SUMMARY
Background:
Data to inform positioning of biologic therapy, both first line and after previous biologic exposure in the treatment of moderate-to-severe Crohn’s disease (CD) is required. We assessed the comparative efficacy and safety of biologics in CD.
Methods:
A systematic review and network meta-analysis including induction (≥14 days) or maintenance (≥22 weeks) randomized controlled trials (RCTs) in adults (≥18 years) with moderate-to-severe CD (Crohn’s Disease Activity Index CDAI 220–450) treated with tumor necrosis factor (TNF) antagonists, anti-integrin, anti-interleukin (IL)-12/23p40, or anti-IL23p19 agents, relative to placebo or an active comparator, was conducted. MEDLINE, EMBASE, CENTRAL, conference proceedings, trial registries and unpublished data were searched from inception to 03/06/2021. The primary endpoint was induction of clinical remission (CDAI <150), extracted from published reports. Network meta-analysis using multivariate consistency model random-effects meta-regression was performed, with rankings based on surface under the cumulative ranking (SUCRA) probability.
Findings:
Based on 15 RCTs in 2,931 biologic-naïve patients, infliximab monotherapy (OR 4.53 [95% CI: 1.49, 13.79]) or combined with azathioprine (OR 7.49 [95% CI: 2.04, 27.49]), adalimumab (OR 3.01 [95% CI: 1.25, 7.27]), and ustekinumab (OR 2.63 [95% CI: 1.10, 6.28]), were associated with higher odds of inducing remission compared to certolizumab pegol; infliximab and azathioprine was also associated with higher odds of inducing remission compared to vedolizumab (OR 3.76 [95% CI: 1.01, 14.03]). There was no significant heterogeneity for any of the estimates. Based on 10 RCTs in 2,479 patients with previous biologic exposure, adalimumab after infliximab loss of response (OR 2.82 [95% CI: 1.20, 6.62]) and risankizumab (OR 2.10 [95% CI: 1.12, 3.92]) were associated with higher odds of inducing remission compared to vedolizumab, without significant heterogeneity. Most trials were at low or uncertain risk of bias.
Interpretation:
Although biologic treatment choices in patients with moderate-to-severe CD must be individualized for each patient, this analysis suggests that infliximab with azathioprine and adalimumab may be preferred first-line therapy, and adalimumab (after infliximab loss of response) or risankizumab may be preferred second-line therapy for induction of clinical remission.
Funding:
Siddharth Singh is supported by the NIH/NIDDK K23DK117058 and R03DK129631 and IOIBD Operating Grant 2019 (grants not specifically for this unfunded study). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No authors are employees of NIH. This study was not funded by industry.
Keywords: GRADE, inflammatory bowel disease, tumor necrosis factor, pharmacotherapy, remission
INTRODUCTION
Over the past two decades, the therapeutic armamentarium for the medical management of patients with moderate-to-severe Crohn’s disease (CD) has substantially expanded. While tumor necrosis factor (TNF) antagonists became the mainstay of treatment after their introduction in the late 1990s for patients with CD refractory to conventional therapies, approximately 30–40% patients are either primary non-responders or lose response to TNF blockade over time.1 Consequently, considerable investment in drug development for CD has resulted in the approval of two efficacious and safe biologic agents since 2015: vedolizumab, an anti-integrin monoclonal antibody, and ustekinumab, an interleukin (IL)-12/23p40 antagonist.2,3 Furthermore, positive phase 3 induction and maintenance trials for risankizumab, a monoclonal antibody targeting IL23p19, in both biologic-naïve and biologic-experienced patients with CD have recently been reported, representing a potential fourth class of advanced therapy for this patient population.
As more therapeutic options with differing efficacy and safety profiles become available, an important clinical question that remains unanswered is the appropriate positioning of different agents in the disease course.4 These choices can be complex and involve shared decision-making models that incorporate patient preferences, considerations of disease phenotype, the presence of extra-intestinal manifestations, and patient comorbidity.5 Choosing the right biologic at the right time has implications for a patient’s probability of response. For example, it is well recognized that poorer outcomes are achieved in patients who have previously failed a biologic therapy, potentially both as a surrogate measure of a more refractory phenotype, more bowel damage, or related to changes in the immune response induced by biologic exposure.6 Network meta-analyses, relying primarily on indirect comparisons, have helped inform clinical guidelines and health technology assessments on treatment sequence for first- and second-line biologics in patients with moderate-to-severe CD, especially in the absence of a predictive biomarker of treatment response, treatment futility, or direct head-to-head comparisons.7–9
Since the previous network meta-analysis on the topic, several important changes in the CD landscape have occurred. First, the understanding of the mechanistic role of IL23 in the pathogenesis of CD has been better elucidated.10 Several trials have now demonstrated that more specific IL23p19 blockade is more effective than anti-IL12/23p40 for other immune-mediated disorders, including psoriasis and psoriatic arthritis.11 Second, the first head-to-head trial in patients with moderate-to-severe CD (Safety and Efficacy of Adalimumab Versus Ustekinumab for One Year [SEAVUE]), comparing adalimumab and ustekinumab has recently been reported.12 Third, landmark ‘treatment strategy’ trials have confirmed that early combined immunosuppression with a TNF antagonist and azathioprine, as well as escalating to combination therapy to maintain tight control of inflammatory activity, results in better long-term patient outcomes.13,14 Taken together, these observations highlight the need to re-evaluate the optimal biologic sequence in CD.
Therefore, we conducted an updated systematic review with pairwise and network meta-analyses, comparing the relative efficacy and safety of TNF antagonists (infliximab, adalimumab, certolizumab pegol), anti-integrins (vedolizumab), anti-IL12/23p40 agents (ustekinumab), and anti-IL23p19 agents (risankizumab), either alone or in combination with immunosuppressants, for the treatment of moderate-to-severe CD in biologic-naïve and biologic-exposed populations.
METHODS
Search Strategy and Selection Criteria
We conducted a systematic review with network meta-analysis. The review was not registered. A comprehensive search of multiple electronic databases, including MEDLINE, EMBASE, and the Cochrane CENTRAL Register of Controlled Trials, from inception to June 3, 2021, without language restrictions was conducted. The full search strategy is detailed in the Appendix (p.2), was conducted by the senior author (CM), and captures terms relevant to randomized controlled trials and CD. Conference proceedings from Digestive Disease Week, the European Crohn’s and Colitis Organization Congress, and United European Gastroenterology Week (2016–2021) were also hand searched, and we reached out to experts in the field for any unpublished data. Two investigators independently searched the study title, abstract, and full texts of relevant studies, to identify articles of interest based on inclusion and exclusion criteria listed below. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analyses for health care interventions (Appendix p.23–25).15 Good research practices from the International Society for Pharmacoeconomics and Outcomes Research report for interpreting indirect treatment comparisons for healthcare decision-making were followed.16
Studies included in this meta-analysis were phase II or III randomized controlled trials (RCTs) meeting the following criteria: (1) including adult (age ≥18 years) patients with moderate-to-severe CD (defined by Crohn’s Disease Activity Index [CDAI] 220–450); (2) receiving a biologic treatment with TNF antagonists (infliximab, adalimumab, certolizumab pegol), anti-integrin (vedolizumab), anti-IL12/23p40 (ustekinumab) or anti-IL23p19 (risankizumab), either alone or in combination with immunosuppressants, as their first-line biologic or after previous biologic exposure; (3) minimum duration of therapy of 14 days for trials reporting induction of remission in active disease and 22 weeks in trials reporting maintenance of remission; (4) compared to another biologic agent or placebo; (5) reporting induction of clinical remission (CDAI <150), maintenance of remission (among patients with clinical response to induction therapy in re-randomization trials and among all patients in treat-through trials), or safety (serious adverse events [SAE] and/or infections as defined by the study authors). Only trials in immunosuppressant-naïve patients, in which patients were started on biologics and immunosuppressants in combination and compared with biologic monotherapy alone, were classified as trials of combination therapy. In contrast, trials specifically evaluating biologic therapy as an intervention in which some patients were previously or concurrently exposed to, and failed immunosuppressants at trial entry, were considered trials of respective biologic monotherapy.
We excluded studies where: (1) trial results were not stratified by biologic exposure status for induction therapy; (2) pediatric trials; and (3) trials of either novel therapies without reported phase III data or trials of advanced therapies not frequently used for the treatment of moderate-to-severe CD in clinical practice (examples include natalizumab, etrolizumab, abrilumab, brazikumab, mirikizumab, secukinumab, tofacitinib, filgotinib, upadacitinib, brodalumab, ozanimod, etrasimod, abatacept, mongersen). In a sensitivity analysis, we included interim results from the phase II induction data from the phase II/III GALAXI (A Study of the Efficacy and Safety of Guselkumab in Participants with Moderately to Severely Active Crohn’s Disease, NCT03466411).
Data Analysis
A standardized data extraction form was used to capture study-, participant-, disease-, and treatment-related study characteristics, and was done independently by two investigators (SS and CM). Any discrepancies were resolved by consensus or in consultation with a third reviewer (WJS). Summary estimates of the primary and secondary outcomes were extracted; individual patient-level data was not sought. The most complete report of trial data, based on intent-to-treat analysis principles for efficacy endpoints and last observation carried forward for safety endpoints, was used for extraction if study results were reported in multiple publications. Two study investigators also independently rated the risk of bias of included RCTs using the Cochrane risk-of-bias tool for randomized trials.
The primary efficacy outcome chosen in induction trials was achievement of clinical remission, defined by a CDAI<150. Secondary endpoints included achievement of clinical response (defined by a reduction in the CDAI ≥100 points compared to baseline). Safety endpoints in induction trials were not assessed due to the limitations of capturing adverse events in short-term studies. For maintenance trials, the primary efficacy outcome was maintenance of clinical remission (CDAI <150); both responder re-randomization and treat-through trials were included (although data informing remission rates among induction responders was preferentially used when available for the primary maintenance endpoint in treat-through studies). A sensitivity analysis was conducted excluding treat-through trials. The safety outcomes assessed in maintenance trials were SAEs and infections, as defined by the primary study authors. An inadequate number of trials reported endoscopic response and remission rates with consistent endpoint definitions to allow pooling in meta-analysis. We have summarized findings on endoscopic outcomes for each intervention based on clinical trials in the Appendix (p18).
Data from a therapy’s approved dose and administration was used when multiple doses were evaluated. For guselkumab, in the absence of data on approved dose of therapy being pursued for late-stage trials, we combined data on efficacy and safety for all doses. The timing of assessment for induction trials varied up to 16 weeks; when multiple time points were reported, data was preferentially used from week 6 or 8. For maintenance trials, outcomes were assessed at one year (preferentially at week 52). Unblinded long-term extension data were not used. The denominator used in all trials was based on intention-to-treat analysis with dropouts or missing data treated as non-responders for remission and response outcomes; last-observation-carried-forward was used for safety endpoints.
