Abstract
Given the increasing number of gene transfer therapy studies either completed or underway, there is growing attention to the importance of preexisting adaptive immunity to the viral vectors used. The recombinant viral vectors developed for gene transfer therapy share structural features with naturally occurring wild-type virus. Antibodies generated against viral vectors obtained through a previous exposure to wild-type virus can potentially compromise transgene expression by blocking transduction, thereby limiting the therapeutic efficacy of the gene transfer therapy; they may also pose potential safety concerns. Therefore, systemic gene transfer delivery requires testing patients for preexisting antibodies. Two different assays have been used: (1) binding assays that focus on total antibodies (both neutralizing and non-neutralizing) and (2) neutralizing assays that detect neutralizing antibodies. In this review we focus on adeno-associated virus-based gene therapies, describing the immune response that occurs to naturally occurring adeno-associated viruses, the implications for patients with this exposure, the assays used to detect preexisting immune responses, and strategies to circumvent preexisting adaptive immunity to expand the patient base that could benefit from such therapies.
Keywords: adeno-associated virus, antibodies, assays, binding assays, efficacy, gene transfer therapy, immunity, neutralizing assays, safety, titer
Graphical abstract

Preexisting immunity to adeno-associated virus (AAV)-based gene therapies may compromise therapeutic efficacy and pose potential safety concerns. Systemic gene transfer therapy requires screening patients for preexisting antibodies. This review describes the immune responses to wild-type AAVs and implications for patients, the assays used, and potential strategies to circumvent these responses.
Introduction
The aim of gene transfer therapy is to treat or prevent a disease by adding a functional gene to compensate for a mutated or absent gene, allowing restoration of a functional protein product with the potential for a long-lasting treatment effect after a single administration (Figure 1). Gene transfer therapies include both ex vivo and in vivo approaches. In ex vivo approaches, the transgene is introduced to target cells isolated and maintained in culture.1 Once the culture has been expanded, the cells are reintroduced into the patient. In vivo approaches directly deliver a transgene into the body via local or systemic administration.1
Figure 1.
AAV gene transfer therapy mechanism of action
Following gene transfer therapy administration, the capsid binds to the cell membrane of target cells (1), where it is internalized through endocytosis (2). Following release from the endosome (3), the vector transits to the nucleus (4) and is imported through a nuclear pore (5), where the capsid is thought to be degraded (uncoating steps not shown), exposing vector DNA to the nucleus (6). Once the vector DNA transforms into episomal DNA (7), it is transcribed (8), and the resultant mRNA is translocated to the cytoplasm (9), where it is translated, thereby producing the protein of interest (10).
Different systems are being explored to deliver the transgene. Of these, viral vectors are one of the most studied, as viruses have evolved to effectively interact with human cells, deliver their genetic material, and express their proteins. As such, multiple investigations are using recombinant viral vectors, which do not have the components associated with pathogenicity and maintain only those needed to direct transduction and thus transgene expression in target cells. Different vectors have been tested for gene transfer therapy, including adenoviruses, retroviruses, lentiviruses, and adeno-associated viruses (AAVs). The experience in early clinical trials highlighted the important role of the viral vector in the safety of gene transfer therapies, as illustrated in 1999 by the unfortunate death of a patient who suffered from a massive inflammatory response following adenoviral gene therapy for ornithine transcarbamylase deficiency.2 Subsequent gene therapy studies using retroviral vectors also reported cases of leukemias caused by insertional mutagenesis.3, 4, 5, 6
AAVs are considered an efficient delivery vehicle for in vivo gene transfer therapy and have emerged as a preferred vector because of currently available safety data related to their absence of pathogenicity, low immunogenicity, and minimal genome integration.7 Another advantage of AAV vectors over other viral vector delivery systems is their wide tissue tropism (both proliferative and nonproliferative cells). Within AAVs, different serotypes have varying tissue tropism with some widely expressed in multiple tissue types (e.g., transduction of brain, retina, lung, liver, and muscle by AAV2) whereas others are more tissue specific (e.g., CNS transduction by AAV9 and muscle transduction by AAV8, AAV9, and AAVrh74).8,9
Limitations of AAV gene transfer therapy include their limited packaging size (maximum ∼4.7 kb) and the presence of preexisting adaptive immunity (hereafter referred to as preexisting immunity), both cellular and humoral.10 Previous exposure to naturally occurring (wild-type) AAVs results in preexisting immunity that can potentially compromise transgene expression by blocking transduction, limiting the therapeutic efficacy of the gene transfer therapy,11,12 and represents a potential safety concern. Immunity developed in seronegative patients after gene transfer may also limit the ability to re-administer treatment if necessary.
