Abstract
Background
There is growing interest in the antidepressant potential of statins. We tested whether statin use is associated with cognitive markers previously found to indicate psychological vulnerability to depression within the context of the COVID-19 pandemic.
Methods
Between April 2020 and February 2021, we conducted an observational online study of 2043 adults in the United Kingdom. Participants completed cognitive tasks assessing processes related to depression vulnerability, including affective bias and reward processing. We also measured working memory, medication use, and current psychiatric symptoms. Using mixed analysis of covariance and regression models, we compared participants on statins alone (n = 81), antihypertensive medication alone (n = 126), both medications (n = 111), and on neither medication (n = 1725).
Results
Statin use was associated with reduced recognition of angry and fearful faces (F1 = 9.19, p = .002; F1 = 6.9, p = .009) and with increased misclassification of these expressions as positive. Increased recognition of angry faces at baseline predicted increased levels of depression and anxiety 10 months later (β = 3.61, p = .027; β = 2.37, p = .002). Statin use was also associated with reduced learning about stimuli associated with loss (F1,1418 = 9.90, p = .002). These indicators of reduced negative bias were not seen in participants taking antihypertensive medication alone, suggesting that they were related to statin use in particular rather than nonspecific demographic factors. In addition, we found no evidence of an association between statin use and impairment in working memory.
Conclusions
Statin use was associated with cognitive markers indicative of reduced psychological vulnerability to depression, supporting their potential use as a prophylactic treatment for depression.
Keywords: Affective bias, Cognitive neuroscience, Depression, Emotional processing, Experimental medicine, Statins
SEE COMMENTARY ON PAGE 528
Statins are widely prescribed cholesterol-lowering medications (1). Their ability to modulate immune response, as well as improve blood flow and reduce oxidative damage, is believed to contribute to additional neuroprotective effects (2). Increasing evidence indicates that inflammatory processes are associated with the development and maintenance of depression and the response of depressive symptoms to different interventions (3,4). As such, there is growing interest in developing or repurposing interventions with anti-inflammatory properties (5), including interest in the idea that statins may provide benefits in management of depressive disorders. The association between vascular disease and depression (6,7) provides further interest in the development of interventions that may target putative shared mechanisms. Despite early concerns that statins may increase depression rates (8), converging evidence across large population studies (9, 10, 11), meta-analyses of randomized controlled trials (12,13), and animal studies (14) consistently indicates that statins are associated with both reduced risk of developing depression in healthy participants and reduced depressive symptoms in patients. To date, no studies have investigated the psychological mechanisms of these antidepressant-like effects.
In 2020, people worldwide faced the challenge of the COVID-19 pandemic and the unprecedented social restrictions implemented to curb transmission (15,16). Many people were confronted with a constellation of psychosocial risk factors for poor mental health during this time, including loneliness, financial uncertainty, health anxiety, and bereavement (17,18), with consequent increased rates of psychiatric disorders (19,20). National lockdowns—including the lockdown implemented by the UK government on March 23, 2020—therefore provide a unique context for identifying factors, such as medication use, associated with psychiatric risk and resilience.
Previous studies identifying predictors of mental health vulnerability during the COVID-19 pandemic have focused on self-reported psychiatric symptoms; however, the use of cognitive biomarkers of depression risk may provide greater sensitivity to early changes in vulnerability, reduce susceptibility to demand characteristics, and elucidate underlying mechanisms. Negative affective bias is a key mechanism underlying depression and the action of conventional antidepressants and is a valuable cognitive biomarker for evaluating potential antidepressant interventions (21). Similarly, impaired performance on reward learning tasks has been associated with anhedonia and predicts clinical outcomes in depression (22).
We carried out a large, online, observational study to test the hypothesis that statin use would be associated with reduced negative affective bias and altered sensitivity to reward/loss during the UK COVID-19 lockdown, indicating reduced psychological vulnerability to depression. Because there has also been considerable discussion over whether statins impair or enhance cognition (23, 24, 25), we also measured working memory.
Methods and Materials
Study Design and Participants
The Oxford COSIE study (COvid-19, Social Isolation and Emotion) was conducted online, with 4 phases of data collection between April 2020 and February 2021. In phase 1, we recruited 2043 UK adults via an online research participant pool (Prolific Academic). All participants who completed phase 1 of data collection were recontacted three times over the following 10 months and invited to take part in subsequent phases. Of the original sample, 1925 participants (94.2%) completed phase 2 of data collection, 1832 participants (89.7%) completed phase 3, and 1707 participants (83.55%) completed phase 4.
Inclusion criteria were age over 18 years, currently living in the UK, access to a computer/internet connection, and passing all task-understanding checks at baseline.
This study received ethical approval from the University of Oxford CUREC (R69299/RE001) on April 17, 2020. Participants provided informed consent at each time point.
Procedures
Questionnaires and tasks were programed and completed online using Gorilla (https://gorilla.sc/). Full details can be found online on OSF (https://osf.io/9yv5j/).
