Abstract
Light at night in adults suppresses melatonin in a non-linear intensity-dependent manner. In children, bright light of a single intensity before bedtime has a robust melatonin suppressing effect. To our knowledge, whether evening light of different intensities is related to melatonin suppression in young children is unknown. Healthy, good sleeping children (n = 36; 3.0 – 4.9 years; 39% male) maintained a stable sleep schedule for seven days followed by a 29.5-h in-home dim-light circadian assessment (~1.5 lux). On the final night of the protocol, children received a 1-h light exposure (randomized to one of 15 light levels, ranging 5 – 5,000 lux, with ≥2 participants assigned to each light level) in the hour before habitual bedtime. Salivary melatonin was measured to calculate the magnitude of melatonin suppression during light exposure compared with baseline levels from the previous evening, as well as the degree of melatonin recovery 50 min after the end of light exposure. Melatonin levels were suppressed between 69.4% and 98.7% (M = 85.4 +/− 7.2%) during light exposure across the full range of intensities examined. Overall, we did not observe a light intensity-dependent melatonin suppression response; however, children exposed to the lowest quartile of light intensities (5 – 40 lux) had an average melatonin suppression (77.5 +/− 7.0%) which was significantly lower than that observed at each of the three higher quartiles of light intensities (86.4 +/− 5.6%, 89.2 +/− 6.3%, and 87.1 +/− 5.0%, respectively). We further found that melatonin levels remained below 50% baseline for at least 50 min after the end of light exposure for the majority (62%) of participants, and recovery was not influenced by light intensity. These findings indicate that preschool-aged children are highly sensitive to light exposure in the hour before bedtime and suggest the lighting environment may play a crucial role in the development and maintenance of behavioral sleep problems through impacts on the circadian timing system.
Keywords: Circadian rhythm, melatonin, light, sleep, child, preschool, child development
Introduction
Circadian timing is determined by an individual’s biology (e.g., circadian period) and their lighting environment. Light influences the circadian clock chiefly through stimulation of the eye’s intrinsically photosensitive retinal ganglion cells (ipRGCs), melanopsin-expressing photoreceptors with a peak sensitivity to light of ~480 nm 1,2. When stimulated, the signal from the ipRGCs is transmitted via the retinohypothalamic tract to the suprachiasmatic nucleus (SCN), the master circadian clock. In turn, the SCN controls the pineal gland’s production of the sleep-promoting hormone melatonin 3,4. Several findings suggest that this mechanism emerges early in mammalian development. In rodent models, the ipRGCs are functional and light sensitive from birth 5–7. Additionally, melanopsin is present in human eye tissue at eight weeks post-conception 8. Lastly, in pre-term infants, the pupillary light reflex is evoked by 470 nm blue light but not 635 nm red light, suggesting activation of the ipRGCs 9. Yet, despite the early development of this pathway, few experimental studies have examined the circadian response to light in early childhood.
What is known about children’s photosensitivity during the first decade of life suggests a strong melatonin suppression response to light. Compared with their parents, school-aged children (aged ~9 years) demonstrated nearly twice the melatonin suppression during an evening bright light exposure (580 lux) 10. Additionally, 9-year-olds’ melatonin was suppressed significantly more under home light levels (~140 lux) compared to dim light conditions (<30 lux), a difference not observed in their parents 10. In preschoolers, we previously demonstrated that a 1-h exposure to bright light (1,000 lux) in the hour before bedtime resulted in robust melatonin suppression (~90%) and that melatonin levels remained attenuated 50 min after the end of the light stimulus 11. Children’s photosensitivity is likely related to developmental changes in the eye, including larger pupils and clearer lenses than adults, allowing for greater light transmission 10,12–14. Together, these findings point to the importance of understanding the effects of the lighting environment on the maturing circadian clock.
The adult circadian response to light is intensity-dependent, and even low levels of evening light can suppress melatonin production 15–18. Zeitzer and colleagues 19 established illuminance response curves to a 6.5-h experimental light stimulus of varying intensities with light exposure centered 3.5 h before the fitted minimum of the endogenous core body temperature. They reported that 50% of the maximal melatonin suppression response occurred at ~50–130 lux, within the range of typical indoor room light. Recent data from a 5-h evening light exposure protocol suggest that the adult circadian system may be highly sensitive to evening light, with 50% of the melatonin suppression response occurring at an average of only ~25 lux 15. To date, young children’s sensitivity to evening light intensity has not been examined and was the objective of this research.
Employing a rigorous, experimentally controlled, randomized research design, healthy, preschool-aged children maintained a stable sleep schedule for seven days and then entered an in-home dim-light environment (29.5 h). On the final night of the dim-light protocol, they received a 1 h light exposure in the hour before their habitual bedtime, a time chosen to reflect when children are often exposed to artificial light in their everyday lives. Salivary melatonin was collected in order to calculate baseline dim-light melatonin onset (DLMO), melatonin suppression during the light exposure, and melatonin recovery following light termination. We hypothesized that evening light exposure would induce acute melatonin suppression in a non-linear intensity-dependent manner.