Other covariables which were extracted included location of trial conduct, number of trial sites, trial design (induction/maintenance, treat-through vs. induction responder re-randomization), sample size (and biologic naïve proportion), outcome timing and definitions, proportion of male participants, mean or median age and disease duration, disease distribution (proportion ileal, colonic, ileocolonic), proportion of patients on concomitant therapy (thiopurines, methotrexate, aminosalicylates, corticosteroids), treatment dosing. The Cochrane Risk of Bias tool for randomized controlled trials was used to assess the risk of selection, performance, detection, attrition, reporting, and other biases.
Pooled odds ratios (OR) with 95% confidence intervals (CI) were estimated using the DerSimonian and Laird random-effects method. A random effects model was selected given the anticipated differences between trials with respect to patient populations and interventions. Statistical heterogeneity was evaluated using the I2 statistic, interpreted as 0%−40% (heterogeneity may not be important), 30%−60% (moderate heterogeneity), 50%−90% (substantial heterogeneity), and 75%−100% (considerable heterogeneity). Small study effects and reporting bias were examined by assessing for funnel plot asymmetry. Direct comparisons were performed using Review Manager 5.4 (Cochrane Collaboration, Copenhagen, Denmark). Indirect comparisons in network meta-analysis were conducted using frequentist methods, using a multivariate consistency model random-effects meta-regression, conducted in STATA v15.0 (College Station, TX). The relative ranking of agents for each outcome was expressed using the surface under the cumulative ranking (SUCRA), which represents the percentage of efficacy or safety achieved by an agent compared to a hypothetical comparator that is always the best without uncertainty (i.e., SUCRA=100%). Higher SUCRA scores correspond to higher ranking for induction/maintenance of efficacy outcomes and higher ranking for safety (i.e., lowest risk of SAE or infection).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria were used to appraise confidence in estimates derived from direct and indirect comparisons of efficacy outcomes. In this approach, direct evidence from RCTs starts at high confidence, and is rated down by risk of bias, indirectness, imprecision, inconsistency/heterogeneity, and/or publication bias to moderate, low, and very low confidence. Indirect evidence starts at the lowest rating of the two pairwise estimates that contribute as first-order loops to the indirect estimate but can be further down-rated for imprecision or intransitivity between direct and indirect comparisons.
Role of the Funding Source
This was an unfunded study. No sponsors had any role in study design, data collection, analysis, interpretation, or writing. All authors had full access to the data and accept responsibility for publication.
RESULTS
Search Results and Study Characteristics
The search strategy yielded 18,382 citations, of which 7,584 were duplicates and removed. Of the remaining 10,798 records that were screened, 569 full text articles were reviewed, of which 31 trials were eligible for inclusion (Appendix p.3). A total of 15 RCTs evaluated induction efficacy in biologic-naïve patients,12,17–29 10 RCTs evaluated induction efficacy in biologic-experienced patients,20–22,24,28–32 and 15 RCTs evaluated maintenance of remission in patients with moderate-to-severe CD.12,20–22,25,26,28,31,33–40 Patient and trial-level characteristics are summarized in the appendix (p.14–15); most trials included an even distribution of patients by sex, with mean age approximately in the 30–40 years range, although disease duration and location were more heterogeneous. Most trials were at low risk for bias or were at uncertain risk for bias if only published in abstract form (Appendix p.19). One head-to-head active comparator trial of two biologic therapies (ustekinumab versus adalimumab, SEAVUE) was identified; this trial did not allow concomitant immunosuppression with thiopurines or methotrexate.12 Two head-to-head active comparator trials of combination therapy (biologic + azathioprine vs. biologic alone) were identified.25,26 The network of included trials is shown in Figure 1. All other included trials required conventional treatment failure as an inclusion criterion for enrolment. All outcomes were uniformly assessed based on standard definitions of the CDAI as previously described, between weeks 4 and 12 for induction therapy and weeks 22 and 60 for maintenance therapy. Funnel plots did not show evidence of small study effects (Appendix p.4).