Questionnaires
Participants reported demographic information via multiple choice questions (Figure S1). They were also asked to report the following: current and past psychiatric diagnoses (including major depressive disorder [MDD] and generalized anxiety disorder [GAD] when asked “Do you currently have a diagnosis of/Have you ever previously been diagnosed with any of the following?”), current use of medication (statins, antihypertensive medications, and antidepressants) and psychological therapies, weekly intake of alcohol units, average daily hours of physical activity, and use of cigarettes/tobacco. Changes in employment status, degree of social distancing, diagnoses, and use of medication or psychological therapy were recorded at all follow-up.
Self-reported symptoms of depression and anxiety were assessed using the 20-item Center for Epidemiologic Studies Depression Scale and the 7-item General Anxiety Disorder Scale at all time points.
Cognitive Tasks
Our primary affective bias task was the facial expression recognition task (FERT) (26), in which participants identify the expressions of faces presented on screen (for 500 ms) via a mouse click. Faces show an expression of fear, happiness, sadness, disgust, anger, or surprise or are neutral, with variation in the degree of emotion shown (for screenshots of all tasks, see Figure S2). There were 250 trials across four blocks, in a fixed pseudorandomized order, with emotions and actors balanced across blocks.
Additional affective bias tasks included the emotional categorization task and the emotional recall task (26) (see Additional Methods in the Supplement). All affective bias tasks were completed at phases 1, 2, and 3 of data collection, with different stimuli (words and faces) used at each time point.
During phase 2, participants completed an adapted probabilistic instrumental learning task (PILT) to assess reward learning (27,28). Participants are simultaneously presented with two stimuli (abstract symbols) that have reciprocal probabilities (0.7 vs. 0.3) of a gain versus a no gain outcome in gain trials or a loss versus no loss outcome in loss trials. Participants used their mouse to choose one of the two stimuli onscreen, following which they received visual feedback on the trial outcome (gain, loss, or no change in points) and their current total. They were informed of the probabilistic nature of the task and told that the aim was to win as many points as possible. There were 180 trials across three blocks and a new randomization of trial order for each participant.
During phase 3, participants completed an n-back task to assess working memory (29). Participants were presented with a series of letters (for 2000 ms) and asked to respond via a button press if the presented stimulus matched the stimulus presented n trials ago and given visual feedback (a tick/cross for correct/incorrect answers) after each response. One-back, 2-back, and 3-back blocks were included, with 0-back as a control condition. Each block consisted of 25% target and 75% nontarget letters. There were 160 trials across eight blocks, consisting of two blocks of each condition, in a fixed order.
Understanding of the task instructions was assessed before each task using multiple choice questions (see Additional Methods in the Supplement), and participants completed practice trials before each task.
Statistical Analysis
A full description of the analysis approach and outlier removal is provided in the Supplement. Data processing and analysis were conducted in R Studio (version 3.6.0; R Core Team, 2019) on Windows 10 ×64 (build 19042). All scripts are available on OSF.
Group Comparisons
Owing to a high proportion of participants taking statins and antihypertensive medication concurrently, and to provide a demographically similar comparison group, participants were grouped into those taking statins only (3.96%, n = 81), antihypertensive medication only (6.17%, n = 126), both medications (5.43%, n = 111), and neither medication (84.43%, n = 1725).
Any group differences in demographic and self-report measures were identified using regression models, controlling for age, gender, and ethnicity, and were included in subsequent maximally corrected analyses as covariates (Tables S2 and S3).
Task Analysis
Facial expression recognition task outcomes were analyzed with mixed analysis of covariance (ANCOVA), assessing the main effect of medication group (between-subject factor), emotion of facial expression (within-subject factor), and the interaction between group and emotion on task performance. Significant interactions were further analyzed using one-way ANCOVAs focusing on contrasts between specific emotions and medication groups.
For the facial expression recognition task, the primary outcome was unbiased hit rate—a measure of emotion identification accuracy that accounts for response bias, i.e., any general tendency to identify the emotion (30). We also assessed misclassifications (the number of times a participant incorrectly identified an emotion as an alternative emotion) for any emotion with a significant group difference in unbiased hit rate. Reaction time (in milliseconds) for trials with correct responses was also assessed. Data from phases 1 to 3 were combined, with time point added as a covariate, to increase the number of trials for each condition and reduce potential noise from task version.
PILT outcomes for loss and gain trials were analyzed separately with one-way ANCOVAs and focused on the last 20 trials as per analysis of the original task, where learning typically plateaus (27). Analyses including all trials were also conducted. The primary outcome was the proportion of trials for which the participant chose the advantageous stimulus, i.e., whether participants correctly chose the stimulus with the 0.7 probability of gaining points in gain trials and the stimulus with the 0.7 probability of not losing points in loss trials. Participants with low performance (advantageous symbol chosen in under 60% of trials) were excluded, because they were presumed to not adequately understand the task structure (n = 295)1 .
N-back outcomes were analyzed with mixed ANCOVAs. The within-subject factor was n-back condition (0-back, 1-back, 2-back, 3-back); the primary outcome was hit rate (%). False alarms (%), misses, and correct rejections were used to calculate d′ and beta values (30). Reaction time (ms) for trials with correct responses was also assessed.