Methods
Participants
This study included 36 healthy children aged 3.0 – 4.9 years (M = 4.2 +/− 0.5 years; 39% male; 34 Caucasian, 2 mixed race) recruited from the greater Boulder, CO area. Two additional participants were enrolled, but did not complete the study due to accidental light exposure (n = 1) or illness (n = 1). Interested parents were screened through online questionnaires and an in-depth phone interview to assess eligibility. Children were excluded for parental report of any of the following: clinical sleep disorders; behavioral/emotional problems; preterm or post-term delivery (term = 35–45 weeks) or low birth weight (<5.5 lbs.); current use of caffeine or medications affecting the sleep/circadian systems or light sensitivity; developmental disabilities; neurological or metabolic disorders; chronic medical conditions; lead poisoning; head injury involving loss of consciousness; migraine or frequent headaches; oral disease or injury; travel beyond two time zones in the two months before circadian assessments; nighttime sleep opportunity (time in bed) of < 10 h/night; parent-reported child’s sleep schedule varying >2 h between weekdays and weekends; regular daytime napping (>2 times/week); visual impairment, eye disorders, or color blindness (confirmed with Ishihara Color Vision Test). Parents completed written informed consent. All study procedures were approved by the University of Colorado Boulder Institutional Review Board and in accordance with the Declaration of Helsinki. Families were compensated for their participation.
Protocol
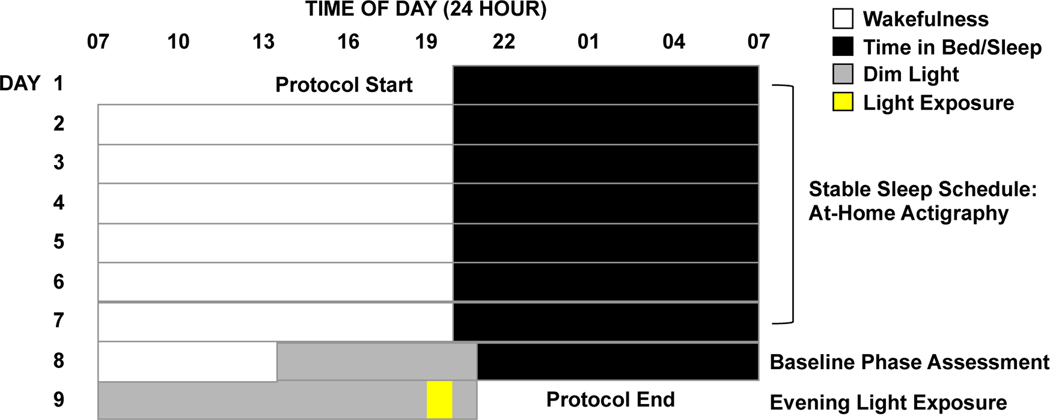
Data were collected during the summer months (mid-May to mid-August) of 2017, 2018, and 2019 to control for variations in photoperiod throughout the year. All study procedures took place in participants’ homes. Children completed a 9-day protocol (Fig. 1). Throughout the study, children wore an actigraph (Spectrum Plus, Philips Respironics, Pittsburgh, PA, USA) on their non-dominant wrists to objectively measure sleep and light exposure. For the first seven days of the protocol, children followed a strict parent-selected sleep schedule (bedtime and wake time) of at least 10 h time in bed per night. Parents completed a sleep diary and were contacted by researchers daily in order to confirm adherence to the schedule.
Fig. 1: 9-day study protocol.
Children maintained a strict parent-selected sleep/wake schedule for seven days, followed by a 29.5-h dim-light assessment. On Day 8, a baseline DLMO was determined. On Day 9, children received a 1-h light exposure in the hour before habitual bedtime, with salivary melatonin measured before, during, and after the exposure. The times in the figure are intended as an example; actual parent-selected bedtimes and wake times varied across participants.
On Day 8, researchers transformed the participant’s home into a dim-light environment by covering windows with black plastic and installing low wattage bulbs and dimmer switches, achieving an average light level of ~1.5 lux. Participants entered the dim light environment 4.5 h before their scheduled bedtime and remained in dim-light through the completion of the protocol (1 h past scheduled bedtime on Day 9).
During the evening of Day 8, we assessed children’s baseline DLMO. Starting 3 h and 20 min before habitual bedtime, saliva samples were collected in 20- or 30-min intervals, continuing 1 h past habitual bedtime. In cases where a participant exhibited a long sleep onset latency, as measured by the first 7 days of actigraphy, collection of saliva samples was extended for an additional hour each night, in order to account for the possibility of a later DLMO (n=3). Saliva samples were obtained by having the child mouth and chew on one end of a braided cotton roll for ~2 min. Children remained in a sitting posture for 5 min prior to and during each sample collection 20 and did not eat or drink for 15 min before each sample. Light levels were obtained during each sample using a research photometer (ILT2400; International Light Technologies, Inc., Peabody, MA, USA) held approximately 5 cm adjacent to the child’s eye and directed in the angle of gaze. Samples were immediately centrifuged and stored on ice in coolers on-site, then transferred to the laboratory and stored in a −20°C freezer. Following the completion of each data collection period, samples were shipped and assayed offsite at Solid Phase, Inc. (Portland, Maine, USA) by technicians who were blind to the study conditions.