Figure 1.
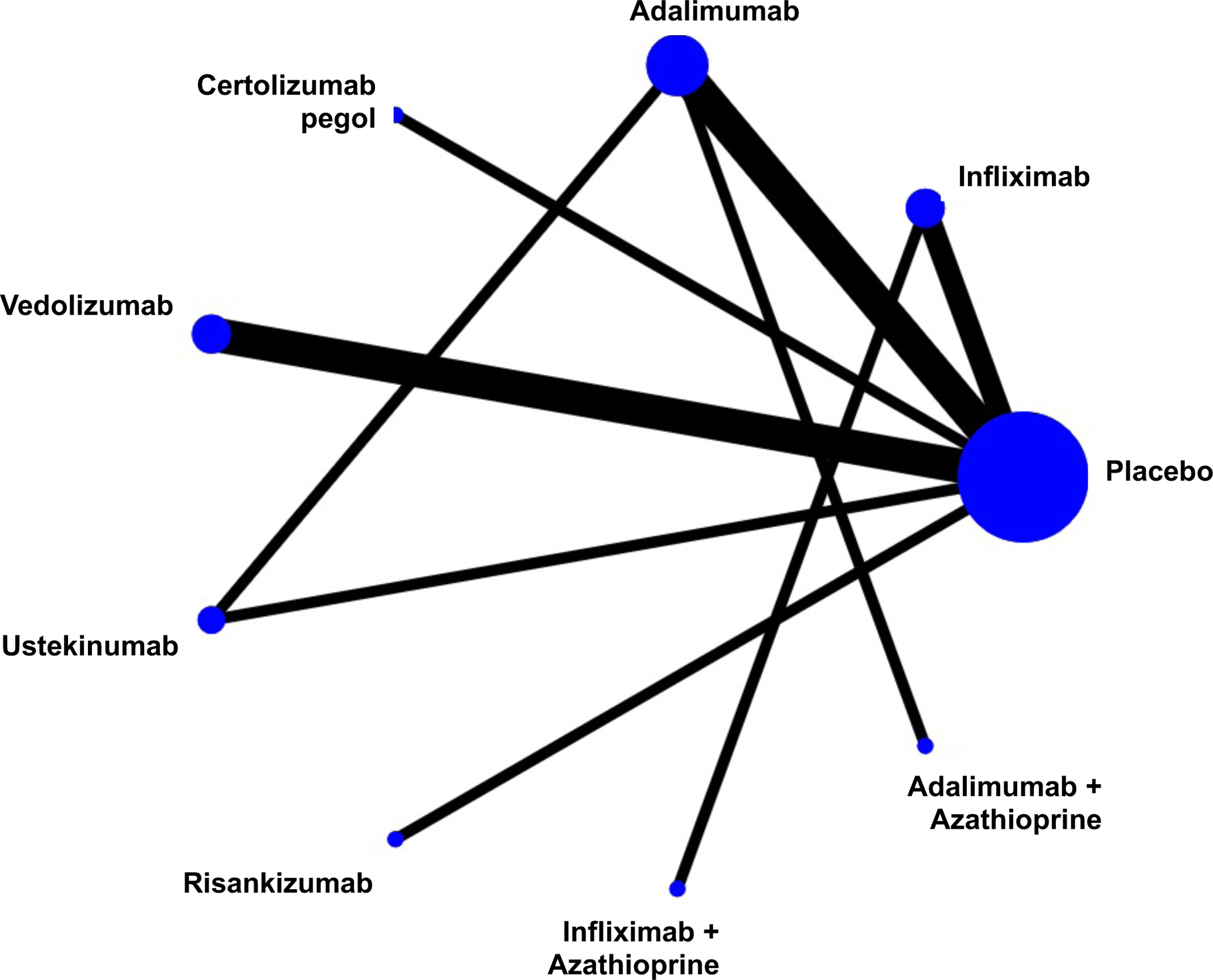
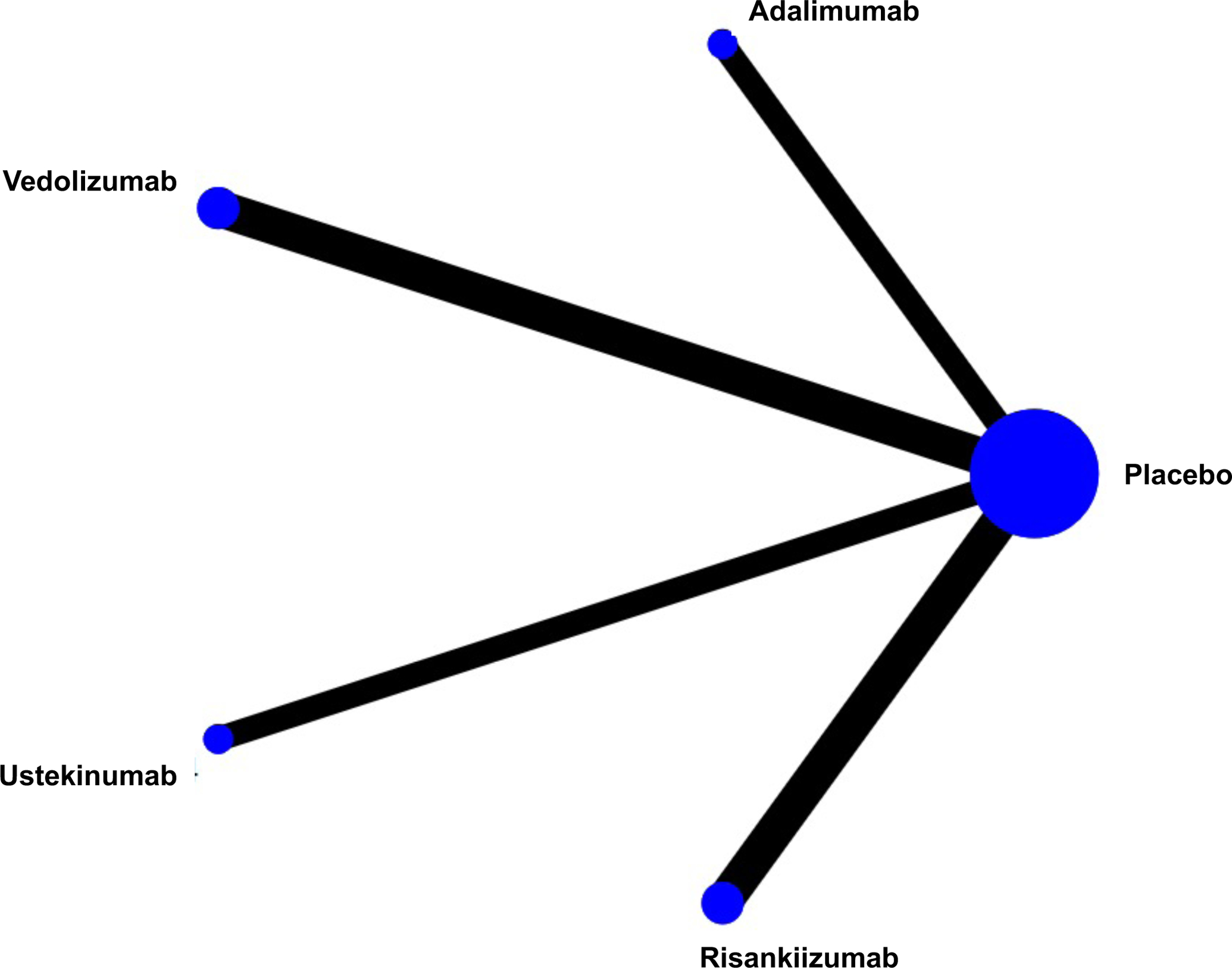
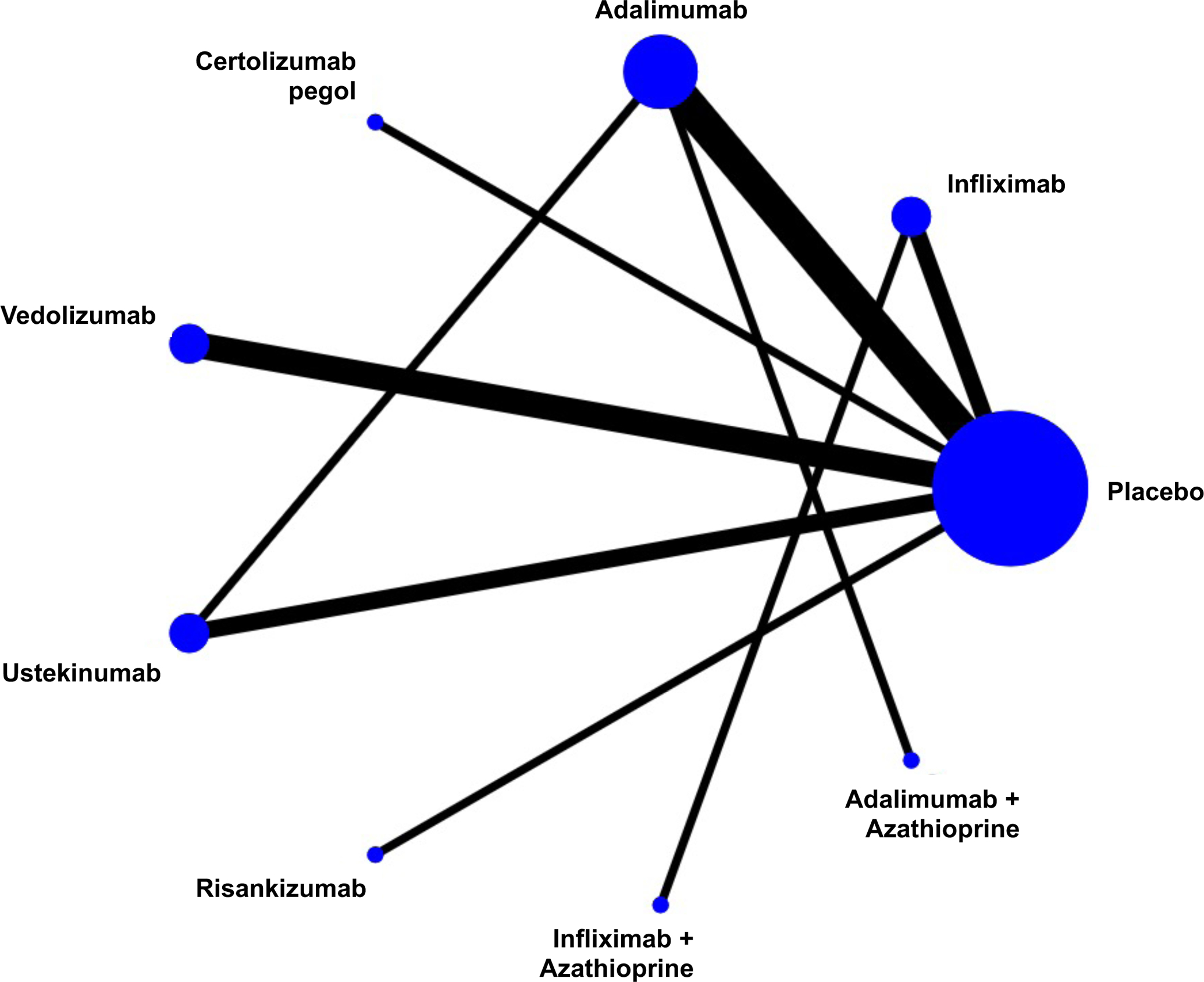
Network of included studies with the available direct comparisons for (A) induction of clinical remission in biologic-naïve patients, (B) induction of clinical remission in biologic-exposed patients, and for (C) maintenance of remission in patients with moderate-severe Crohn’s Disease. The size of the nodes and the thickness of the edges are weighted according to the number of studies evaluating each treatment and direct comparison, respectively.
Induction Therapy in Biologic-Naïve Patients
A total of 15 RCTs including 2,931 biologic-naïve patients with moderate-to-severe CD assessed induction response after treatment with infliximab (n=3 trials), adalimumab (n=5 trials), certolizumab pegol (n=1 trial), vedolizumab (n=3 trials), ustekinumab (n=3 trials), and risankizumab (n=1 trial). On direct meta-analysis, all agents except certolizumab pegol, were significantly superior to placebo for inducing clinical remission (Appendix p.6). The largest effect sizes compared to placebo were for infliximab (OR 6.55 [95% CI: 2.31, 18.55]) and adalimumab (OR 5.31 [95% CI: 2.99, 9.43]). There was no benefit of ustekinumab over adalimumab in one head-to-head trial for inducing clinical remission (OR 1.11 [95% CI: 0.74, 1.65]), and no benefit of combination azathioprine with adalimumab (OR 0.57 [95% CI: 0.29, 1.10]) over adalimumab alone. In one direct comparison, infliximab and azathioprine in combination was superior to infliximab alone (OR 1.65 [95% CI: 1.07, 2.54]). There was no significant heterogeneity for any of the estimates for induction of clinical remission.
On network meta-analysis, there was moderate confidence in estimates supporting the use of infliximab monotherapy (OR 4.53 [95% CI: 1.49, 13.79]), infliximab with azathioprine (OR 7.49 [95% CI: 2.04, 27.49], adalimumab (OR 3.01 [95% CI: 1.25, 7.27]), or ustekinumab (OR 2.63 [95% CI: 1.10, 6.28]) over certolizumab pegol for induction of remission (Figure 2). There was low confidence in estimates supporting the use of infliximab combination therapy over vedolizumab (OR 3.76 [95% CI: 1.01, 14.03]) and certolizumab pegol (OR 7.49 [95% CI: 2.04, 27.49] (rated down for very serious indirectness being derived from second order loops), and very low confidence in estimates supporting the use of infliximab combination therapy over adalimumab (monotherapy or in combination), ustekinumab or risankizumab in biologic-native patients (rated down for very serious indirectness being derived from second order loops, and for serious imprecision) (Appendix p.20–21). There was no significant difference between ustekinumab and vedolizumab, nor risankizumab and ustekinumab. On loop-specific analysis where both direct and indirect evidence was available, no inconsistency was identified. Overall, infliximab in combination with azathioprine (SUCRA 0.96) and infliximab alone (SUCRA 0.81) were ranked highest, followed by adalimumab (SUCRA 0.67), ustekinumab (SUCRA 0.58), risankizumab (SUCRA 0.49), vedolizumab (SUCRA 0.45) and certolizumab pegol (SUCRA 0.15) (Appendix p.5). The proportion of biologic-naïve patients expected to achieve remission with each treatment is summarized in Appendix (p.22).
Figure 2.
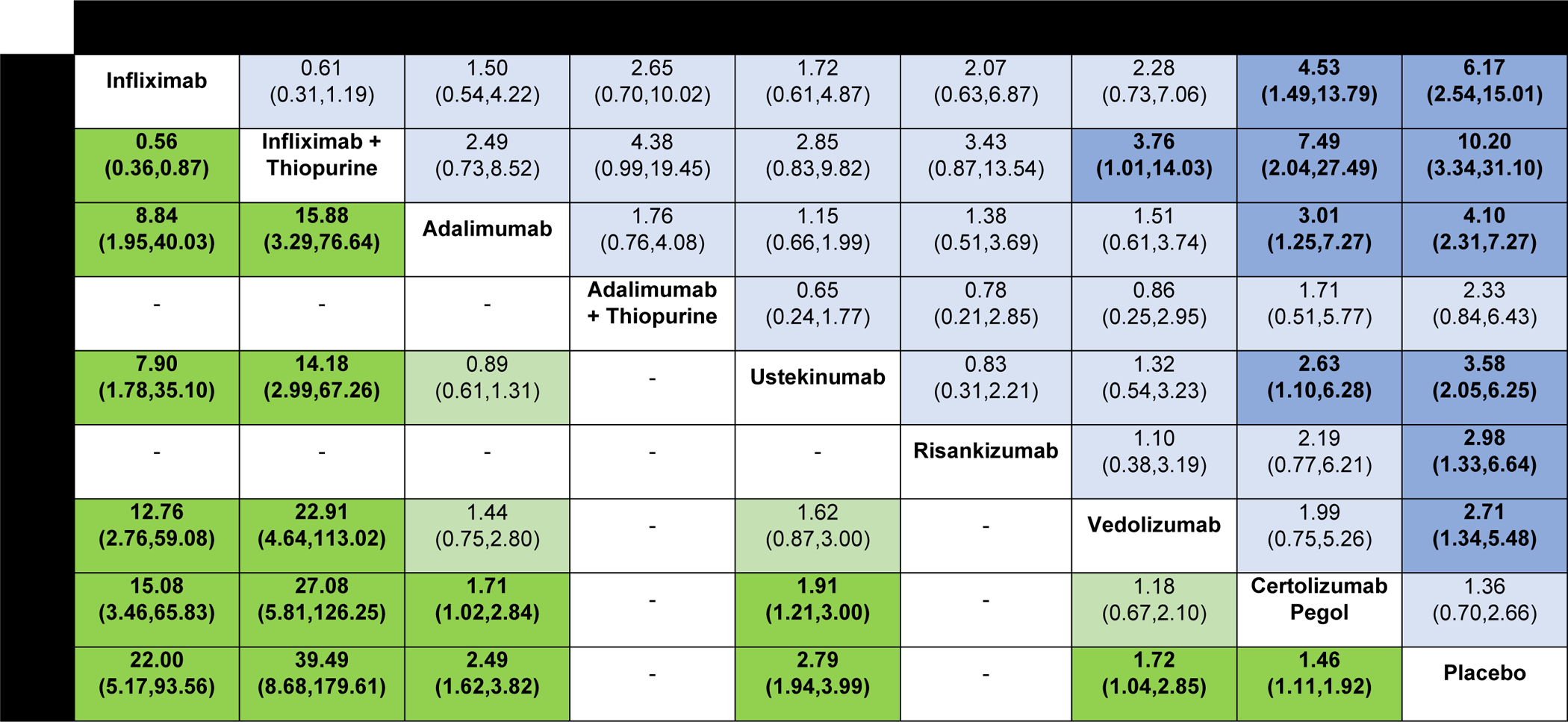
Comparative efficacy of biologic agents for induction of clinical remission and clinical response in biologic-naïve patients with moderate-to-severe Crohn’s disease using network meta-analysis, expressed as odds ratios with 95% confidence intervals. Comparisons read from left-to-right; odds ratios for comparisons in the cell in common between column- and row-defining treatment. Odds ratios >1 favor row-defining treatment. For induction of clinical remission, odds ratio >1 favors row-defining treatment. For induction of clinical response, odds ratio >1 favors column defining treatment.
