Abstract
This study aimed to identify the importance of ecological factors to distribution patterns of the invasive Clam (Corbicula fluminea) relative to native mussels (family: Unionidae) across seven rivers within the Mobile and Tennessee basins, Southeast United States. We quantitatively surveyed dense, diverse native mussel aggregations across 20 river reaches and estimated mussel density, biomass, and species richness along with density of invasive C. fluminea (hereafter Corbicula). We measured substrate particle size, velocity, and depth in quadrats where animals were collected. Additionally, we characterized reach scale environmental parameters including seston quantity and quality (% Carbon, % Nitrogen, % Phosphorous), water chemistry (ammonium [], soluble reactive phosphorous [SRP]), and watershed area and land cover. Using model selection, logistic regression, and multivariate analysis, we characterized habitat features and their association to invasive Corbicula within mussel beds. We found that Corbicula were more likely to occur and more abundant in quadrats with greater mussel biomass, larger substrate size, faster water velocity, and shallower water depth. At the reach scale, Corbicula densities increased where particle sizes were larger. Mussel richness, density, and biomass increased with watershed area. Water column increased at reaches with more urban land cover. No land cover variables influenced Corbicula populations or mussel communities. The strong overlapping distribution of Corbicula and mussels support the hypothesis that Corbicula are not necessarily limited by habitat factors and may be passengers of change in rivers where mussels have declined due to habitat degradation. Whether Corbicula is facilitated by mussels or negatively interacts with mussels in these systems remains to be seen. Focused experiments that manipulate patch scale variables would improve our understanding of the role of species interactions (e.g., competition, predation, facilitation) or physical habitat factors in influencing spatial overlap between Corbicula and native mussels.
Keywords: filter‐feeders, invasive species, niche overlap, niche partitioning, positive associations, rivers, species interactions
Identifying environmental factors associated with the distribution of invasive species relative to native species is fundamental to conservation and management. Our quantitative surveys of dense, diverse native mussel beds in rivers across a broad geographic area showed that invasive Corbicula fluminea were more likely to occur and more abundant where mussels are. The strong positive association between invasive and native bivalves support the hypothesis that C. fluminea are not necessarily limited by habitat factors and may be passengers of change in rivers where mussels have declined due to habitat degradation.
![]()
1. INTRODUCTION
Invasive species are a leading threat to native biodiversity (Clavero & García‐Berthou, 2005; Wilcove et al., 1998). Invasive species often exert negative pressures on native species through predation and competition (Davis, 2003) which can contribute to their successful establishment (Gamradt & Kats, 1996; Simberloff et al., 2020). When invasive species are functionally similar to native species, their competitive effects can be particularly harmful because of their overlapping resource requirements (Booth et al., 2003; David et al., 2017). However, theory predicts that competitive exclusion will limit the coexistence of functionally similar species (Levin, 1970; Macarthur & Levins, 1967); thus, native communities may also suppress invasion by functionally similar species due to limiting similarity (Fargione et al., 2003; Tilman, 2004). In either case, non‐overlapping distributions between invasive and native species have been used to support speculations of competitive exclusion, suppression, or biotic homogenization (Fargione & Tilman, 2005; Padial et al., 2020). However, the Anthropocene is characterized by significant declines in native biodiversity, making it equally possible that patterns of overlap between native and invasive species are driven by functionally similar species invading habitats once occupied by their native counterparts (MacDougall & Turkington, 2005; Strayer et al., 1999). Therefore, characterizing the spatial overlap of invasive species relative to native communities and the habitat that control invasive species populations is essential for designing appropriate control measures for invasive species and recovery plans for native species (Pergl et al., 2020).
Freshwater ecosystems are particularly vulnerable to species introductions and extinctions because of their high degree of isolation and endemism (Reid et al., 2019). Consequently, invasive species are a prominent component of contemporary freshwater ecosystems and are implicated in populations declines or extirpations of many species (Strayer et al., 1999; Strayer & Dudgeon, 2010). In particular, invasive bivalves such as zebra mussels (Dreissenia polymorpha) and the invasive Clam (Corbicula fluminea) continue to spread and negatively affect freshwater ecosystems worldwide (Sousa et al., 2014; Strayer et al., 2014). This can be problematic for effected ecosystems with diverse and abundant communities of functionally similar native bivalves because of apparent similarities in niche requirements (Atkinson et al., 2010). Once established, invasive bivalves can dominate communities and physically alter benthic habitats (Ilarri et al., 2015; Sousa et al., 2009). Thus, quantifying biotic and abiotic controls over their invasive range is a fundamental first step in identifying potential effects on native communities.
Freshwater mussels (Bivalvia: Unionidae) are long‐lived, benthic, filter‐feeding bivalves (Haag, 2012). Mussels are common in eastern North American streams where they are patchily distributed at multiple spatial scales. Mussel life‐histories are unique, such that adults are sedentary and release larvae that are ectoparasites on fish (Barnhart et al., 2008). Therefore, mussel distributions at regional scales are partially influenced by fish host populations (Schwalb et al., 2013; Vaughn & Taylor, 2000), but once settled persistence is largely governed by environmental factors (Sansom et al., 2018). Mussels often occur as dense, species‐rich aggregations called mussel beds where mussels are 10–100× denser than in areas outside of beds (Strayer, 2008). Further, mussel densities within these beds can vary with stream size (Atkinson et al., 2012; Hopper et al., 2018). Mussel beds exist in river channels that experience significant sediment mobility, but beds can persist in the same stream sites and have similar abundance and species composition for decades (Sansom et al., 2018). Mussels are also heterogeneous within beds, with individual mussels aggregating in dense patches separated by areas with few or no mussels (Atkinson & Forshay, 2022; Vaughn & Spooner, 2006b). Mussels are crucial for ecosystem function as they filter particles from the water column and excrete and egest nutrients that are important to green (Vaughn et al., 2008) and brown food webs (Atkinson et al., 2021; Hopper et al., 2021). Unfortunately, mussels account for nearly half of imperiled species in freshwater ecosystems (Lopes‐Lima et al., 2018) and their declines have been influenced by habitat destruction, disease, climate change, and invasive species (Böhm et al., 2020). In North American rivers, of the more than 300 species of mussels, 74% are considered imperiled, and at least 35 are considered extinct (Patterson et al., 2018). Although freshwater mussels are effected by several factors involving habitat degradation and modification, and sometimes unknown reasons (Haag, 2019), the increase of invasive populations of functionally similar bivalves of the genus Corbicula further threatens freshwater mussel populations (Haag et al., 2020).
Bivalves of the genus Corbicula are native to Southeast Asia, the Middle East, Australia, and Africa (Araujo et al., 1993). Specifically, Corbicula fluminea (hereafter Corbicula) is distributed across all continents except Antarctica and was introduced in the United States on the west coast in the early 1900s (Crespo et al., 2015). Human‐mediated dispersal for various reasons (e.g., food, fish bait, aquarium releases) has promoted Corbicula introduction and establishment in new ecosystems (Ferreira‐Rodríguez et al., 2019; Strayer, 2010). Rapid growth, early sexual maturity, short lifespan, and high fecundity (Sousa et al., 2008) are traits that have aided in its successful establishment. Corbicula colonization can alter biogeochemical cycles controlled by native mussels, reduce phytoplankton abundance, alter benthic communities (Atkinson et al., 2011; Hakenkamp et al., 2001; Novais et al., 2017), or directly compete for habitat with native filter‐feeders (Ferreira‐Rodríguez et al., 2018; Ferreira‐Rodríguez & Pardo, 2017). Experimental evidence supports these observations, demonstrating that growth, physiological condition, and behavior of a native mussel (Unio delphinus) is reduced under increased densities of co‐occurring Corbicula (Ferreira‐Rodríguez et al., 2018), suggesting the displacement of native mussels to less favorable habitats by Corbicula may drive mussel declines. Although research conducted on Corbicula shows negative effects on native communities, dense populations of Corbicula often co‐occur with native freshwater mussels (Bódis et al., 2011; Modesto et al., 2019; Vaughn & Spooner, 2006a). Because the effects of habitat loss and disturbance also weigh heavily in many invaded systems, it is conceivable that invasive species success may be less attributable to competitive ability than expected. Indeed, the native mussel communities may not interact strongly with Corbicula in such a way that causes change to mussel communities, but rather Corbicula may be passengers of more fundamental environmental change that is most limiting to native mussels (MacDougall & Turkington, 2005; Strayer et al., 1999, 2004). Thus, identifying habitat characteristics that support Corbicula in habitats where mussels are abundant is key to understanding the causes and consequences of spatial overlap between native mussels and Corbicula.
