Highlights
-
•
Versatility of CD4 T cells enables different attack modes towards cancer cells.
-
•
Cooperation of CD4 and CD8 T cells renders anti-tumor responses most efficient.
-
•
Integrating CD4 T cells in cancer therapy will improve clinical outcome.
Abstract
The focus in cancer immunotherapy has mainly been on CD8 T cells, as they can directly recognize cancer cells. CD4 T cells have largely been neglected, because most cancers lack MHC II expression and cannot directly be recognized by CD4 T cells. Yet, tumor antigens can be captured and cross-presented by MHC II-expressing tumor stromal cells. Recent data suggest that CD4 T cells act as a swiss army knife against tumors. They can kill cancer cells, if they express MHC II, induce tumoricidal macrophages, induces cellular senescence of cancer cells, destroy the tumor vasculature through cytokine release and help CD8 T cells in the effector phase. We foresee a great future for CD4 T cells in the clinic, grafted with tumor antigen specificity by T cell receptor gene transfer, either alone or in combination with engineered CD8 T cells.
Current Opinion in Immunology 2022, 74:18–24
This review comes from a themed issue on Tumor Immunology (April 2022)
Edited by Hans Stauss
For complete overview of the section, please refer to the article collection, “Tumor Immunology (April 2022)”
Available online 4th October 2021
https://doi.org/10.1016/j.coi.2021.09.005
0952-7915/© 2021 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Immunotherapy has entered the cancer treatment landscape successfully in the last decade. Checkpoint blockade and adoptive T cell therapy (ATT) have brought great benefit to patients with high mutational burden cancers or hematological malignancies, respectively. For immunotherapy of solid cancers, responses are mainly partial and of short duration. The focus in immunotherapy has been mainly on CD8 T cells, because they can directly kill cancer cells through recognition of peptide-presenting MHC class I (pMHC I) on the cell surface. In recent years, however, CD4 T cells and their role in anti-tumor immunity have received more attention. CD4 T cells are not a uniform cell population but exist as T regulatory cells (Tregs) or conventional T helper cells of different subtypes [1]. Hence, their role in cancer immunity is controversial. Endogenous CD4 T cell responses can augment immune tolerance in the tumor microenvironment and enhance tumor growth [2,3]. Naïve tumor-specific T cells were shown to be tolerated in the tumor draining lymph nodes to become Tregs and reinforce tumor immune tolerance [4]. On the other hand, adoptively transferred CD4 T cells have demonstrated the ability to mount successful immune responses [5]. Here, we illuminate the potential of CD4 T cells to enhance anti-tumor immune responses for more effective and sustained responses in ATT.
Mechanisms of CD4 T cell involvement
While it has become clear that CD4 T cells are involved in anti-tumor immunity, the exact mechanism how they exert their effects is less clear. Most cancer cells do not express MHC class II molecules (MHC II) and can thus not be recognized by CD4 T cells directly but rather through cross-presentation of tumor antigens by tumor stromal cells. It can be assumed that cross-presentation of tumor antigens on MHC II is more effective than MHC I, as necrotic tumor cells or vesicles released by cancer cells that are taken up by stromal cells primarily enter the classical processing pathway for MHC II [6]. In most solid tumors, monocytes/macrophages are the most abundant MHC II-positive cells. Dependent on the presence or absence of MHC II on the cancer cells and the CD4 subset involved, several different mechanisms of tumor rejection have been suggested for CD4 T cells (Figure 1).
Figure 1.
Mechanisms how CD4 T cells contribute to anti-tumor immunity.
(a) How CD4 T cells get activated. Tumor stromal cells, especially macrophages, internalize antigens from cancer cells via taking up necrotic cancer cells or secreted antigen. Upon presentation of the contained antigens on MHC II on the macrophage, antigen-specific CD4 T cells get activated by recognizing their target and in turn activate the macrophage, for example, through CD40/CD40L interaction. High cytokine levels can occur by IFNγ and TNFα secretion by CD4 T cells and macrophages. (b) Effector mechanisms by activated CD4 T cells. 1) Direct cytotoxicity by CD4 T cells can occur when cancer cells themselves express MHC II on their surface. A cytotoxic phenotype involving granzymes and cytokines renders CD4 T cells cytotoxic [7,8]. 2) Macrophages become effectors when activated by CD4 T cells and destroy tumor cells by upregulating nitric oxide synthetase (iNOS) and producing NO [11•,12]. 3) Growth arrest of cancer cells can be achieved by inducing senescence through IFNγ and TNFα produced by CD4 T cells [14]. 4) IFNγ and TNFα produced by CD4 T cells and macrophages synergize to destruct blood vessels in an early phase of tumor rejection [23••].