A sensitivity analysis including data from the phase II GALAXI induction trial with the placebo and ustekinumab comparator arms was also conducted. Guselkumab was highly ranked (SUCRA 0.79) although this was based on small numbers (45/73, 61.6% patients achieving clinical remission after induction). The ranking of the other agents was otherwise unchanged.
Direct and network comparisons for induction of clinical response were also similar and summarized in Figure 2 and the appendix (p.7). There was low quality evidence that both infliximab and infliximab with azathioprine were significantly superior to other agents and to placebo for inducing clinical response (downgraded due to imprecision of estimates).
Induction Therapy in Biologic-Experienced Patients
Overall, 10 RCTs including 2,479 patients with moderate-to-severe CD who had previous biologic exposure were identified. Therapies that were evaluated included adalimumab (n=2 trials), vedolizumab (n=3 trials), ustekinumab (n=2 trials), and risankizumab (n=2 trials) (Figure 1B). The number of permitted biologic failures was highest for patients treated with risankizumab: in the MOTIVATE phase III trial, 51.8% (99/191) of patients randomized to risankizumab 600 mg IV had failed up to 5 biologics including 18.8% (36/191) who had failed ustekinumab prior to enrolment. One trial of adalimumab (GAIN) included only patients with prior infliximab response or intolerance to infliximab but excluded primary infliximab non-responders. The GEMINI-III trial included ~75% of patients with prior TNF antagonist failure, of whom 28.2% (59/209) had failed one, 39.2% (82/209) had failed two, and 6.7% (14/209) had failed three TNF antagonists at enrolment. No data from direct head-to-head active comparator trials in biologic-exposed patients were available.
On direct meta-analysis, ustekinumab (OR 2.55 [95% CI: 1.39, 4.69]) and risankizumab (OR 2.64 [95% CI: 1.89, 3.68]) were associated with a significantly higher odds of inducing clinical remission compared to placebo (Appendix p.9). Adalimumab (OR 3.55 [95% CI: 1.82, 6.93]) was also associated with higher odds of inducing clinical remission after loss of response or intolerance to infliximab compared to placebo; this was primarily driven by the GAIN trial where patients with primary non-response to infliximab were excluded. There was no significant heterogeneity for any of the estimates. Vedolizumab was significantly associated with induction of clinical response (OR 1.77 [95% CI: 1.06, 2.94]) but not clinical remission (OR 1.25 [95% CI: 0.63, 2.49]) compared to placebo in patients with previous biologic exposure.
On network meta-analysis, there was low quality evidence supporting the use of adalimumab (OR 3.55 [95% CI: 1.82, 6.93]) in biologic exposed patients; the quality of evidence was downgraded due to selective inclusion of patients with prior infliximab response or intolerance in the GAIN trial and imprecision due to a small number of events. There was also low confidence in estimates supporting the use of vedolizumab (OR 1.26 [95% CI: 0.74, 2.14]) for inducing clinical remission over placebo (very serious imprecision with wide confidence intervals, crossing unity) (Figure 3). There was moderate confidence in estimates supporting risankizumab (OR 2.10 [95% CI: 1.12, 3.92]) over vedolizumab for inducing remission in biologic-exposed patients. There was low confidence in estimates supporting adalimumab (OR 2.82 [95% CI: 1.20, 6.62]) over vedolizumab for biologic-exposed patients (downgraded due to imprecision due to small number of events and indirectness due to previously identified selective inclusion criteria in the GAIN trial). Overall, adalimumab (SUCRA 0.92), risankizumab (SUCRA 0.77), and ustekinumab (SUCRA 0.53) were ranked higher than vedolizumab (SUCRA 0.23) for inducing clinical remission in this patient population (Appendix p.5). The proportion of biologic-exposed patients anticipated to achieve clinical remission with each treatment is summarized in the appendix (p.22)
Figure 3.
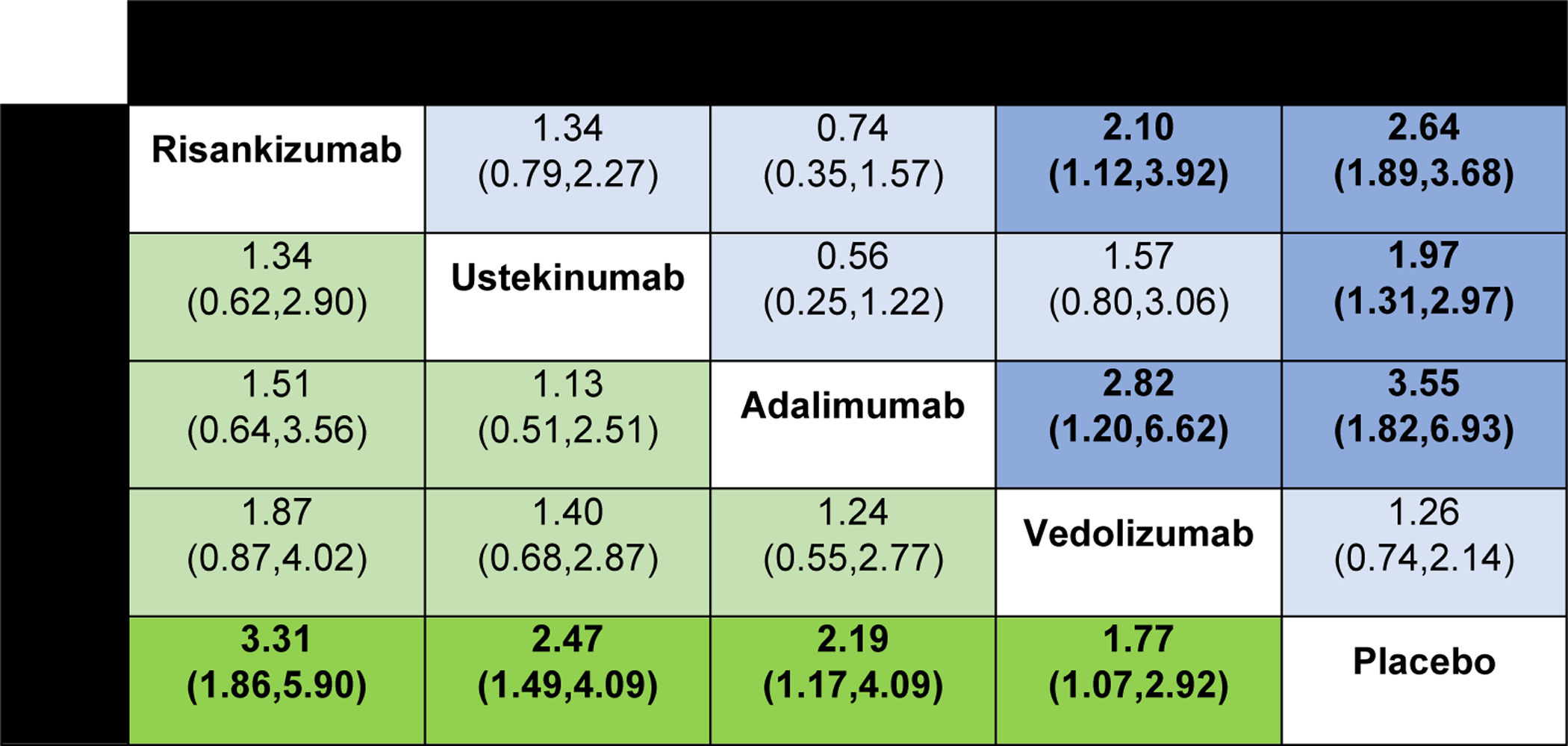
Comparative efficacy of biologic agents for induction of clinical remission and clinical response in biologic-exposed patients with moderate-to-severe Crohn’s disease using network meta-analysis, expressed as odds ratios with 95% confidence intervals. Comparisons read from left-to-right; odds ratios for comparisons in the cell in common between column- and row-defining treatment. Odds ratios >1 favor row-defining treatment. For induction of clinical remission, odds ratio >1 favors row-defining treatment. For induction of clinical response, odds ratio >1 favors column defining treatment.
On network meta-analysis, no agent was clearly superior to others for induction of clinical response among biologic-exposed patients (Figure 3). For clinical response, the overall ranking was highest for risankizumab (SUCRA 0.87) and ustekinumab (SUCRA 0.67).
Maintenance Therapy
A total of 15 maintenance RCTs including 3,786 patients were included, treated with infliximab (n=3 trials), adalimumab (n=5 trials), certolizumab pegol (n=1 trial), vedolizumab (n=3 trials), ustekinumab (n=3 trials), and risankizumab (n=1 trial). One head-to-head trial of ustekinumab compared to adalimumab (SEAVUE) was identified. Most maintenance trials were designed as responder re-randomization studies, in which only patients with clinical response to induction therapy were re-randomized to intervention or comparator; for the SEAVUE trial, week 52 outcomes among week 12 induction responders were used to assess maintenance efficacy although this was designed as a treat-through trial. Although three treat-through trials were included (EXTEND, SONIC, and DIAMOND), a sensitivity analysis excluding treat-through maintenance studies was performed and did not demonstrate a significant difference in outcomes. Similarly, there was no difference when excluding a 22-week trial of maintenance therapy with ustekinumab. Only one trial of risankizumab maintenance therapy (FORTIFY) was identified, with pivotal findings having been presented in a press release.