Given that preexisting immunity may interfere with the effectiveness of gene transfer therapy12 and potentially cause safety issues, systemic delivery requires testing patients for preexisting antibodies. In this review we describe the importance of screening for preexisting immunity prior to gene transfer therapies, methods used for accurate testing, and strategies to overcome it.
Importance of testing
Immune response to AAV
The capsid is the protein shell enclosing the viral genome and therefore can be detected and stimulate immune responses. Although wild-type AAVs generally induce a mild immune response compared with other viral vectors, such as adenovirus, an immune response is generated nonetheless.13 Because of the high degree of homology between the capsid of a wild-type virus and the vector capsid used in gene transfer therapy, patients who have been in contact with wild-type AAVs can have preexisting immunity against the vector and neutralize it before the transgene is transferred.14, 15, 16 Immune responses to foreign antigens, such as the protein capsid, include two collaborative pathways: the innate immune system and the adaptive immune system (Figure 2). These immune responses may have important safety implications and reduce clinical efficacy.
Figure 2.
Timing of immunological responses to AAV gene transfer therapy
Following systemic administration of AAV gene transfer therapy, the innate immune response is active within hours to days. This response includes complement activation, neutralization by preexisting NAbs, and macrophage and neutrophil activation. Acquired immunity, including B cell and T cell activation, occurs weeks later following administration. Both immunological responses decrease the systemic vector concentration. AAV, adeno-associated virus; NAbs, neutralizing antibodies.
Innate immunity
Upon detection of a foreign antigen, the innate immune response is first activated within hours or days (Figure 2). Innate immunity is characterized by its rapid, non-specific response to foreign antigens without inducing immunological memory via recognition of pathogen-associated molecular patterns by immune cells. This molecular recognition leads to activation of NF-κB, induction of interferon (IFN) signaling, or expression of pro-inflammatory cytokines and IFNs.17,18 In addition, complement activation represents an important aspect of innate immunity. The complement system is composed of highly regulated enzymatic cascades that are activated by three different pathways, including the lectin, the alternative, and the classical pathways, all of which cleave C3 into C3a and C3b, the critical regulator. Complement activation results in opsonization of microorganisms, white blood cell release of inflammatory mediators, and target-cell lysis.
Although the impact of these responses in the clinical setting is largely not known beyond the absence of acute clinical responses (changes in vital signs, nausea, vomiting) in most patients following AAV gene transfer,12 a recent U.S. Food and Drug Administration (FDA) advisory committee meeting highlighted the importance of immune-mediated toxicities following AAV gene transfer, including both renal and hepatic toxicities, related to complement activation.19 Also, it is known that innate immunological responses (e.g., inflammation and complement activation) will activate the adaptive response. Inhibiting innate immunity blocked both cytotoxic20,21 and humoral22 immune responses to AAV capsids.
Adaptive or acquired immunity
Adaptive immune responses to a foreign antigen take longer to be initiated (weeks) compared with innate immunity, as it requires multiple steps along with overcoming several check points to prevent inappropriate responses to nonpathogenic foreign molecules (Figure 2). This adaptive response differs from innate responses in that it is specific for the particular antigen and provides immunological memory to the host for long-term protection from reinfection with the same type of pathogen. Immunological memory is mediated by persisting T (CD4+ and CD8+) and B (CD19+) cells that, once primed for a specific antigen, persist in the human body as “sentinels” that can elicit a faster and stronger response resulting in rapid, efficient clearance of antigens with subsequent exposures. This specificity is what makes the adaptive response more efficacious and must be highly regulated to avoid inappropriate activation.
T cell activation (CD4+) is an initial step required to activate all other cells involved in the adaptive immune response, including the cell-mediated cytotoxic response (CD8+ T cells) and the humoral response (B cells), which includes the production of antibodies that bind specific antigens. One aspect of the adaptive immune response that is particularly relevant to gene transfer therapy is the generation of anti-AAV antibodies in response to infection by wild-type virus. Antibodies directly bind the pathogens, preventing their interaction with host cell receptors by neutralization16,23,24 and clearing them from the host by multiple mechanisms, including opsonization followed by macrophage-mediated phagocytosis (Figure 3).25
Figure 3.
Potential innate immunologic responses to viral capsid
Innate responses to the AAV capsid may lead to neutralization because of the inhibition of vector entry into the cell. Additional mechanisms by which anti-AAV antibodies neutralize AAVs may also exist. Transduction can also be inhibited by opsonization. In addition, inflammation induced by opsonization and complement activation. Innate responses also have potential systemic effects resulting from complement activation, including thrombocytopenia, atypical hemolytic uremic syndrome, and immune complex disposition.24, 25, 26, 27, 28, 29 AAV, adeno-associated virus; NAbs, neutralizing antibodies.