Physical habitat variables and species interactions can influence species distributions differently depending on the spatial scale. Indeed, biotic effects are more often quantifiable and observed at fine spatial scales where species interact, whereas physical environmental variables are often more important at regional scales (Bengtsson, 1989). Here, we tested whether Corbicula populations were associated with physical habitat variables and native mussels across two spatial scales to identify characteristics associated with patterns of Corbicula distribution in native mussel beds across a wide range of physiography. Our specific objectives were to address: (1) how Corbicula occurrence and density vary with mussel species richness, density, and biomass at the patch and reach scale; and (2) how stream benthic habitat characteristics such as depth, particle size, and water velocity influence Corbicula occurrence and density at the patch and reach scale. We hypothesized that in patches and reaches where mussels are more abundant or had greater species richness Corbicula would be absent or at low densities due to lack of space, physical displacement by burrowing activities, and potentially reduced patch scale food resources due to competitive effects. Alternatively, Corbicula may invade habitats where mussel communities are already in decline because of anthropogenic activities, such as land use practices that increase non‐point source nutrient loading (MacDougall & Turkington, 2005). In this case, we expected Corbicula occurrence and density to be greatest in patches and reaches with more mussel species and greater densities. Next, we hypothesized that Corbicula would be associated with physical habitat characteristics where mussels occur due to functional similarity. Overall, our findings provide a more nuanced view of the abiotic factors underlying Corbicula occurrence and abundance and the potential spatial overlap with native bivalve communities.
2. MATERIALS AND METHODS
2.1. Study region
North American rivers contain the greatest known diversity of freshwater mussels (~360 species), and the Mobile and Tennessee River Basins represent ~60% of that diversity (Williams et al., 2008). Various human activities have degraded rivers in this region, and ~95% of U.S. federally protected mussels can be found in this region. While Corbicula is suspected to harm mussels and was established in these rivers more than 50 years ago, invasion timing and quantitative population estimates are not widely available (Benson & Williams, 2021). We selected three rivers in the Tennessee Basin and four in the Mobile Basin with variable mussel densities and species composition to evaluate how Corbicula populations are distributed across ecological gradients (Figure 1). The Paint Rock River, Bear Creek, and Duck River are tributaries to the Tennessee River and support high mussel diversity (Paint Rock 58 species, Bear Creek 34 species, Duck 68 species) and vary in the watershed area (Paint Rock 1191 km2, Bear Creek 2450 km2, Duck 8100 km2). The Sipsey (watershed area 2044 km2) and Buttahatchee River (watershed area 2252 km2) occur in the Mobile basin as tributaries to the Tombigbee River and maintain historical mussel communities (Sipsey 42 species, Buttahatchee 43 species). The Cahaba River (watershed area 3009 km2) and Bogue Chitto Creek (watershed area 937 km2) are tributaries to the Alabama River before meeting the Mobile River in southwestern Alabama. Both have been effected negatively (e.g., recent droughts, habitat degradation, and invasive species), but historically had diverse mussel communities with 50 species in the Cahaba (Onorato et al., 2000) and 20 species in Bogue Chitto Creek (Sánchez González et al., 2021).
FIGURE 1.

Map of the study area with defined Level III ecoregions. Sample sites are the black points. Focal watersheds are highlighted within major drainage basins
2.2. Bivalve sampling
We sampled quadrats (patches) nested within sites (mussel bed reaches) to make comparisons across rivers and two spatial scales. We intentionally surveyed sites encompassing a wide range of mussel abundance and richness to examine the range of Corbicula densities within areas where unionids occur and to quantify the effects of variation in mussel abundance on Corbicula. We quantified Corbicula and mussel densities at five sites in the Sipsey River, two in Bear Creek, one in the Paint Rock River, two in the Buttahatchee River and two in Bogue Chitto Creek in 2018 and 2019, and four sites in the Duck River and Cahaba River during 2020 all at base‐flow (Figure 1). We excavated 0.25 m2 quadrats to 15 cm deep traversing the river's width every 2.5 m at four random transects every 20 m along the entire reach (as in Hopper et al., 2021). Total reach length varied (range 40–150 m). We measured the length of all mussels and a minimum of 100 Corbicula found in quadrats along the longest shell axis (mm) at each site. Length‐mass regressions were used to estimate soft tissue dry mass for mussels (STDM (g); Atkinson et al., 2020), and reach level areal biomass was based on averages of the quadrat estimates (g m−2).
2.3. Patch‐scale environmental factors
We measured depth (m) and velocity (m s−1) at each quadrat using a Hach FH950 flow meter (Hach Company, Loveland, CO). We calculated D50 from pebble counts (Wolman, 1954) within quadrats (n = 10 pebbles/quadrat) to describe substrate heterogeneity across spatial scales (patch and reach). Substrate data was not collected at one site in the Sipsey River (Sipsey 2) and is therefore excluded from the analysis of abiotic drivers.
2.4. Reach‐scale environmental factors
To address watershed characteristics that might mediate Corbicula abundances at mussel aggregations, we determined the percentage of watershed land cover (e.g., agriculture, forest) for each site using data from the National Land Cover Database (NLCD) clipped to the watershed area upstream of each site. During low flow conditions in 2019 and 2020, we collected triplicate filtered water samples (ashed, pre‐weighed GF/F; 0.7‐µm pore size; Millipore) to quantify variation in background nutrient concentrations. Water samples were kept in a cooler with ice until arrival to the lab, where samples were frozen at −20°C until nutrient analysis. We used a Seal AQ300 discrete analyzer (Seal Analytical) to analyze SRP (hereafter P) using the colorimetric method (Murphy & Riley, 1962) and (hereafter N) using the phenol method. We measured pH once using a YSI professional plus multiparameter meter (YSI Inc. Yellow Springs, Ohio, USA). We also measured seston quality and quantity at each site in 2019 and 2020. For seston quantity, we filtered one liter of stream water (n = 3/site/year) on ashed, pre‐weighed filters (GF/F; 0.7‐µm pore size; Millipore). The filtered materials were taken to the lab, dried at 50 °C in a convection oven (VWR 414005–106) for 48 h, weighed for dry mass (mg), followed by combustion at 500°C for two hours, and reweighed for ash‐free dry mass (AFDM). For seston quality, we filtered 1–3 L of water on ashed filters (GF/F; 0.7‐µm pore size; Millipore) for the determination of %C, %N, and %P. We subsampled our dried filters and measured %C and %N using a Carlo Erba CHNS‐O EA1108‐Elemental Analyzer (Isomass Scientific, Calgary, Alberta, Canada). For percent P, subsamples were weighed, combusted at 500°C for two hours, and analyzed with HCl digestion followed by soluble reactive P analysis. Lastly, we calculated D50 from pebble counts (n=100) to describe substrate heterogeneity at each site (Wolman, 1954).
2.5. Statistical analysis
Of the 1775 quadrats sampled across rivers, 154 had incomplete abiotic data (~8%). We used only quadrats with complete data fields for modeling with both abiotic and biotic factors. Sample sizes for these models across rivers were as follows: Bear Creek (n = 164), Bogue Chitto Creek (n = 76), Buttahatchee River (n = 225), Cahaba River (n = 292), Duck River (n = 329), Paint Rock River (n = 56), and Sipsey River (n = 479).