On direct meta-analysis, all agents were superior to placebo for maintenance of remission (Appendix, p.12), with similar effect sizes. On network meta-analysis, there was moderate confidence in estimates supporting the efficacy of adalimumab or infliximab (either as monotherapy or as combination therapy), ustekinumab, risankizumab, vedolizumab, and certolizumab pegol over placebo as maintenance therapy (Figure 4). No individual agent was superior to others for maintenance of remission. There was very low confidence in estimates supporting the use infliximab with azathioprine over vedolizumab or risankizumab for maintenance of remission. Overall, infliximab with azathioprine (SUCRA 0.87), and adalimumab (SUCRA 0.78) were highest ranked, followed by infliximab monotherapy (SUCRA 0.63). A sensitivity analysis was conducted excluding treat-through trials: the ranking of individual agents was similar (adalimumab SUCRA 0.83, infliximab SUCRA 0.74).
Figure 4.
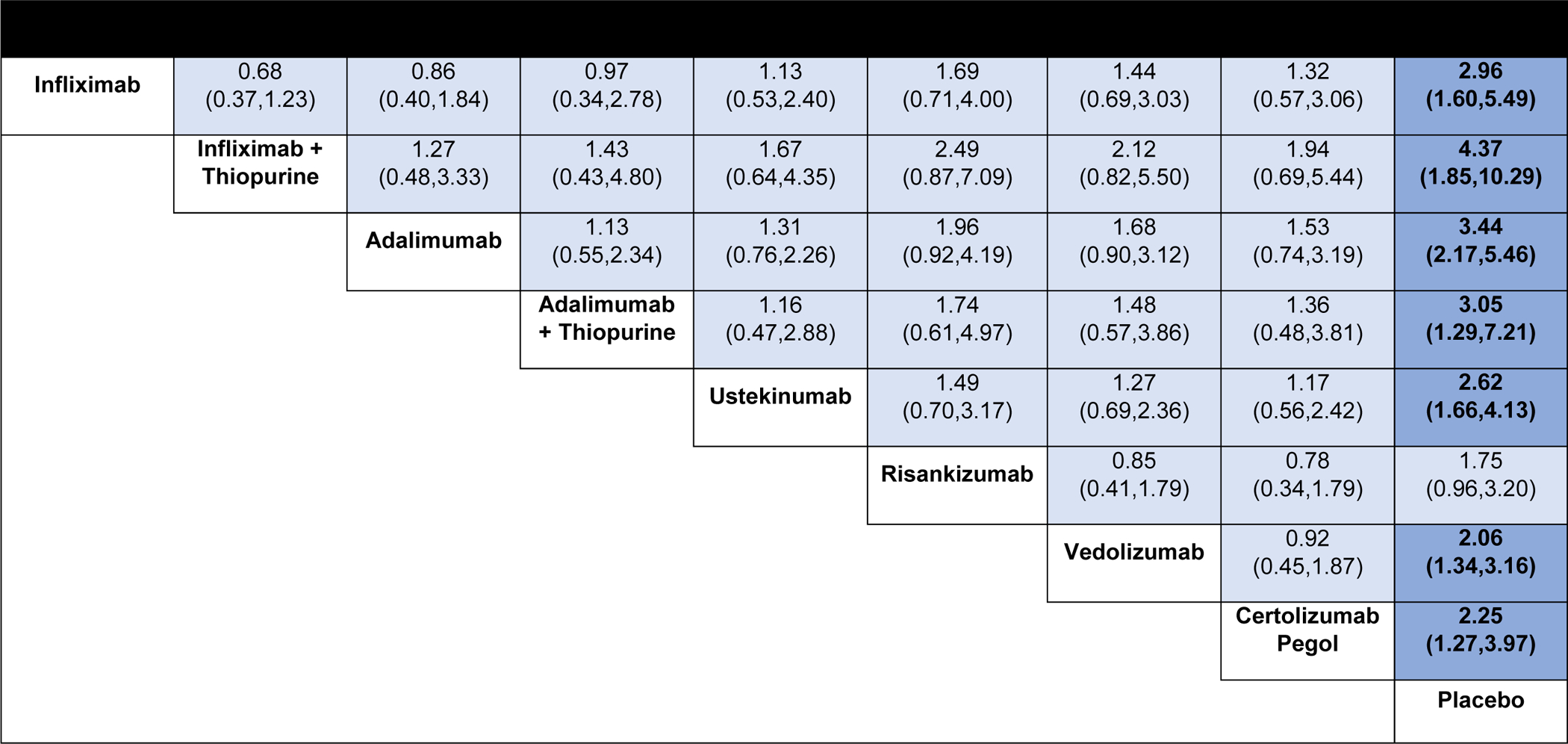
Comparative efficacy of biologic agents for maintenance of clinical remission in patients with moderate-to-severe Crohn’s disease using network meta-analysis, expressed as odds ratios with 95% confidence intervals. Comparisons read from left-to-right; odds ratios for comparisons in the cell in common between column- and row-defining treatment. For maintenance of clinical remission, odds ratio >1 favors row-defining treatment.
Safety
Serious adverse events:
Serious adverse events in trials were defined using the original author definitions and included worsening of underlying CD. On network meta-analysis, risk of SAEs was lower in patients treated with infliximab and azathioprine compared to infliximab monotherapy (OR 0.56 [95% CI: 0.33, 0.97]) (Figure 5). Infliximab and azathioprine (SUCRA 0.86), adalimumab (SUCRA 0.78), and ustekinumab (SUCRA 0.57) were highest ranked (lowest risk of SAE).
Figure 5.
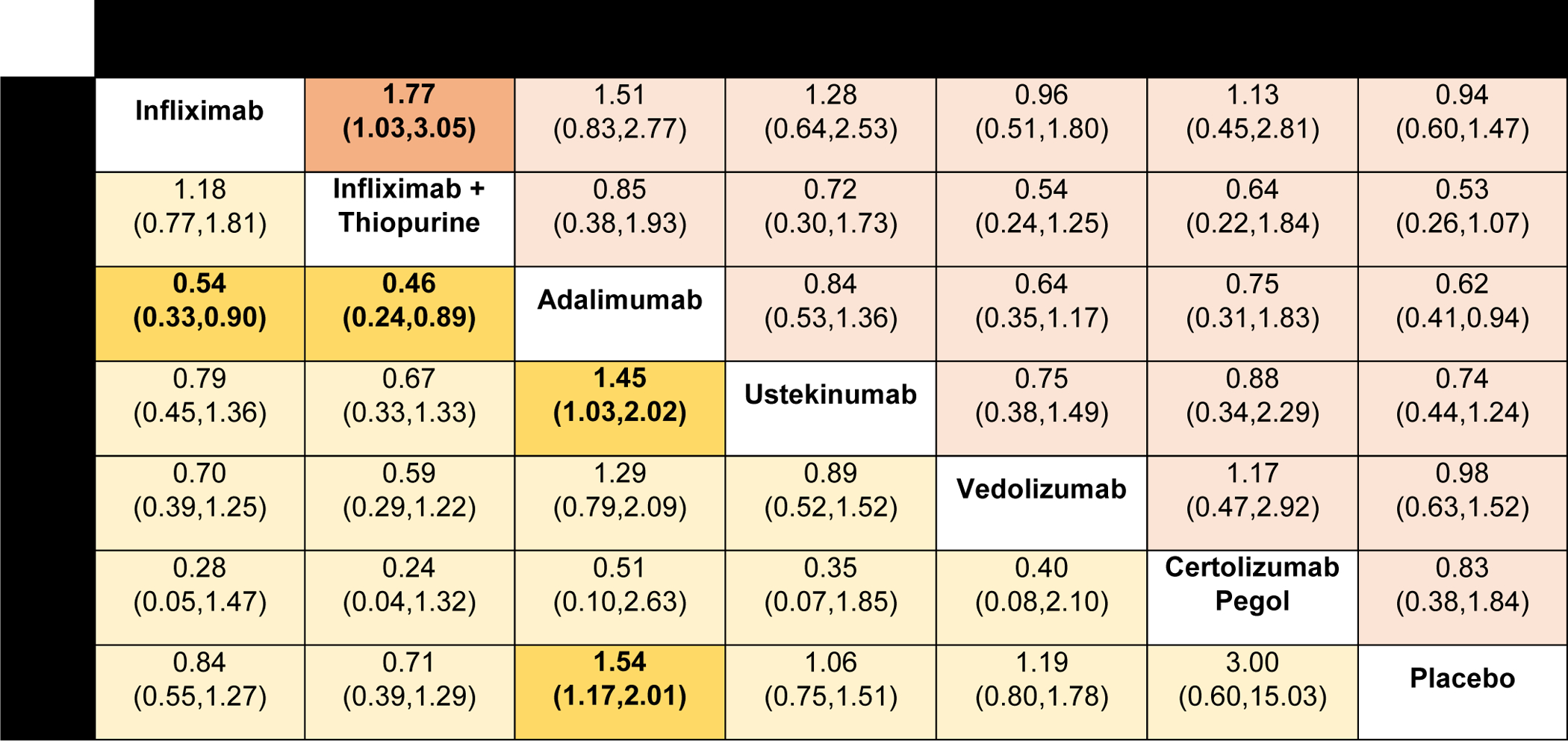
Comparative safety of biologic agents for risk of serious adverse events and any infections in patients with moderate-to-severe Crohn’s disease, using network meta-analysis, expressed as odds ratios with 95% confidence intervals. Comparisons read from left-to-right; odds ratios for comparisons in the cell in common between column- and row-defining treatment. For serious adverse events, odds ratio <1 favors row-defining treatment. For risk of infections, odds ratio <1 favors column-defining treatment.
Infections:
In trials of maintenance therapy, adalimumab therapy was associated with a higher infection rate compared to placebo (OR 1.54 [95% CI: 1.17, 2.01]), whereas none of the other interventions was associated with increased risk of infections (Figure 5). Infliximab alone (OR 0.54 [95% CI: 0.33, 0.90]), infliximab and azathioprine (OR 0.46 [95% CI: 0.24, 0.89]), and ustekinumab (OR 0.69 [95% CI: 0.50, 0.97]) were associated with fewer infections compared to adalimumab. Overall, for the risk of infection, infliximab combination therapy with azathioprine (SUCRA 0.90), infliximab alone (SUCRA 0.77), and ustekinumab (SUCRA 0.55) were highest rated (lowest risk of infection), followed by vedolizumab (SUCRA 0.41), adalimumab (SUCRA 0.16), and certolizumab pegol (SUCRA 0.10).