Cancer cell elimination by CD4 T cells
Only when cancer cells intrinsically express MHC II, they can become direct targets for CD4 T cells via peptide-MHC II (pMHC II) recognition. This is the case for most hematological malignancies, for example, lymphomas. In a model resembling Epstein-Barr Virus-associated lymphoproliferative disease, activation of latent membrane protein 1 in B cells resulted in lymphomas that were efficiently controlled by T cells. CD4 T cells eliminated lymphomas in vivo in a cytotoxicity-independent, IFNγ-independent and TNFα-independent but likely MHC II-dependent fashion [7]. In a melanoma transplantation model, it was shown that tumor rejection by CD4 T cells was dependent on IFNγ and correlated with high granzyme B expression in CD4 T cells [8]. Cancer cell-intrinsic MHC II expression was found in several cancer indications and correlated with favorable disease outcome [9]. Still, the majority of cancers lack intrinsic MHC II expression, even though it may be difficult to exclude low level of expression.
DTH-like tumor rejection
In a mouse model of disseminated leukemia, adoptively transferred CD4 T cells were necessary and sufficient to reject the tumor cells proposedly by a reaction similar to delayed type hypersensitivity (DTH) involving activation of tumoricidal macrophages [10]. Similarly, in a mouse model of MHC II-negative plasmacytoma it was shown that bone marrow-resident macrophages became effectors to kill tumor cells when activated by CD4 T cells through IFNγ [11•]. As mechanism, it was proposed that upon activation of macrophages, they upregulated inducible nitric oxide synthetase (iNOS) and the thereby produced NO eventually destroyed the tumor cells [12]. The tumor-specific antigen (an immunoglobulin V region-derived idiotypic peptide) was secreted by the cancer cells, which was proposed a prerequisite for the described mechanism. In a B cell lymphoma model, where the tumor antigen (idiotypic lambda light chain) was either secreted or expressed as truncated intracellular version, lymphoma rejection in TCR-transgenic mice occurred only when the antigen was secreted [13]. Nevertheless, tumor-resident macrophages showed similar tumor-specific activation for both variants, thus showing that cross-presentation of intracellular antigen occurred efficiently in the antigen-retaining variant [12]. This is promising for adoptive transfer of effector CD4 T cells as many tumor antigens are not secreted. Together these data show an effector function for tumor-resident macrophages in anti-tumor immunity mediated by CD4 T cells.
CD4 T cells induce senescence in cancer cells
Activated CD4 T cells often secrete simultaneously IFNγ and TNFα, typical for Th1 cells. It was shown that IFNγ and TNFα produced by adoptively transferred CD4 T cells caused cytokine-induced senescence resulting in growth arrest of cancer cells [14]. Inhibition of tumor growth depended on the senescence-inducing cell cycle regulators p16Ink4a/p19Arf (Cdkn2a) or p21Cip1 (Cdkn1a) in the cancer cells. Human melanoma metastases that progressed after immune checkpoint blockade therapy were defective of senescence-inducing genes [15•]. In this mechanism, cancer cells are the direct targets of IFNγ and TNFα, but recognition of pMHC II is mediated by stromal cells requiring cross-presentation of cognate tumor antigens. Whether such a cancer cell intrinsic mechanism allows rapid immune escape, remains to be seen.