Predicting Long-Term Outcomes
Linear regression was used to assess baseline predictors of follow-up self-reported depression and anxiety.
Statistical Checks
To assess statistical robustness, we confirmed all significant post hoc ANCOVAs with an equivalent linear regression model and conducted sensitivity checks, excluding nonlipophilic statin use, nonbinary gender (due to small n), PILT understanding check failures2 , and poor performers on the emotional categorization task and the emotional recall task and including all PILT trials. Only results consistent across analyses are reported below. All results from uncorrected models, minimally corrected models (correcting for demographic covariates only), and maximally corrected models (corrected for all covariates identified by the above group comparison analyses) are reported in Table S3, alongside additional analyses investigating the effects of specific antihypertensive medications and exploring interactions with age and gender.
Results
Sample Characteristics
Participants (N = 2043) recruited in phase 1 were 59.52% female, mean age was 45.09 (SD = 14.49, range = 18−79) years, all were UK residents, and the racial diversity of the sample was largely reflective of UK demographics (Table S1). Over a quarter of participants reported a current psychiatric diagnosis (9.94% MDD, 9.25% GAD), and approximately a third of participants reported a past psychiatric diagnosis (17.33% MDD, 10.33% GAD). Owing to COVID-19 restrictions, over half of participants at baseline (59.81%) were leaving their house only for essential supplies and exercise, 14.68% were shielding with access to outside space, and 2.01% were shielding without access to outside space. The most common antihypertensive medications were angiotensin-converting enzyme inhibitors and β-blockers, and the most common statin medications were lipophilic (simvastatin or atorvastatin).
Group Differences in Demographics and Self-report Measures
After controlling for age, gender, and ethnicity, significant group differences were found in household income, alcohol use, weekly physical activity, current antidepressant use, current depression scores, current MDD and GAD diagnoses, and history of any psychiatric diagnosis (Table S2). Age, gender, ethnicity, household income, alcohol use, weekly physical activity, current antidepressant use, current depression scores, and past psychiatric diagnosis were included as covariates in all maximally corrected analyses, reported below. See Table S3 for results of all models.
In an uncorrected model, statin use was negatively associated with baseline depression severity (β = −4.06, p = .004); however, this association was no longer significant when corrected for demographic covariates (β = 0.86, p = .529).
Group Differences in Task Performance
Facial Expression Recognition Task (n = 2033)
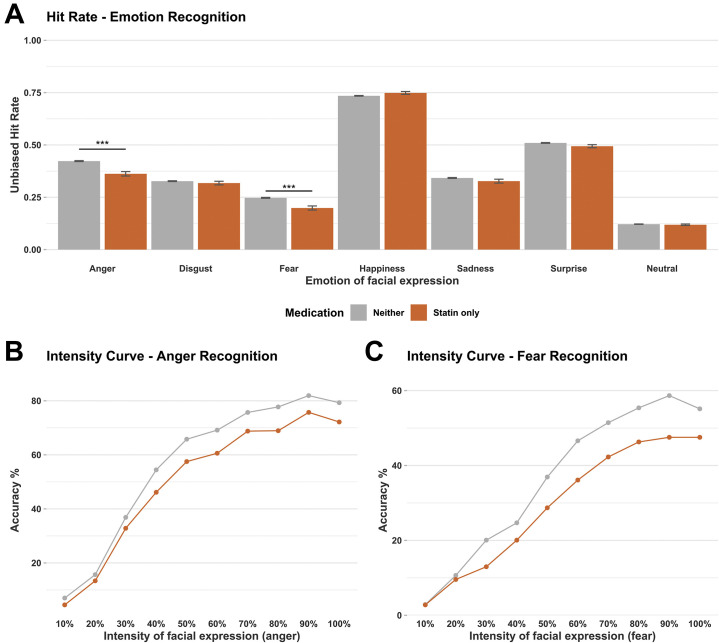
Statin use was associated with reduced recognition of two negative emotional expressions, angry and fearful faces, and with misclassification of these facial expressions as positive (Figure 1 ).
Figure 1.
Emotional processing task performance (facial expression recognition task) by medication group. (A) Mean unbiased hit rate for each emotion displayed during facial expression recognition task. (B) Mean group accuracy for identification of angry faces at each intensity level of anger. (C) Mean group accuracy for identification of fearful faces at each intensity level of fear. ∗∗∗p < .001. Error bars show ±1 standard error.
For unbiased hit rate, there was a significant interaction between facial emotion and medication group (F 18 = 5.41, p < .001) and a significant main effect of medication group (F 3 = 3.44, p = .016). In post hoc tests, participants on a statin alone had a significantly lower hit rate for identifying both angry (F 1 = 9.19, p = .002) and fearful (F 1 = 6.9, p = .009) facial expressions than participants on neither medication; this reduction was also evident in participants on both a statin and antihypertensive medication (anger, F 1 = 6.48, p = .011; fear, F 1 = 5.66, p = .017) but not in participants on antihypertensive medication alone (anger, F 1 = 1.37, p = .242; fear, F 1 = 1.69, p = .194). No significant group differences in hit rate were found for the other facial expressions. These results remained consistent in minimally corrected models.