DISCUSSION
In this updated systematic review and network meta-analysis, we combined available direct and indirect evidence from 31 randomized controlled trials to evaluate the efficacy of different pharmacologic therapies for induction and maintenance of clinical remission, in both biologic-naïve and biologic-exposed populations. Several important findings from this network meta-analysis may influence therapeutic decisions. First, TNF antagonist treatment with either infliximab or adalimumab consistently ranks highly for induction and maintenance of clinical remission. This is especially relevant for patients with moderate-to-severe biologic-naïve CD treated with combination infliximab and azathioprine, which was superior to infliximab alone in a direct head-to-head comparison, and probably superior to vedolizumab and certolizumab pegol in indirect comparisons. Second, IL23 blockade may be the preferred mechanism of action in patients who have been previously exposed to TNF antagonists. Both ustekinumab and risankizumab have demonstrated efficacy in this patient population, and risankizumab was observed to be likely superior to vedolizumab for inducing clinical remission in network meta-analysis. Adalimumab is probably efficacious in a subset of patients with secondary loss of response to infliximab due to immunogenicity or intolerance. Finally, no specific therapy was identified to be superior to other agents for maintaining clinical remission or having a lower SAE risk, although these results should be interpreted cautiously given that most clinical trials are underpowered for rare adverse events. Taken together, these findings will help inform the optimal sequencing of first- and second-line biologic therapy for patients with moderate-to-severe CD.
Despite the introduction of several new classes of biologic therapy, TNF antagonists still play a central role in the treatment of moderate-to-severe CD. Notwithstanding the potential cost benefits associated with biosimilar TNF antagonists compared to novel treatments such as ustekinumab, vedolizumab, or risankizumab, there is a clear role for TNF antagonists in patients with high-risk phenotypes such as fistulizing or penetrating disease41,42, emerging evidence for use in stricturing CD43,44, and advantages for patients with associated TNF-sensitive extraintestinal manifestations.45 In our analysis, both infliximab and adalimumab were highly ranked for induction and maintenance of clinical remission in biologic naïve CD. In contrast, certolizumab pegol was ranked inferior to infliximab, adalimumab, and ustekinumab for induction of clinical remission although we acknowledge that there are no direct comparator studies including certolizumab pegol, initial dose finding studies may have been inadequate, and there is low confidence in estimates supporting large effect sizes for infliximab due to a lack of similarly designed induction trials.46
Our findings in biologic-naïve CD patients were strengthened by the addition of the first head-to-head trial of biologic therapies in this population: in the SEAVUE trial, biologic-naïve CD patients were randomized 1:1 in a double-blind, double-dummy trial to either standard dose ustekinumab or adalimumab.12 Although patients in both treatment groups achieved high rates of clinical and endoscopic remission, there were no difference observed between the ustekinumab or adalimumab-treated patients for the primary endpoint of clinical remission at Week 52 (64.9% ustekinumab vs. 61.0% adalimumab, p=0.417), nor any of the major secondary endpoints, including corticosteroid-free remission, clinical response, Week 16 clinical remission, and endoscopic remission or response. The high rates of clinical and endoscopic remission observed in the SEAVUE trial are most likely related to treatment of an early disease (median duration 2.58 years) population with more moderate disease burden at baseline (only a single ulcer required for enrolment, median Simple Endoscopic Score for CD 8.0).47,48
In addition to including the SEAVUE trial to anchor our comparative network, an important strength of our analysis is the inclusion of two additional active comparator trials (SONIC and DIAMOND) evaluating combination TNF antagonists and azathioprine with infliximab or adalimumab monotherapy. Our network meta-analysis highlights that infliximab in combination with azathioprine is the highest ranked treatment for induction of clinical remission in biologic-naïve patients and maintenance of remission long-term. Combination thiopurine use has been demonstrated to mitigate the risk of immunogenicity and increase serum drug trough concentrations.49 Early combination treatment with infliximab and azathioprine has been associated with improvements in achieving corticosteroid- and surgery-free remission at Week 26 in the Step Up Top Down trial50, and in the REACT cluster randomized trial, it was demonstrated that early combined immunosuppression with adalimumab and an anti-metabolite reduced the two-year risk of major CD-related adverse outcomes, including surgery, hospitalization, or serious disease-related complications (hazard ratio 0.73 [95% CI: 0.62–0.86, p<0.0001) compared to conventional therapy, even though no differences were observed in rates of clinical remission at 12 months.13 However, it should be noted that the DIAMOND trial did not show a significant benefit to adalimumab combination therapy with azathioprine.26 While this trial was limited by the open-label nature and low dose of azathioprine exposure (25–50 mg/day starting dose, maximum dose 100 mg), other authors have similarly found variable benefits to combination treatment with adalimumab.51
In biologic-exposed patients, our network meta-analysis supports the use of ustekinumab or risankizumab for induction of clinical remission. Although adalimumab was highest ranked for this endpoint, this estimate was primarily driven by results from the GAIN trial, which selectively recruited patients with secondary loss of response or intolerance to infliximab failure (quality of evidence rated down for intransitivity and imprecision).52 The GAIN trial also only permitted failure of a single biologic, whereas more recent trials such as UNITI allowed failure of multiple TNF antagonists and ADVANCE/MOTIVATE permitted failure of multiple TNF antagonists as well as other mechanisms of action. Our understanding of the mechanisms underlying failure of TNF antagonist has since improved over the past decade and switching within class is more effective for patients with pharmacokinetic failure leading to secondary loss of response as opposed to primary non-response from mechanistic pharmacodynamic resistance.53 In contrast, both ustekinumab and risankizumab have been demonstrated to be effective after either primary or secondary loss of response to other biologics. Furthermore, approximately half of patients enrolled in the UNITI-I trial had failed multiple TNF antagonists21, and 28.3% (95/336) and 51.8% (99/191) of patients receiving risankizumab 600 mg IV had failed multiple biologics in the ADVANCE and MOTIVATE trials, respectively.29 This reflects an increasing proportion of patients managed in clinical practice, who have failed multiple advanced therapies. Our network meta-analysis suggests that IL23 blockade is superior to anti-integrin therapy for inducing clinical remission in moderate-to-severe CD patients who have failed TNF antagonists. Although we acknowledge that no direct head-to-head comparisons have been conducted in this population, these results are consistent with multiple recent real-world observations that suggest ustekinumab is more effective than vedolizumab after TNF antagonists failure.54–56 For example, Biemans et al. reported results from the Dutch Initiative on Crohn’s and Colitis, a national prospective registry including 128 vedolizumab- and 85 ustekinumab-treated patients who had failed a TNF antagonist; ustekinumab-treated patients were approximately 2.5 times more likely to achieve corticosteroid-free clinical remission compared to vedolizumab-treated patients, even after adjusting for disease- and patient-related confounders and after propensity score matching.56 Potential differences in response to IL23 blockade versus anti-integrin therapy after TNF antagonists failure may be putatively explained by molecular and immunologic changes induced by prior treatment: in a seminal paper by Schmitt et al., the investigators demonstrated that TNF antagonist exposure was associated with higher expression of TNF receptor 2 (TNFR2) in responders, but significant upregulation of mucosal IL23p19, IL23 receptor (IL23R), and IL-17A in TNF antagonist non-responders.57 Furthermore, non-responders demonstrated an expansion of apoptosis-resistant intestinal TNFR2+IL23R+ T-cells, with IL23 exposure in the context of TNF antagonist treatment activating a Th1/Th17-like phenotype and abrogating TNF antagonist-induced apoptosis in mucosal T-cells. Overall, these findings suggest that targeting IL23 may be an effective strategy for TNF-antagonist resistant patients based on molecular mechanisms.
No individual agents were clearly superior for maintenance of remission or for the risk of SAEs in our meta-analysis. However, these findings should be interpreted cautiously for several reasons. First, the maintenance RCTs included in this analysis evaluated endpoints at one year. Longer term data from extension studies are not included here as these are generally open label without a comparator group, although have demonstrated long-term maintenance of remission for multiple agents, including ustekinumab and vedolizumab.58,59 Long-term persistence of therapy with these agents has often been attributed to the low risk of immunogenicity, especially in comparison to TNF antagonist monotherapy.60 Second, definitions of SAEs were based on the original authors, and includes worsening CD: this likely explains the lower risk of SAEs observed with infliximab combination therapy compared to infliximab monotherapy, since the former was more efficacious and hence, was associated with lower risk of worsening CD. A previous systematic review and meta-analysis demonstrated that combination TNF antagonists and immunosuppressive therapy was associated with a higher risk of serious infections compared to TNF antagonist monotherapy alone (relative risk 1.19 [95% CI: 1.03, 1.37]).61 Third, RCTs designed for registrational purposes are generally underpowered to detect rare adverse events. For example, although none of the agents except adalimumab monotherapy were associated with an increased risk of serious infections over placebo in this meta-analysis, registry studies have confirmed an increased risk of serious infections with all TNF antagonists (not just adalimumab). Therefore, safety should be considered based on the totality of both randomized trial, registry, long-term pharmacovigilance, and real-world evidence, and in the context of effectiveness of treatment approach in achieving corticosteroid-free remission. Though mechanistically vedolizumab may cause a lower degree of systemic immunosuppression compared with TNF antagonists, real-world studies have suggested that risk of serious infections is similar in vedolizumab- and TNF antagonist-treated patients with CD.62,63 This may be related to differences in treatment effectiveness as observed in our analyses. Safety surveillance data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) study demonstrated lower numerical rates of serious infections for ustekinumab- (0.93 per 100 patient-years) compared to infliximab-treated patients (2.91 per 100 patient-years), and a significantly lower hazard ratio overall for serious infections compared to other biologics; similarly, integrated safety analyses from phase II and III RCTs in UC and CD demonstrate a comparable safety profile of ustekinumab to placebo.64,65
Our study has several strengths. To our knowledge, this is the first meta-analysis in patients with moderate-to-severe CD to: (1) include direct head-to-head evidence comparing different biologic therapies, which substantially strengthens our comparative network; (2) incorporate the impact of combination azathioprine with infliximab and adalimumab therapy, a strategy which is increasingly important in clinical practice for maintaining tight disease control and preventing long-term disease-related complications; and (3) evaluate the relative efficacy and safety of a novel IL23p19 antagonist using phase III data, which will help inform the positioning of risankizumab as it becomes available in the therapeutic landscape.