On Day 9, researchers arrived at the participant’s home shortly before their scheduled wake time and remained with the child throughout the day in order to confirm adherence to study protocol and maintenance of the dim-light environment. In the evening, children were scheduled to a 1-h light exposure in the hour before their habitual bedtime. Participants sat at a low table playing at a dimmable illuminated flat LED panel (5000K; Beghelli USA). The spectral power distribution of the experimental light source at the maximum set point (5,000 lux) is provided in supplemental data (Fig. S3). A hazard analysis confirmed that the experimental light source emitted no UV radiation below 400nm and had blue-light hazard and burn hazard radiances several orders of magnitude less than the published limits, thereby posing no photobiological risk to participants21,22. Neutral density filters (LEE Filters, Burbank, CA, USA) of varying transmissivities were wrapped over the panel, in conjunction with active dimming controls, to achieve the desired intensity. In order to direct the child’s gaze downwards towards the light source continuously during the 1-h light exposure, researchers engaged the child in activities such as coloring on transparencies or playing with translucent blocks. Light intensity at the child’s angle of gaze was recorded every 10 min throughout the light exposure. Additional readings were taken whenever the child shifted position, and the intensity was modified accordingly with a dimmer knob if needed to ensure constant exposure to the assigned intensity. Saliva samples were collected 20 min before and 10, 30, and 50 min after the start of the light exposure, as well as 20 and 50 min after the end of the light exposure. All samples taken on Day 9 were time-anchored to those collected on the previous evening.
Participants were randomly assigned to a single illuminance (ranging from 10 to 5,000 lux). Sampling intensities were chosen to optimally characterize the features of the illuminance-response curves. To determine sampling intensities, we divided the range of the log(illuminance) into four subintervals: [1.0 – 1.6]; [1.6 – 2.2]; [2.2 – 2.9]; [2.9 – 3.7] corresponding to approximate illuminance ranges for dim (10–40 lux), indoor (60–150 lux), bright indoor (200–750 lux), and bright outdoor light (1000–5000 lux), respectively. Within each of these ranges we identified 3–4 representative sampling intensities, with the highest sampling frequency occurring in the region from 1.8 (~63 lux) to 3 (1,000 lux) where we expected the illuminance-response curve to have its steepest slope and half-maximal response. To account for interindividual variability, at least two participants were assigned to each illuminance level. Participants were assigned illuminance exposure levels using quasi-block randomization to ensure a range of illuminance exposures during each collection period. After reviewing the preliminary findings, data from two final participants were collected at 5 lux.
The melanopic equivalent daylight illuminance (EDI), irradiance, and photon density are presented in Table 1 for each experimental light intensity.
Table 1.
Melanopic equivalent daylight illuminance (EDI), irradiance, and photon density of experimental light intensities.
| Intensity set point (lux) | Melanopic EDI (lux) | Irradiance (W m−2) | Photon Density (photons s−1 m−2) |
|---|---|---|---|
|
| |||
| 5 | 4.5 | 0.03 | 1.03×1017 |
| 10 | 8.5 | 0.04 | 1.30×1017 |
| 20 | 16.8 | 0.08 | 2.56×1017 |
| 40 | 31.5 | 0.13 | 3.90×1017 |
| 60 | 45.4 | 0.20 | 5.66×1017 |
| 80 | 61.4 | 0.26 | 7.68×1017 |
| 100 | 76.9 | 0.33 | 9.64×1017 |
| 150 | 116.1 | 0.51 | 1.47×1018 |
| 200 | 140.6 | 0.57 | 1.59×1018 |
| 350 | 247.2 | 1.00 | 2.83×1018 |
| 500 | 351.6 | 1.43 | 4.03×1018 |
| 750 | 517.2 | 2.12 | 5.89×1018 |
| 1000 | 686.5 | 2.81 | 7.82×1018 |
| 2000 | 1335.9 | 5.51 | 1.53×1019 |
| 5000 | 3275.7 | 13.62 | 3.79×1019 |
Analysis
Salivary melatonin levels were assayed using radioimmunoassay (Bühlmann Laboratories AG, Schöenbuch, Switzerland). The limits of detection of the melatonin assays were 0.5 to 50.0 pg/mL. Any sample measured above the upper limit was recorded as 50.0 pg/mL (n=7). The inter-assay coefficients of variation for samples from 2017 and 2018 were between 11.4% and 12.7% (n=22). The intra-assay coefficients of variation for these samples ranged from 6.3% to 11.0%. For the samples collected during 2019, a different lot of controls was employed resulting in inter-assay coefficients of variation between 8.2% and 8.7%, and intra-assay coefficients of variation from 5.0% to 9.9% (n=22). DLMO was calculated as the linear interpolated clock time at which salivary melatonin levels reached 4 pg/mL, provided melatonin levels remained above the threshold for ≥2 consecutive samples20,23. One child was a high melatonin secretor, and thus an adjusted threshold of 10 pg/mL was used to calculate the DLMO for this participant 24. Analyses were run both including and excluding this participant’s data. The only change in results that occurred by excluding this participant was in the comparison of the average melatonin suppression in the lowest 2 quartiles of assigned light intensity, which became a non-significant trend when the participant was excluded.