Given the rapid and strong immune responses that occur once immunological memory has been established, individuals with preexisting adaptive immunity to the specific vector capsid used in gene transfer therapy must be identified and excluded to avoid any clinical complication due to preexisting adaptive immunity. From the total antibodies (TAbs) generated against the wild-type virus, a subset categorized as neutralizing antibodies (NAbs) are able to inhibit transduction, reducing the efficacy of gene transfer therapy often by blocking the attachment of the rAAV to receptors on target cells (Figure 3).16,23,24 All the other antibodies generated against the wild-type virus that do not block transduction but still bind to the rAAV are called non-neutralizing antibodies (nNAbs). nNAbs also affect the efficacy of gene transfer therapy by reducing transduction through vector clearance via opsonization or complement activation, resulting in phagocytosis and elimination of the circulant rAAV by macrophages and neutrophils (Figure 3).25, 26, 27 These processes can be trigered by both NAbs and nNAbs and have important safety implications that may lead to inflammation. In addition, complications resulting from complement effectors include thrombocytopenia, atypical hemolytic uremic syndrome (aHUS), and immune complex deposition (Figure 3).28,29 aHUS, a type of thrombotic microangiopathy (TMA), is characterized by thrombocytopenia and microangiopathy hemolytic anemia, often resulting in renal involvement and immune complex deposition. Indeed, TMA, acute kidney injury, and complement activation have been reported in patients following systemic AAV gene therapy.30,31
Concept of antibody titers
Preexisting immunity is measured by determining antibody titers to a specific antigen. Antibody titers are determined by serially diluting the serum or plasma fraction of blood until the endpoint (i.e., lack of fluorescent signal) is achieved (Figure 4). Greater dilutions are indicative of greater levels of antibody in the sample.
Figure 4.
Determination of antibody titer by ELISA
Antibody titer is the maximum dilution at which the fluorescent signal stops; it is determined by serially diluting a fraction of plasma and testing for the presence of antibody. The last dilution that produces a signal and the first dilution that ceases to produce a signal determine the titer. AAV, adeno-associated virus.
Titer cut-offs are used in gene transfer therapy studies as part of the eligibility criteria to maximize efficacy and minimize potential safety concerns (Table 1). The titer selected as a cut-off is variable between assays; variations can also potentially exist using the same assay if it is optimized differently. Changes to the assay can influence the cut-offs even within the same program. As an example, there are two different facilities in the United States that test infants with spinal muscular atrophy for preexisting anti-AAV9 antibodies prior to treatment with Zolgensma. In one facility, samples >1:50 dilution are reported as positive, whereas samples ≥1:25 dilution are reported as elevated at the other laboratory.32 Given the high variability, clinicians emphasize the importance of being positive or negative under each specific assay cut-off. Of note, high antibody titers in some infants may arise from passive transfer of maternal neutralizing antibodies. For these infant to toddler patients, retesting is important, as titers may eventually fall within titer thresholds in the following weeks or months, permitting eventual treatment.32 Retesting older children typically yields results similar to the previous test unless the initial result was within borderline range.
Table 1.
Determination of preexisting immunity in clinical trials of intravenous AAV gene transfer
| Clinical study | Vector | Cut-offa | Assay used |
|---|---|---|---|
| DMD, phase I/II (NCT03368742) | SGT-001, AAV9 | not disclosed | not disclosed |
| DMD, phase I (NCT03362502) | PF-06939926, AAV9 | not disclosed | neutralizing assay |
| DMD, phase I/II (NCT03375164)33 | AAVrh74.MHCK7.micro-dystrophin, AAVrh74 | >1:400 | binding assay |
| SMA1, phase I/II (NCT02122952),34 phase III (NCT03306277, NCT03461289, NCT03505099) | AVXS-101, AAV9 | >1:50 | binding assay |
| Hemophilia A, phase I/II (NCT02576795)35 | AAV5 | not disclosed | neutralizing and binding assays |
| Hemophilia A, phase I/II (NCT03370172)35,36 | BAX 888, AAV8 | ≥1:5 | neutralizing assay |
| Hemophilia A, phase I/II (NCT03520712) | BMN270, AAV5 | not disclosed | binding assay |
| Hemophilia A, phase III (NCT04370054) | PF-07055480, recombinant AAV2/6 | not disclosed | neutralizing assay |