However, we acknowledge several important limitations, in addition to any limitations from the original trials. First, there remains a paucity of head-to-head trials in this space that can inform direct treatment comparisons. However, several studies now include active comparator arms, and a phase III head-to-head trial comparing ustekinumab and risankizumab in patients previously failing TNF antagonists is currently recruiting (SEQUENCE, NCT04524611). These results will be eagerly awaited. We did include induction data from the phase II/III GALAXI trial as part of a sensitivity analysis, which includes an ustekinumab comparator arm; these results did not appreciably change treatment rankings. Second, we used clinical remission as the primary outcome. Although achieving corticosteroid-free remission and endoscopic remission have been increasingly recognized as important therapeutic objectives in patients with CD, we felt the substantial heterogeneity in the reporting and definitions of these endpoints made pooling of these endpoints challenging.66 The regulatory co-primary endpoint including clinical remission and endoscopic response is a relatively recent innovation, only used in the risankizumab phase III RCTs. Third, although not statistically significant for any of the outcomes evaluated, we acknowledge that there is between-study heterogeneity in the characteristics of enrolled patients and trial design features such primary assessment timepoint (which can influence treatment efficacy), particularly as studies have evolved over the last two decades. This is perhaps most evident when comparing the landmark infliximab trials conducted in the late 1990s with more recent phase III registrational programs. Over time, an increasingly refractory population of patients have been enrolled in CD RCTs, not only reflected in the proportion of patients failing previous biologics (including multiple mechanisms of action or multiple TNF antagonists in recent trials), but also with respect to other surrogate measures of disease activity. For example, while the median disease duration in patients enrolled the Lemann et al infliximab trial ranged from 3–7 years,18 patients in the UNITI phase III ustekinumab program had mean disease durations ranging from 8.7 to 12.7 years at induction.21 In the absence of individual patient data from clinical trials, these changes in trial populations over time influence the interpretation of relative efficacy and safety of treatment. Besides these, there may be differences in unmeasured confounders across studies which modify relative treatment effect. We conservatively used a random effects model, and performed sensitivity analyses to adjust for differences in maintenance trial designs (treat-through vs. responder re-randomization). Finally, we included two trials that directly compared infliximab/adalimumab monotherapy with combination azathioprine and TNF antagonist treatment, recognizing that most other trials permit concomitant azathioprine use at a stable dose. However, these trials also required failure of conventional immunosuppressants as an enrolment criterion; hence, we do not believe that this threatens the validity of our findings.
In conclusion, therapeutic decisions for patients with moderate-to-severe CD are complex and prominently feature considerations of treatment efficacy and safety. This updated systematic review and network meta-analysis highlights the important role of infliximab and adalimumab (either in combination with azathioprine or as monotherapy) as first-line agents for inducing and maintaining clinical remission and identifies IL23 blockade with ustekinumab or risankizumab as a potentially more effective strategy in patients with previous TNF antagonist exposure. This network meta-analysis will help inform conversations around the risk-benefit profile of different agents and supplement physician experience, cost/resource constraints, and patient values and preferences in making therapeutic decisions.
Supplementary Material
Supplemental File 1. Search strategy
Supplemental File 2. PRISMA Checklist
Supplemental Figure 1. PRISMA flow diagram
Supplemental Figure 2. Funnel plots for assessment of small study effects and publication bias.
Supplemental Figure 3. Relative efficacy of different interventions for induction and maintenance of clinical remission in biologic-naïve and biologic-exposed patients with moderate-to-severely active Crohn’s disease based on surface under the cumulative ranking (SUCRA)
Supplemental Figure 4. Efficacy of pharmacologic agents for inducing clinical remission (A) and clinical response (B) in biologic-naïve patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 5. Cumulative ranking probability of each intervention for induction of remission in biologic-naïve patients with moderate-to-severe Crohn’s disease
Supplemental Figure 6. Efficacy of pharmacologic agents for inducing clinical remission (A) and clinical response (B) in biologic-exposed patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 7. Cumulative ranking probability of each intervention for induction of remission in biologic-exposed patients with moderate-to-severe Crohn’s disease
Supplemental Figure 8. Efficacy of pharmacologic agents for maintaining clinical remission in patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 9. Cumulative ranking probability of each intervention for maintenance of remission in patients with moderate-to-severe Crohn’s disease with clinical response to induction therapy
Supplemental Table 1. Characteristics of included randomized controlled trials comparing biologic agents for induction of remission in patients with moderate-to-severe Crohn’s disease
Supplemental Table 2. Characteristics of included randomized controlled trials comparing biologic agents for maintenance of remission in patients with moderate-to-severe Crohn’s disease.
Supplemental Table 3. Endoscopic outcomes in randomized controlled trials comparing biologic agents in patients with moderate-to-severe Crohn’s disease
Supplemental Table 4. Risk of bias assessment.
Supplemental Table 5. Certainty of evidence based on GRADE for network meta-analysis. Where there was moderate certainty evidence available based on head-to-head direct comparisons, we assigned that certainty of evidence to the network estimate. Where only low or very low certainty of evidence was available based on head-to-head comparisons, we calculated certainty of evidence for indirect estimates. Higher of the two estimates (certainty of evidence based on direct and indirect comparisons) was assigned to the network estimate. No intransitivity was observed between direct and indirect comparisons where both were available.
Supplemental Table 6. Proportion of biologic-naïve and biologic-exposed patients with moderate-to-severe Crohn’s disease anticipated to achieve induction and maintenance of clinical remission with different therapies, based on observed placebo remission rates
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed, conference proceedings, trial registries and unpublished data (by contacting content experts) on June 3, 2021 for previously published network meta-analysis on pharmacological interventions for moderate-to-severe Crohn’s disease using the search terms ‘Crohn’s disease’ combined with ‘network meta-analysis’. We identified 8 network meta-analyses focusing primarily on comparative efficacy and safety of tumor necrosis factor (TNF)-α antagonists and vedolizumab, relying on indirect treatment comparisons in the absence of head-to-head trials and frequently combined biologic-naïve and biologic-exposed patients together in the synthesis. One prior network meta-analysis was used to inform clinical guidelines published by the American Gastroenterological Association on positioning biologic therapies for management of moderate-to-severe Crohn’s disease.
Added value of this study
Our study updates prior network meta-analyses of all biologic therapies either approved or in advanced stages of regulatory approval, for management of moderate-to-severe Crohn’s disease, including three head-to-head of active comparator trials, with systematic assessment of quality of the evidence using GRADE methodology. Through a systematic review with network meta-analysis, including 31 RCTs (8020 participants) comparing 8 active interventions for induction and maintenance of remission in patients with moderate-to-severe CD, we are able to inform the treatment approach in these patients. Based on 15 RCTs including 2,931 biologic-naïve patients with moderate-to-severe CD, we observed that infliximab monotherapy or combined with azathioprine, adalimumab monotherapy, and ustekinumab were more efficacious than certolizumab pegol for inducing remission (low to moderate certainty of evidence); infliximab combined with azathioprine was more efficacious than vedolizumab for inducing remission (low certainty of evidence). Based on 10 RCTs in 2,479 patients with moderate-to-severe CD with previous biologic exposure, risankizumab and adalimumab (in a subset of patients with secondary loss of response to infliximab) were more efficacious than vedolizumab for inducing remission (moderate certainty of evidence). For maintenance of remission, infliximab combined with azathioprine was ranked highest. No single agent had a significantly higher risk of serious adverse events.
Implications of all the available evidence
Contextualizing findings on comparative efficacy and tolerability in biologic-naïve and biologic-exposed patients with moderate-to-severe CD, coupled with patients’ values and preference as well cost and resource utilization considerations, our findings can directly inform treatment guidelines. Patients with moderate-to-severe CD who are naïve to biologic therapy are most likely to achieve remission infliximab monotherapy or in combination with thiopurines and adalimumab monotherapy. With cost considerations, biosimilars of infliximab and adalimumab may provide cost-effective alternatives. In patients with moderate-to-severe CD who have failed TNFα antagonists, risankizumab and ustekinumab are most likely to achieve remission. In a subset of patients who develop immunogenicity or intolerance to infliximab, adalimumab may be a reasonable alternative as second-line therapy.
Disclosures:
SS’ institute has received research grants from AbbVie and Janssen; SS has received personal fees from Pfizer for ad hoc grant review
MHM has no relevant disclosures
MF has received consulting fees and/or lecture fees from AbbVie, Amgen, Ferring, Fresenius Kabi, Janssen, Takeda, Pfizer, Tillots, Celgene, Celltrion, MSD, Biogen, Gilead, and Galapagos research support from Pfizer, Sandoz, Abbvie and Takeda
RS has received consulting fees from Alimentiv Inc (formerly Robarts Clinical Trials).
VJ has received consulting/advisory board fees from AbbVie, Alimentiv Inc (formerly Robarts Clinical Trials), Arena pharmaceuticals, Asieris, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genetech, Gilead, Janssen, Merck, Mylan, Pandion, Pendopharm, Pfizer, Reistone Biopharma, Roche, Sandoz, Takeda, Topivert; speaker’s fees from, Abbvie, Ferring, Galapagos, Janssen Pfizer Shire, Takeda
RP has received consulting. advisory board fees from AbbVie, Abbott, Alimentiv(formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pandion Pharma, Pendopharm, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics,Theravance Biopharma, UCB, Takeda Pharmaceuticals Speaker Fees: AbbVie, Arena Pharmaceuticals, Celgene, Eli Lilly, Ferring, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sandoz, Shire, Takeda Pharmaceuticals Research/Educational Support: AbbVie, Ferring, Janssen, Pfizer, Takeda
WJS has received research grants from Abbvie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo Smith Kline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from Abbvie, Abivax, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), Beigene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma (acquired by Ventyx Biosciences), Otsuka, Pandion Therapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, Zealand Pharma; stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma (acquired by Ventyx Biosciences), Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences; and employee at Shoreline Biosciences. Spouse: Iveric Bio - consultant, stock options; Progenity - stock; Oppilan Pharma (acquired by Ventyx Biosciences) - consultant, stock options; Prometheus Biosciences - employee, stock, stock options; Prometheus Laboratories – stock, stock options, consultant; Ventyx Biosciences – stock, stock options; Vimalan Biosciences – stock, stock options.
CM has received consulting fees from AbbVie, Amgen, AVIR Pharma Inc, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pfizer, Roche, Alimentiv (formerly Robarts Clinical Trials Inc.); speaker’s fees from AbbVie, Amgen, AVIR Pharma Inc, Janssen, Takeda, and Pfizer; research support from Pfizer.
DATA SHARING STATEMENT
All data collected for this study is publicly available. Other data, coding, protocol, or study materials are available upon request.
