Abstract
Background:
Thirty percent of women who seek professional breastfeeding support require assistance with ongoing breast and nipple pain and < 50% of women report resolution of their pain. It is unknown if there is a molecular risk for ongoing breast and nipple pain during breastfeeding.
Aim –
To evaluate associations among breast and nipple pain sensitivity and candidate pain sensitivity single-nucleotide polymorphisms [SNPs], (COMT rs6269, rs4633, rs4818, rs4680 and OXTR rs2254298, rs53576) in breastfeeding women.
Design -
A secondary analysis of a pilot randomized controlled trial of a pain self-management intervention conducted over 6 weeks postpartum. Setting and Participants - Sixty women were recruited from two hospital settings after birth.
Methods –
All participants underwent standardized mechanical somatosensory testing for an assessment of pain sensitivity and provided baseline buccal swabs for genetic analysis. At 1, 2, and 6 weeks postpartum, women self-reported breast and nipple pain severity using a visual analogue scale.
Results -
Women with the minor allele OXTR rs53576 reported 8.18-fold higher breast and nipple pain severity over time. For every 1-unit increase in Mechanical detection threshold and windup ratio, women reported 16.51-fold and 4.82-fold higher breast and nipple pain severity respectively. Six women with the OXTR rs2254298 minor allele reported allodynia.
Conclusion -
The presence of OXTR alleles in women with enhanced pain sensitivity suggests a phenotype of genetic risk for ongoing breast and nipple with potential for pain-associated breastfeeding cessation. Somatosensory testing identified women who reported higher breast and nipple pain during the first weeks of breastfeeding.
Background
Most women report breast and nipple discomfort in the first weeks of initiating breastfeeding (Declercq et al., 2013; Lucas & McGrath, 2016). However, 30% of new mothers will cease breastfeeding in the first 3 weeks due to intolerable pain (Odom et al., 2013). Among women who seek professional lactation support during the first 30 days of breastfeeding, 30% of women require assistance with ongoing breast and nipple pain, and ≤50% report resolution of their pain (Kent et al., 2015). Intolerable or ongoing breast and nipple pain during breastfeeding is thus a clinically significant issue and a major barrier for continued breastfeeding. It is unknown if there is a molecular risk for intolerable or ongoing breast and nipple pain during breastfeeding that may influence the likelihood of breastfeeding cessation.
Breastfeeding is the most effective preventative measure for protecting infant health, supports mother and infant emotional communication, and provides health promotion and protection to women and infants across the lifespan (Quigley et al., 2016; Victora et al., 2016). In 2018, 83.2% of women initiated breastfeeding, but within 2–3 weeks after delivery the rate of exclusive breastfeeding dropped to 60%, and by 2 months it dropped to 53% as women returned to work (Center for Disease Control and Prevention, 2018). However, in order to have adequate milk supply for mothers and infants to breastfeed to 6 months, the public health goal for optimal health benefit for women and infants, women need to routinely breastfeed or pump for at least 9 weeks (Dozier et al., 2018). Although the reasons for early breastfeeding cessation can be complex, including psychological and social determinants of health, women most often report ceasing breastfeeding due to the lack of infant satiation, perception of maternal milk insufficiency, and intolerable or ongoing breast and nipple pain (Center for Disease Control and Prevention, 2018).
Pain during breastfeeding arises from complex stimulation of the glandular, somatic, and visceral tissues. Sensations are transmitted via nociceptors pathways that can become sensitized, leading to altered sensation such as allodynia or spontaneous pain (Eriksson et al., 1996; Jackson et al., 2019). Biologic factors (infection) and anatomic factors (positioning challenges, infant anatomic issues such as ankyloglossia or torticollis, abnormal nipple shape or size) are among the triggers for sensitization of nociceptor pathways and ongoing pain (Berens et al., 2016; Lucas & McGrath, 2016). In addition, breastfeeding occurs during a period of intense emotional and neurohormonal changes known to impact an individuals’ pain sensitivity (Amir, Jones, & Buck, 2015; Office of the Assistant Secretary for Health, 2019; Schug et al., 2019). Emotional reactions to pain can suppress or amplify the perception of painful stimuli (Edwards et al., 2009; Russell et al., 2018). All of these factors together may contribute to ongoing pain that interferes with milk ejection and nipple expansion, and creates a negative neurohormonal feedback loop leading to maternal milk insufficiency and a lack of infant satiation (Eriksson et al., 1996; Francis & Dickton, 2019; Newton & Newton, 1948). However, less studied has been the role of individual variation in pain sensitivity and its influence on breastfeeding pain and duration.
Experimental pain methods using quantitative sensory testing (QST) can be used to precisely measure somatosensory function and pain sensitivity (Backonja et al., 2013; Rolke et al., 2006). Using QST, various types of noxious stimuli can be applied, including mechanical, thermal, and electrical stimuli, to determine the individual’s pain tolerance, thresholds, and other indicators of somatosensory function. Muddana et al. (2018) recently described cases of persistent breast and nipple pain without resolution using an attenuated QST assessment to identify differences in somatosensory functioning. The findings suggested a potential role for pain sensitization in the development of breast and nipple pain, as has been identified in various other experimental and clinical pain outcomes (Diatchenko et al., 2005; Fillingim et al., 2005; Muddana et al., 2018). While the emergence of painful conditions may be affected by development and environmental experiences, genetic factors contribute to individual differences in pain susceptibility across the lifespan. The present study was designed to assess differences in somatosensory functioning (using QST) as well as determine the impact of pain-relevant genetic polymorphisms that may contribute to intolerable or ongoing breast and nipple pain susceptibility in breastfeeding women.