| Hemophilia A, phase I/II (NCT03734588) | SPK-8016 | not disclosed | not disclosed |
| Hemophilia B, phase II (NCT02396342)37 | AMT-060, AAV5 | 29% inhibition of transduction from 1:50 dilution | neutralizing assay |
| Hemophilia B, phase II (NCT02484092) | SPK-9001 | not disclosed | neutralizing assay |
| Hemophilia B, phase I/II (NCT04394286) | SHP648, AAV8 | ≥1:5 | neutralizing assay |
| Hemophilia B, phase I/II (NCT02618915) | DTX101, AAVrh10 | >1:5 | neutralizing assay |
| Hemophilia B, phase I/II (NCT03489291) | AMT-061, AAV5 | not included in the eligibility criteria | N/A |
| Hemophilia B, phase I/II (NCT03369444) | FLT180a | not disclosed | in vivo transduction inhibition assay |
| X-linked myotubular myopathy, phase I/II (NCT03199469) | AT132, AAV8 | not disclosed | neutralizing assay |
| GM1 gangliosidosis, phase I/II (NCT03952637) | AAV9-GLB1 | >1:50 | binding assay |
| Danon disease, phase I/II (NCT03882437) | RP-A501 | >1:40 | neutralizing assay |
| Krabbe disease, phase I/II (NCT04693598) | FBX-101, AAVrh10 | not included in the eligibility criteria | NA |
| Pompe disease, phase I/II (NCT04093349) | SPK-3006 | not disclosed | neutralizing assay |
| Late-onset Pompe disease, phase I/II (NCT04174105) | AT845, AAV8 | not disclosed | neutralizing assay |
| Glycogen storage disease type Ia (GSDIa), phase I/II (NCT03517085) | DTX401, AAV8 | ≥1:5 | neutralizing assay |
| Fabry disease, phase I/II (NCT04040049) | FLT190 | not disclosed | neutralizing assay |
| Acute intermittent porphyria, phase I (NCT02082860) | rAAV2/5-PBGD, AAV5 | not disclosed | neutralizing assay |
| Wilson disease, phase III (NCT04884815) | UX701, AAV9 | not disclosed | not disclosed |
| Wilson disease, phase I/II (NCT04537377) | VTX-801 | not included in the eligibility criteria | N/A |
| Sanfilippo syndrome, phase I/II (NCT04088734) | ABO-102, AAV9 | >1:100 | binding assay |
| Mucopolysaccharidosis II, phase I/II (NCT03041324) | SB-913, rAAV2/6 | not disclosed | neutralizing assay |
| Homozygous familial hypercholesterolemia, phase I/II (NCT02651675) | AAV-directed hLDLR gene therapy, AAV8 | >1:10 | neutralizing assay |
DMD, Duchenne muscular dystrophy; N/A, not applicable; SMA1, spinal muscular atrophy type 1.
Every program has its own assay to determine eligibility that has variability between laboratories.
Titer cut-offs are often determined using preclinical studies and validated after for clinical use. For example, analysis of transgene transduction in rhesus macaques following isolated limb perfusion of AAVrh74.MCK.micro-dys.FLAG showed that those with preexisting AAV antibody titers >1:400 had reduced transgene expression comparedwitho animals with AAV antibody titers ≤1:400 (transgene expression 20% and 48%, respectively).38 These preclinical non-human primate (NHP) studies informed the eligibility criteria for subsequent clinical studies, which used a preexisting antibody titer of 1:400 as the cut-off.33
Prevalence of different AAV serotype antibodies in clinical populations
Gene transfer therapy could potentially trigger strong and rapid immune responses via immunological memory because of previous exposure to a wild-type virus. Therefore, seroprevalence studies are crucial to identify vectors with lower potential to have preexisting immunity as candidates for gene transfer therapy studies. Seroprevalence studies indicate that exposure to wild-type AAVs and subsequent development of humoral immunity typically occurs in childhood as early as 2 years of age.39 In another study, the presence of anti-AAV antibodies to AAV2 and AAV8 in the participants was moderate at birth from maternal antibodies and decreased between 7 and 11 months of age, increasing again after 3 years of age.39
The prevalence of anti-AAV antibodies, TAbs, or only NAbs varies on the basis of serotype17,26,39, 40, 41 as well as geographical location.28 In healthy participants, a greater percentage of individuals were seropositive for anti-AAV1 NAbs in Australia, Europe, and Africa, compared with the United States.41 In the hemophilia A population, 47% of patients in Russia were seropositive for anti-AAV5 antibodies compared with 21% in the United States.42
Anti-AAV antibodies to one serotype can also potentially cross-react with other AAV serotypes because of the presence of conserved residues that could lead to overlapping epitopes.17,26,40 This high homology between AAV serotypes also predicts that patients are likely to have antibodies to multiple serotypes.43 Figure 5 shows the phylogenetic relationship between AAV vectors that are used in gene transfer therapy trials; serotypes that are further away from each other are less likely to be similar.