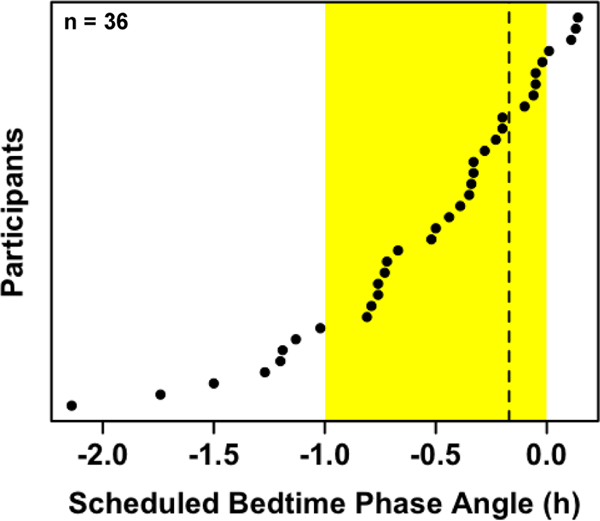
Actigraphy data were scored with our previously published standard procedures25. Averages were calculated across the first 7 days of actigraphy for the following sleep parameters: bedtime (lights out), sleep start time, midsleep time (the midpoint between sleep start and sleep end), sleep end time, wake time (lights on), and sleep onset latency (number of minutes between bedtime and sleep start time). Phase angles of entrainment were computed as the difference between DLMO and each sleep timing parameter (i.e., scheduled bedtime, bedtime, sleep start, midsleep, sleep end, and wake time). Data from participants with a scheduled bedtime phase angle greater than −0.17 h, indicating that baseline DLMO had not occurred by the time of the final saliva sample during light exposure, were removed from the analysis (n=9; Fig. 2). Minute-by-minute measurements of light exposure were recorded by the actigraph and used to compute average light exposure between wake time and bedtime. Photometer readings collected during saliva samples at the child’s angle of gaze were averaged, excluding samples collected during the experimental light exposure.
Fig. 2: Participants’ scheduled bedtime phase angles.
Phase angle was calculated as clock time of baseline DLMO minus scheduled bedtime. Data are organized from smallest to largest phase angle. The shaded yellow area represents the 1-h light exposure in the hour before scheduled bedtime. The dashed line denotes the inclusion cutoff (the time of the final saliva sample collected during the light exposure), with participants to the right of the line excluded from analysis (n=9).
Melatonin suppression resulting from the light exposure was determined using area under the curve (AUC; trapezoidal method) of the melatonin profile during the light exposure and the corresponding 1-h time window on the baseline night 11. Melatonin levels at the beginning and end of the light exposure were interpolated for each participant. Suppression was calculated as the following normalized quantity:
Melatonin recovery was calculated as the ratio between the melatonin levels 50 min after the end of the light exposure and the levels at the same clock time on the previous evening (baseline).
Group averages of melatonin levels at each sample time were compared across the two evenings through paired-samples t-tests. Independent samples t-tests were used to compare average melatonin suppression across quartiles of light intensity. Effect sizes for all t-tests are presented as Cohen’s d. Bivariate correlations were used to examine the relationship between light intensity and melatonin suppression for the participants with baseline DLMO before the start of the light exposure, as well as between melatonin recovery and both light intensity and melatonin suppression across the full sample. All significance testing was performed with an α -level of 0.05.
Results
Table 2 provides means and standard deviations for sleep and circadian variables for this cohort of preschool-aged, healthy children. Average bedtime during the seven days leading up to the dim-light assessment was 20:04 +/− 0:36. The timing of baseline DLMO ranged from 17:48 to 21:16 (M = 19:18 +/− 0:47), occurring on average 45.9 +/− 28.7 min before children’s average bedtime. Baseline DLMO ranged from 68 min before to 48 min after the timing of light onset. Depictions of individual DLMO and sleep variable averages are provided in supplemental data (Fig. S1).
Table 2:
Means and standard deviations of sleep and circadian variables.
| M | SD | |
|---|---|---|
|
| ||
| Sleep variables | ||
| Bedtime | 20:04 | 0:36 |
| Sleep start time | 20:21 | 0:37 |
| Midsleep time | 1:35 | 0:36 |
| Sleep end time | 6:47 | 0:39 |
| Wake time | 6:59 | 0:37 |
| Sleep onset latency (min) | 17.9 | 7.7 |
| Circadian variables | ||
| Dim light melatonin onset time | 19:18 | 0:47 |
| Bedtime phase angle (min) | 45.9 | 28.7 |
| Sleep start phase angle (min) | 62.5 | 27.7 |
| Midsleep phase angle (h) | 6.3 | 0.5 |
| Sleep end phase angle (h) | 11.5 | 0.6 |
| Wake time phase angle (h) | 11.7 | 0.6 |
| Light exposure phase angle (min) | 14.4 | 30.0 |
Note: For dim light melatonin onset time and light exposure phase angle, n = 27. For all other variables, n = 26 due to an actigraph technical failure.
Participants were exposed to an average of 2,241 +/− 1,665 lux per day from wake time to bedtime during the week before the circadian assessment. Across the 29.5 h in the dim-light environment, children were exposed to an average of 1.5 +/− 0.8 lux. During collection of saliva samples (not including those obtained during the experimental light exposure), intensity at the child’s angle of gaze averaged 0.7 +/− 0.3 lux.