As this is the first study to explore the genetic factors contributing to ongoing breast and nipple pain, we chose to investigate relatively common pain-relevant genetic variants within catechol-o-methyltransferase (COMT) and the oxytocin receptor gene (OXTR) known to play a pivotal role in the neurohormonal cascade for breastfeeding. COMT and OXTR both have single-nucleotide polymorphisms (SNPs) with established associations with a variety of health behaviors (Baribeau et al., 2017; Diatchenko et al., 2005; Hu et al., 2018; Slane et al., 2014). The COMT SNPs rs4680 A>G (Val158Met), rs6269: A>G (promoter region), rs4633: C>T (His62His) and rs4818: C>G (Leu136Leu) have well-established associations with experimental pain sensitivity in both the normal healthy population and clinical populations with inflammatory, neuropathic, or postsurgical pain (Diatchenko et al., 2005; Hu et al., 2018). Given its role in catecholamine metabolism, it is perhaps unsurprising that COMT genotype is also associated with risk for depression, anxiety, and stress reactivity (Antypa, Drago, & Serretti, 2013; Young et al., 2017). Relevant to the current study, the COMT genotype has also been implicated as a genetic determinant of the length of the first stage of labor (Terkawi et al., 2012). Three haplotypes for COMT, composed of the combined genotypes for these four SNPs (rs4680 (A>G), rs6269 (A>G), rs4633 (C>T), and rs4818 (C>G) and strongly associated with pain sensitivity, have been identified as follows: low pain sensitivity (LPS) for G_C_G_G, average pain sensitivity (APS) for A_T_C_A, and high pain sensitivity (HPS) for A_C_C_G (Diatchenko et al., 2005). Oxytocin (OXT) has the potential to modulate pain through central and peripheral psychological and physiological mechanisms (Tracy, Georgiou-Karistianis, Gibson, & Giummarra, 2015; Xin, Bai, & Liu, 2017). The OXTR gene, encoding the receptor for OXT, contains two SNPs, rs2254298 (G>A) and rs53576 (G>A), known to affect parents’ sensitivity to their infants’ behavioral cues, and is associated with depressive symptoms across the lifespan (Bakermans-Kranenburg & van Ijzendoorn, 2008; Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011). Both OXTR rs53576 and COMT rs4633 SNPs contribute to increased length of early labor (Terkawi et al., 2012). In two large samples of early postpartum mothers, the OXT and OXTR polymorphisms were not associated with breastfeeding duration (Colodro-Conde et al., 2018), although a relationship with breast and nipple pain during breastfeeding was not explicitly assessed.
Clinically, the evaluation of breastfeeding pain is almost exclusively dependent on self-report measures by breastfeeding mothers. The most common evaluation used in breastfeeding studies to effectively capture changes in breast and nipple pain across time is the visual analogue scale (VAS; Coca et al., 2018; McClellan et al., 2012). While the VAS is a validated global measure of pain severity and intensity, it may not capture modality-specific differences in somatosensory aberrations that may contribute to pain sensitivity (i.e., pressure, mechanical, etc.) and may not accurately reflect the impact of pain (i.e., pain burden or pain interference) on function. A well-established objective measurement to detect somatosensory aberrations that may contribute to acute and chronic pain susceptibility is QST. QST has been used in chronic pelvic and low back pain conditions experienced in women (As-Sanie et al., 2013; Starkweather et al., 2016). In lactation, only one study attempted mechanical sensitivity testing using a tooth-pick on the breast (Muddana et al., 2018). However, no study has used a standardized mechanical QST protocol in women initiating breastfeeding to comprehensively evaluate breastfeeding pain.
Research Question
The aim of this secondary analysis was to examine associations among self-reported breast and nipple pain, experimental pain sensitivity using QST, and candidate pain sensitivity single-nucleotide polymorphisms [SNPs], (COMT rs6269, rs4633, rs4818, rs4680 and OXTR rs2254298, rs53576) in breastfeeding women.
Methods
Study Design
This study was a secondary analysis of selected pain-related data from a pilot randomized control trial that tested the feasibility and acceptability of a breastfeeding self-management (BSM) intervention among breastfeeding women (Lucas et al., 2019). The study was approved by the University Institutional Review Board in 2017, and registered with Clinical Trials.gov (NCT03392675). Primary results of the study were used to determine effect sizes for future studies, and have been reported elsewhere (Lucas et al., 2019).
Setting
Women were recruited after delivery from one research-intensive tertiary medical center and one teaching community hospital in the northeast region of the United States.
Sample
A convenience sample of 80 participants were approached, and a total of 65 women were enrolled. Five women withdrew before data collection for a total of 60 women who completed data collection at entry, 1, 2, and 6 weeks. At 6 weeks, 56 women (26 BSM intervention, 30 control) were breastfeeding, and their complete pain data set was used for this analysis. The pilot RCT was a feasibility study with a sample size goal of 60 mothers, which was large enough to report significant differences in average breastfeeding pain severity scores between the BSM and control groups (Erdfelder, Faul, & Buchner, 1996).
To be included, women had to 1) be 18–45 years of age; 2) be English proficient; 3) have delivered a full-term infant (38–42 weeks gestation); 4) have planned to breastfeed, and 5) have daily access to a smartphone or computer. Women were excluded if they had a history of potential changes in pain sensorium, a significant mental health disorder (i.e., schizophrenia, bipolar disorder), or health conditions not associated with pregnancy (i.e., sickle cell anemia, HIV, diabetes, history of seizures), or if they had delivered an infant with medical complications, congenital anomalies, or ankyloglossia. In addition, participants were monitored throughout the study for infection, positioning challenges, infant ankyloglossia or torticollis, and abnormal nipple shape or size, and were withdrawn if they developed any of these conditions.
Breastfeeding Self-Management Intervention
Group assignment of women was based on a randomization schedule created by the study statistician. The BSM intervention was delivered via cloud-based educational modules, biweekly nurse-lead texting, and weekly follow-up to women in their homes at 1, 2, and 6 weeks after discharge. A full description of the BSM intervention has been provided in a previous publication (Lucas et al., 2019). Members of the study team were blinded to group assignment.