The first event during tumor rejection: IFNγ and TNFα destroy the tumor vasculature
IFNγ has multiple biological activities. Its role during tumor rejection has mainly been associated with upregulation of MHC I, resulting in more efficient cancer cell recognition. There is ample evidence for this assumption and in this regard, it is interesting that IFNγ produced by T cells diffuses widely into the tumor microenvironment and alters cell signaling in remote tumor cells [16•,17•]. The necessity of IFNγ to act on the cancer cells is illustrated by the observation that IFNγ-unresponsiveness has been implicated in escape from CD8 T cell attack in mouse models and humans [18,19]. Yet, experiments some time ago had shown that IFNγ needed to act on non-bone marrow-derived cells to inhibit blood vessel formation in the tumor [20,21]. This was the case in models of both, CD4 and CD8 T cell mediated tumor cell rejection, also when the cancer cells were IFNγ receptor (IFNγ-R)-deficient [21,22]. In a model of large vascularized tumors in mice with selective IFNγ-R expression in individual cell types/organs, it was shown that endothelial cells were a necessary and sufficient target of IFNγ to initiate tumor regression [23••]. In this set of experiments, IFNγ was locally induced in the solid tumors. Ischemia-like blood vessel regression preceded cancer cell death, compatible with findings showing that perforin-deficient but not IFNγ-deficient T cells eradicated large established tumors, raising doubts about a series-killing mechanism [24]. Furthermore, IFNγ and TNFα synergized in vessel destruction. Using intravital microscopy, it was shown that the mechanism for IFNγ involves controlled blood vessel regression resembling physiological remodeling, while TNFα caused bursting of the blood vessels, both leading to collapse of the tumor as secondary event [23••]. In conclusion, IFNγ and TNFα are both produced by effector CD4 T cells and cause blood vessel and hence tumor destruction.
It should be noted that the effector molecules required for solid tumor destruction are largely overlapping for CD4 and CD8 T cells: both produce IFNγ and TNFα upon antigen recognition, when appropriately activated. If CD4 T cells reject solid tumors by cutting the blood supply, which then leads to collapse of the entire tumor tissue (except perhaps of the rim of the tumor) without directly recognizing the cancer cells, the cancer cells should undergo apoptosis independent of whether they express the target antigen or not. This phenomenon, termed bystander elimination of antigen-negative variants, was first shown for CD8 T cells recognizing overexpressed model antigens cross-presented through MHC I on tumor stromal cells, resulting in increased effector function [25]. Assuming more efficient cross-presentation through MHC II compared to MHC I, one might expect more pronounced bystander elimination of CD4 T cells compared to CD8 T cells. An advantage of CD4 T cells over CD8 T cells is also that they will not select tumor antigen-negative or MHC I-negative variants and remain active as long as there is sufficient antigen expressed by the cancer cells to feed MHC II-positive stromal cells. One should not expect, however, that the last cancer cell is eradicated by CD4 T cells alone.
Cooperation of CD4 and CD8 T cells in the effector phase
While it has been appreciated that CD4 T cells are essential to mediate a fully integrated immune response, focus has been primarily on helper functions during the priming phase [26]. By recognizing their antigen on the same dendritic cell, CD4 T cells enhance priming of specific CD8 T cells through activation of dendritic cells by CD40/CD40L interaction and thereby stimulate an effective immune response [26]. In addition to help in the priming phase, which is primarily relevant for endogenous CD8 T cell responses, there is evidence that the combined action of CD4 and CD8 T cells locally at the tumor side leads to enhanced anti-tumor immunity [27•,28,29•,30••] (Figure 2). In a model of transplanted fibrosarcoma, bystander killing of antigen-negative cancer cell variants through stromal targeting was observed only when antigen targets for both CD4 and CD8 T cells were expressed by the same cancer cells and not when cancer cells expressing only one antigen target were mixed [27•]. Thus, local cooperation of CD4 and CD8 T cells is required in the effector phase to exert a bystander killing effect [27•]. In a different model, escape of antigen-negative variants was prevented by administering polyclonal CD4 T cells recognizing a cross-presented alloantigen [29•]. Although those tumors were resistant to checkpoint blockade, CD4 T cells restored CD8 T cell function regarding downregulation of PD-1, proliferation, and IFNγ secretion [29•]. Of note, mice were treated with T cells, when large tumors had established.