To test whether species interactions, habitat characteristics or the combined influence of habitat and species interactions most strongly regulate Corbicula abundance at the patch scale, we fit generalized linear mixed models (GLMM; glmer function) in R (R Core Team, 2021; Zuur, 2019) and compared them using AIC (Burnham & Anderson, 2009). We exclusively used mussel biomass (STDM m−2) to test the hypothesis related to biotic interactions because variance inflation factors (VIF) for mussel biomass, density, and mussel species richness were >5. Biomass incorporates aspects of both density and mussel species richness as species‐specific length‐mass models were used to estimate biomass (Atkinson et al., 2020). However, we present correlations between mussel biomass, density, and richness for completeness (see below). The first set of models evaluated the probability of Corbicula occurrence (i.e., detection/non‐detection) in quadrats as a function of mussel biomass; Corbicula occurrence in quadrats as a function of substrate particle size, velocity, and depth; and Corbicula occurrence as a function of mussel biomass and abiotic factors using a binomial distribution (link = logit). Following model selection, we quantitatively tested the effects of each predictor by running separate GLMMs and visualized them using scatter plots. Next, we constructed a set of models that included Corbicula density (individuals m−2) as a function of mussel biomass; Corbicula density as a function of substrate particle size, velocity, and depth; Corbicula density as a function of mussel biomass and abiotic factors. We also fit null models for comparison. Corbicula density was square‐root transformed in each model to better conform to the assumption of normality and heterogeneity. River was treated as a random effect in all models. We used AIC (function aictab; package AICcmodavg) to determine the best‐supported model (Burnham & Anderson, 2009). Variance described by the random effect was considered as the difference between marginal R² and conditional R 2 (MuMIn Bartoń, 2019; Nakagawa & Schielzeth, 2013).
2.6. Multivariate analysis of general patterns
Last, we examined combined abiotic and biotic drivers to determine the spatial overlap of invasive and native species. We used principal components analysis (PCA) to visualize scaled abiotic and biotic variable relationships for quadrats among sites using the function prcomp. We calculated 95% confidence ellipses to show quadrats “typical” of each river using the function stat_conf_ellipse from the package ggpubr (Kassambara, 2020; Wickham, 2011). We used adonis to perform permutational multivariate analysis of variance (PERMANOVA, 999 permutations (Oksanen et al., 2019), to test for differences in quadrat scale variables visualized in the PCA and betadisper to test for heterogeneity of variance (Anderson, 2006; Oksanen et al., 2019). In addition, we calculated and visualized a correlation matrix using ggcorplot in the package corrplot (Kassambara, 2019; Wickham, 2011) to assess global relationships among variables in the quadrat level GLMM analysis. We evaluated relationships between variables measured at the reach scale by calculating and plotting an additional correlation matrix.
3. RESULTS
3.1. Biotic variables
We collected a total of 12,411 Corbicula and 3892 mussels from 1775 quadrats. Total mussel species richness ranged from 0 to 12 in quadrats and 4 to 32 for reaches. Corbicula densities ranged from 0 to ~2000 individuals m−2 in quadrats (Figure 2a), and mean densities for reaches ranged from 1.70 to 131.60 individuals m−2 (Figure 2b). Mussel densities in quadrats ranged from 0 to 148 individuals m−2 (Figure 2a) and at the reach scale ranged from 0.50 to 23.86 individuals m−2 (Figure 2c). Mussel biomass ranged from 0 to 403 g STDM m−2 in quadrats, and mean mussel biomass for reaches ranged from 0.46 to 40.24 g STDM m−2 (Appendix S1).
FIGURE 2.

Scatter plot of Corbicula density and mussel biomass with square‐root transformed axes (a). Box plots showing variation in substrate particle size (d), velocity (e), Depth (f), Corbicula density (b), and mussel biomass (c) measured in quadrats across seven rivers in the southeastern USA. Boxes cover the first through third quartile of the data; horizontal black line in each box is the median. One data point representing the greatest Corbicula density (2088 individuals m−2) is excluded from panels a and b
3.2. Environmental variables
Quadrats varied in depth from 0 to 1.87 m (Figure 2d). Quadrats with a zero depth were typically wetted, but at the edge of the river and made up ~1% of samples. Water velocity measured in quadrats ranged from 0 to 1.05 m s−1 (Figure 2e). Substrate particle sizes ranged from <2 to 180 mm (Figure 2f) and calculated D50 for quadrats was between 1 and 65 mm (Figure 3). Bedrock and large wood represented the dominate substrate in some quadrats, but no Corbicula or mussels were collected from those quadrats.
FIGURE 3.

Corbicula probability of occurrence increases in quadrats with more mussel biomass (a), faster velocity (b), and larger particle sizes (c), but decreases in deeper quadrats. Points represent quadrats and are vertically jittered to avoid complete overlap. X axis in (a) is square‐root transformed
Watershed area for reaches ranged 564–3119 km2, with agriculture land cover comprising 16.9–1453 km2 and developed land being 0.5–373 km2 (Appendix S2). Nutrient concentrations were also variable with concentrations from 5.73 to 28.41 µg L−1 and SRP from 7.04 to 112.29 µg L−1. The sites had pH values ranging from 6.08 to 7.96. Seston AFDM varied from 1.75 to 20.62 mg L−1. Seston % C ranged from 2.19 to 10.01, while seston % N ranged from 0.24 to 1.32, and seston % P varied from 0.07 to 0.91. D50 summarized for reach was between 8 and 16.
3.3. Ecological drivers of spatial overlap between Corbicula and mussels
Corbicula was present at all sites and was detected in 55% of sampled quadrats (Figure 3). Each hypothesis‐driven model explaining the probability of Corbicula occurrence in quadrats had considerably lower AIC values than the null model (Table 1), indicating each was an improvement over the null model. The model including only mussel biomass performed worst, followed by the model containing abiotic factors. The model with both abiotic factors and mussel biomass explained the most variance and performed the best (AIC = 1672.5) even with penalization for having the most variables (Table 1). All terms included in the best model were strong predictors of Corbicula probability of occurrence. This result indicates that Corbicula probability of occurrence increased in shallower quadrats with more mussel biomass, relatively larger substrate particle sizes, and faster velocities (Table 1; Figure 3). Variance attributed to the random effect of river was strong in all models (R 2 marginal – R 2 Conditional), suggesting unmeasured factors associated with ecological gradients across rivers influence model outcomes at the patch scale.
TABLE 1.
Generalized linear models fit for each hypothesis regarding Corbicula presence or density as the response variable including river as a random effect
| Model | AIC | Variable | χ2 | Estimate | p‐Value | R 2 marginal | R 2 conditional |
|---|---|---|---|---|---|---|---|
| Corbicula presence | |||||||
| Null | 1788.4 | – | – | 0.49 | .21 | .00 | .20 |
| Biotic | 1758.2 | Mussel biomass | 30.21 | 0.16 | <.0001 | .03 | .21 |
| Abiotic | 1687.7 | Substrate (D50) | 35.7 | 0.05 | <.0001 | .09 | .27 |
| Velocity | 18.84 | 1.85 | <.0001 | ||||
| Depth | 39.94 | −1.24 | <.0001 | ||||
| Biotic and abiotic | 1672.5 | Mussel biomass | 16.44 | 0.12 | <.0001 | .10 | .26 |
| Substrate (D50) | 27.54 | 0.04 | <.0001 | ||||
| Velocity | 14.61 | 1.63 | <.0001 | ||||
| Depth | 38.73 | −1.27 | <.0001 | ||||
| Corbicula density | |||||||
| Null | 7468 | – | – | 3.09 | .00 | .34 | |
| Biotic | 7357.3 | Mussel biomass | 46.057 | 0.25 | <.0001 | .026 | .33 |
| Abiotic | 7429.4 | Substrate (D50) | 68.65 | 0.07 | <.0001 | .07 | .37 |
| Velocity | 7.59 | 1.44 | .005 | ||||
| Depth | 50.97 | −1.91 | <.0001 | ||||
| Biotic and abiotic | 7332.4 | Mussel biomass | 32.1 | 0.21 | <.0001 | .09 | .36 |
| Substrate (D50) | 56.69 | 0.07 | <.0001 | ||||
| Velocity | 4.67 | 1.13 | .03 | ||||
| Depth | 54.49 | −1.96 | <.0001 | ||||
AIC values were used to compare hypothesis‐driven models to the null model. Variables included in the model had VIF < 5. Chi‐square values, model coefficients, and p‐values are shown for each variable. Marginal and conditional R 2 are given for each model.