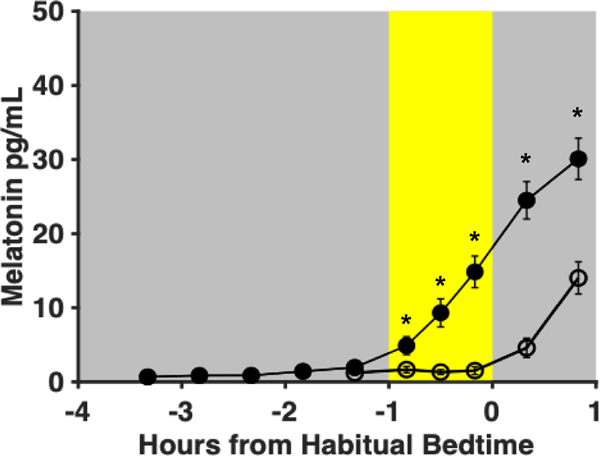
Group averages of melatonin levels at each of the sample times are depicted in Fig. 3. Melatonin levels 10 min (p = 0.001, d = 0.72), 30 min (p < 0.001, d = 0.96), and 50 min (p < 0.001, d = 1.43) after light onset were significantly lower than melatonin levels at the same clock time on the previous night. Additionally, melatonin levels were significantly lower 20 min (p < 0.001 d = 1.75) and 50 min (p < 0.001, d = 1.50) after the end of light exposure compared to melatonin levels at the same clock time on the previous night. Across the wide range of experimental light intensities, melatonin levels during the light exposure were low with little variability between participants, suggesting a consistent, robust melatonin suppression effect.
Fig. 3: Group averages of melatonin levels (n=27).
Filled circles represent saliva samples collected during the baseline night (Day 8) and open circles represent those collected during the light exposure night (Day 9). Error bars denote standard error. The yellow shaded area represents the timing of the light exposure (1 h before habitual bedtime). Asterisks denote significant differences between melatonin levels of the light exposure and baseline days (p<0.05). Melatonin levels on Day 9 were significantly lower 10, 30, and 50 min after light onset, as well as 20 and 50 min after the end of light exposure, compared with the same clock time on Day 8.
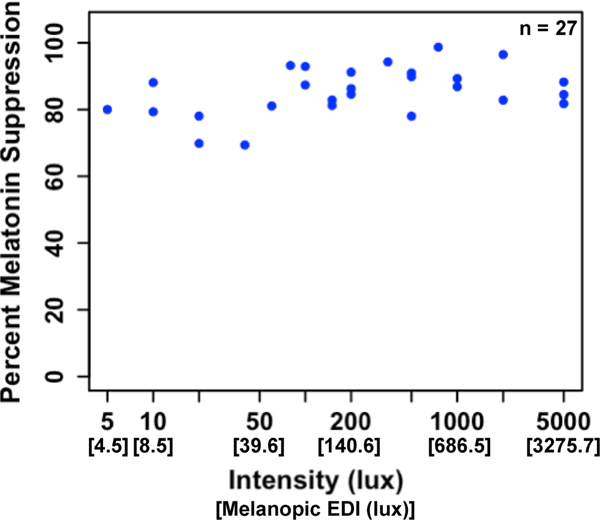
Melatonin suppression during the 1-h light exposure ranged from 69.4% to 98.7% (M = 85.4 +/− 7.2%; Fig. 4). A single sample t-test revealed that melatonin levels were significantly suppressed across the sample, t(26) = 61.64, p < 0.001. A relationship between light intensity and the magnitude of melatonin suppression was not observed. Rather, suppression values were high across the full range of light intensities.
Fig. 4: Melatonin suppression as a function of light intensity across all participants.
Melatonin suppression ranged from 69.4% to 98.7% with an average of 85.4 +/− 7.2%. Across the full sample, no relationship between light intensity and the magnitude of melatonin suppression was observed.
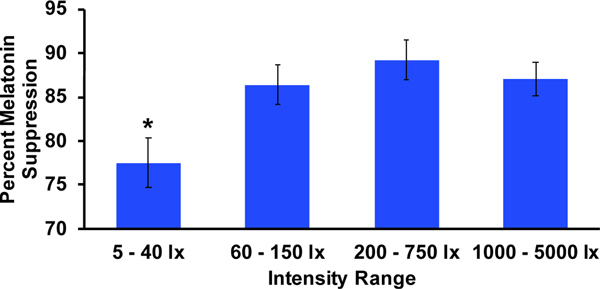
Intensities were divided into quartiles (5 – 40 lux, 60 – 150 lux, 200 – 750 lux, and 1,000 – 5,000 lux) in order to further examine any differences in melatonin suppression between those exposed to high or low intensity light. Illustrations of the group averages of melatonin levels within each quartile are provided in the supplemental data (Fig. S2). The average percent melatonin suppression within each quartile is depicted in Fig. 5. Average suppression within the lowest quartile was significantly lower than the 2nd (p = 0.03, d = 1.41), 3rd (p = 0.01, d = 1.77), and 4th (p = 0.01, d = 1.59) quartile ranges of intensity (M = 77.5 +/− 7.0%, 86.4 +/− 5.6%, 89.2 +/− 6.3%, and 87.1 +/− 5.0%, respectively). Comparisons among the three higher quartiles were non-significant. These results indicate that participants assigned to a light intensity of 40 lux or lower had less melatonin suppression compared to those exposed to higher intensities.
Fig. 5: Average melatonin suppression (%) within each quartile of light intensity.
Error bars represent standard error. The average melatonin suppression across the lowest quartile (M = 77.5+/−7.0%) was significantly less than each of the three higher quartiles (M = 86.4 +/− 5.6%, 89.2 +/− 6.3%, 87.1+/− 5.0%; all p<0.05).