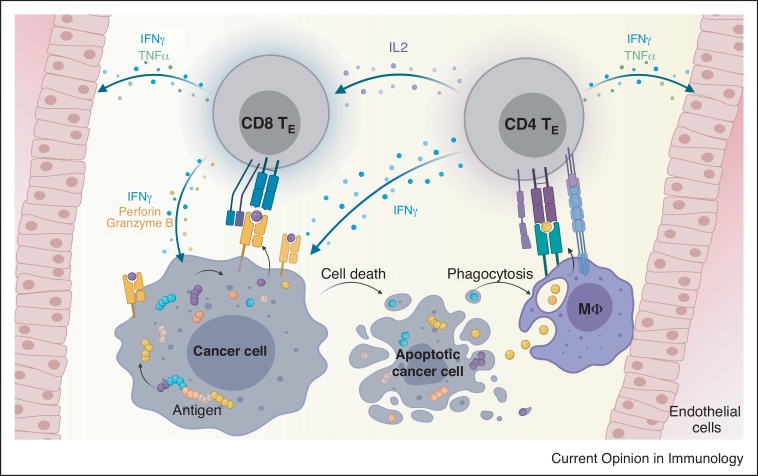
Figure 2.
CD4 and CD8 T cell cooperation in the effector phase.
CD4 effector T cells (CD4 TE) recognize their target cross-presented on macrophages (Mφ) leading to cytokine production. CD8 effector T cells (CD8 TE) recognize their target on the cancer cell and get additionally activated by neighboring CD4 T cells. CD8 T cells exert direct kill on cancer cells and high cytokine levels by CD4 and CD8 T cells lead to vessel destruction and changes in the tumor microenvironment, for example, MHC upregulation. Apoptotic cancer cells are taken up by macrophages that cross-present tumor antigens to CD4 T cells to feed the circle.
CD4-TCRs specific for human tumor antigen restricted to human MHC II
Most experimental models employed surrogate antigens with the goal to translate the knowledge into the clinic by grafting a new antigen specificity on patient’s T cells through T cell receptor (TCR) gene transfer. In a translational model employing a therapeutically relevant antigen, NY-ESO-1, TCRs were generated [30••]. NY-ESO-1 is a cancer testis antigen with broad expression in different tumor indications but limited expression in healthy tissue [31]. Hence, it is an attractive target for TCR-T cell therapy, if NY-ESO-1 expression is high and relatively homogenous. Clinical studies using an affinity-enhanced (naturally low-affine, because isolated from an antigen-positive human cancer patient [32]) TCR recognizing an HLA-A2-restricted epitope of NY-ESO-1 showed objective response rates of around 50% in metastatic or recurrent synovial sarcoma or melanoma [33,34]. The responses, however, were mainly partial and despite persistence of TCR-transgenic T cells for at least six months no selection for antigen-negative variants occurred, suggesting suboptimal activity [33]. Recently, novel TCRs were isolated from mice with a diverse human TCR repertoire, either restricted to HLA-A2 (CD8-TCR) or HLA-DRA/DRB1*0401 (HLA-DR4) (CD4-TCR) [30••,35]. Because NY-ESO-1 is a foreign antigen for the mice, both TCRs were of optimal affinity. Compared to CD4-TCRs isolated from humans, the mouse-derived human CD4-TCR were more sensitive in peptide recognition and recognition of human melanoma cells, which expressed HLA-DR4. Such TCRs of optimal affinity are likely better suited to target NY-ESO-1-positive cancers either in a monotherapy with a CD4-TCR or in a combined approach with CD8-TCR.
For analyzing human CD4-TCRs against human tumor antigens in vivo, one has to take into account that the cancer cells are usually MHC II-negative and any therapeutical effect is mediated by CD4 T cells recognizing the tumor antigen cross-presented by MHC II-positive tumor stromal cells, likely monocytes/macrophages. Xenograft models are, therefore, not suitable to analyze their mode of action. To analyze the combined effect of CD4-TCR and CD8-TCR in vivo, a model was established, in which HLA-DR4 transgenic Rag1-deficient mice bearing an NY-ESO-1/HLA-A2 expressing tumor, both with a C57Bl/6 genetic background, were treated with CD4-TCR and/or CD8-TCR. The TCRs were introduced into OT-II (CD4) or P14 (CD8) T cells, which are TCR transgenic with tumor-unrelated specificity. In this syngeneic model, CD4 T cells recognize NY-ESO-1 cross-presented by HLA-DR4 on stromal cells, while CD8 T cells recognize NY-ESO-1 on the cancer cells. While CD8 T cells alone achieved tumor regression in some mice, CD4 T cells alone only slightly impaired tumor growth [30••]. The relatively weak effect of the CD4-TCR is likely caused by OT-II cells producing insufficient levels of cytokines compared to wildtype mice (unpublished observation). We hypothesize that transgenic CD4 T cells generated from a mouse line with physiological cytokine secretion can achieve a more significant effect when administered as a monotherapy. Nevertheless, only when mice were treated with both CD4 and CD8 T cells, CD8 T cells were found in higher numbers in the blood and in the tumor and tumors were rejected in all mice. Macrophages isolated from the tumor cross-presented NY-ESO-1 and stimulated TCR-transduced CD4 T cells in a HLA-DR4 restricted manner [30••]. In summary, TCR-transduced CD4 and CD8 T cells synergize in rejecting tumors.
