Abstract
A platform for automated screening of zebrafish larvae in high throughput should allow detection of phenotypic changes in single cells.
In the last 20 years, the zebrafish has emerged as a powerful vertebrate model organism for both genetic and chemical screens. In this issue of Nature Methods, Yanik and colleagues overcome some of the current obstacles in carrying out such screens in high throughput1.
Screening in zebrafish has afforded major discoveries in the fields of embryogenesis, organogenesis and neuroscience2. The benefits of the zebrafish as a model include the availability of genetic mutants that cover a range of defects and a large collection of tissue-specific transgenic reporter lines. A high degree of genetic conservation to mammals, rapid development, and the ability to collect embryos in the thousands, make zebrafish an ideal model for high-throughput in vivo screening.
Despite these many benefits, the usefulness of zebrafish in screens has been limited by the need to manually handle and process embryos, to make observations, and then to record and analyze the data. Large-scale screens in zebrafish have relied on mutations or small molecules that produce obvious morphological or behavioral phenotypes2. The need to observe and record complex data under many different conditions has surpassed what a user can perform manually3,4. This need has led to the development of automated imaging and analysis platforms that usually rely on reading multiwell plates. Most of these systems are limited to low-resolution brightfield or fluorescence imaging, which allows measurement of movement3,4 or the pattern of a transgenic reporter5 but does not allow observations at the cellular level. Thus, although current platforms represent important advances, there are still many requirements that could be fulfilled.
In this issue of Nature Methods, Yanik and colleagues tackle the challenges of working with a vertebrate organism at high throughput1. Imaging and laser surgery of a zebrafish larva at cellular resolution requires that it be possible to orient the larva along all three axes, which they achieved by loading the larva into a small capillary tube. Additionally, the platform allows rapid loading of larvae from tanks or multiwell plates, prescreening to exclude bubbles and debris, and multiple options for imaging and laser surgery before ejection from the system—all in less than 30 seconds (Fig. 1). The authors demonstrated the utility of this system using the neuronal robo2 astray mutant6 and convincingly detected subtle axon-guidance defects. This demonstration also showed that even a small error in the orientation of a larva could prevent scoring of cellular morphology (Fig. 1). Yanik and colleagues then performed laser surgery on a single axon, followed by observation of its regeneration and recovery of the zebrafish.
Figure 1 |.
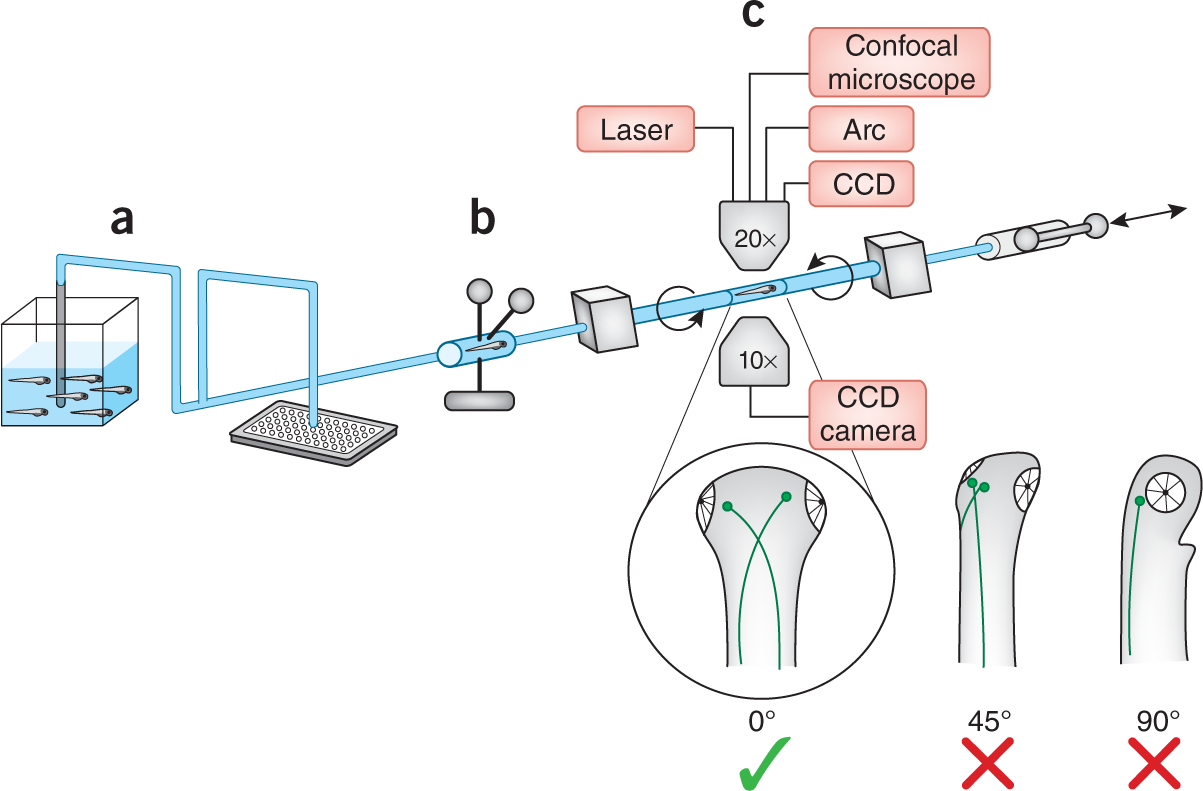
A high-throughput platform for zebrafish screening. Larvae are introduced from a tank or a multiwell plate (a), prescreened to distinguish them from bubbles or debris (b), oriented in a capillary tube (c), imaged or subjected to microsurgery and then ejected from the system. The schematic of larvae illustrates that correct orientation is essential for observation of Mauthner axons. CCD, charge-coupled device; Arc, mercury arc lamp; laser, femtosecond laser.