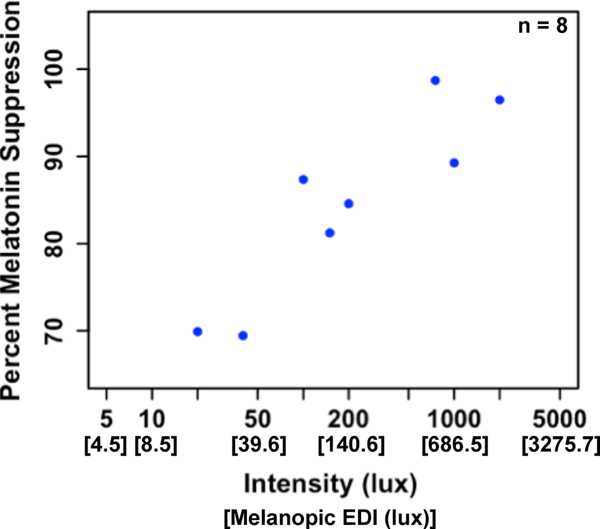
As depicted in Fig 2., we observed a wide variation in the circadian timing of the light stimulus across participants. Baseline DLMO occurred before the clock time of light onset in eight participants, indicating that the full 1-h light exposure occurred during their biological night. Analyzing melatonin suppression in only those 8 children revealed a significant relationship (r = 0.72, p = 0.046; Fig. 6), such that brighter light intensities resulted in greater melatonin suppression. However, even at the lower assigned intensities (i.e., 20 and 40 lux), we still observed robust melatonin suppression of ~70%.
Fig. 6: Melatonin suppression as a function of light intensity across participants with baseline DLMO occurring before light onset.
We observed a significant relationship between light intensity and percent melatonin suppression (r = 0.72, p = 0.046).
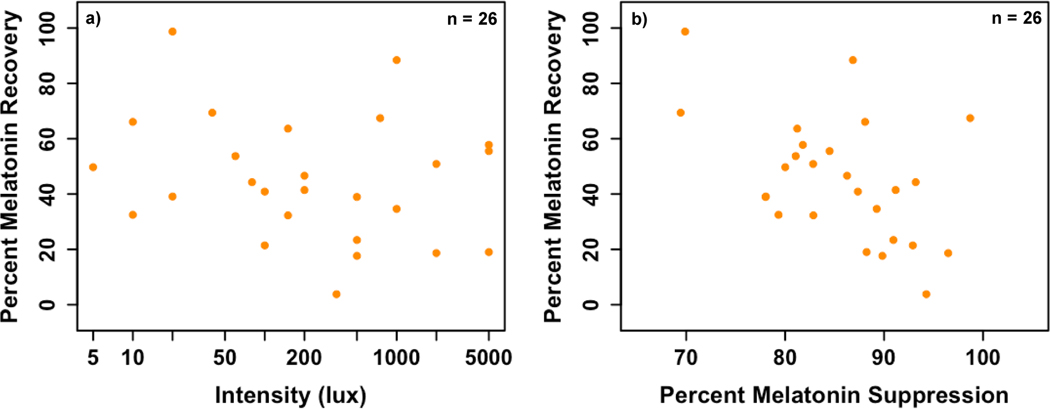
Finally, we compared melatonin levels 50 min after the end of light exposure to melatonin levels at the same clock time on the prior evening in order to assess melatonin recovery following suppression. Melatonin levels remained below 50% of baseline melatonin levels in the majority (62%) of participants. Melatonin recovery ranged from 3.8% to 98.7% (M = 45.2 +/− 22.3%). No association was observed between melatonin recovery and light intensity (r = −0.06, p = 0.78; Fig. 7a); however, larger melatonin suppression was significantly correlated with smaller melatonin recovery (r = −0.50, p = 0.01; Fig. 7b). This association remained significant after controlling for light intensity (r = −0.50, p = 0.01).
Fig. 7: Associations between percent melatonin recovery and a) light intensity and b) melatonin suppression.
Percent melatonin recovery was not associated with the intensity of the light exposure (r = −0.06, p = 0.78), but was inversely associated with percent melatonin suppression (r = −0.50, p = 0.01). One participant did not provide a final sample on the baseline night; thus, this analysis includes data from 26 participants.
Discussion
In this well-controlled, randomized research design, we examined young children’s melatonin suppression responses to a wide range of light intensities (5 – 5,000 lux). Contrary to our hypothesis and prior findings observed in adults 15,19, we did not observe a light intensity-dependent effect on melatonin suppression. Rather, a 1-h light exposure in the hour before bedtime resulted in consistently high melatonin suppression across a wide range of light intensities. These suppression data are not well-described by a logistic dose-response curve as previously reported in adult illuminance-response curves, and, given that the minimum suppression was 69%, we are not able to calculate the intensity of the half-maximal response15,19. However, average melatonin suppression across the lowest quartile of light intensities (5 – 40 lux) was significantly lower than that measured across each of the 3 higher quartiles of intensity. This finding may reflect a large range of light intensities that result in a saturated melatonin suppression response. It is possible that a finer resolution at lower intensities could reveal an intensity-dependent melatonin suppression response curve. However, as we observed large melatonin suppression (~82%) in response to 5 and 10 lux intensities, our findings suggest that significant melatonin suppression would likely occur at very dim intensities. We also observed a sustained effect of light exposure, such that melatonin levels were lower 50 min after the end of light exposure compared to baseline the night prior. Overall, our findings suggest that young children are highly sensitive to light exposure at night prior to habitual bedtime with regards to melatonin suppression. Our findings among the eight participants who received the full 1-h light exposure during their biological night suggest that the melatonin suppression response to light exposure in the hour after DLMO may demonstrate a dose-dependent relationship with intensity. However, even at low intensities, we still observed melatonin suppression of ~70%, indicating that young children are highly sensitive to dim light levels in the hour before bedtime.