Clinical evidence of CD4 T cell efficacy
Although clinical data on CD4 T cells in immunotherapy are limited, several studies show the therapeutic potential of CD4 T cells. In a case study, a patient with metastatic melanoma experienced a durable clinical response following treatment with an expanded NY-ESO-1-specific CD4 T cell clone, while subsequent patients did not show a response [36,37]. Reasons could be that the extensive culturing period of the T cell clone resulted in an unfavorable T cell phenotype or, alternatively, due to tolerance mechanisms CD4 T cells with suboptimal affinity were selected, since NY-ESO-1 is expressed in human thymus [38]. Furthermore, treatment with ex vivo expanded tumor-infiltrating lymphocytes (TILs) achieved remarkable response rates, especially in melanoma [39]. In several cases, neoantigen-specific CD4 T cells occurred within the TILs suggesting that they were involved in anti-tumor immunity [40, 41, 42, 43]. Moreover, PD1-high or neoantigen-specific CD4 T cells in the memory pool were identified in the peripheral blood suggesting their previous activation [44•,45•]. Involvement of CD4 T cells in anti-tumor immunity is further supported by a case study, in which a cholangiocarcinoma patient experienced regression of all lung and liver metastases following transfer of TILs containing CD4 T cells specific for mutated ERBB2IP. The patient showed ongoing remission at six months following a second TIL transfer, which consisted of 95% ERBB2IP-specific CD4 T cells [46••]. In a further case study, a melanoma patient experienced a complete response after transfer of TILs that contained a small proportion of BRAFV600E-specific CD4 T cells, which were enriched in the periphery 1–2 years following transfer [47•]. Finally, in a TCR gene therapy trial, 17 patients were treated with CD4 T cells engineered to express a MAGE-A3-reactive TCR [48•]. Three objective partial responses among nine high dose-treated patients and one complete response among eight low dose-treated patients were observed. However, as clinical effects did not correlate with persistence of T cells, more patients are needed to clearly attribute the clinical effects to the T cell therapy.
Conclusion
CD4 T cells as effectors during regression of solid MHC II-negative solid tumors have been underestimated. Which of the multiple mechanisms leading to tumor regression by CD4 T cells is prevalent, is currently unknown. The prerequisite for CD4 T cells to recognize tumor antigens and being activated is the uptake of the tumor antigens by stromal cells from dying tumor cells and presentation on MHC II molecules. Little is known about which tumor antigens, expressed at natural levels, are cross-presented through MHC II molecules. To better judge the suitability of tumor antigens for CD4-TCR gene therapy, MHC II-positive tumor stromal cells could be isolated from human tumors or xenografts grown in respective human MHC II-transgenic mice and tested for recognition by CD4-TCR transduced CD4 T cells. Overexpression of the target antigen of CD4-TCRs is certainly an advantage, but other factors like efficiency of processing of the peptide may vary and are difficult to predict. Neoantigens are often not overexpressed as compared to for example cancer-testis antigens, therefore it remains to be shown whether CD4-TCR gene therapy is more suitable for certain classes of tumor antigens. Peptide-MHC I binding affinity is critical for therapeutic efficacy by CD8 T cells. The same may be true for peptide-MHC II binding affinity for the therapeutic efficacy of CD4 T cells. Current problems are that bioinformatic tools are relatively poor in predicting peptide-MHC II binding affinity and that MHC II epitopes are rarely precisely defined for their length. An advantage of CD4-TCRs compared to CD8-TCR may be that they less likely select antigen-negative variants and may mediate sustained regression but unlikely will they completely eradicate the tumor. If CD4 T cells can induce regression of MHC II-negative tumors, as shown in experimental models, bystander elimination of antigen-negative cancer cells is a vital option, yet its significance for the clinic remains to be shown.