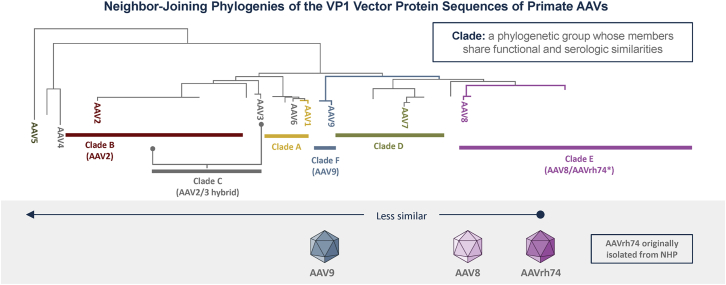
Figure 5.
Phylogenetic relationship of AAV vectors used in gene transfer therapy clinical trials
Neighbor-joining phylogenies of the VP1 capsid protein sequence of AAVs are shown. The further away one clade is from another, the less functional and serological similarities exist.44,45 AAV, adeno-associated virus; NHP, non-human primate.
As with preexisting humoral responses, a natural exposure to AAV can produce preexisting cell-mediated immunity. Prevalence studies suggest that preexisting cell-mediated responses range from 4% to 60% in peripheral blood mononuclear cells (PBMCs) and 7% to 62.5% in splenocytes; however, they may be less prevalent than preexisting humoral immunity.17 To monitor cell-mediated preexisting immunity, the enzyme-linked immunospot (ELISpot) assay can be used to measure the level of cytokines, including IFN-γ, secreted by activated antigen-specific B or T cells in the presence or absence of stimuli.44
As a way to circumvent preexisting immunity but maintain the advantages of AAV vectors, some gene transfer platforms use vectors derived from non-human species as sources with lower seroprevalence. For example, AAVrh10 and AAVrh74 are recombinant vectors isolated from rhesus macaques.46,47 In a healthy population, 59% had TAbs against AAVrh10 and 21% had NAbs.48 Analysis of TAbs against AAVrh74 in patients with Duchenne muscular dystrophy (DMD) and limb girdle muscular dystrophy (LGMD) showed that 83% were seronegative.49 In a separate interim analysis of 101 patients with DMD aged >4 to <18 years, the majority of patients (84.9%) were seronegative (<1:400) for anti-rAAVrh74 TAbs.50
Previous exposure to antigen will compromise safety and efficacy of the therapy: Clinical trials
In addition to compromising safety, studies in animal models have shown that preexisting antibodies can reduce transgene expression. Transgene expression can also be reduced by non-antibody serum factors.51 Both mouse and non-human primate studies have shown that preexisting AAV antibody titers as low as 1:5 can block AAV vector transduction of liver completely.16,52
Preexisting immunity has a similar impact in humans.15 In the first AAV gene transfer therapy trial with systemic delivery (through the hepatic artery), immune-mediated destruction of transduced cells through preexisting humoral immunity to AAV capsid antigens limited the therapeutic efficacy to ∼8 weeks, highlighting the importance of preexisting immunity to efficacy.15 Two patients were enrolled in the high-vector-dose cohort (2 × 1012 viral genome [vg]/kg); one patient with greater levels of preexisting immunity (NAb titer 1:17) to the AAV2 capsid did not express any detectable circulating factor IX (F.IX). In contrast, the patient, who had a low titer of 1:2 to the AAV2 capsid developed circulating levels of F.IX approximately 10% of normal.15 Similarly, in the first limb girdle muscular dystrophy 2D (LGMD2D) gene transfer therapy clinical trial, in which six patients received intramuscular rAAV1.tMCK.hSGCA, efficacy evaluation showed that only one patient had extremely low transgene copy numbers per nucleus and immunostaining for alpha sarcoglycan was unable to distiguish the treated muscle from the control.53 Subsequent analysis revealed an exagerated immune response (both humoral and cell mediated) to the AAV1 capsid, suggesting that this patient had preexisting immunity (baseline NAb titer 1:1,600).53
As with wild-type virus, administration of AAV vectors into seronegative individuals induces seroconversion via induction of adaptive immune responses.17 Thus, it is important to obtain therapeutic levels of transgene expression with the first dose given the challenge of seroconversion postdosing, thereby currently limiting re-administration of the same, and potentially other, AAV vector subtypes. Compared with baseline, persistence of high titers of circulating NAbs to AAV2 has been demonstrated up to 9 years following gene transfer therapy administration.54
Assays used to detect anti-AAV antibodies
Given the potential safety and efficacy risks associated with preexisting immunity to AAV vectors, antibody titers have been included as inclusion or exclusion criteria for gene transfer therapy protocols although the cut-offs and types of assays used differ (Table 1). Preclinical studies are essential in determining the best cut-off titer to maximize efficacy and minimize potential safety concerns.