In our previous published work, an evening light exposure to 1,000 lux resulted in significant melatonin suppression that did not return to baseline levels 50 min after the end of the light exposure 11. These findings were replicated and extended in the present study, in which group average melatonin levels 50 min after the end of light exposure were significantly lower than those at the same clock time of the previous evening (baseline). Additionally, more than half of participants (62%) failed to reach 50% of their baseline melatonin levels at 50 min after the end of the light exposure. No association was observed between percent melatonin recovery and light intensity although percent melatonin recovery was inversely correlated with percent melatonin suppression. Together, our findings indicate that in preschool-aged children, exposure to light before bedtime, even at low intensities, results in robust and sustained melatonin suppression.
These findings add to a growing body of literature demonstrating that children are highly sensitive to evening light, as demonstrated by the magnitude of melatonin suppression 10,26. One possible mechanism underlying children’s sensitivity to light is their ophthalmological features. Children have larger pupil diameters compared to their parents, under both dim and bright light conditions 10, and larger baseline pupil diameter in adults is predictive of greater light-induced melatonin suppression 27. Additionally, the human ocular lens becomes increasingly yellow and opaque with age, limiting the effectiveness of light to suppress melatonin in older individuals 12. At 10 years of age, the transmission of light through the lens at 480 nm, peak sensitivity of melanopsin, is 72% more than at 80 years 28. Children’s larger pupils and clearer lenses allow for greater light transmission and likely contribute to the high photosensitivity observed in this population. Although little is known about the ontogeny of human ipRGCs, findings from rodent models suggest substantial changes throughout early development. Newborn mice have nearly five times more light-responsive retinal ganglion cells than adults 5. The response of the ipRGCs to light stimuli increases throughout early development as the ipRGCs start to receive input from the rods and cones 7. Additionally, projections from the ipRGCs to the SCN via the retinohypothalamic tract strengthen during the first few weeks of life 29. Understanding how developmental changes in the human ipRGCs or other downstream processes contribute to photosensitivity in early childhood is an important area for future research. Additionally, the contribution of other photoreceptors (e.g., S-cones) to the melatonin suppression pattern that we observed needs to be elucidated30.
Prior studies in adults on the relationship between light intensity and melatonin suppression have employed light exposures lasting several hours and/or anchored to a circadian phase marker 15,19. In contrast, we utilized an ecologically valid light exposure time (1 h before habitual bedtime) in order to simulate how children are exposed to evening light in their everyday environment. For instance, a recent survey found that nearly half of children under age 8 use screen media in the hour before bedtime 31. Furthermore, children in our study directed their gaze downwards to the light throughout the 1-h exposure, similarly to how they would use a mobile electronic device. Additionally, in contrast to adults who typically self-select bedtimes on average ~2 h after DLMO 32, bedtimes for children in this age group are parent- or caregiver-selected and may differ widely with respect to a child’s DLMO 25. It is challenging to compare our results to those reported in previous adult studies due to these differences in methodology. Under the conditions of the present study, however, we observed high sensitivity in young children.
The present study provides strong evidence for the high sensitivity of the developing circadian system to evening light exposure. However, some limitations of this research should be noted. First, our strict eligibility criteria resulted in a homogenous sample of healthy, good-sleeping children, limiting the generalizability of our findings to broader populations. A recent study with adults demonstrated that photosensitivity can vary greatly across individuals, with the light intensity needed to achieve 50% melatonin suppression ranging from 6 to 350 lux 15. Given that each of our participants was assigned to only one light intensity, we are not able to analyze potential interindividual differences in children’s photosensitivity. Furthermore, our interpretation of percent melatonin suppression was complicated by variability in the timing of light exposure relative to melatonin onset. Consistent with our previous findings with young children25, parent-selected bedtime (used to set the timing of the light exposure) varied with respect to melatonin onset, with a much narrower phase angle than those observed in adults. Intrinsic circadian periods longer than 24h could have contributed to our findings for calculated melatonin suppression due to drift in the dim-light environment. As there are currently no reliable estimates of circadian period for this age group and we did not include a within-subject control condition at 0 lux for each participant, we cannot disregard the possibility that drift may have contributed to the high melatonin suppression observed, particularly for participants with later melatonin onsets. Although we identified a modest relationship between light intensity and melatonin suppression among the eight participants who received the full 1-h light exposure after melatonin onset, variability in the timing of light exposures limited our ability to establish this relationship across the full cohort. Finally, photic history influences sensitivity to nighttime light exposure, such that prior adaptation to a dim-light environment results in greater light-induced melatonin suppression compared with adaptation to typical room light 33,34. Given that our subjects spent 27.5 h in dim light prior to the light exposure, the dark adaptation may have increased their sensitivity compared to their typical light environment. Future studies should explore the effects of prior light history on photosensitivity in this population.