Conflict of interest statement
LP and TB are named inventors on a patent (PCT/ EP2016/055242) covering MHC II-restricted T cell receptors held by the Max-Delbrück-Center for Molecular Medicine.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
Work described here was supported by ERC Advanced Grant 882963 — NeoTs.
References
- 1.Sallusto F. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol. 2016;34:317–334. doi: 10.1146/annurev-immunol-032414-112056. [DOI] [PubMed] [Google Scholar]
- 2.Siegel C.T., Schreiber K., Meredith S.C., Beck-Engeser G.B., Lancki D.W., Lazarski C.A., Fu Y.-X., Rowley D.A., Schreiber H. Enhanced growth of primary tumors in cancer-prone mice after immunization against the mutant region of an inherited oncoprotein. J Exp Med. 2000;191:1945–1956. doi: 10.1084/jem.191.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeNardo D.G., Barreto J.B., Andreu P., Vasquez L., Tawfik D., Kolhatkar N., Coussens L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso R., Flament H., Lemoine S., Sedlik C., Bottasso E., Péguillet I., Prémel V., Denizeau J., Salou M., Darbois A., et al. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Diez A., Joncker N.T., Choi K., Chan W.F.N., Anderson C.C., Lantz O., Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B., Kracker S., Yasuda T., Casola S., Vanneman M., Hömig-Hölzel C., Wang Z., Derudder E., Li S., Chakraborty T., et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148:739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A., et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelrod M.L., Cook R.S., Johnson D.B., Balko J.M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res. 2019;25:2392–2402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg P.D., Kern D.E., Cheever M.A. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Haabeth O.A.W., Hennig K., Fauskanger M., Løset G.Å, Bogen B., Tveita A. CD4+ T-cell killing of multiple myeloma cells is mediated by resident bone marrow macrophages. Blood Adv. 2020;4:2595–2605. doi: 10.1182/bloodadvances.2020001434. [DOI] [PMC free article] [PubMed] [Google Scholar]; By using a mouse model of established multifocal myeloma, the authors demonstrate that CD4 T cells exert anti-tumor immune responses through interaction with tumor-resident macrophages.
- 12.Bogen B., Fauskanger M., Haabeth O.A., Tveita A. CD4+ T cells indirectly kill tumor cells via induction of cytotoxic macrophages in mouse models. Cancer Immunol Immunother. 2019;68:1865–1873. doi: 10.1007/s00262-019-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthay A., Lundin K.U., Lorvik K.B., Hofgaard P.O., Bogen B. Secretion of tumor-specific antigen by myeloma cells is required for cancer immunosurveillance by CD4+ T cells. Cancer Res. 2009;69:5901–5907. doi: 10.1158/0008-5472.CAN-08-4816. [DOI] [PubMed] [Google Scholar]
- 14.Braumüller H., Wieder T., Brenner E., Aßmann S., Hahn M., Alkhaled M., Schilbach K., Essmann F., Kneilling M., Griessinger C., et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 15•.Brenner E., Schörg B.F., Ahmetlić F., Wieder T., Hilke F.J., Simon N., Schroeder C., Demidov G., Riedel T., Fehrenbacher B., et al. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat Commun. 2020;11 doi: 10.1038/s41467-020-14987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adding on previous results on cytokine-induced senescence in cancer cells, the authors show that deleting senescence-inducing cell cycle regulators in cancer cells abrogates T cell-mediated rejection.
- 16•.Hoekstra M.E., Bornes L., Dijkgraaf F.E., Philips D., Pardieck I.N., Toebes M., Thommen D.S., van Rheenen J., Schumacher T.N.M. Long-distance modulation of bystander tumor cells by CD8+ T cell-secreted IFNγ. Nat Cancer. 2020;1:291–301. doi: 10.1038/s43018-020-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Back to back with Ref. [17], the authors show that IFNγ secreted by T cells acts over long distances in the tumor. By employing an IFNγ-sensing reporter and multiday intravital imaging they show that tumor-reactive T cells cause IFNγ-induced modulation of antigen-negative tumor cells.