Sensitive and reliable assays to measure anti-AAV titers are crucial for screening patients prior to enrollment in a clinical trial or treatment with an approved drug. Assays currently used to measure anti-AAVs include both TAb assays and neutralizing assays (NAb), both of which have advantages and disadvantages.
TAb assays detect all binding antibodies, both NAbs and nNAbs, and include ELISA-based binding assays in which serum anti-AAV antibodies are bound to whole capsids or peptides that are coated onto a plate and subsequently detected using a secondary antibody (Figure 6). TAb assays screen out any patient with any antibody titer greater than the cut-off (current range ≥1:5 to >1:400; Table 1). The ELISA technique allows rapid detection of most anti-AAV antibodies of even low avidity.44 ELISAs are convenient to set up, robust, reproduceable, and implementable in a commercial setting. Although this method is sensitive in determining anti-AAV antibodies in total and convenient,55 it is more conservative and may screen out a larger number of patients than if measuring only neutralizing activity.56 For example, analysis of both TAbs and NAbs against AAVrh10 in a healthy population showed that 59% were positive for TAbs, while 21% were positive for NAbs.48
Figure 6.
Assays used to measure preexisting immunity to AAVs
(A) Binding assays use ELISA to detect TAbs that can bind to AAV antigens, including both NAbs and nNAbs. (B) Neutralizing assays detect NAbs. In vitro neutralizing assays measure reporter gene expression following transduction with an AAV similar to the gene transfer therapy that has been pre-incubated with a subject’s serum or plasma. AAV, adeno-associated virus; NAbs, neutralizing antibodies; nNAbs, non-neutralizing antibodies; TAbs, total antibodies.
In vitro cell-based neutralizing assays detect antibodies that can interfere with transduction (NAbs) by measuring residual expression of a reporter gene following transduction with an AAV similar to the gene transfer therapy that has been pre-incubated with a subject’s serum or plasma (Figure 6).56 The dilution that detects <50% of the reporter signal is determined; the cut-off is defined as the threshold above which a sample is considered positive for neutralizing activity.57 This method gives consistent results and may be able to detect the presence of non-antibody neutralization factors;58 however, because AAVs are inefficient in transducing cells in vitro and subtypes differ in their transduction efficiencies, greater multiplicities of infection (MOIs) are required (range, 103–105 viral genome/cell59), which can underestimate the titer of NAbs.56,57 In addition, cell culture conditions may induce variability into the assay (e.g., specific cell line used and cell density and passage).56 Furthermore, non-antibody neutralizing factors, including blood proteins (e.g., human serum albumin and galectin 3 binding protein), can also bind the vector and disturb transduction.18,60, 61, 62, 63
It is important to note that this assay uses plasma or serum samples, which might not fully reflect all the other components of the immune system that can influence transduction and are only present in vivo, such as complement, macrophages, and cytokines. This assay does not measure nNAbs that might not block transduction in vitro but have the potential to affect the efficacy of gene transfer therapy in vivo by reducing transduction through vector clearance via opsonization or complement activation, resulting in phagocytosis and elimination of the circulant rAAV by macrophages and neutrophils (Figure 3).25,26 These processes may have important safety implications, which may further lead to severe adverse events,28,29 suggesting that TAb assays may be more robust and safer for use as a screening assay.
Complications resulting from complement effectors include thrombocytopenia, atypical hemolytic uremic syndrome, and immune complex deposition (Figure 3).28,29 aHUS, a type of thrombotic microangiopathy, is characterized by thrombocytopenia and microangiopathy hemolytic anemia, often resulting in renal involvement and immune complex deposition. Indeed, TMA, acute kidney injury, and complement activation have been reported in patients following systemic AAV gene therapy.30,31
Although in vivo neutralizing assays overcome some of the limitations of in vitro assays, they have their own limitations, including high variability and lack of validation; they are also difficult to standardize and scale up.57