Measurements
Participants completed a demographic questionnaire, rated their breast and nipple pain intensity (0–100) using a visual analogue scale (Breivik et al., 2008), underwent QST using mechanical stimuli (cutaneous, vibration, pressure), and provided a buccal swab for genetic analysis. Both the BSM intervention and the control group received a text/email at 1, 2, and 6 weeks with a link to complete assessments for maternal report of breast and nipple pain severity.
Data Collection
The QST testing was conducted in each participant’s hospital room before discharge. QST testing assessed peripheral and central pain sensitivity using a range of mechanical stimuli to measure Mechanical detection threshold, pain threshold, pain sensitivity, Windup ratio (repeated mechanical pressure which captures temporal summation of pain), Vibration detection threshold, and Pain pressure threshold. The QST protocol and equipment followed standards of the German Neuropathic Pain Network (Rolke et al., 2006) with the exclusion of thermal testing to decrease participant burden.
Within 48 hours after delivery and before discharge, women were tested on the nondominant arm for cutaneous, vibration, and pressure sensitivity and pressure pain thresholds. Women were asked to rate the severity of their experimental pain using a numerical rating scale (0 indicating no pain to 10 indicating “most intense pain imaginable”). Cutaneous mechanical pain threshold and the Windup ratio were assessed using von Frey fibers (Opti-hair2-Set, MarstockNervtest, Germany), which can exert a force between 0.25 and 512 mN upon bending. Dynamic mechanical allodynia was tested using a light brush stroke over 1 cm in length. The Vibration detection threshold used a Rydel-Seiffer tuning fork (64 Hz, 8/8 scale), and participants were asked to indicate when they no longer felt vibration. Lastly, pressure pain thresholds were assessed by applying pressure manually at a range between 50 and 600 kPa using a Medoc algometer (Medoc Algomed, 2020). Participants held a button, which they pushed when they felt pain. The procedure took 10 minutes to complete.
Candidate pain sensitivity SNPs genotyping was conducted using buccal cell samples collected at baseline before discharge. Participants were instructed to rinse their mouth twice with water and then roll the sterile buccal brush firmly on the inside of the cheek. Samples were immediately transported to Dr. Young’s biobehavioral lab for processing and storage in a −80°C freezer for batch analyses. Genomic DNA was extracted from buccal cells using Gentra® Puregene® Buccal Cell Kit according to the manufacturer’s instructions (Qiagen, #158845). SNP genotyping was completed for seven SNPs: four SNPs within COMT and three within OTXR (Table 2) using Taqman SNP genotyping assays (VIC/FAM) and allelic discrimination analysis according to the manufacturer’s directions using an Applied Biosystems StepOne Plus PCR machine and ABI allelic discrimination software (ThermoFisher Scientific, Waltham, MA). Dr. Young verified a 95% ≤ call rate for all SNPs included using 10uL samples and manufacturer’s running parameters.
Table 2.
Estimated Coefficients and LRT Result of Each SNP
| SNPs (Minor Allele) | Estimate | Standard Error | CI | p Value |
|---|---|---|---|---|
| rs6269(G) | 0.73 | 3.62 | [−5.88 to 7.35] | .825 |
| rs4633(T) | 0.62 | 3.32 | [−5.43 to 6.67] | .839 |
| rs4818(G) | 1.97 | 3.68 | [−4.75 to 8.68] | .560 |
| rs4680(G) | −0.83 | 3.20 | [−6.67 to 5.01] | .777 |
| rs2254298(A) | 2.85 | 5.21 | [−6.67 to 12.38] | .551 |
| rs53576(A) | 8.18 | 4.19 | [0.55–15.82] | .036 |
Data Analysis
Descriptive statistics were generated with mean and standard deviation for continuous variables, and frequency and proportion for discrete variables. Demographic characteristics, seven QST measurements, and six SNPs for the 56 participants were included in the descriptive statistics table.
To evaluate the association between the SNP genotypes and pain sensitivity, we performed the Kruskal-Wallis one-way analysis of variance on ranks to confirm if the distribution of a continuous QST was consistent in each genotype of a SNP. For the discrete QST measurement dynamic mechanical allodynia, we used the Fisher exact test instead.
Breast and nipple pain severity was the main outcome measurement considered in this secondary-analysis study. To visualize the effect of each SNP on breast and nipple pain severity, we generated graphs with nonparametric smoothing curves for pain severity over time by groups of alleles. Lucas et al. (2019) used a linear mixed model (LMM) to evaluate the effect of the BSM intervention on reducing breastfeeding pain over 6 weeks. The model included pain severity at weeks 1, 2, and 6 as the outcome, and baseline pain severity, week, BSM intervention group, and time by group interaction as covariates (Lucas et al., 2019). In this secondary analysis, a similar LMM model with adjusted demographic variables was considered. We put each QST and SNP into the model alternatively, and conducted the likelihood-ratio test (LRT) to verify the significant effect of each QST and SNP on reducing breastfeeding and nipple pain over time. We coded the number of minor alleles (0–2) as the covariate of SNP in order to minimize the degrees of freedom due to the small sample size. All the analyses were performed with the statistical software R 3.6.2.
Results
Demographics and Allelelic Frequency
There was no significant difference in demographic characteristics (age, race, or ethnicity, delivery or parity) between the two intervention groups (see Table 1). The mean age of the participants was 30.38 years old (SD = 4.86 years). Participants were mainly non-Hispanic Caucasian (76.8%) or Latino (76.8%), and 80.4% had a vaginal delivery. Fewer than half (46.4%) were breastfeeding-naive. Means and standard deviations of QST measures (see Table 1) were reported, except for dynamic mechanical allodynia; the majority of patients scored a 0 (83.9%), so we treated it as a categorical variable (=0 or 0<). Allelic frequencies and relative proportions for all SNPs are reported in Table 1. Sample OXTR rs2254298 and rs53576 and COMT rs6269 and rs4818 minor allele frequency were similar to global frequency, but COMT rs4633 (sample 0.51, global 0.37), and rs4680 (sample 0.49, global 0.37) minor allele frequencies were higher than global values.