3.4. Multivariate analysis of general patterns
The first axis of the PCA explained 45.2% of the variation in biotic and abiotic factors measured at the quadrat scale, and the second axis explained 16.8% (Figure 4A). Ordination of the quadrat level factors were supported by PERMANOVA with centroids clearly separated (F6,1445 = 48.81, p = .001, R 2 = .17) and clear heterogeneity among rirvers (F6,1445 = 30.03, p = .001). PC1 was positively associated with the quadrat level mussel variables (richness, density, and biomass) and particle size and velocity. PC2 was positively associated with Corbicula density and negatively with water depth. Quadrats with more mussels, relatively larger substrates, and faster velocity fell out on the positive end of PC1 and were generally characteristic of quadrats sampled from the Duck River (95% confidence ellipse). Bogue Chitto Creek quadrats had the lowest density of mussels and were the most negative on the first axis. Quadrats from the Cahaba and Paint Rock River were the most positive on the second axis, where Corbicula densities were greatest, while the Sipsey and Buttahatchee Rivers both had the lowest densities of Corbicula and some of the deepest quadrats.
FIGURE 4.

Ordination of principle components analysis of biotic and abiotic variables measured at the quadrat scale. River names are written in italics and correspond to 95% confidence ellipses for each river and vector arrows indicate latent biotic and abiotic gradients among rivers. “Corbicula” is Corbicula density (individuals m−2) and “Mussels” is mussel biomass (g m−2), richness, and density (individuals m−2). A single vector is shown for mussels because density and richness were strongly collinear (a). Plot of correlation matrix of variables included in quadrat scale generalized linear mixed models. Green indicates significant positive correlations, while pink indicated significant negative correlations. Boxes with an “X” are not statistically significant at p = .05 (b)
3.5. Correlation matrices at the patch‐ and reach‐scales
The correlation matrix of biotic and abiotic quadrat data showed that the mussel species richness, density, and biomass were positively correlated with each other, supporting the conclusion of the VIF analysis. Corbicula densities were positively correlated with mussel density (r = .14), biomass (r = .19), and richness (r = .22), suggesting strong overlap with mussels at the quadrat scale (Figure 4b). Velocity (r = .07) and substrate particle size (r = .20) were also positively correlated with Corbicula density, but depth was negatively correlated (r = −17).
The correlation matrix of mussel and habitat variables measured at the reach scale showed mean Corbicula density was positively correlated with D50 (Figure 5), suggesting it increases in reaches with larger particles sizes (r = .59). Mussel density (r = .54), biomass (r = .59), and richness (r = .51) were all positively correlated with watershed area. Mussel biomass (r = .58) was positively correlated with SRP. Mussel biomass (r = .55) and SRP (r = .59) were correlated with pH. Seston % C and % N were strongly and positively correlated with each other (r = .97). Water column was positively correlated with the proportion of developed landcover (r = .48). Larger watersheds were negatively related with the proportion of agricultural development (r = −.56).
FIGURE 5.
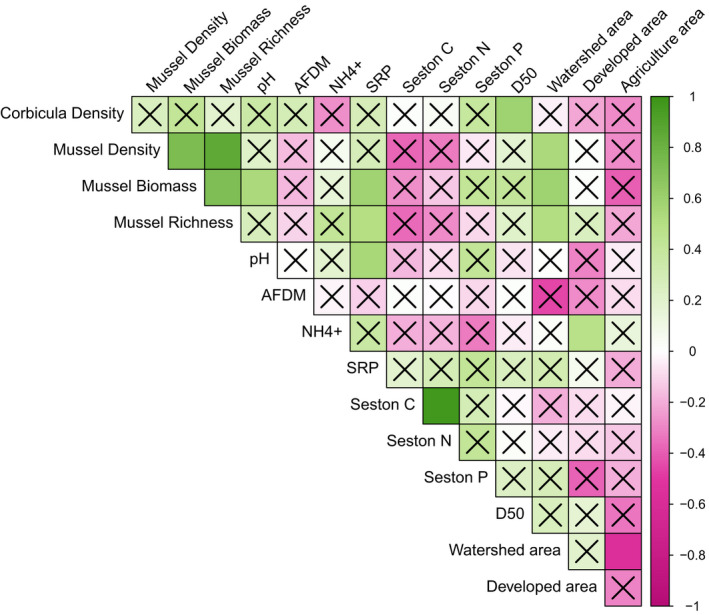
Correlation matrix of variables measured at mussel bed reaches. Green indicates significant positive correlations, while pink indicates significant negative correlations. Boxes with an “X” are not statistically significant at p = .05
4. DISCUSSION
In the rivers we studied, Corbicula was widespread, suggesting that its effects on the structure and functions of these ecosystems may be strong. Given the high density of Corbicula within mussel habitats and its functional similarity to native mussels, the potential for interactions with mussels is great. Studying the factors that influence the distribution of invasive species relative to native communities is important to understand the potential for positive and negative interactions (Ricciardi et al., 2020). We characterized factors associated with invasive Corbicula populations in seven environmentally heterogeneous rivers with diverse native mussel communities that have been differentially effected by anthropogenic pressures. We generated three hypothesis‐driven models to explain these occurrence and density patterns. Whereas all models were improvements over the null model, the best supported one included the full set of abiotic and biotic factors, suggesting that native mussels and Corbicula occupy similar stream habitats. This is contrary to our expectations that native mussels and Corbicula would limit each other's distribution as predicted by limiting similarity hypothesis (Macarthur & Levins, 1967) and previous works showing negative interactions (Ferreira‐Rodríguez, Fandiño, et al., 2018; Ferreira‐Rodríguez, Sousa, et al., 2018; Modesto et al., 2019, 2021; Vaughn & Spooner, 2006a). Thus, suggesting that niche requirements in these contemporary river habitats may not be limiting to either group or other mechanisms, such as positive interactions, may be at play (Silknetter et al., 2020).
Species interactions and physical habitat factors were important in explaining Corbicula occurrence and density in quadrats but varied strongly across the seven rivers even with our targeted sampling design within aggregations of mussels. Mussels and Corbicula share similar feeding and habitat requirements, therefore we expected competitive interactions to result in non‐overlapping distributions (Haag et al., 2020; Vaughn & Spooner, 2006a). Our analysis focused on mussel biomass because of the strong collinearity between species richness and density in the rivers we studied, and accounts for trade‐offs in space occupancy by many small‐bodied or few large‐bodied individuals. Using biomass as a metric to evaluate potential species interaction outcomes, we found only positive associations between mussels and Corbicula occurrence and density. Interestingly, most research addressing interactions between Corbicula and mussels has highlighted those with negative outcomes for mussels, but the strong positive association between each group in our study warrants investigation of positive interactions, such as the potential for mussels to facilitate Corbicula invasion. For example, Corbicula settlement and persistence within patches of mussels may be facilitated by the reduced turbulent shear stresses generated by high densities of mussels protruding from the sediment (Sansom et al., 2020). Nevertheless, facilitation of Corbicula still could result in harm to mussel populations through mechanisms other than competition. Indeed, recent efforts showed a negative relationship between survival of mussel larvae (glochidia) and Corbicula densities and hypothesized that the high filtration capacity of Corbicula may increase mortality of larval mussels by damaging larval shells when filtered by co‐occurring Corbicula or the high excretion capacity of Corbicula may lead to mortality of larval mussels by increasing local ammonia concentration (Modesto et al., 2019). The high potential for negative interaction outcomes for mussels highlights the need for further investigation into the potential for mussels to facilitate Corbicula invasions especially in regard to environmental context in which the interaction occurs (Ferreira‐Rodríguez et al., 2022), because the strength and direction (e.g., negative, neutral, positive) of the interactions may be context dependent (Albertson et al., 2021; Silknetter et al., 2020).