In summary, although we were unable to extend prior research with adults by establishing a non-linear illuminance-response curve for melatonin suppression, our data indicate that melatonin secretion in preschool-aged children is highly sensitive to light in the 1 h before bedtime across a wide range of intensities. Children’s evening lighting environments can disrupt the regular production of melatonin, which contributes to physiological changes that prepare the body for sleep in humans35, and may contribute to the development of evening settling problems (i.e., sleep onset delay, bedtime resistance) in early childhood. These findings highlight the importance of reducing light levels in the home before bedtime in order to support healthy sleep and circadian rhythms in young children.
Supplementary Material
Acknowledgements
We thank the children and families who participated in this study. We also thank the staff and students of the Sleep and Development Lab at the University of Colorado Boulder who assisted with collecting these data. Thank you to Shelby Stowe for her assistance in processing light history data.
Funding
This research was supported with funds from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01-HD087707), the University of Colorado Boulder Undergraduate Research Opportunities Program, and the University of Colorado Boulder Biological Sciences Initiative Scholars Program.
Conflicts:
LEH, LDA, and NS have no financial or personal conflicts to declare. CDB reports receiving research support from the National Science Foundation and LumosTech, outside the submitted work. MKL reports receiving travel funds from the Australian Research Council and research support from the National Institutes of Health, beyond the submitted work. KPW reports being a consultant to and/or receiving personal fees from Circadian Therapeutics, Inc., Circadian Biotherapies, Inc., Philips, Inc, and U.S. Army Medical Research and Materiel Command - Walter Reed Army Institute of Research; and receiving research support from the National Institutes of Health and the PAC-12 conference, outside the submitted work.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. [DOI] [PubMed] [Google Scholar]
- 2.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythm. 2005;20(4):314–325. [DOI] [PubMed] [Google Scholar]
- 4.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. [DOI] [PubMed] [Google Scholar]
- 5.Sekaran S, Lupi D, Jones SL, et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15(12):1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu DC, Zhang D, Demas J, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–999. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100(1):371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarttelin EE, Bellingham J, Bibb LC, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76(3):393–396. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda T, Ishikawa H, Shimizu K, Asakawa K, Goseki T. Pupillary size and light reflex in premature infants. Neuro-Ophthalmology. 2015;39(4):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014;99(9):3298–3303. [DOI] [PubMed] [Google Scholar]
- 11.Akacem LD, Wright KP Jr., LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Rep. 2018;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charman W. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthal Phys Opt. 2003;23(2):181–187. [DOI] [PubMed] [Google Scholar]
- 13.Weale R. Human lenticular fluorescence and transmissivity, and their effects on vision. Exp Eye Res. 1985;41(4):457–473. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Thompson K, Burns SA. Pupil location under mesopic, photopic, and pharmacologically dilated conditions. Invest Ophth Vis Sci. 2002;43(7):2508–2512. [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips AJK, Vidafar P, Burns AC, et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci U S A. 2019;116(24):12019–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy JF, Wright KP Jr., Entrainment of the human circadian system by light. J Biol Rhythm. 2005;20(4):326–338. [DOI] [PubMed] [Google Scholar]
- 17.Prayag AS, Najjar RP, Gronfier C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 2019;66(4):e12562. [DOI] [PubMed] [Google Scholar]
- 18.Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(3):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167(1–2):191–194. [DOI] [PubMed] [Google Scholar]
- 21.Protection ICoN-IR. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Phys. 2013;105(1):74–96. [DOI] [PubMed] [Google Scholar]
- 22.IEC 62471. Photobiological safety of lamps and lamp systems. In: International Electrotechnical Commission; Geneva; 2006. [Google Scholar]
- 23.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythm. 1997;12(3):278–289. [DOI] [PubMed] [Google Scholar]
- 24.Crowley SJ, Eastman CI. Human adolescent phase response curves to bright white light. J Biol Rhythm. 2017;32(4):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBourgeois MK, Carskadon MA, Akacem LD, et al. Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythm. 2013;28(5):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocr Metab. 2015;100(11):4067–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higuchi S, Ishibashi K, Aritake S, et al. Inter-individual difference in pupil size correlates to suppression of melatonin by exposure to light. Neurosci Lett. 2008;440(1):23–26. [DOI] [PubMed] [Google Scholar]
- 28.Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refr Surg. 2010;36(2):308–312. [DOI] [PubMed] [Google Scholar]
- 29.Brooks E, Canal MM. Development of circadian rhythms: role of postnatal light environment. Neurosci Biobehav Rev. 2013;37(4):551–560. [DOI] [PubMed] [Google Scholar]
- 30.Brown TM, Thapan K, Arendt J, Revell VL, Skene DJ. S-cone contribution to the acute melatonin suppression response in humans. J Pineal Res. 2021:e12719. [DOI] [PubMed] [Google Scholar]
- 31.Rideout V, Robb MB. The Common Sense census: Media use by kids age zero to eight. San Francisco, CA: Common Sense Media;2020. [Google Scholar]
- 32.Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1(2):102–114. [DOI] [PubMed] [Google Scholar]
- 33.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589(Pt 5):1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89(7):3610–3614. [DOI] [PubMed] [Google Scholar]
- 35.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.