For reaction time, there was no main effect of medication group (F 3 = 1.49, p = .216), but there was a significant interaction between facial emotion and medication group (F 18 = 5.01, p < .001); post hoc tests indicated that participants on a statin alone took significantly longer to correctly identify angry (F 1 = 7.93, p = .005) and disgusted (F 1 = 3.88, p = .049) facial expressions.
Follow-up analysis of misclassifications for angry (F 15 = 3.31, p < .001) and fearful faces (F 15 = 3.47, p < .001) identified a significant interaction between emotion of the misclassification and medication group; post hoc tests indicated that participants on a statin alone were significantly more likely to misclassify angry faces as surprised (F 1 = 11.52, p < .001).
Probabilistic Learning Task (n = 1636)
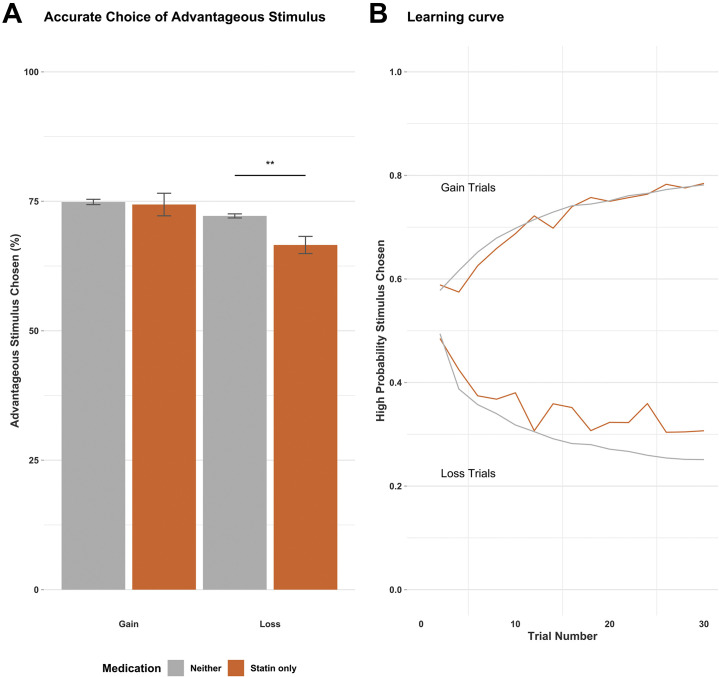
Statin use was associated with reduced learning about loss, with participants less likely to avoid stimuli with a high probability of loss (Figure 2 ).
Figure 2.
Reward learning task performance (probabilistic instrumental learning task) by medication group. (A) Mean percentage of trials during which participants choose the advantageous stimuli in a reward learning task (probabilistic instrumental learning task). (B) Learning curve showing proportion of participants in each group choosing the symbol associated with a high probability of loss or gain, trial by trial. High probability stimuli are advantageous in gain trials and not advantageous in loss trials. ∗∗p < .01. Error bars show ±1 standard error.
For gain trials, there was no main effect of medication group on choice of the high probability stimulus (F 3,1610 = 0.06, p = .979). For loss trials, there was a significant main effect of medication group on choice of the high probability stimulus (F 3,1610 = 4.73, p = .003); post hoc tests found that those on statins alone were more likely to choose the stimulus with a high probability of loss (F 1,1418 = 9.90, p = .002), and this was not consistently seen in the other medication groups. These results remained consistent in minimally corrected models and when analyses included either data from all trials or the last 20 trials.
Working Memory (N-back; n = 1767)
Statin use was not associated with any reduction in working memory (Figure S3).
For hit rate, there was no main effect of group (F 3 = 1.60, p = .187), but there was a significant interaction between n-back type and medication group (F 6 = 3.77, p < .001); post hoc tests found significantly reduced hit rates in the 1-back condition for those on antihypertensive medication alone (F 1,1536 = 11.38, p < .001) and significantly reduced hit rates for those on both a statin and antihypertensive medication in the 2-back condition (F 1,1521 = 12.65, p < .001). Compared with those on neither medication, no differences were found in the statin group (1-back, F 1,1494 = 0.29, p = .589; 2-back, F 1,1494 = 0.54, p = .462; 3-back, F 1,1494 = 1.22, p = .270). For false alarms, there was no main effect of medication group (F 3 = 0.25, p = .863) or interaction between n-back type and medication group (F 6 = 1.939, p = .073).
Similar patterns were seen for misses, correct rejections, d′, and beta (Table S3). For all outcomes, there was no group difference on the 0-back control condition and no main effect of group or interaction for reaction time. All results remained consistent in minimally corrected models.