Table 1.
Demographic Characteristics
| Characteristic | Sample (N = 56) | |
|---|---|---|
| Mean | SD | |
| Age | 30.38 | 4.86 |
| QST | ||
| Mechanical detection threshold | 3.43 | 0.30 |
| Mechanical pain threshold | 6.41 | 0.34 |
| Mechanical pain sensitivity | 1.26 | 1.28 |
| Windup ratio | 1.08 | 1.13 |
| Vibration detection threshold | 9.05 | 5.00 |
| Pain pressure threshold | 258.6 | 91.73 |
| n | % | |
| Group (BSM intervention group) | 26 | 46.4 |
| Race | ||
| White | 43 | 76.8 |
| Asian | 3 | 5.4 |
| Black or African American | 6 | 10.7 |
| Not reported | 4 | 7.1 |
| Ethnicity | ||
| Hispanic or Latino | 7 | 12.5 |
| Not Hispanic or Latino | 43 | 76.8 |
| Unknown or not reported | 6 | 10.7 |
| No. of BF (1 = Yes) | 26 | 46.4 |
| Delivery (Vaginal) | 45 | 80.4 |
| QST dynamic mechanical allodynia (0 = Yes) | 47 | 83.9 |
| SNP (alleles, minor allele frequency) | ||
| rs6269 (A > G, 0.35) | ||
| A/A | 26 | 46.4 |
| G/A | 21 | 37.5 |
| G/G | 9 | 16.1 |
| rs4633 (C > T, 0.51) | ||
| C/C | 16 | 28.6 |
| C/T | 22 | 39.3 |
| T/T | 17 | 30.4 |
| Missing | 1 | 1.8 |
| rs4818 (C > G, 0.34) | ||
| C/C | 27 | 48.2 |
| G/C | 20 | 35.7 |
| G/G | 9 | 16.1 |
| rs4680 (A > G, 0.49) | ||
| A/A | 18 | 32.1 |
| A/G | 20 | 35.7 |
| G/G | 17 | 30.4 |
| Missing | 1 | 1.8 |
| rs2254298 (G > A, 0.20) | ||
| G/G | 35 | 62.5 |
| G/A | 20 | 35.7 |
| A/A | 1 | 1.8 |
| rs53576 (G > A, 0.28) | ||
| G/G | 27 | 48.2 |
| G/A | 25 | 44.6 |
| A/A | 3 | 5.4 |
| Missing | 1 | 1.8 |
BSM = breastfeeding self-management; SD = standard deviation.
Pain Severity Over Time vs. SNPs
Pain severity was plotted over time for each SNP genotype using the local polynomial regression fitting method provided by the “geom_smooth” function in the “ggplot2” package (Figure 1). Only rs53576 genotype was significantly associated with pain severity reports over time. Participants who were heterozygotes (A/G; green curve) and homozygous (G/G; blue curve) reported similar baseline pain severity, but the G/G group reported significantly lower pain severity compared to the A/G group at weeks 1, 2, and 6.
Figure 1.
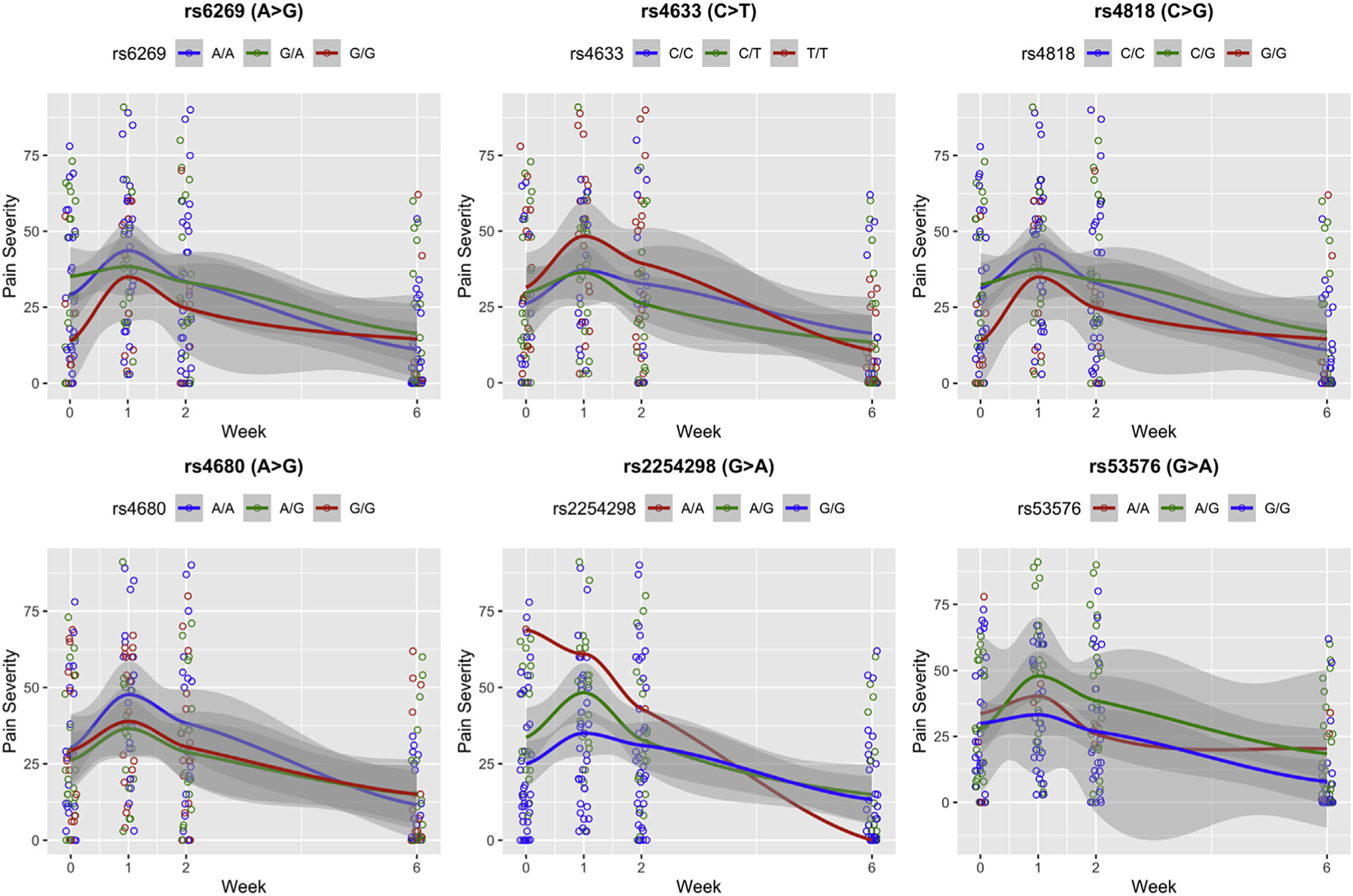
The red curve in each plot represents the trend of mean pain severity for the homozygous minor (rs6269, rs4818, rs4680, GG; rs4633, TT; rs2254298, rs53576, AA); the green curve represents the heterozygous minor alleles (rs6269, G/A; rs4818 C/G; rs4680 A/G; rs4633, C/T; rs2254298, rs53576, A/G); the blue curve represents the homozygous major alleles (rs6269, rs4680, A/A; rs4818, rs4633, C/C; rs2254298, rs53576, A/A). The grey shade represents the confidence interval of the trend. Note that there was only one A/A of rs2254298 in the sample, so the red curve in the rs2254298 graph represents the pain severity trend for that single individual.
Table 2 presents the findings of the LRT result for each SNP, respectively. Only the minor allele of rs53576 had a significant effect on reducing breast and nipple pain severity over time (p = .036). The estimated coefficient of the number of minor allele of rs53576 is 8.18, which can be interpreted to mean that, on average, the women experienced an 8.18 increase in breastfeeding and nipple pain scores over time, with the addition of one minor allele A of rs53576. We also checked the time by number of allele interaction effect of rs53576, but it was not significant. No significant effect was found for the other SNPs.