In a seroprevalence study, samples from healthy participants were tested using ELISA to measure TAbs as well as a neutralizing assay to determine NAbs. TAb prevalence was higher for AAV1 and AAV2 than AAV5, AAV6, AAV8, and AAV9.26 Similarly, the percentage of samples seropositive for neutralizing factors was greatest for AAV2 and AAV1 with AAV8 and AAV5 being the least prevalent.41 Of participants who were seropositive for TAb AAV5, AAV8, and AAV9 serotypes, approximately 70%–100% had low NAb titers (1:20).26
Overcoming preexposure challenges
Beyond the current approaches of excluding seropositive patients and selecting those with low to undetectable preexisting antibodies to AAV, particularly when the gene transfer therapy is administered systemically, possible solutions to avoid or suppress the immune response to AAV gene therapies include transient immunosuppression (e.g., rituximab, eculizumab, sirolimus) in the immediate days following infusion, although data on this approach are inconclusive. Other studies have also highlighted the potential to modulate vector immunogenicity; co-administration of tolerogenic rapamycin nanoparticles with AAV vectors suppressed induction of adaptive immune responses, both cell-mediated and humoral.64 The suppression of these responses permitted re-administration of AAV vectors in both mice and NHPs.64 However, complications of currently available immunosuppressants, including infections and malignancies, may limit their use, especially in the long term.65
Other methods for overcoming preexisting immunity include capsid engineering approaches to produce novel, antigenically distinct AAV variants66,67 and the removal of NAbs by degradation or plasmapheresis. Specifically, transient degradation of NAbs using an IgG-degrading enzymes has been proposed as a means to treat seropositive patients.68,69 This approach permitted AAV transduction of seropositive mice68 as well as the re-administration of AAV vectors in NHPs.69 In addition, plasmapheresis has also been proposed.38 In seropositive NHPs that underwent plasmapheresis to remove AAV NAbs, transduction following isolated limb perfusion was similar to that observed in seronegative animals and higher than non-apheresed seropositive NHPs (61% vs. 54% and 10%, respectively).38 Because plasmapheresis nonspecifically removes all circulating IgG, more recent studies have used AAV-specific plasmapheresis.70
Conclusions and future directions
At present, there is no universal test or titer used to detect anti-AAV antibodies in patients (Table 1). Both total binding and neutralizing assays are generally difficult to standardize, especially with respect to thresholds indicative of positivity, which can result in differences in the prevalence of anti-AAV antibodies as well as different cut-off values.17 Neutralizing assays can detect NAbs as well as other non-antibody neutralizing factors (although their relationship to safety is unclear). However, these assays are more difficult to validate and have a higher risk for underestimating total anti-AAV antibody titer, which may have safety implications for including/excluding patients for gene transfer therapy.
TAb assays are more robust and screen out all antibodies without discriminating between NAbs and nNAbs; therefore, the titer obtained is more conservative and perhaps safer. The conservative aspect of TAb assays was recently demonstrated in patients with hemophilia B treated with AAV5-hFIX gene transfer therapy, who were initially selected using a neutralizing assay.71 Subsequent analysis of TAbs with ELISA showed that 2 of 10 treated patients were positive to low levels of total anti-AAV5 IgG antibodies despite being classified as negative using the original neutralizing assay.71 Finally, until there is a standard method for analyzing preexisting neutralization capacity and the implications of non-antibody factors are clarified, TAb assays may be more robust and safer.
Acknowledgments
Medical writing and editorial support were provided by Marjet Heitzer, PhD of 360 Medical Writing and funded by Sarepta Therapeutics, Inc.
Author contributions
Conceptualization, S.D.D., L.Q.-G., and L.R.R.-K.; Investigation, J.R.M., A.M.C., K.J.L., D.A.G., S.Z.K., S.D.D., and L.R.R.-K.; Writing – Original Draft, S.Z.K., S.D.D., L.Q.-G., and L.R.R.-K.; Writing – Review & Editing, J.R.M., A.M.C., D.A.G., K.J.L., S.Z.K., S.D.D., L.Q.-G., and L.R.R.-K.; Supervision, L.R.R.-K.
Declaration of interests
J.R.M. has received funding from the National Institute of Neurological Disorders and Stroke (NINDS), Sarepta Therapeutics, Inc., and Novartis Pharmaceuticals. A.M.C. has served on advisory boards for Sarepta Therapeutics, Inc., Novartis Pharmaceuticals, Genentech Roche, Edgewise Therapeutics, and Dyne Therapeutics; and is a site principal investigator for Fibrogen Inc. D.A.G., S.Z.K., S.D.D., L.Q.-G., and L.R.R.-K. are employees of Sarepta Therapeutics, Inc.