Predicting Long-Term Mental Health Outcomes
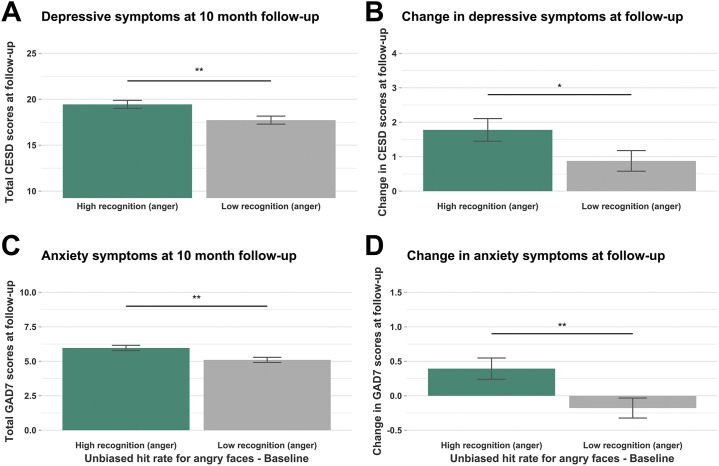
Baseline statin use did not predict self-reported depression (β = 1.35, p = .216) or anxiety (β = −0.35, p = .505) at phase 4. However, reduced recognition of angry faces at baseline was associated with lower levels of self-reported depression (β = 3.61, p = .027) and anxiety (β = 2.37, p = .002) at phase 4 and lower increases in severity between baseline and follow-up for both depression (β = 3.61, p = .027) and anxiety (β = 2.37, p = .002) (Figure 3 ). Medication use and anger recognition were not significant predictors of self-reported depression or anxiety at earlier follow-up (Table S3). However, a growth curve analysis in which depression and anxiety were modeled as longitudinal outcomes across all time points found that recognition of angry faces significantly predicted the intercepts for both depression (β = 1.064, p = .046) and anxiety (β = 0.682, p = .007).
Figure 3.
Baseline predictors of depression and anxiety at follow-up. Analysis was conducted on continuous measures of anger recognition. For these plots, participants were split into those above (high) and below (low) the median anger recognition performance. (A) Mean depressive symptoms at 10-month follow-up, split by those with high and low anger recognition at baseline. (B) Mean change in depressive symptoms between baseline and 10-month follow-up, split by those with high and low anger recognition at baseline. (C) Mean anxiety symptoms at 10-month follow-up, split by those with high and low anger recognition at baseline. (D) Mean change in anxiety symptoms between baseline and 10-month follow-up, split by those with high and low anger recognition at baseline. ∗p < .05, ∗∗p < .01. Error bars show ±1 standard error. CESD, Center for Epidemiologic Studies Depression Scale; GAD7, 7-item General Anxiety Disorder scale.
Pathway analyses in which statin use predicted recognition of angry faces and recognition of angry faces predicted follow-up depression or anxiety found that (as above) the associations between recognition of angry faces and statin use, follow-up depression, and follow-up anxiety (statin use, β = −0.052, p = .002; depression, β = 3.797, p = .014; anxiety, β = 2.214, p = .002) were significant, but there was no direct significant association between statin use and follow-up depression (β = 1.592, p = .140) or anxiety (β = 0.002, p = .998). The models, incorporating all covariates, led to reasonable parameters of model fit (depression, comparative fit index = 0.984, Tucker-Lewis index = 0.963; anxiety, comparative fit index = 0.980, Tucker-Lewis index = 0.954).
Discussion
In a diverse sample of over 2000 participants, including 192 participants taking statin medication, statin use was associated with reduced negative affective bias and reduced learning about loss. This pattern of task performance (associated with reduced vulnerability to depression) provides support for the possible use of statins as a prophylactic depression intervention, particularly in high-stress scenarios, and adds to growing evidence of their antidepressant properties (9, 10, 11, 12, 13). In addition, these findings provide a potential psychological mechanism to explain reported associations between statin use and reduced risk of depression or depressive symptoms.
Statin use was associated with reduced negative affective bias in the recognition of facial expressions. Specifically, participants on statins showed reduced recognition of angry and fearful faces, instead classifying these negative facial expressions as positive. Negative affective bias is a key cognitive mechanism targeted by common antidepressants such as selective serotonin reuptake inhibitors (31) and has previously been shown to predict clinical outcomes in depression (32).
In our sample, increased negative affective bias (indicated by greater recognition of angry faces) at baseline was associated with increased depressive and anxious symptoms 10 months later, supporting the clinical and predictive relevance of our findings and of measuring affective bias more broadly. Growth curve analysis indicated that negative bias significantly predicted changes in self-reported mental health over the study period but negative bias did not predict these scores at individual earlier follow-ups. For context, over the first three data collection phases, the UK was relaxing pandemic restrictions, and our sample reported improvement in mental health with significant decreases in depression severity at both phase 2 and phase 3. In contrast, phase 4 was during a period of increased restrictions in winter months of early 2021, with self-reported depression severity at the highest level throughout data collection, and so potentially represents the time point with greatest mental health risk. This may be where we see the effects of psychological vulnerability, as indicated by negative affective bias, become particularly relevant.
Statin use was also associated with reduced learning about loss in a learning task. Specifically, participants on statins showed reduced avoidance of stimuli associated with high likelihood of loss. Reduced reward sensitivity has been predominantly considered the core impairment in depression (33); however, heightened loss sensitivity has also been reported (34), and conventional antidepressants have previously been found to increase the ability to disregard negative interference (35) and specifically reduce loss sensitivity (36). Our identified association between statin use and reduced sensitivity to loss may therefore provide further support for consideration of statins as a promising intervention in depression.
