Circulating Tumor Cells Identifies a Chromosomal Instability Biomarker Associated with Poor Outcome in Castration-Resistant Prostate Cancer. Cancer Res. 80, 4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schukken KM, and Sheltzer JM (2021). Extensive protein dosage compensation in aneuploid human cancers. BioRxiv 2021.06.18.449005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmecki A, Gerami-Nejad M, Paulson C, Forche A, and Berman J (2008). An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol 68, 624–641. [DOI] [PubMed] [Google Scholar]
- Selmecki AM, Dulmage K, Cowen LE, Anderson JB, and Berman J (2009). Acquisition of Aneuploidy Provides Increased Fitness during the Evolution of Antifungal Drug Resistance. PLOS Genet. 5, e1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, et al. (2014). Genome-Scale CRISPR-Cas9 Knockout Screening in Human Cells. Science. 343, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM (2013). A Transcriptional and Metabolic Signature of Primary Aneuploidy Is Present in Chromosomally Unstable Cancer Cells and Informs Clinical Prognosis. Cancer Res. 73, 6401–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, and Amon A (2011). The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet. TIG 27, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Torres EM, Dunham MJ, and Amon A (2012). Transcriptional consequences of aneuploidy. Proc. Natl. Acad. Sci 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Ko JH, Replogle JM, Habibe Burgos NC, Chung ES, Meehl CM, Sayles NM, Passerini V, Storchova Z, and Amon A (2017). Single-chromosome Gains Commonly Function as Tumor Suppressors. Cancer Cell 31, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Nguyen THM, Moka SB, Ellis JJ, Grady JP, Oey H, Cristino AS, Khanna KK, Kroese DP, Krause L, et al. (2020). Chromosome arm aneuploidies shape tumour evolution and drug response. Nat. Commun 11, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, and Sheltzer JM (2018). Systematic identification of mutations and copy number alterations associated with cancer patient prognosis. ELife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, and Sheltzer JM (2021). Genome-wide identification and analysis of prognostic features in human cancers. BioRxiv 2021.06.01.446243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingele S, Stoehr G, Peplowska K, Cox J, Mann M, and Storchova Z (2012). Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol 8, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RJ, and Flaherty KT (2013). Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 49, 1297–1304. [DOI] [PubMed] [Google Scholar]
- Swanton C, Nicke B, Schuett M, Eklund AC, Ng C, Li Q, Hardcastle T, Lee A, Roy R, East P, et al. (2009). Chromosomal instability determines taxane response. Proc. Natl. Acad. Sci 106, 8671–8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, and Compton DA (2008). Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol 180, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, and Swanton C (2016). Metastasis as an evolutionary process. Science 352, 169–175. [DOI] [PubMed] [Google Scholar]
- Vasudevan A, Baruah PS, Smith JC, Wang Z, Sayles NM, Andrews P, Kendall J, Leu J, Chunduri NK, Levy D, et al. (2020). Single-Chromosomal Gains Can Function as Metastasis Suppressors and Promoters in Colon Cancer. Dev. Cell 52, 413–428.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Schukken KM, Sausville EL, Girish V, Adebambo OA, and Sheltzer JM (2021). Aneuploidy as a promoter and suppressor of malignant growth. Nat. Rev. Cancer 21, 89–103. [DOI] [PubMed] [Google Scholar]
- Viganó C, von Schubert C, Ahrné E, Schmidt A, Lorber T, Bubendorf L, De Vetter JRF, Zaman GJR, Storchova Z, and Nigg EA (2018). Quantitative proteomic and phosphoproteomic comparison of human colon cancer DLD-1 cells differing in ploidy and chromosome stability. Mol. Biol. Cell 29, 1031–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang M, Liang D, Sun W, Zhang C, Jiang M, Liu J, Li J, Li C, Yang X, et al. (2019). Molecular design and anticancer activities of small-molecule monopolar spindle 1 inhibitors: A Medicinal chemistry perspective. Eur. J. Med. Chem 175, 247–268. [DOI] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, and Amon A (2008). Aneuploidy Affects Proliferation and Spontaneous Immortalization in Mammalian Cells. Science 322, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Verma A, Naveed U, Bakhoum S, Khosravi P, and Elemento O (2021). Deep Learning Predicts Chromosomal Instability from Histopathology Images. IScience 24, 102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Teoh F, Tan ASM, Cao Y, Pavelka N, Berman J, and Malik H (2019). Aneuploidy Enables Cross-Adaptation to Unrelated Drugs. Mol. Biol. Evol 36, 1768–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimonjic D, Brooks MW, Popescu N, Weinberg RA, and Hahn WC (2001). Derivation of Human Tumor Cells in Vitro without Widespread Genomic Instability. Cancer Res. 61, 8838–8844. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw short read sequencing data generated for this study have been deposited in the NCBI Short Read Archive and are available under BioProject accession number PRJNA725851.
Clinical data for TCGA patients were acquired from (Liu et al. 2018). FGA data for TCGA samples were acquired from cBioportal (Gao et al. 2013). Patient-derived xenograft drug response data were acquired from (Gao et al. 2015).
All original code is available at https://github.com/joan-smith and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper will be made available from the lead contact upon request.