Ecological characteristics measured within quadrats reflected a suite of mussel assemblages and habitats occurring in the Mobile and Tennessee River basins that are determined by underlying physiography (Parmalee & Bogan, 1998; Williams et al., 2008). Benthic characteristics are an important factor in the distribution of benthic species, including mussels and Corbicula which live buried in benthic habitats and therefore may partition habitat at fine spatial scales. We hypothesized increasing probability of Corbicula occurrence and density in habitats where mussels already exist if habitat requirements are similar, but not limiting to either group. Our results show that larger substrate particle sizes (e.g., gravel and cobble), faster water velocity, and shallower depths positively influence Corbicula occurrence and density within mussel aggregations. Corbicula appears to be successful in similar habitats as native mussels within mussel beds suggesting a preference for the same habitat despite wide ecological gradients covered by our study. Whether Corbicula favor similar habitats outside of mussel bed reaches remains to be seen because our sampling sites may not represent the complete set of ecological conditions needed for maximum population growth. For example, most studies in North America of Corbicula habitat use are performed in sites with mussels (Ferreira‐Rodríguez, Sousa, et al., 2018; Vaughn & Spooner, 2006a). Although fine‐scale variables appear important to the distribution of Corbicula in these rivers, it could still be influenced by other factors not measured in this study. For example, another study of Corbicula habitat preference in the River Minho estuary in Spain showed a positive relationship with Corbicula biomass and the organic matter (OM) content of the sediment (Sousa et al., 2008), but did not mention the presence of mussels. Sediment organic matter produced by mussels via biodeposition may be an important factor influencing the distribution of Corbicula, because OM can be an alternative food source that is accessed through pedal feeding (Hakenkamp & Palmer, 1999; Hakenkamp et al., 2001) and may provide them with beneficial gut microbiota (Chiarello et al., 2022). It seems likely high densities of Corbicula may be supported in habitats with high organic matter content, such as mussel beds (Atkinson & Forshay, 2022; Vaughn & Hakenkamp, 2001). Thus, Corbicula invading into mussel bed habitats may not be limited by habitat parameters in these rivers and begs the question of whether OM biodeposition may facilitate Corbicula invasion into mussel beds.
Freshwater habitats of the Anthropocene are characterized by excessive nutrient loads due to difficulties managing non‐point source pollution inputs (e.g., fertilizer runoff) and may affect species interactions (Strayer, 2014). Studies of the trophic niche of Corbicula often conclude that it is highly flexible and overlaps with the trophic niche of native mussel species, but the extent varies with ecological context (Atkinson et al., 2010; Modesto et al., 2021), and such flexibility can facilitate invasion success (Moyle & Light, 1996; Olsson et al., 2009). We anticipated that nutrient loading would increase quality (increased nutrient content) and quantity of particulate food sources, alleviating competition for food resources between Corbicula and mussels, thereby allowing co‐occurrence. Whereas watersheds with more urban land cover did have increased water column , our seston data did not indicate food resource quantity or quality was related to land use differences among watersheds. Moreover, bivalve variables were not correlated with nutrient or seston data. This supports the hypothesis that food quantity may not limit either Corbicula or mussel production in these rivers, and that Corbicula may be passengers of change in degraded mussel habitats (MacDougall & Turkington, 2005). Whether food quantity or quality limits mussel or Corbicula production in habitats where they co‐occur remains to be seen, particularly in low‐productivity habitats where mussel restoration efforts often focus (Strayer et al., 2019). Future efforts should systematically evaluate Corbicula population dynamics in habitats without mussels to separate the influence of mussels from nutrient context. Additionally, in situ or controlled lab experiments altering the seston quantity and C: nutrient ratios could be used to identify food threshold elemental ratios (Frost et al., 2006) that optimize Corbicula or mussel species growth.
The unexpected positive association between Corbicula and mussel distributions represents a snapshot in time that may not reflect the temporal variability of Corbicula and mussel population dynamics, which unfold at different time scales, which is needed to understand their direct or indirect interactions. For example, Corbicula is quite vulnerable to high temperatures and low dissolved oxygen which can lead to mass mortality events resulting in water quality issues that can harm mussels (McDowell et al., 2017; McDowell & Sousa, 2019). Yet, Corbicula can recover quickly (within a year) from such disturbances, while mussel populations take decades to recover from disturbances due to their slow maturation (Haag, 2012). Future efforts that combine life history traits and population estimates could be used to assess mussel and Corbicula population responses to disturbances related to global change. Further experimental work is warranted to disentangle interactions between Corbicula and mussels to address how their interactions change across environmental gradients.
5. CONCLUSION
Disentangling the factors that control invasive species’ distribution and abundance is challenging, especially once a species is established. Our data across two spatial scales indicated high spatial overlap when considering the occurrence of an invasive species in targeted native communities and highlights support for our alternative hypotheses of non‐limiting resources (i.e., low competition), or that Corbicula may be passengers of change in degraded rivers. Additionally, our study brings to light the hypothesis of facilitation of Corbicula into mussel beds via mussel activities, but the underlying mechanisms are unknown or speculative and warrant further investigation. When invasive species co‐occur with functionally similar species, the potential competitive outcome may be especially harmful to the native fauna because competitive interactions should be strong. Large‐scale and long‐term monitoring programs in place for imperiled species, such as native mussels, should incorporate systematic sampling of functionally similar invasive species, such as Corbicula, to provide data on range overlaps, population growth trajectories, potential interactions with native communities, and altered ecosystem function, and to inform future management and conservation strategies.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Taylor E. Kelley: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Investigation (equal); Methodology (supporting); Visualization (supporting); Writing – original draft (equal); Writing – review & editing (lead). Garrett W. Hopper: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (supporting); Visualization (lead); Writing – original draft (equal); Writing – review & editing (supporting). Irene Sánchez González: Conceptualization (supporting); Investigation (supporting); Methodology (supporting); Visualization (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). Jamie R. Bucholz: Conceptualization (supporting); Investigation (supporting); Methodology (supporting); Writing – original draft (supporting); Writing – review & editing (supporting). Carla L. Atkinson: Conceptualization (equal); Funding acquisition (lead); Investigation (supporting); Methodology (supporting); Resources (supporting); Supervision (supporting); Writing – original draft (supporting); Writing – review & editing (supporting).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the Alabama Aquatic Biodiversity Center, Todd Amacker, Austin Beets, Marlène Chiarello, Ansley Hamid, Madison Knapp, Megan Kubala, Matt Lodato, Mark McCauley, Josh Millwood, Austin Omer and Brian van Ee for help in the field, Anne Bell analyzed water samples for N and P. The Alabama Department of Natural Resources and Conservation, The Alabama Water Institute, and the National Science Foundation DEB‐1831512 provided support for this project. Freshwater mussel collection was conducted under USFWS permit #TE68616B‐1, TWRA permit #1807, ALCDNR permit # 2019118497068680, and MS permit #0715191
Kelley, T. E. , Hopper, G. W. , Sánchez González, I. , Bucholz, J. R. , & Atkinson, C. L. (2022). Identifying potential drivers of distribution patterns of invasive Corbicula fluminea relative to native freshwater mussels (Unionidae) across spatial scales. Ecology and Evolution, 12, e8737. 10.1002/ece3.8737
DATA AVAILABILITY STATEMENT
Data supporting the results of this paper can be found at the Open Science Framework https://doi.org/10.17605/OSF.IO/M2U9J.