In addition, while previous literature has reported mixed findings for the cognitive effect of statins (23, 24, 25), we found no association between statin use and any reduction in working memory on an n-back task, only impaired performance in participants taking antihypertensive medication. This concurs with other studies reporting that statins do not impair cognitive ability (24).
Taken collectively, our findings are most consistent with a pattern of reduced negative bias as opposed to cognitive impairment. If participants on statins were showing general reductions in cognitive ability, we would expect to see reduced recognition of facial expressions across both negative and positive facial expressions, reduced learning about both rewards and losses, and reduced working memory performance. In contrast, the reduction in the recognition of facial expressions was specific to negative faces and the misclassifications of these negative facial expressions was as positive, indicating a positive affective bias. Additionally, there was no impaired learning in reward conditions and no impairment on even the most challenging condition of the working memory task (some analyses indicated improved performance but this was not consistent; Table S3).
The mechanisms by which statins may reduce depression risk are unclear, and previous research has predominantly focused on physiological mechanisms. Preclinical studies support an anti-inflammatory effect of statins on both the central and peripheral nervous system, including in brain regions such as the hippocampus; however, there are also effects on blood flow, neurotransmission, and markers of plasticity (37). This study suggests that cognitive changes in emotional processing are particularly associated with statin use and may play a role in their protective action in depression.
Several studies have reported that negative affective bias (such as bias toward angry and sad faces) is associated with elevated inflammatory markers, such as C-reactive protein (38). In addition, experimentally induced stress, shown to elevate interleukin 1β and interleukin 6 (inflammatory cytokines) levels, has been associated with subsequent increases in negative attentional bias (39,40), with similar findings of increased sensitivity to loss in a PILT when vaccine challenge was used to manipulate inflammation (41). Consistent with this, neuroimaging studies have demonstrated that experimentally increasing inflammation is associated with increased activation in response to negative social cues or facial expressions in brain regions such as the amygdala and anterior cingulate cortex (42, 43, 44); reduced activation in these regions, in response to negative faces, has been shown to predict therapeutic response to antidepressants (32). In addition, reducing inflammation via therapies such as anti–tumor necrosis factor α has been associated with increased attentional bias toward positive words (45). Therefore, it is possible that our observed association between statin use and reduced negative affective bias may occur via reductions in inflammatory cascades, which in turn have been associated with indirect effects on serotonin and hypothalamic-pituitary-adrenal axis function via cytokine regulation (46). In the light of the extreme social disruption seen throughout this study, it is worth noting that inflammation-based theories of depression typically highlight social stress as a particularly relevant cause of inflammation (47).
A limitation of this study is that, despite having an overall sample of 2043 participants, the number of participants taking statin and/or antihypertensive medication was smaller (318 participants), preventing potential subanalyses of interest. The use of online data collection is another potential limitation, relying on self-report measures and lacking the controlled lab setting typically desired for cognitive tasks. We took several steps to mitigate this risk, including requiring mandatory successful completion of task-understanding checks at baseline, repeating these checks at all time points, including engagement checks during self-report measures, conducting sensitivity analyses to ensure results were not unduly affected by poor performance, and maintaining regular communication with the cohort to foster engagement. An additional limitation is that the reward learning task and working memory task were not included at all time points due to practical restraints, limiting our ability to assess baseline group differences or consistency over time.
One concern when interpreting these observational data is that nonspecific differences between the groups might have driven our findings. We addressed this in two main ways, first by controlling for demographic differences (age, gender, ethnicity, and household income) and other potential confounders (past psychiatric history, antidepressant use, and current depression severity) in our analysis, and second by comparing participants on statins with participants on antihypertensive medication, who provided an additional control group with a similar demographic and elevated cardiovascular risk. Critically, the differences in facial expression recognition were only seen in participants taking statins and not in participants taking antihypertensive medication, and the differences in reward learning were also only consistently seen in participants taking statins, providing strong evidence that our findings are specific to use of statin medication.
In summary, we report results from an online, observational study indicating that statin use is associated with reduced psychological vulnerability to depression, as demonstrated by reduced negative affective bias and reduced sensitivity to loss. These reductions in psychological risk factors for depression corroborate several sources of evidence indicating that statins may be protective against depression. Depression is a common and disabling psychiatric disorder, and rates of depressive symptoms in the general population have only been exacerbated during the COVID-19 pandemic, making the need for cheap, novel interventions particularly acute. A priority for future research is confirming these findings with controlled, randomized studies to establish the utility of statin treatment in depression, their potential prophylactic effects during high-stress periods, and the physiological and cognitive mechanisms involved.
Acknowledgments and Disclosures
The investigators acknowledge the philanthropic support of the donors to the University of Oxford’s COVID-19 Research Response Fund, which funded this study. The study was further supported by the National Institute for Health Research Oxford Biomedical Research Centre.