Pain Severity and Somatosensory Function (QST)
Table 3 presents the findings of LRT for each QST variable, respectively. Significant effects were found for two QST standardized tests, Mechanical detection threshold (sensation; p = .036) and Windup ratio (repeated mechanical stimulation; p = .033). The estimated coefficients of the Mechanical detection threshold and the Windup ratio were 16.51 and 4.82, respectively, indicating that participants experienced 16.51-fold higher breast and nipple pain scores over time if they were 1 unit more sensitive in the measurement of Mechanical detection threshold. Women also experienced 4.82-fold higher breast and nipple pain severity scores over time if they were 1 unit more pain-sensitive in the measurement of the Windup ratio. There was no significant interaction effect with time for these two QSTs. No significant effect was found for the other QST tests.
Table 3.
Estimated Coefficients, and LRT Result of Each QST
| QST | Estimate | Standard Error | CI | p Value |
|---|---|---|---|---|
| Mechanical detection threshold | 16.51 | 8.42 | [1.14–31.89] | .036 |
| Mechanical pain threshold | 15.01 | 8.70 | [−0.89 to 30.92] | .064 |
| Mechanical pain sensitivity | −0.62 | 2.17 | [−4.58 to 3.34] | .755 |
| Windup ratio | 4.82 | 2.41 | [0.42–9.22] | .033 |
| Vibration detection threshold | −0.61 | 0.54 | [−1.59 to 0.37] | .219 |
| Pain pressure threshold | −0.02 | 0.03 | [−0.07 to 0.04] | .502 |
| Dynamic mechanical allodynia | −0.58 | 7.56 | [−14.39 to 13.24] | .934 |
SNP Associations With Somatosensory Function (QST)
Owing to the nonparametric distribution of QST measurements, Kruskal-Wallis one-way analysis of variance was performed for the association between QSTs and SNPs. For the discrete QST measurement dynamic mechanical allodynia, we performed Fisher exact test to check its association with each SNP. Only dynamic mechanical allodynia and rs2254298 had a significant association (p = .03; Table 4). Thirty-two women with G/G and 15 women with A/G had a dynamic mechanical allodynia value of 0; three women with G/G, five women with A/G, and one woman with A/A had dynamic mechanical allodynia values greater than 0. This shows that the number of minor alleles of rs2254298 increased the likelihood of a nonzero dynamic mechanical allodynia value. No other significant associations were found between QST measures and SNP genotypes.
Table 4.
p Values of Hypothesis Testing QST vs. SNP
| rs6269 | rs4633 | rs4818 | rs4680 | rs2254298 | rs53576 | |
|---|---|---|---|---|---|---|
| Mechanical detection threshold | .181 | .179 | .257 | .221 | .327 | .309 |
| Mechanical pain threshold | .343 | .205 | .567 | .056 | .272 | .300 |
| Mechanical pain sensitivity | .557 | .858 | .492 | .943 | .171 | .182 |
| Windup ratio | .557 | .482 | .715 | .650 | .929 | .127 |
| Vibration detection threshold | .073 | .321 | .087 | .387 | .576 | .745 |
| Pain pressure threshold | .164 | .333 | .339 | .218 | .704 | .240 |
| Dynamic mechanical allodynia | .404 | .375 | .491 | .226 | .030 | .1211 |
The Kruskal Wallis test is applied to the first 6 QSTs; The Fisher exact test is applied to the dynamic mechanical allodynia.