REFERENCES
- Albertson, L. K. , MacDonald, M. J. , Tumolo, B. B. , Briggs, M. A. , Maguire, Z. , Quinn, S. , Sanchez‐Ruiz, J. A. , Veneros, J. , & Burkle, L. A. (2021). Uncovering patterns of freshwater positive interactions using meta‐analysis: Identifying the roles of common participants, invasive species and environmental context. Ecology Letters, 24(3), 594–607. 10.1111/ele.13664 [DOI] [PubMed] [Google Scholar]
- Anderson, M. J. (2006). Distance‐based tests for homogeneity of multivariate dispersions. Biometrics, 62(1), 245–253. 10.1111/j.1541-0420.2005.00440.x [DOI] [PubMed] [Google Scholar]
- Araujo, R. , Moreno, D. , & Ramos, M. (1993). The Asiatic clam Corbicula fluminea (Müller, 1774) (Bivalvia : Corbiculidae) in Europe. American Malacologia Bulletin. Retrieved from http://www.fauna‐iberica.mncn.csic.es/CV/rafa_PDF_2/Amer_Malac_Bull.pdf [Google Scholar]
- Atkinson, C. L. , First, M. R. , Covich, A. P. , Opsahl, S. P. , & Golladay, S. W. (2011). Suspended material availability and filtration–biodeposition processes performed by a native and invasive bivalve species in streams. Hydrobiologia, 667(1), 191–204. 10.1007/s10750-011-0640-5 [DOI] [Google Scholar]
- Atkinson, C. L. , & Forshay, K. J. (2022). Community patch dynamics governs direct and indirect nutrient recycling by aggregated animals across spatial scales. Functional Ecology, 36(3), 595–606. 10.1111/1365-2435.13982 [DOI] [Google Scholar]
- Atkinson, C. L. , Halvorson, H. M. , Kuehn, K. A. , Winebarger, M. , Hamid, A. , & Waters, M. N. (2021). Filter‐feeders have differential bottom‐up impacts on green and brown food webs. Oecologia, 195(1), 187–198. 10.1007/s00442-020-04821-7 [DOI] [PubMed] [Google Scholar]
- Atkinson, C. L. , Julian, J. P. , & Vaughn, C. C. (2012). Scale‐dependent longitudinal patterns in mussel communities. Freshwater Biology, 57(11), 2272–2284. 10.1111/fwb.12001 [DOI] [Google Scholar]
- Atkinson, C. L. , Opsahl, S. P. , Covich, A. P. , Golladay, S. W. , & Conner, L. M. (2010). Stable isotopic signatures, tissue stoichiometry, and nutrient cycling (C and N) of native and invasive freshwater bivalves. Journal of the North American Benthological Society, 29(2), 496–505. 10.1899/09-083.1 [DOI] [Google Scholar]
- Atkinson, C. L. , Parr, T. B. , van Ee, B. C. , Knapp, D. D. , Winebarger, M. , Madoni, K. J. , & Haag, W. R. (2020). Length‐mass equations for freshwater unionid mussel assemblages: Implications for estimating ecosystem function. Freshwater Science, 39(3), 377–390. 10.1086/708950 [DOI] [Google Scholar]
- Barnhart, M. C. , Haag, W. R. , & Roston, W. N. (2008). Adaptations to host infection and larval parasitism in Unionoida. Journal of the North American Benthological Society, 27(2), 370–394. 10.1899/07-093.1 [DOI] [Google Scholar]
- Bartoń, K. (2019). MuMIn: Multi‐Model Inference, Version 1.43.6. R Package.
- Bengtsson, J. (1989). Interspecific competition increases local extinction rate in a metapopulation system. Nature, 340(6236), 713–715. 10.1038/340713a0 [DOI] [Google Scholar]
- Benson, A. J. , & Williams, J. D. (2021). Review of the invasive Asian Clam Corbicula spp. (Bivalvia: Cyrenidae) distribution in North America, 1924–2019. S. Geological Survey Scientific Investigations Report 2021–5001. Retrieved from https://www.researchgate.net/deref/https%3A%2F%2Fdoi.org%2F10.3133%2Fsir20215001 [Google Scholar]
- Bódis, E. , Nosek, J. , Oertel, N. , Tóth, B. , Hornung, E. , & Sousa, R. (2011). Spatial distribution of bivalves in relation to environmental conditions (middle Danube catchment, Hungary). Community Ecology, 12(2), 210–219. 10.1556/ComEc.12.2011.2.9 [DOI] [Google Scholar]
- Böhm, M. , Dewhurst‐Richman, N. I. , Seddon, M. , Ledger, S. E. H. , Albrecht, C. , Allen, D. , Bogan, A. E. , Cordeiro, J. , Cummings, K. S. , Cuttelod, A. , Darrigran, G. , Darwall, W. , Fehér, Z. , Gibson, C. , Graf, D. L. , Köhler, F. , Lopes‐Lima, M. , Pastorino, G. , Perez, K. E. , … Collen, B. (2020). The conservation status of the world’s freshwater molluscs. Hydrobiologia, 848(12‐13), 3231–3254. 10.1007/s10750-020-04385-w [DOI] [Google Scholar]
- Booth, M. S. , Caldwell, M. M. , & Stark, J. M. (2003). Overlapping resource use in three Great Basin species: Implications for community invasibility and vegetation dynamics. Journal of Ecology, 91(1), 36–48. 10.1046/j.1365-2745.2003.00739.x [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2009). Model selection and multi‐model inference: A practical information‐theoretic approach, 2nd ed. Springer. [Google Scholar]
- Chiarello, M. , Bucholz, J. R. , McCauley, M. , Vaughn, S. N. , Hopper, G. W. , Sánchez González, I. , Atkinson, C. L. , Lozier, J. D. , & Jackson, C. R. (2022). Environment and co‐occurring native mussel species, but not host genetics, impact the microbiome of a freshwater invasive species (Corbicula fluminea). Frontiers in Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavero, M. , & García‐Berthou, E. (2005). Invasive species are a leading cause of animal extinctions. Trends in Ecology and Evolution, 20(3), 110. 10.1016/j.tree.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Crespo, D. , Dolbeth, M. , Leston, S. , Sousa, R. , & Pardal, M. Â. (2015). Distribution of Corbicula fluminea (Müller, 1774) in the invaded range: a geographic approach with notes on species traits variability. Biological Invasions, 17(7), 2087–2101. 10.1007/s10530-015-0862-y [DOI] [Google Scholar]
- David, P. , Thébault, E. , Anneville, O. , Duyck, P.‐F. , Chapuis, E. , & Loeuille, N. (2017). Impacts of invasive species on food webs. Advances in Ecological Research, 56, 1–60. 10.1016/bs.aecr.2016.10.001 [DOI] [Google Scholar]
- Davis, M. (2003). Biotic globalization: Does competition from introduced species threaten biodiversity. BioScience, 53(5), 481–489. 10.1641/0006-3568(2003)053[0481:BGDCFI]2.0.CO;2 [DOI] [Google Scholar]
- Fargione, J. , Brown, C. S. , & Tilman, D. (2003). Community assembly and invasion: An experimental test of neutral versus niche processes. Proceedings of the National Academy of Sciences of the United States of America, 100(15), 8916–8920. 10.1073/pnas.1033107100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargione, J. E. , & Tilman, D. (2005). Diversity decreases invasion via both sampling and complementarity effects. Ecology Letters, 8(6), 604–611. 10.1111/j.1461-0248.2005.00753.x [DOI] [Google Scholar]
- Ferreira‐Rodríguez, N. , Defeo, O. , Macho, G. , & Pardo, I. (2019). A social‐ecological system framework to assess biological invasions: Corbicula fluminea in Galicia (NW Iberian Peninsula). Biological Invasions, 21(2), 587–602. 10.1007/s10530-018-1846-5 [DOI] [Google Scholar]
- Ferreira‐Rodríguez, N. , Laura, F. , Pedreira, A. , & Pardo, I. (2018). First evidence of asymmetric competition between the non‐native clam Corbicula fluminea and the native freshwater mussel Unio dolphinus during a summer heat wave. Aquatic Conservation: Marine and Freshwater Ecosystems, 28(5), 1105–1113. 10.1002/aqc.2964 [DOI] [Google Scholar]
- Ferreira‐Rodríguez, N. , Gangloff, M. , Shafer, G. , & Atkinson, C. L. (2022). Drivers of ecosystem vulnerability to Corbicula invasions in southeastern North America. Biological Invasions. 10.1007/s10530-022-02751-4 [DOI] [Google Scholar]
- Ferreira‐Rodríguez, N. , & Pardo, I. (2017). The interactive effects of temperature, trophic status, and the presence of an exotic clam on the performance of a native freshwater mussel. Hydrobiologia, 797(1), 171–182. 10.1007/s10750-017-3170-y [DOI] [Google Scholar]