- 17•.Thibaut R., Bost P., Milo I., Cazaux M., Lemaître F., Garcia Z., Amit I., Breart B., Cornuot C., Schwikowski B., et al. Bystander IFN-γ activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer. 2020;1:302–314. doi: 10.1038/s43018-020-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Back to back with Ref. [16], the authors show that IFNγ secreted by T cells acts over long distances in the tumor. By analyzing STAT-1 nuclear translocation as a proxy for IFNγ signaling and intravital imaging, the authors show the effect of IFNγ on antigen-negative tumor cells.
- 18.Textor A., Schmidt K., Kloetzel P.-M., Weißbrich B., Perez C., Charo J., Anders K., Sidney J., Sette A., Schumacher T.N.M., et al. Preventing tumor escape by targeting a post-proteasomal trimming independent epitope. J Exp Med. 2016;213:2333–2348. doi: 10.1084/jem.20160636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Z., Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFNg receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 21.Qin Z., Schwartzkopff J., Pradera F., Kammertoens T., Seliger B., Pircher H., Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 22.Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D., Schreiber H. CD4+ T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFNg. Proc Natl Acad Sci U S A. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Kammertoens T., Friese C., Arina A., Idel C., Briesemeister D., Rothe M., Ivanov A., Szymborska A., Patone G., Kunz S., et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017;545:98–102. doi: 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using conditional IFNγ receptor knock-out mice and multiday intravital imaging the authors demonstrate that IFNγ acting on tumor endothelial cells leads to non-apoptotic blood vessel destruction and tumor regression.
- 24.Listopad J.J., Kammertoens T., Anders K., Silkenstedt B., Willimsky G., Schmidt K., Kuehl A.A., Loddenkemper C., Blankenstein T. Fas expression by tumor stroma is required for cancer eradication. Proc Natl Acad Sci U S A. 2013;110:2276–2281. doi: 10.1073/pnas.1218295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiotto M.T., Rowley D.A., Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 26.Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 27•.Schietinger A., Philip M., Liu R.B., Schreiber K., Schreiber H. Bystander killing of cancer requires the cooperation of CD4+ and CD8+ T cells during the effector phase. J Exp Med. 2010;207:2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a series of tumor challenge experiments, the authors show that cancer cells can be rejected by combined action of CD4 and CD8 T cells targeting the stroma. The results showed that local cooperation of CD4 and CD8 T cells is required for elimination of antigen-loss variants.
- 28.Bos R., Sherman L.A. CD4 + T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8 + T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Arina A., Karrison T., Galka E., Schreiber K., Weichselbaum R.R., Schreiber H. Transfer of allogeneic CD4 + T cells rescues CD8 + T cells in anti-PD-L1–resistant tumors leading to tumor eradication. Cancer Immunol Res. 2017;5:127–136. doi: 10.1158/2326-6066.CIR-16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, the authors show that relapse of regressed large tumors upon CD8 T cell therapy can be prevented by administering allogeneic CD4 T cells either together with the CD8 T cells or at later time points.
- 30••.Poncette L., Chen X., Lorenz F.K., Blankenstein T. Effective NY-ESO-1-specific MHC II-restricted T cell receptors from antigen-negative hosts enhance tumor regression. J Clin Invest. 2019;129:324–335. doi: 10.1172/JCI120391. [DOI] [PMC free article] [PubMed] [Google Scholar]; Employing a mouse model of T cell therapy of cancer targeting a clinically relevant antigen, NY-ESO-1, the authors demonstrate that only the combined action of CD8 and CD4 T cells leads to tumor regression in all mice.