Discussion
The present study explored associations among self-reported breast and nipple pain, somatosensory function (QST), and pain sensitivity polymorphisms in women who were initiating breastfeeding. In our pilot sample, only women with the minor allele OXTR rs53576 reported significantly higher breast and nipple pain severity over time, with 8.18-fold higher pain scores compared with women with the major allele. In addition, the QST standardized test, Mechanical detection threshold, and Windup ratio (repeated mechanical stimulation) were predictive of greater self-report pain severity. For every 1-unit increase in sensitivity to the Mechanical detection threshold, women experienced 16.51 more breast and nipple pain, and for the Windup ratio, women reported 4.82 higher breast and nipple scores. Lastly, women who had one or more minor alleles at OXTR rs2254298 were more likely to experience hypersensitivity to brush stimulation in the dynamic mechanical allodynia test.
OXT is a significant neurohormone throughout the perinatal period and breastfeeding initiation (Augustine et al., 2018). Oxytocin signaling through OXTR genotype in the peripheral and central nervous system plays a critical role in sensory processing and pain perception (Xin et al., 2017). Although no prior studies have directly linked OXT or OXTR genotype to breastfeeding outcomes, our study suggests that OXTR genotype has a moderating effect on breast and nipple pain, and thereby may contribute to breastfeeding success and duration. Women carrying one or more of the minor alleles at OXTR rs53576 had a significantly higher pain score and reported earlier sensitivity to mechanical pressure and repeated sensations for the Windup ratio test. For women with OXTR rs53576 minor alleles, the increased pain scores at 1 and 2 weeks after delivery may be a response to the 8 to 12 times a day breastfeeding sessions, in which women potentially experience repeated pressure and somatosensory painful simulation until the lactation tissue becomes desensitized or infant positioning has been corrected (Lucas & McGrath, 2016). Women with OXTR rs2254298 regardless of heterozygous or homozygous major and minor alleles were more likely to experience allodynia.
In our study, we anticipated that differences in self-report pain scores would be associated with COMT gene minor alleles. Although the COMT rs4633 and rs4680 alleles were higher in our sample than global frequency, there was no significant relationship between women’s pain severity scores and the COMT gene. Future studies will need to extend the present findings to explore the impact of genotype on breast and nipple pain in breastfeeding beyond the acute pain of breastfeeding compared to ongoing chronic pain at 3 and 6 months.
Clinical Implications
In the present study, QST provided a simple and noninvasive technique by which to identify women at risk of high levels of pain during breastfeeding. This may serve as a method for nurses to identify, counsel, and support women at risk as they begin breastfeeding, with the goal of promoting exclusive breastfeeding throughout the 6-month period.
Women can experience ongoing pain during breastfeeding owing to mechanical pain of a poor infant latch or positioning, or shearing pain from the wrong size of flange of their pump (Lucas & McGrath, 2016). In alignment with these prior findings, infant-related issues were indicated as the reason for discontinuation of breastfeeding in all four women who stopped breastfeeding during the study: three women from the control group owing to infant ankyloglossia (tongue-tie) and one woman from the intervention group owing to poor infant latch. All four women reported ongoing elevated pain scores and transitioned to pumping breast milk.
Women reporting breast and nipple pain should first be assessed for other potential pain triggers including infection (e.g., Candida, bacterial mastitis) or underlying medical conditions, (e.g., psoriasis or Reynaud’s syndrome; Berens et al., 2016; Lucas & McGrath, 2016). If treatment of these conditions does not resolve pain, clinicians should consider the presence of allodynia or other neuropathic pain syndrome and prescribe medications recommended by American Breastfeeding Medicine protocol (Berens et al., 2016). In addition, as emotional regulation of anxiety and depression contribute to women’s pain sensorium, clinicians should consider referring women for counseling or peer support groups, encouraging nonpharmacological interventions such as creative imagery and massage therapy, or pharmacological intervention in the form of a breastfeeding-safe antidepressant (Sriraman et al., 2015; Subnis et al., 2016). Even with clinical interventions to manage ongoing pain, many women cease breastfeeding due to returning to work or a lack of social support (Center for Disease Control, 2018). These environmental and social factors may differentially affect those with increased pain susceptibility, further adding to the conflict women experience regarding ceasing breastfeeding and their role as a mother providing optimal nutrition to their infant (Jackson et al., 2019).
The U.S. Preventative Task Force recognizes that women experience pain of greater severity and frequency than men (U.S. Preventive Task Force, 2016). Because breastfeeding is considered a “normal” behavior, breastfeeding pain as a “pain” condition has not been explored. If a woman has increased susceptibility to breast and nipple pain as a result of her genotype and she experiences ongoing pain, this could lower her mechanical pain threshold and put her at risk for chronic pain in the future. In addition, for women who present with a nonbreastfeeding but chronic pain condition, a breastfeeding history with targeted questions about breastfeeding being an ongoing pain experience should be explored in the individual’s pain history.
Limitations
This is a secondary analysis of a pilot study evaluating pain related to genetic risk. As a small study, this has a risk to produce false positive and negative results; a larger study is needed to validate the results. In addition, we used the visual analogue scale (0–100), which is reliable to measure acute pain before and after an intervention but is not as sensitive to small differences in pain scores (Breivik et al., 2008). Owing to the small sample size, group was not factored into the results; the report of pain might be higher without the moderation of the breastfeeding pain intervention.
A confounding factor of women’s self-report of pain is the standard of care during delivery: Many women receive synthetic oxytocin during and after delivery. Women receiving synthetic oxytocin during labor experience a downstream effect of decreased endogenous oxytocin, affecting breast milk supply for several weeks (Cadwell & Brimdyr, 2017). We hypothesize that some women may present with allodynia as a result of labor-related exogenous oxytocin owing to the blockage of OXTR receptor sites and barriers to endogenous oxytocin. In our pilot study, 75% of women received oxytocin augmentation. Our data suggest that their threshold of mechanical sensitivity, pain, and allodynia could be affected by the action of exogenous oxytocin. We also hypothesize that women with minor OXTR rs53576 and rs2254298 alleles lack the moderating effect of oxytocin, and with the frequent number of feedings, have greater pain. In future studies, we will collect data on the total amount of exogenous oxytocin mothers receive during labor to see if there is an effect on the endogenous expression of oxytocin related to OXTR alleles.