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
CJH and SEM conceptualized the study and acquired the funding for this study. CJH, SEM, and ALG designed the methodology. ALG constructed the online experiment and oversaw data collection. ALG, CW, and IVA accessed and verified the underlying data. ALG, CW, and IVA wrote the scripts and did the formal analyses and visualizations. ALG takes responsibility for the integrity of the data and accuracy of data analysis. ALG wrote the original manuscript draft. All authors contributed to reviewing and editing the manuscript. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
We thank all the COSIE study participants who took part in the study over a tumultuous year and who brightened our inbox with messages, the patient and public contributors who advised on which variables to measure, the department administrators who helped process requests at very short notice, and the colleagues who contributed to the setup of the online tasks and questionnaires (Calum Guinea, James Carson, Jess Scaife) and who advised on interpretation of the probabilistic instrumental learning task (Chamith Halahakoon and Michael Browning).
Unrelated to this work, ALG has received consultancy fees from Zogenix; CJH has received consultancy fees from P1vital Ltd, Janssen Pharmaceuticals (Antwerp, Belgium), Sage Therapeutics, Pfizer, Lundbeck, and Zogenix; and SEM has received consultancy fees from Janssen Pharmaceuticals, Zogenix, and Sumitomo Dainippon Pharma. CJH and SEM hold grant income from UCB Pharma, Janssen Pharmaceuticals, Zogenix, and Pfizer. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Low performance on the probabilistic instrumental learning task was not associated with medication group (χ23 [n = 1893] = 0.7523, p = .861).
Completion of facial expression recognition task, emotional categorization task, and emotional recall task understanding checks was mandatory at phase 1, and only 1 participant failed n-back understanding checks, so understanding checks for these tasks were not used in sensitivity analyses.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2022.03.009.
Supplementary Material
References
- 1.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–486. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 2.van der Most P.J., Dolga A.M., Nijholt I.M., Luiten P.G.M., Eisel U.L.M. Statins: Mechanisms of neuroprotection. Prog Neurobiol. 2009;88:64–75. doi: 10.1016/j.pneurobio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Orlovska-Waast S., Köhler-Forsberg O., Brix S.W., Nordentoft M., Kondziella D., Krogh J., Benros M.E. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: A systematic review and meta-analysis [published correction appears in Mol Psychiatry 2019; 24:929–934] Mol Psychiatry. 2019;24:869–887. doi: 10.1038/s41380-018-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valkanova V., Ebmeier K.P. Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biol Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Harrison R.W., Ashton C.H. Do cholesterol-lowering agents affect brain activity? A comparison of simvastatin, pravastatin, and placebo in healthy volunteers. Br J Clin Pharmacol. 1994;37:231–236. doi: 10.1111/j.1365-2125.1994.tb04268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redlich C., Berk M., Williams L.J., Sundquist J., Sundquist K., Li X. Statin use and risk of depression: A Swedish national cohort study. BMC Psychiatry. 2014;14:348. doi: 10.1186/s12888-014-0348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessing L.V., Rytgaard H.C., Gerds T.A., Berk M., Ekstrøm C.T., Andersen P.K. New drug candidates for depression—A nationwide population-based study. Acta Psychiatr Scand. 2019;139:68–77. doi: 10.1111/acps.12957. [DOI] [PubMed] [Google Scholar]
- 11.Molero Y., Cipriani A., Larsson H., Lichtenstein P., D’Onofrio B.M., Fazel S. Associations between statin use and suicidality, depression, anxiety, and seizures: A Swedish total-population cohort study. Lancet Psychiatry. 2020;7:982–990. doi: 10.1016/S2215-0366(20)30311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salagre E., Fernandes B.S., Dodd S., Brownstein D.J., Berk M. Statins for the treatment of depression: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235–242. doi: 10.1016/j.jad.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 13.Yatham M.S., Yatham K.S., Ravindran A.V., Sullivan F. Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J Affect Disord. 2019;257:55–63. doi: 10.1016/j.jad.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Yu X.B., Zhang H.N., Dai Y., Zhou Z.Y., Xu R.A., Hu L.F., et al. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord. 2019;245:939–949. doi: 10.1016/j.jad.2018.11.086. [DOI] [PubMed] [Google Scholar]
- 15.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383:510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 16.Moreno C., Wykes T., Galderisi S., Nordentoft M., Crossley N., Jones N., et al. How mental health care should change as a consequence of the COVID-19 pandemic [published correction appears in Lancet Psychiatry 2021; 8:e16] Lancet Psychiatry. 2020;7:813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killgore W.D.S., Cloonan S.A., Taylor E.C., Dailey N.S. Loneliness: A signature mental health concern in the era of COVID-19. Psychiatry Res. 2020;290:113117. doi: 10.1016/j.psychres.2020.113117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groarke J.M., Berry E., Graham-Wisener L., McKenna-Plumley P.E., McGlinchey E., Armour C. Loneliness in the UK during the COVID-19 pandemic: Cross-sectional results from the COVID-19 Psychological Wellbeing Study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L.Z., Wang S. Prevalence and predictors of general psychiatric disorders and loneliness during COVID-19 in the United Kingdom. Psychiatry Res. 2020;291:113267. doi: 10.1016/j.psychres.2020.113267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith L.E., Amlot R., Lambert H., Oliver I., Robin C., Yardley L., Rubin G.J. Factors associated with self-reported anxiety, depression, and general health during the UK lockdown; a cross-sectional survey. medRxiv. 