- 31.Chen Y.T., Scanlan M.J., Sahin U., Türeci O., Gure A.O., Tsang S., Williamson B., Stockert E., Pfreundschuh M., Old L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins P.F., Li Y.F., El-Gamil M., Zhao Y., Wargo J.A., Zheng Z., Xu H., Morgan R.A., Feldman S.A., Johnson L.A., et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Angelo S.P., Melchiori L., Merchant M.S., Bernstein D., Glod J., Kaplan R., Grupp S., Tap W.D., Chagin K., Binder G.K., et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 c259T cells in synovial sarcoma. Cancer Discov. 2018;8:944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins P.F., Kassim S.H., Tran T.L.N., Crystal J.S., Morgan R.A., Feldman S.A., Yang J.C., Dudley M.E., Wunderlich J.R., Sherry R.M., et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1–reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obenaus M., Leitão C., Leisegang M., Chen X., Gavvovidis I., van der Bruggen P., Uckert W., Schendel D.J., Blankenstein T. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat Biotechnol. 2015;33:402–407. doi: 10.1038/nbt.3147. [DOI] [PubMed] [Google Scholar]
- 36.Hunder N.N., Wallen H., Cao J., Hendricks D.W., Reilly J.Z., Rodmyre R., Jungbluth A., Gnjatic S., Thompson J.A., Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muranski P., Restifo N.P. Adoptive immunotherapy of cancer using CD4+ T cells. Curr Opin Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotter J., Brors B., Hergenhahn M., Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohaan M.W., van den Berg J.H., Kvistborg P., Haanen J.B.A.G. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6 doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linnemann C., van Buuren M.M., Bies L., Verdegaal E.M.E., Schotte R., Calis J.J.A., Behjati S., Velds A., Hilkmann H., el Atmioui D., et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 41.Friedman K.M., Prieto P.A., Devillier L.E., Gross C.A., Yang J.C., Wunderlich J.R., Rosenberg S.A., Dudley M.E. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J Immunother. 2012;35:400–408. doi: 10.1097/CJI.0b013e31825898c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assadipour Y., Zacharakis N., Crystal J.S., Prickett T.D., Gartner J.J., Somerville R.P.T., Xu H., Black M.A., Jia L., Chinnasamy H., et al. Characterization of an immunogenic mutation in a patient with metastatic triple-negative breast cancer. Clin Cancer Res. 2017;23:4347–4353. doi: 10.1158/1078-0432.CCR-16-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran E., Ahmadzadeh M., Lu Y.-C., Gros A., Turcotte S., Robbins P.F., Gartner J.J., Zheng Z., Li Y.F., Ray S., et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Cafri G., Yossef R., Pasetto A., Deniger D.C., Lu Y.-C., Parkhurst M., Gartner J.J., Jia L., Ray S., Ngo L.T., et al. Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients. Nat Commun. 2019;10 doi: 10.1038/s41467-019-08304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; By analyzing peripheral memory T cells from epithelia cancer patients, CD4 and CD8 T cells were isolated reactive with unique or shared KRAS mutations.
- 45•.Gros A., Tran E., Parkhurst M.R., Ilyas S., Pasetto A., Groh E.M., Robbins P.F., Yossef R., Garcia-Garijo A., Fajardo C.A., et al. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J Clin Invest. 2019;129:4992–5004. doi: 10.1172/JCI127967. [DOI] [PMC free article] [PubMed] [Google Scholar]; By analyzing PD1 high peripheral T cells from gastrointestinal cancer patients, CD4 and CD8 T cells were isolated reactive with unique or shared mutations.
- 46••.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.-C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this case study, a patient with metastatic cholangiocarcinoma was treated subsequently with two tumor infiltrating lymphocyte products containing mutation-specific CD4 T cells. Tumor regression of all metastases after both transfers occurred.
- 47•.Veatch J.R., Lee S.M., Fitzgibbon M., Chow I.-T., Jesernig B., Schmitt T., Kong Y.Y., Kargl J., Houghton A.M., Thompson J.A., et al. Tumor-infiltrating BRAFV600E-specific CD4+ T cells correlated with complete clinical response in melanoma. J Clin Invest. 2018;128:1563–1568. doi: 10.1172/JCI98689. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this case study, an acral melanoma patient which showed a complete response upon treatment with infiltrating lymphocytes contained a minority of BRAFV600E-specific CD4 T cells in the infusion product. Those T cells were found enriched in peripheral blood at 12 and 24 months post treatment suggesting contribution to anti-tumor immunity by CD4 T cells.
- 48•.Lu Y.-C., Parker L.L., Lu T., Zheng Z., Toomey M.A., White D.E., Yao X., Li Y.F., Robbins P.F., Feldman S.A., et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. 2017;35:3322–3329. doi: 10.1200/JCO.2017.74.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this phase I/II study, 17 patients were treated with CD4 T cells engineered to express a MAGE-A4-reactive TCR, of which 4 patients showed a clinical response. This was the first study employing CD4 T cells engineered with an MHC II-restricted TCR.