Although the system described in this issue1 is a major advance in vertebrate screening, there are potential limitations that must still be overcome. The system can process a single larva in less than 30 seconds and an entire 96-well plate in approximately 30 minutes, but the rapid development of zebrafish could create drift in staging between samples. In minutes or hours a zebrafish embryo can progress through developmental stages that would be equivalent to days in the mouse. This could be partially corrected by multiwell treatment plates loaded with embryos from staggered spawnings, which would compensate for some of the differences in stage when the larvae actually arrive in the capillary chamber for imaging or surgery. This would be essential if embryos were treated using large chemical libraries containing thousands of compounds; the stages of treatment and processing would need to be carefully controlled.
The availability of this new technology raises exciting possibilities in the resolution of phenotypic changes that can be detected in a zebrafish screen. In this study1, GFP-positive Mauthner axons in the hindbrain provided an easy readout of a specific mutant phenotype. In the future, tissue-specific transgenic reporters could be used together with chemical screening; this has already been demonstrated at low resolution by treating vascular-specific fli1:gfp embryos with antiangiogenic compounds5. Chemical screening to identify small molecules that suppress gross morphological phenotypes2 could be extended with this platform to detect suppression of subtle cellular defects. There are many available transgenic zebrafish reporter lines, but the difficulty in orienting and imaging these larvae has limited their use in large-scale screening; we hope that this platform will bring more transgenic lines into screening pipelines.
In addition to the imaging options on this platform, the laser for subcellular surgery could also have other applications. By disrupting neurons or other cell types in a mutagenized background or in the presence of chemicals, this laser could become a screening tool for cell-specific regeneration; this would only be possible with automation. Additionally, the laser could be tuned to perform other manipulations, making use of the extensive toolbox of photoconvertible substrates that can be activated in single cells of the zebrafish. Common reagents include caged fluorophores for short-term lineage tracing7, caged morpholinos (that is, oligonucleotides that block translation or splicing) for cell-specific knockdown8 and, more recently, caged small molecules9 or photoconvertible proteins that switch their emission profile when irradiated with ultraviolet wavelengths10. In fact, a photoconvertible protein has been used to label single neurons one by one to build a small network of cells11; using the automation of this new platform, it may be possible to assemble even larger neural networks by photolabeling. Any one of these photoconvertible tools, used together with this laser-equipped screening platform, has many possibilities for dissecting the function and ontogeny of single cells.
All together, the platform is an exciting step forward in high-throughput vertebrate screening and will allow users to ask questions with a much higher degree of resolution and specificity.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemethods/.
References
- 1.Pardo-Martin C et al. Nat. Methods 7, 634–636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zon LI & Peterson RT Nat. Rev. Drug Discov. 4, 35–44 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Kokel D et al. Nat. Chem. Biol. 6, 231–237 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rihel J et al. Science 327, 348–351 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran TC et al. Cancer Res 67, 11386–11392 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Fricke C, Lee JS, Geiger-Rudolph S, Bonhoeffer F & Chien CB Science 292, 507–510 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski DJ & Weinberg ES Methods Mol. Biol. 135, 349–355 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Esengil H, Chang V, Mich JK, & Chen J K Nat. Chem. Biol. 3, 154–155 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Sinha DK et al. Zebrafish 7, 199–204 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Hatta K, Tsujii H & Omura T Nat. Protoc. 1, 960–967 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Aramaki S & Hatta K Dev. Dyn. 235, 2192–2199 (2006). [DOI] [PubMed] [Google Scholar]