REFERENCES
- 1.Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2019; 15(12): 656–65. [PMC free article] [PubMed] [Google Scholar]
- 2.Kotze PG, Ma C, Almutairdi A, Panaccione R. Clinical utility of ustekinumab in Crohn’s disease. J Inflamm Res 2018; 11: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bressler B Use of Vedolizumab for the Treatment of Crohn’s Disease. Gastroenterol Hepatol (N Y) 2019; 15(4): 204–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NH, Singh S, Sandborn WJ. Positioning Therapies in the Management of Crohn’s Disease. Clin Gastroenterol Hepatol 2020; 18(6): 1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazlewood GS, Pokharel G, Deardon R, et al. Patient preferences for maintenance therapy in Crohn’s disease: A discrete-choice experiment. PLoS One 2020; 15(1): e0227635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalal SR, Cohen RD. What to Do When Biologic Agents Are Not Working in Inflammatory Bowel Disease Patients. Gastroenterol Hepatol (N Y) 2015; 11(10): 657–65. [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA Technical Review on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021; 160(7): 2512–56 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho EY, Singh S, Terdiman JP. Providing the Best Care for Patients With Crohn’s Disease: An Examination of the New AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021; 160(7): 2557–62. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018; 48(4): 394–409. [DOI] [PubMed] [Google Scholar]
- 10.Ma C, Panaccione R, Khanna R, Feagan BG, Jairath V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract Res Clin Gastroenterol 2019; 38–39: 101604. [DOI] [PubMed] [Google Scholar]
- 11.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018; 392(10148): 650–61. [DOI] [PubMed] [Google Scholar]
- 12.Sands BE, Irving PM, Hoops T, et al. USTEKINUMAB VERSUS ADALIMUMAB FOR INDUCTION AND MAINTENANCE THERAPY IN MODERATE-TO-SEVERE CROHN’S DISEASE: THE SEAVUE STUDY. Gastroenterology 2021; 160(6): 775D. [Google Scholar]
- 13.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386(10006): 1825–34. [DOI] [PubMed] [Google Scholar]
- 14.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017. [DOI] [PubMed] [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162(11): 777–84. [DOI] [PubMed] [Google Scholar]
- 16.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011; 14(4): 417–28. [DOI] [PubMed] [Google Scholar]
- 17.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130(2): 323–33; quiz 591. [DOI] [PubMed] [Google Scholar]
- 18.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology 2006; 130(4): 1054–61. [DOI] [PubMed] [Google Scholar]
- 19.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997; 337(15): 1029–35. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. Journal of Crohn’s & colitis 2012; 6(2): 160–73. [DOI] [PubMed] [Google Scholar]
- 21.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016; 375(20): 1946–60. [DOI] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369(8): 711–21. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clinical Gastroenterology & Hepatology 2011; 9(8): 670–8.e3. [DOI] [PubMed] [Google Scholar]
- 24.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147(3): 618–27 e3. [DOI] [PubMed] [Google Scholar]
- 25.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362(15): 1383–95. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab Monotherapy and a Combination with Azathioprine for Crohn’s Disease: A Prospective, Randomized Trial. J Crohns Colitis 2016; 10(11): 1259–66. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Gao X, Zhong J, et al. Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn’s disease: results from a randomized trial. Therap Adv Gastroenterol 2020; 13: 1756284820938960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K, Motoya S, Ogata H, et al. Effects of vedolizumab in Japanese patients with Crohn’s disease: a prospective, multicenter, randomized, placebo-controlled Phase 3 trial with exploratory analyses. J Gastroenterol 2020; 55(3): 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Haens GR, Colombel JF, Bossuyt P, et al. RISANKIZUMAB INDUCTION THERAPY IN PATIENTS WITH MODERATE-TO-SEVERE CROHN’S DISEASE WITH INTOLERANCE OR INADEQUATE RESPONSE TO CONVENTIONAL AND/OR BIOLOGIC THERAPY: RESULTS FROM THE PHASE 3 ADVANCE STUDY. Gastroenterology 2021; 160(6): 775a. [Google Scholar]
- 30.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007; 146(12): 829–38. [DOI] [PubMed] [Google Scholar]
- 31.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012; 367(16): 1519–28. [DOI] [PubMed] [Google Scholar]
- 32.Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017; 389(10080): 1699–709. [DOI] [PubMed] [Google Scholar]
- 33.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359(9317): 1541–9. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117(4): 761–9. [DOI] [PubMed] [Google Scholar]
- 35.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132(1): 52–65. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56(9): 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease.[Erratum appears in N Engl J Med. 2007 Sep 27;357(13):1357]. N Engl J Med 2007; 357(3): 239–50. [DOI] [PubMed] [Google Scholar]
- 38.Vermeire S, Sandborn W, Baert F, et al. OP23 Efficacy and safety of vedolizumab SC in patients with moderately to severely active Crohn’s disease: Results of the VISIBLE 2 study. J Crohn’s Colitis 2020; 14(Supplement_1): S020–S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142(5): 1102–11 e2. [DOI] [PubMed] [Google Scholar]
- 40.AbbVie. Phase 3 Maintenance Results Show Patients with Crohn’s Disease Receiving Risankizumab (SKYRIZI®) Achieved Endoscopic Response and Clinical Remission at One Year. 2021. https://news.abbvie.com/news/press-releases/phase-3-maintenance-results-show-patients-with-crohns-disease-receiving-risankizumab-skyrizi-achieved-endoscopic-response-and-clinical-remission-at-one-year.htm (accessed June 2, 2021.
- 41.Lee MJ, Parker CE, Taylor SR, et al. Efficacy of Medical Therapies for Fistulizing Crohn’s Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018; 16(12): 1879–92. [DOI] [PubMed] [Google Scholar]
- 42.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999; 340(18): 1398–405. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Baraty B, Lee Robertson H, et al. Systematic review: medical therapy for fibrostenosing Crohn’s disease. Aliment Pharmacol Ther 2020; 51(12): 1233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018; 67(1): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF Treatment for Extraintestinal Manifestations of Inflammatory Bowel Disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis 2017; 23(7): 1174–81. [DOI] [PubMed] [Google Scholar]
- 46.Winter TA, Wright J, Ghosh S, Jahnsen J, Innes A, Round P. Intravenous CDP870, a PEGylated Fab’ fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn’s disease: an exploratory study. Aliment Pharmacol Ther 2004; 20(11–12): 1337–46. [DOI] [PubMed] [Google Scholar]
- 47.Ma C, Beilman CL, Huang VW, et al. Anti-TNF Therapy Within 2 Years of Crohn’s Disease Diagnosis Improves Patient Outcomes: A Retrospective Cohort Study. Inflamm Bowel Dis 2016; 22(4): 870–9. [DOI] [PubMed] [Google Scholar]
- 48.Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr Drug Targets 2014; 15(11): 1056–63. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019; 4(5): 341–53. [DOI] [PubMed] [Google Scholar]
- 50.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371(9613): 660–7. [DOI] [PubMed] [Google Scholar]
- 51.Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8(12): 1632–41. [DOI] [PubMed] [Google Scholar]
- 52.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007; 146(12): 829–38. [DOI] [PubMed] [Google Scholar]
- 53.Ma C, Battat R, Jairath V, Vande Casteele N. Advances in Therapeutic Drug Monitoring for Small-Molecule and Biologic Therapies in Inflammatory Bowel Disease. Curr Treat Options Gastroenterol 2019; 17(1): 127–45. [DOI] [PubMed] [Google Scholar]
- 54.Manlay L, Boschetti G, Pereira B, et al. Comparison of short- and long-term effectiveness between ustekinumab and vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor therapy. Aliment Pharmacol Ther 2021; 53(12): 1289–99. [DOI] [PubMed] [Google Scholar]
- 55.Townsend T, Razanskaite V, Dodd S, et al. Comparative effectiveness of ustekinumab or vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther 2020; 52(8): 1341–52. [DOI] [PubMed] [Google Scholar]
- 56.Biemans VBC, van der Woude CJ, Dijkstra G, et al. Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther 2020; 52(1): 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 2019; 68(5): 814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandborn WJ, Rebuck R, Wang Y, et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermeire S, Loftus EV Jr., Colombel JF, et al. Long-term Efficacy of Vedolizumab for Crohn’s Disease. J Crohns Colitis 2017; 11(4): 412–24. [DOI] [PubMed] [Google Scholar]
- 60.Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018; 11: 1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh S, Facciorusso A, Dulai PS, Jairath V, Sandborn WJ. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020; 18(1): 69–81 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh S, Heien HC, Herrin J, et al. Comparative Risk of Serious Infections With Tumor Necrosis Factor alpha Antagonists vs Vedolizumab in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirchgesner J, Desai RJ, Beaugerie L, Schneeweiss S, Kim SC. Risk of Serious Infections With Vedolizumab Versus Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 64.Papp K, Gottlieb AB, Naldi L, et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol 2015; 14(7): 706–14. [PubMed] [Google Scholar]
- 65.Sandborn WJ, Feagan BG, Danese S, et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm Bowel Dis 2021; 27(7): 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma C, Hussein IM, Al-Abbar YJ, et al. Heterogeneity in Definitions of Efficacy and Safety Endpoints for Clinical Trials of Crohn’s Disease: A Systematic Review. Clin Gastroenterol Hepatol 2018; 16(9): 1407–19 e22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1. Search strategy
Supplemental File 2. PRISMA Checklist
Supplemental Figure 1. PRISMA flow diagram
Supplemental Figure 2. Funnel plots for assessment of small study effects and publication bias.
Supplemental Figure 3. Relative efficacy of different interventions for induction and maintenance of clinical remission in biologic-naïve and biologic-exposed patients with moderate-to-severely active Crohn’s disease based on surface under the cumulative ranking (SUCRA)
Supplemental Figure 4. Efficacy of pharmacologic agents for inducing clinical remission (A) and clinical response (B) in biologic-naïve patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 5. Cumulative ranking probability of each intervention for induction of remission in biologic-naïve patients with moderate-to-severe Crohn’s disease
Supplemental Figure 6. Efficacy of pharmacologic agents for inducing clinical remission (A) and clinical response (B) in biologic-exposed patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 7. Cumulative ranking probability of each intervention for induction of remission in biologic-exposed patients with moderate-to-severe Crohn’s disease
Supplemental Figure 8. Efficacy of pharmacologic agents for maintaining clinical remission in patients with moderate-to-severe Crohn’s disease.
Supplemental Figure 9. Cumulative ranking probability of each intervention for maintenance of remission in patients with moderate-to-severe Crohn’s disease with clinical response to induction therapy
Supplemental Table 1. Characteristics of included randomized controlled trials comparing biologic agents for induction of remission in patients with moderate-to-severe Crohn’s disease
Supplemental Table 2. Characteristics of included randomized controlled trials comparing biologic agents for maintenance of remission in patients with moderate-to-severe Crohn’s disease.
Supplemental Table 3. Endoscopic outcomes in randomized controlled trials comparing biologic agents in patients with moderate-to-severe Crohn’s disease
Supplemental Table 4. Risk of bias assessment.
Supplemental Table 5. Certainty of evidence based on GRADE for network meta-analysis. Where there was moderate certainty evidence available based on head-to-head direct comparisons, we assigned that certainty of evidence to the network estimate. Where only low or very low certainty of evidence was available based on head-to-head comparisons, we calculated certainty of evidence for indirect estimates. Higher of the two estimates (certainty of evidence based on direct and indirect comparisons) was assigned to the network estimate. No intransitivity was observed between direct and indirect comparisons where both were available.
Supplemental Table 6. Proportion of biologic-naïve and biologic-exposed patients with moderate-to-severe Crohn’s disease anticipated to achieve induction and maintenance of clinical remission with different therapies, based on observed placebo remission rates
Data Availability Statement
All data collected for this study is publicly available. Other data, coding, protocol, or study materials are available upon request.