2020 doi: 10.1101/2020.06.23.20137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godlewska B.R., Harmer C.J. Cognitive neuropsychological theory of antidepressant action: A modern-day approach to depression and its treatment. Psychopharmacology (Berl) 2021;238:1265–1278. doi: 10.1007/s00213-019-05448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrieze E., Pizzagalli D.A., Demyttenaere K., Hompes T., Sienaert P., de Boer P., et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz B.G., Patten D.K., Berlau D.J. The role of statins in both cognitive impairment and protection against dementia: A tale of two mechanisms. Transl Neurodegener. 2018;7:5. doi: 10.1186/s40035-018-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samaras K., Makkar S.R., Crawford J.D., Kochan N.A., Slavin M.J., Wen W., et al. Effects of statins on memory, cognition, and brain volume in the elderly. J Am Coll Cardiol. 2019;74:2554–2568. doi: 10.1016/j.jacc.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Alsehli A.M., Olivo G., Clemensson L.E., Williams M.J., Schiöth H.B. The cognitive effects of statins are modified by age. Sci Rep. 2020;10:6187. doi: 10.1038/s41598-020-63035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmer C.J., O’Sullivan U., Favaron E., Massey-Chase R., Ayres R., Reinecke A., et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- 27.Pessiglione M., Seymour B., Flandin G., Dolan R.J., Frith C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh A.E.L., Huneke N.T.M., Brown R., Browning M., Cowen P., Harmer C.J. A dissociation of the acute effects of bupropion on positive emotional processing and reward processing in healthy volunteers. Front Psychiatry. 2018;9:482. doi: 10.3389/fpsyt.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner H.L. On measuring performance in category judgment studies of nonverbal behavior. J Nonverbal Behav. 1993;17:3–28. [Google Scholar]
- 31.Harmer C.J., Bhagwagar Z., Perrett D.I., Völlm B.A., Cowen P.J., Goodwin G.M. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- 32.Godlewska B.R., Browning M., Norbury R., Cowen P.J., Harmer C.J. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiatry. 2016;6:e957. doi: 10.1038/tp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halahakoon D.C., Kieslich K., O’Driscoll C., Nair A., Lewis G., Roiser J.P. Reward-processing behavior in depressed participants relative to healthy volunteers: A systematic review and meta-analysis [published correction appears in JAMA Psychiatry 2020; 77:1310] JAMA Psychiatry. 2020;77:1286–1295. doi: 10.1001/jamapsychiatry.2020.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eshel N., Roiser J.P. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Scholl J., Kolling N., Nelissen N., Browning M., Rushworth M.F.S., Harmer C.J. Beyond negative valence: 2-week administration of a serotonergic antidepressant enhances both reward and effort learning signals. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzallah M.M., Moustafa A.A., Natsheh J.Y., Abdellatif S.M., Taha M.B., Tayem Y.I., et al. Learning from negative feedback in patients with major depressive disorder is attenuated by SSRI antidepressants. Front Integr Neurosci. 2013;7:67. doi: 10.3389/fnint.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Giorgi R., Rizzo Pesci N., Quinton A., De Crescenzo F., Cowen P.J., Harmer C.J. Statins in depression: An evidence-based overview of mechanisms and clinical studies. Front Psychiatry. 2021;12:702617. doi: 10.3389/fpsyt.2021.702617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyle C.C., Ganz P.A., Van Dyk K.M., Bower J.E. Inflammation and attentional bias in breast cancer survivors. Brain Behav Immun. 2017;66:85–88. doi: 10.1016/j.bbi.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maydych V., Claus M., Watzl C., Kleinsorge T. Attention to emotional information is associated with cytokine responses to psychological stress. Front Neurosci. 2018;12:687. doi: 10.3389/fnins.2018.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper C.M., Godlewska B., Sharpley A.L., Barnes E., Cowen P.J., Harmer C.J. Interferon-α induces negative biases in emotional processing in patients with hepatitis C virus infection: A preliminary study. Psychol Med. 2018;48:998–1007. doi: 10.1017/S0033291717002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison N.A., Voon V., Cercignani M., Cooper E.A., Pessiglione M., Critchley H.D. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol Psychiatry. 2016;80:73–81. doi: 10.1016/j.biopsych.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muscatell K.A., Dedovic K., Slavich G.M., Jarcho M.R., Breen E.C., Bower J.E., et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray M.A., Chao C.Y., Staudacher H.M., Kolosky N.A., Talley N.J., Holtmann G. Anti-TNFα therapy in IBD alters brain activity reflecting visceral sensory function and cognitive-affective biases. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maydych V. The interplay between stress, inflammation, and emotional attention: Relevance for depression. Front Neurosci. 2019;13:384. doi: 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.