- Ferreira‐Rodríguez, N. , Sousa, R. , & Pardo, I. (2018). Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia, 810(1), 85–95. 10.1007/s10750-016-3059-1 [DOI] [Google Scholar]
- Frost, P. C. , Benstead, J. P. , Cross, W. F. , Hillebrand, H. , Larson, J. H. , Xenopoulos, M. A. , & Yoshida, T. (2006). Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecology Letters, 9(7), 774–779. 10.1111/j.1461-0248.2006.00919.x [DOI] [PubMed] [Google Scholar]
- Gamradt, S. C. , & Kats, L. B. (1996). Effect of introduced crayfish and mosquitofish on California newts. Conservation Biology, 10(4), 155–1162. 10.1046/j.1523-1739.1996.10041155.x [DOI] [Google Scholar]
- Haag, W. R. (2012). North American freshwater mussels: natural history, ecology, and conservation. Cambridge University Press. 10.1017/cbo9781139048217 [DOI] [Google Scholar]
- Haag, W. R. (2019). Reassessing enigmatic mussel declines in the United States. Freshwater Mollusk Biology and Conservation, 22(2), 43– 10.31931/fmbc.v22i2.2019.43-60 [DOI] [Google Scholar]
- Haag, W. R. , Jacob, C. , Drayer, A. N. , McGregor, M. A. , White, D. E. J. , & Price, S. J. (2021). Abundance of an invasive bivalve, Corbicula fluminea, is negatively related to growth of freshwater mussels in the wild. Freshwater Biology, 66(3), 447–457. 10.1111/fwb.13651 [DOI] [Google Scholar]
- Hakenkamp, C. C. , & Palmer, M. A. (1999). Introduced bivalves in freshwater ecosystems: The impact of Corbicula on organic matter dynamics in a sandy stream. Oecologia, 119(3), 445–451. 10.1007/s004420050806 [DOI] [PubMed] [Google Scholar]
- Hakenkamp, C. C. , Ribblett, S. G. , Palmer, M. A. , Swan, C. M. , Reid, J. W. , & Goodison, M. R. (2001). The impact of an introduced bivalve (Corbicula fluminea) on the benthos of a sandy stream. Freshwater Biology, 46(4), 491–501. 10.1046/j.1365-2427.2001.00700.x [DOI] [Google Scholar]
- Hopper, G. W. , Chen, S. , Sanchez‐Gonzalez, I. , Bucholz, J. R. , Lu, Y. , & Atkinson, C. L. (2021). Aggregated filter‐feeders govern the flux and stoichiometry of locally available energy and nutrients in rivers. Functional Ecology, 35(5), 1183–1195. 10.1111/1365-2435.13778 [DOI] [Google Scholar]
- Hopper, G. W. , Gido, K. B. , Vaughn, C. C. , Parr, T. B. , Popejoy, T. G. , Atkinson, C. L. , & Gates, K. K. (2018). Biomass distribution of fishes and mussels mediates spatial and temporal heterogeneity in nutrient cycling in streams. Oecologia, 188(4), 1133–1144. 10.1007/s00442-018-4277-1 [DOI] [PubMed] [Google Scholar]
- Ilarri, M. I. , Souza, A. T. , & Sousa, R. (2015). Contrasting decay rates of freshwater bivalves’ shells: Aquatic versus terrestrial habitats. Limnologica, 51, 8–14. 10.1016/j.limno.2014.10.002 [DOI] [Google Scholar]
- Kassambara, A. (2019). Visualization of a Correlation Matrix using “ggplot2.” Cran R.
- Kassambara, A. (2020). Package ‘ggpubr’: “ggplot2” Based Publication Ready Plots. R Package Version 0.4.0.
- Levin, S. A. (1970). Community equilibria and stability, and an extension of the competitive exclusion principle. The American Naturalist, 104(939), 413–423. 10.1086/282676 [DOI] [Google Scholar]
- Lopes‐Lima, M. , Burlakova, L. E. , Karatayev, A. Y. , Mehler, K. , Seddon, M. , & Sousa, R. (2018). Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia, 810, 1–14. 10.1007/s10750-017-3486-7 [DOI] [Google Scholar]
- Macarthur, R. , & Levins, R. (1967). The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist, 101(921), 377–385. 10.1086/282505 [DOI] [Google Scholar]
- MacDougall, A. S. , & Turkington, R. (2005). Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology, 86(1), 42–55. 10.1890/04-0669 [DOI] [Google Scholar]
- McDowell, W. G. , McDowell, W. H. , & Byers, J. E. (2017). Mass mortality of a dominant invasive species in response to an extreme climate event: Implications for ecosystem function. Limnology and Oceanography, 62(1), 177–188. 10.1002/lno.10384 [DOI] [Google Scholar]
- McDowell, W. G. , & Ronaldo, S. (2019). Mass mortality events of invasive freshwater bivalves: Current understanding and potential directions for future research. Frontiers in Ecology and Evolution, 7, 10.3389/fevo.2019.00331 [DOI] [Google Scholar]
- Modesto, V. , Castro, P. , Lopes‐Lima, M. , Antunes, C. , Ilarri, M. , & Sousa, R. (2019). Potential impacts of the invasive species Corbicula fluminea on the survival of glochidia. Science of the Total Environment, 673, 157–164. 10.1016/j.scitotenv.2019.04.043 [DOI] [PubMed] [Google Scholar]
- Modesto, V. , Dias, E. , Ilarri, M. , Lopes‐Lima, M. , Teixeira, A. , Varandas, S. , Castro, P. , Antunes, C. , & Sousa, R. (2021). Trophic niche overlap between native freshwater mussels (Order: Unionida) and the invasive Corbicula fluminea. Aquatic Conservation: Marine and Freshwater Ecosystems, 31(8), 2058–2071. 10.1002/aqc.3618 [DOI] [Google Scholar]
- Moyle, P. B. , & Light, T. (1996). Biological invasions of fresh water: Empirical rules and assembly theory. Biological Conservation, 78(1‐2), 149–161. 10.1016/0006-3207(96)00024-9 [DOI] [Google Scholar]
- Murphy, J. , & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 2670(00), 31–36. 10.1016/S0003-2670(00)88444-5 [DOI] [Google Scholar]
- Nakagawa, S. , & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution, 4(2), 133–142. 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- Novais, A. , Pascoal, C. , & Sousa, R. (2017). Effects of invasive aquatic carrion on soil chemistry and terrestrial microbial communities. Biological Invasions, 19(8), 2491–2502. 10.1007/s10530-017-1459-4 [DOI] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Friendly, M. , Kindt, R. , Legendre, P. , McGlinn, D. , & Wagner, H. (2019). vegan: Community Ecology Package. R package version 2.5‐2. Cran R.
- Olsson, K. , Stenroth, P. , Nyström, P. , & Granéli, W. (2009). Invasions and niche width: Does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology, 54(8), 1731–1740. 10.1111/j.1365-2427.2009.02221.x [DOI] [Google Scholar]
- Onorato, D. , Angus, R. A. , & Marion, K. R. (2000). Historical changes in the ichthyofaunal assemblages of the upper Cahaba river in Alabama associated with extensive urban development in the watershed. Journal of Freshwater Ecology, 15(1), 47–63. 10.1080/02705060.2000.9663721 [DOI] [Google Scholar]
- Padial, A. A. , Vitule, J. R. S. , & Olden, J. D. (2020). Preface: aquatic homogenocene—understanding the era of biological re‐shuffling in aquatic ecosystems. Hydrobiologia, 847(18), 3705–3709. 10.1007/s10750-020-04413-9 [DOI] [Google Scholar]
- Parmalee, P. W. , & Bogan, A. E. (1998). The Freshwater Mussels of Tennessee (Illustrate). University of Tennessee Press. [Google Scholar]
- Patterson, M. A. , Mair, R. A. , Eckert, N. L. , Gatenby, C. M. , Brady, T. , Jones, J. W. , & Devers, J. L. (2018). In Freshwater mussel propagation for restoration. Cambridge University Press. 10.1017/9781108551120 [DOI] [Google Scholar]
- Pergl, J. , Pyšek, P. , Essl, F. , Jeschke, J. M. , Courchamp, F. , Geist, J. , Hejda, M. , Kowarik, I. , Mill, A. , Musseau, C. , Pipek, P. , Saul, W.‐C. , Schmalensee, M. , & Strayer, D. (2020). Need for routine tracking of biological invasions. Conservation Biology, 34(5), 1311–1314. 10.1111/cobi.13445 [DOI] [PubMed] [Google Scholar]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Reid, A. J. , Carlson, A. K. , Creed, I. F. , Eliason, E. J. , Gell, P. A. , Johnson, P. T. J. , Kidd, K. A. , MacCormack, T. J. , Olden, J. D. , Ormerod, S. J. , Smol, J. P. , Taylor, W. W. , Tockner, K. , Vermaire, J. C. , Dudgeon, D. , & Cooke, S. J. (2019). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews, 94(3), 849–873. 10.1111/brv.12480 [DOI] [PubMed] [Google Scholar]