Conclusion
This study on women with ongoing breast and nipple pain after breastfeeding initiation suggests a phenotype of genetic risk for breastfeeding pain to identify women at high risk of pain-associated breastfeeding cessation. In addition, the novel application of somatosensory testing using QST for sensitivity and pain sensation thresholds in the postpartum period identified women who reported higher breast and nipple pain during the first 6 weeks after delivery. Together these findings provide an objective assessment of women at risk for pain during the postpartum period.
Acknowledgments
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health (NIH-NINR), grant number NIH-NINR P20NR016605. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Deborah McDonald, R.N., Ph.D., for her support and advice.
Footnotes
Conflict of Interest
All authors have no financial relationships relevant to this article to disclose or any conflict of interest relevant to this manuscript.
References
- Amir LH, Jones LE, & Buck ML (2015). Nipple pain associated with breastfeeding: Incorporating current neurophysiology into clinical reasoning. Australian Family Physician, 44(3), 127–132. [PubMed] [Google Scholar]
- Antypa N, Drago A, & Serretti A (2013). The role of COMT gene variants in depression: Bridging neuropsychological, behavioral and clinical phenotypes. Neuroscience and Biobehavioral Reviews, 37(8), 1597–1610. [DOI] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, & Clauw DJ (2013). Increased pressure pain sensitivity in women with chronic pelvic pain. Obstetrics and Gynecology, 122(5), 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RA, Seymour AJ, Campbell RE, Grattan DR, & Brown CH (2018). Integrative neurohumoural regulation of oxytocin neurone activity in pregnancy and lactation. Journal of Neuroendocrinology, 30(8), 1–15. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, & Ziegler D (2013). Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain, 154(9), 1807–1819. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & van Ijzendoorn MH (2008). Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience, 3(2), 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Scherer SW, Schachar RJ, Arnold PD, Szatmari P, Nicolson R, Georgiades S, Crosbie J, Brian J, Iaboni A, Lerch J, & Anagnostou E (2017). Oxytocin receptor polymorphisms are differentially associated with social abilities across neurodevelopmental disorders. Scientific Reports, 7(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Eglash A, Malloy M, & Steube AM (2016). ABM clinical protocol #26: Persistent pain with breastfeeding. Breastfeeding Medicine, 11(2), 46–53. [DOI] [PubMed] [Google Scholar]
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Breivik Hals EK, Kvarstein G, & Stubhaug A (2008). Assessment of pain. British Journal of Anaesthesia, 101(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Cadwell K, & Brimdyr K (2017). Intrapartum administration of synthetic oxytocin and downstream effects on breastfeeding: Elucidating physiologic pathways. Annals of Nursing Research and Practice, 2(3). [Google Scholar]
- Center for Disease Control. (2018). Breastfeeding Report Card. Retrieved from May 29, 2018 https://www.cdc.gov/breastfeeding/data/reportcard.htm.
- Center for Disease Control and Prevention. (2018). Breastfeeding among U.S. children born 2009–2015, CDC National Immunization Survey. [Google Scholar]
- Coca KP, Amir LH, dos Remédiosda Silva Alves M, Barbieri M, Marcacine KO, & de Vilhena Abrão ACF (2018). Measurement tools and intensity of nipple pain among women with or without damaged nipples: A quantitative systematic review. Journal of Advanced Nursing, 75(6). [DOI] [PubMed] [Google Scholar]
- Colodro-Conde L, Sánchez-Romera JF, Lind PA, Zhu G, Martin NG, Medland SE, & Ordoñana JR (2018). No evidence of association of oxytocin polymorphisms with breastfeeding in 2 independent samples. Genes, Brain and Behavior, 17(7), e12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq ER, Sakala C, Corry MP, Applebaum S, & Herrlich A (2013). Listening to mothers III: Pregnancy and birth. Childbirth Connection, 1–43. [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, & Maixner W (2005). Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics, 14(1), 135–143. [DOI] [PubMed] [Google Scholar]
- Dozier AM, Brownell EA, Thevenet-Morrison K, Martin H, Hagadorn JI, & Howard C (2018). Predicting maintenance of any breastfeeding from exclusive breastfeeding duration: A replication study. Journal of Pediatrics, 203, 197–203.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Campbell C, Jamison RN, & Wiech K (2009). The neurobiological underpinnings of coping with pain. Current Directions in Psychological Science, 18(4), 237–241. [Google Scholar]
- Erdfelder E, Faul F, & Buchner A (1996). GPOWER: A General Power Analysis Program. Behavior Researh Methods, Instruments, & Computers, 28, 1–11. [Google Scholar]
- Eriksson M, Lindh B, Uvnäs-Moberg K, & Hökfelt T (1996). Distribution and origin of peptide-containing nerve fibres in the rat and human mammary gland. Neuroscience, 70(1), 227–245. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, & Wallace MR (2005). The A118G single nucleotide polymorphism of the μ-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. Journal of Pain, 6(3), 159–167. [DOI] [PubMed] [Google Scholar]
- Francis J, & Dickton D (2019). Physical analysis of the breast after direct breastfeeding compared with hand or pump expression: A randomized clinical trial. Breastfeeding Medicine, 14(10), 705–711. [DOI] [PubMed] [Google Scholar]
- Hu B, Zhang X, Xu G, Zhang Q, Qian P, Liu S, Zhu J, & Shen R (2018). Association between COMT polymorphism Val158Met and opiod consumption in patients with postoperative pain: A meta-analysis. Neurosignals, 11–21. [DOI] [PubMed] [Google Scholar]
- Jackson KT, Mandler T, & O’Keefe-McCarthy S (2019). Women’s experiences of breastfeeding-related pain. MCN The American Journal of Maternal/Child Nursing, 44(2), 66–72. [DOI] [PubMed] [Google Scholar]
- Kent JC, Ashton E, Hardwick CM, Rowan MK, Chia ES, Fairclough KA, Menon LL, Scott C, Mather-McCaw G, Navarro K, & Geddes DT (2015). Nipple pain in breastfeeding mothers: Incidence, causes and treatments. International Journal of Environmental Research and Public Health, 12(10), 12247–12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RF, Bernier KM, Perry M, Heather E, Ramesh D, Young E, Walsh S, & Starkweather A (2019). Promoting self-management of breast and nipple pain in breastfeeding women: Protocol of a randomized controlled trial. Research in Nursing and Health, 42, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RF, & McGrath JM (2016). Clinical assessment and management of breastfeeding pain. Topics in Clinical Nutrition, 32(3), 1–12. [Google Scholar]
- Lucas RF, Zhang Y, Walsh S, Evans H, Young E, & Starkweather AR (2019). Efficacy of a breastfeeding pain self-management intervention: A pilot randomized controlled trial (RCT). Nursing Research, 68(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan HL, Hepworth AR, Garbin CP, Rowan MK, Deacon J, Hartmann PE, & Geddes DT (2012). Nipple pain during breastfeeding with or without visible trauma. Journal of Human Lactation, 28(4), 511–521. [DOI] [PubMed] [Google Scholar]
- Medoc Algomed. (2020). Computerized Pressure Algomed. In Medoc Advanced Medical Systems. Retrieved from https://www.medoc-web.com/algomed. (Accessed 10 April 2020). [Google Scholar]
- Muddana A, Asbill DT, Jerath MR, & Stuebe AM (2018). Quantitative Sensory Testing, antihistamines, and beta-blockers for management of persistent breast pain: A case series. Breastfeeding Medicine, 13(4), 275–280. [DOI] [PubMed] [Google Scholar]
- Newton M, & Newton NR (1948). The let-down reflex in human lactation. The Journal of Pediatrics, 33(6), 698–704. [DOI] [PubMed] [Google Scholar]
- Odom EC, Li R, Scanlon KS, Perrine CG, & Grummer-Strawn L (2013). Reasons for earlier than desired cessation of breastfeeding. Pediatrics, 131(3), e726–e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Assistant Secretary for Health. (2019). Pain management best practices inter-agency task force: Draft report on pain management best practices: Updates, gaps, inconsistencies, and recommendations draft report overview. Retrieved from https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html. (Accessed 24 January 2019).
- Quigley MA, Carson C, Sacker A, & Kelly Y (2016). Exclusive breastfeeding duration and infant infection. European Journal of Clinical Nutrition, 70(12), 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, & Treede RD (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. European Journal of Pain, 10(1), 77–88. [DOI] [PubMed] [Google Scholar]
- Russell BS, Lincoln CR, & Starkweather AR (2018). Distress tolerance and emotion regulation: Promoting maternal mental health across the transition to parenthood. Journal of Holistic Nursing, 1–13. [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, & Taylor SE (2011). Oxytocin receptor gene (OXTR) is related to psychological resources. Proceedings of the National Academy of Sciences of the United States of America, 108(37), 15118–15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug S, Lavand’homme P, Barke A, Korwisi B, Rief W, Treede R-D, & The IASP Taskforce for the Classification of Chronic Pain. (2019). The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain, 160(1). manuscript accepted for publication. [DOI] [PubMed] [Google Scholar]
- Slane MM, Lusk LG, Boomer KB, Hare AE, King MK, & Evans DW (2014). Social cognition, face processing, and oxytocin receptor single nucleotide polymorphisms in typically developing children. Developmental Cognitive Neuroscience, 9, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman NK, Melvin K, & Meltzer-Brody S (2015). ABM clinical protocol #18: Use of antidepressants in breastfeeding mothers. Breastfeeding Medicine, 10(6), 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, & Dorsey SG (2016). Methods to measure peripheral and central sensitization using quantitative sensory testing: A focus on individuals with low back pain. Applied Nursing Research, 29, 237–241. [DOI] [PubMed] [Google Scholar]
- Subnis UB, Starkweather A, & Menzies V (2016). A current review of distraction-based interventions for chronic pain management. European Journal of Integrative Medicine, 8(5), 715–722. [Google Scholar]
- Terkawi AS, Jackson WM, Thiet MP, Hansoti S, Tabassum R, & Flood P (2012). Oxytocin and catechol-O-methyltransferase receptor genotype predict the length of the first stage of labor. American Journal of Obstetrics and Gynecology. [DOI] [PubMed] [Google Scholar]
- Tracy LM, Georgiou-Karistianis N, Gibson SJ, & Giummarra MJ (2015). Oxytocin and the modulation of pain experience: Implications for chronic pain management. Neuroscience and Biobehavioral Reviews, 55, 53–67. [DOI] [PubMed] [Google Scholar]
- US Preventive Task Force. (2016). Primary care interventions to support breastfeeding: An updated systematic review for the U.S. Preventive Services Task Force. JAMA, 316(16), 1688–1693. [DOI] [PubMed] [Google Scholar]
- Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, & Richter L (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. [DOI] [PubMed] [Google Scholar]
- Xin Q, Bai B, & Liu W (2017). The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochemistry International, 103, 57–64. [DOI] [PubMed] [Google Scholar]
- Young EE, Kelly DL, Shim I, Baumbauer KM, Starkweather A, & Lyon DE (2017). Variations in COMT and NTRK2 influence symptom burden in women undergoing breast cancer treatment. Biological Research for Nursing, 19(3), 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]