- Ricciardi, A. , Iacarella, J. C. , Aldridge, D. C. , Blackburn, T. M. , Carlton, J. T. , Catford, J. A. , Dick, J. T. A. , Hulme, P. E. , Jeschke, J. M. , Liebhold, A. M. , Lockwood, J. L. , MacIsaac, H. J. , Meyerson, L. A. , Pyšek, P. , Richardson, D. M. , Ruiz, G. M. , Simberloff, D. , Vilà, M. , & Wardle, D. A. (2020). Four priority areas to advance invasion science in the face of rapid environmental change. Environmental Reviews, 29(2), 119–141. 10.1139/er-2020-0088 [DOI] [Google Scholar]
- Sanchez Gonzalez, I. , Hopper, G. W. , Bucholz, J. , & Atkinson, C. L. (2021). Long‐term monitoring reveals differential responses of mussel and host fish communities in a biodiversity hotspot. Diversity, 13(3), 122. 10.3390/d13030122 [DOI] [Google Scholar]
- Sansom, B. J. , Bennett, S. J. , Atkinson, J. F. , & Vaughn, C. C. (2018). Long‐term persistence of freshwater mussel beds in labile river channels. Freshwater Biology, 63(11), 1469–1481. 10.1111/fwb.13175 [DOI] [Google Scholar]
- Sansom, B. J. , Bennett, S. J. , Atkinson, J. F. , & Vaughn, C. C. (2020). Emergent hydrodynamics and skimming flow over mussel covered beds in rivers. Water Resources Research, 56(8), e2019WR026252. 10.1029/2019WR026252 [DOI] [Google Scholar]
- Schwalb, A. N. , Morris, T. J. , Mandrak, N. E. , & Cottenie, K. (2013). Distribution of unionid freshwater mussels depends on the distribution of host fishes on a regional scale. Diversity and Distributions, 19(4), 446–454. 10.1111/j.1472-4642.2012.00940.x [DOI] [Google Scholar]
- Silknetter, S. , Creed, R. P. , Brown, B. L. , Frimpong, E. A. , Skelton, J. , & Peoples, B. K. (2020). Positive biotic interactions in freshwaters: A review and research directive. Freshwater Biology, 65(4), 811–832. 10.1111/fwb.13476 [DOI] [Google Scholar]
- Simberloff, D. , Ricciardi, A. , & Elton, C. S. (2020). The ecology of invasions by animals and plants. Springer International Publishing. [Google Scholar]
- Sousa, R. , Gutiérrez, J. L. , & Aldridge, D. C. (2009). Non‐indigenous invasive bivalves as ecosystem engineers. Biological Invasions, 11(10), 2367–2385. 10.1007/s10530-009-9422-7 [DOI] [Google Scholar]
- Sousa, R. , Nogueira, A. J. A. , Gaspar, M. B. , Antunes, C. , & Guilhermino, L. (2008). Growth and extremely high production of the non‐indigenous invasive species Corbicula fluminea (Müller, 1774): Possible implications for ecosystem functioning. Estuarine, Coastal and Shelf Science, 80(2), 289–295. 10.1016/j.ecss.2008.08.006 [DOI] [Google Scholar]
- Sousa, R. , Novais, A. , Costa, R. , & Strayer, D. L. (2014). Invasive bivalves in fresh waters: Impacts from individuals to ecosystems and possible control strategies. Hydrobiologia, 735(1), 233–251. 10.1007/s10750-012-1409-1 [DOI] [Google Scholar]
- Sousa, R. , Rufino, M. , Gaspar, M. , Antunes, C. , & Guilhermino, L. (2008). Abiotic impacts on spatial and temporal distribution of Corbicula fluminea (Müller, 1774) in the River Minho Estuary, Portugal. Aquatic Conservation: Marine and Freshwater Ecosystems, 18(1), 98–110. 10.1002/aqc.838 [DOI] [Google Scholar]
- Strayer, D. L. (2010). Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology, 55, 152–174. 10.1111/j.1365-2427.2009.02380.x [DOI] [Google Scholar]
- Strayer, D. L. (2014). Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia, 735(1), 277–292. 10.1007/s10750-013-1461-5 [DOI] [Google Scholar]
- Strayer, D. L. , Caraco, N. F. , Cole, J. J. , Findlay, S. , & Pace, M. L. (1999). Transformation of freshwater ecosystems by bivalves: A case study of zebra mussels in the Hudson River. BioScience, 49(1), 19– 10.2307/1313490 [DOI] [Google Scholar]
- Strayer, D. L. , Cole, J. J. , Findlay, S. E. G. , Fischer, D. T. , Gephart, J. A. , Malcom, H. M. , Pace, M. L. , & Rosi‐Marshall, E. J. (2014). Decadal‐scale change in a large‐river ecosystem. BioScience, 64(6), 496–510. 10.1093/biosci/biu061 [DOI] [Google Scholar]
- Strayer, D. L. , Downing, J. A. , Haag, W. R. , King, T. L. , Layzer, J. B. , Newton, T. J. , & Nichols, S. J. (2004). Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience, 54(5), 429–439. 10.1641/0006-3568(2004)054[0429:cpopmn]2.0.co;2 [DOI] [Google Scholar]
- Strayer, D. L. , & Dudgeon, D. (2010). Freshwater biodiversity conservation: Recent progress and future challenges. Journal of the North American Benthological Society, 29(1), 344–358. 10.1899/08-171.1 [DOI] [Google Scholar]
- Strayer, D. L. , Geist, J. , Haag, W. R. , Jackson, J. K. , & Newbold, J. D. (2019). Essay: Making the most of recent advances in freshwater mussel propagation and restoration. Conservation Science and Practice, 1(7), e53. 10.1111/csp2.53 [DOI] [Google Scholar]
- Tilman, D. (2004). Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 10854–10861. 10.1073/pnas.0403458101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn, C. C. , Allen, D. C. , Irmscher, P. , & Miller, C. J. (2008). Freshwater mussel ecology: A multifactor approach to distribution and abundance. Journal of the North American Benthological Society, 28(2), 515–516. 10.1899/28.2.BR.515.1 [DOI] [Google Scholar]
- Vaughn, C. C. , & Hakenkamp, C. C. (2001). The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology, 46(11), 1431–1446. 10.1046/j.1365-2427.2001.00771.x [DOI] [Google Scholar]
- Vaughn, C. C. , Nichols, S. J. , & Spooner, D. E. (2008). Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society, 27(2), 409–423. 10.1899/07-058.1 [DOI] [Google Scholar]
- Vaughn, C. C. , & Spooner, D. E. (2006a). Scale‐dependent associations between native freshwater mussels and invasive Corbicula. Hydrobiologia, 568(1), 331–339. 10.1007/s10750-006-0210-4 [DOI] [Google Scholar]
- Vaughn, C. C. , & Spooner, D. E. (2006b). Unionid mussels influence macroinvertebrate assemblage structure in streams. Journal of the North American Benthological Society, 25(3), 691–700. 10.1899/0887-3593(2006)25[691:UMIMAS]2.0.CO;2 [DOI] [Google Scholar]
- Vaughn, C. C. , & Taylor, C. M. (2000). Macroecology of a host‐parasite relationship. Ecography, 23(1), 11–20. 10.1111/j.1600-0587.2000.tb00256.x [DOI] [Google Scholar]
- Wickham, H. (2011). ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics, 3(2), 180–185. 10.1002/wics.147 [DOI] [Google Scholar]
- Wilcove, D. S. , Rothstein, D. , Dubow, J. , Phillips, A. , & Losos, E. (1998). Quantifying threats to imperiled species in the United States. BioScience, 48(8), 607–615. 10.2307/1313420 [DOI] [Google Scholar]
- Williams, J. D. , Bogan, A. E. , & Garner, J. T. (2008). Freshwater mussels of Alabama and the Mobile Basin in Georgia, Mississippi and Tennessee. University of Alabama Press. [Google Scholar]
- Wolman, M. G. (1954). A method of sampling coarse river‐bed material. Eos, Transactions American Geophysical Union, 35(6), 951– 10.1029/TR035i006p00951 [DOI] [Google Scholar]
- Zuur, A. , Ieno, E. N. , Walker, N. , Saveliev, A. A. , & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R, 2009th ed. Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data supporting the results of this paper can be found at the Open Science Framework https://doi.org/10.17605/OSF.IO/M2U9J.