References
- 1.Collins M., Thrasher A. Gene therapy: progress and predictions. Proc. Biol. Sci. 2015;282:20143003. doi: 10.1098/rspb.2014.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 3.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 6.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D., et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6:35–47. doi: 10.3390/diseases6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 10.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaiss A.K., Liu Q., Bowen G.P., Wong N.C., Bartlett J.S., Muruve D.A. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 2002;76:4580–4590. doi: 10.1128/jvi.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 16.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 17.Vandamme C., Adjali O., Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaiss A.K., Cotter M.J., White L.R., Clark S.A., Wong N.C., Holers V.M., Bartlett J.S., Muruve D.A. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration (FDA) Cellular, tissue, and gene therapies advisory committee (CTGTAC) meeting #70. Toxicity risks of adeno-associated virus (AAV) vectors for gene therapy (GT) https://www.fda.gov/media/151599/download
- 20.Rogers G.L., Shirley J.L., Zolotukhin I., Kumar S.R.P., Sherman A., Perrin G.Q., Hoffman B.E., Srivastava A., Basner-Tschakarjan E., Wallet M.A., et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017;129:3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirley J.L., Keeler G.D., Sherman A., Zolotukhin I., Markusic D.M., Hoffman B.E., Morel L.M., Wallet M.A., Terhorst C., Herzog R.W. Type I IFN sensing by cDCs and CD4+ T cell help are both requisite for cross-priming of AAV capsid-specific CD8+ T cells. Mol. Ther. 2020;28:758–770. doi: 10.1016/j.ymthe.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuranda K., Jean-Alphonse P., Leborgne C., Hardet R., Collaud F., Marmier S., Costa Verdera H., Ronzitti G., Veron P., Mingozzi F. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J. Clin. Invest. 2018;128:5267–5279. doi: 10.1172/JCI122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcedo R., Wilson J.M. Humoral immune response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nidetz N.F., McGee M.C., Tse L.V., Li C., Cong L., Li Y., Huang W. Adeno-associated viral vector-mediated immune responses: understanding barriers to gene delivery. Pharmacol. Ther. 2020;207:107453. doi: 10.1016/j.pharmthera.2019.107453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 27.Schmaljohn A.L. Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. Curr. HIV Res. 2013;11:345–353. doi: 10.2174/1570162x113116660057. [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G., Lambris J.D. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin. Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chand D.H., Zaidman C., Arya K., Millner R., Farrar M.A., Mackie F.E., Goedeker N.L., Dharnidharka V.R., Dandamudi R., Reyna S.P. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J. Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 31.https://www.pfizer.com/news/press-release/press-release-detail/pfizers-new-phase-1b-results-gene-therapy-ambulatory-boys.
- 32.Day J.W., Finkel R.S., Mercuri E., Swoboda K.J., Menier M., van Olden R., Tauscher-Wisniewski S., Mendell J.R. Adeno-associated virus serotype 9 antibodies in patients screened for treatment with onasemnogene abeparvovec. Mol. Ther. Methods Clin. Dev. 2021;21:76–82. doi: 10.1016/j.omtm.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Systemic gene delivery clinical trial for Duchenne muscular dystrophy. https://clinicaltrials.gov/ct2/show/NCT03375164
- 34.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 35.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 36.Spark Therapeutics Corporate Overview Presentation. August 2018.
- 37.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwable J., Bonig H., et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chicoine L.G., Montgomery C.L., Bremer W.G., Shontz K.M., Griffin D.A., Heller K.N., Lewis S., Malik V., Grose W.E., Shilling C.J., et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 2014;22:338–347. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccin. Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majowicz A., Ncete N., van Waes F., Timmer N., van Deventer S.J., Mahlangu J.N., Ferreira V. Seroprevalence of pre-existing nabs against AAV1, 2, 5, 6 and 8 in South African hemophilia B patient population. Blood. 2019;134:3353. [Google Scholar]
- 41.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klamroth R., Hayes G., Andreeva T., Suzuki T., Hardesty B., Shima M., et al. Res Pract Thromb Haemost. 2021; 5 (Suppl 2). https://abstracts.isth.org/abstract/global-seroprevalence-of-pre-existing-immunity-against-aav-serotypes-in-people-with-hemophilia-a/. Accessed March 11, 2022. Res Pract Thromb Haemost; 2021. Global Seroprevalence of Pre-existing Immunity against AAV Serotypes in People with Hemophilia A. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piechnik M., Sawamoto K., Ohnishi H., Kawamoto N., Ago Y., Tomatsu S. Evading the AAV immune response in mucopolysaccharidoses. Int. J. Mol. Sci. 2020;21:3433. doi: 10.3390/ijms21103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino A.T., Herzog R.W., Anegon I., Adjali O. Measuring immune responses to recombinant AAV gene transfer. Methods Mol. Biol. 2011;807:259–272. doi: 10.1007/978-1-61779-370-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zygmunt D.A., Crowe K.E., Flanigan K.M., Martin P.T. Comparison of serum rAAV serotype-specific antibodies in patients with Duchenne muscular dystrophy, becker muscular dystrophy, inclusion body myositis, or GNE myopathy. Hum. Gene Ther. 2017;28:737–746. doi: 10.1089/hum.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin P.T., Xu R., Rodino-Klapac L.R., Oglesbay E., Camboni M., Montgomery C.L., Shontz K., Chicoine L.G., Clark K.R., Sahenk Z., et al. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am. J. Physiol. Cell Physiol. 2009;296:C476–C488. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thwaite R., Pages G., Chillon M., Bosch A. AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Ther. 2015;22:196–201. doi: 10.1038/gt.2014.103. [DOI] [PubMed] [Google Scholar]
- 49.Griffin D.A., Potter R.A., Pozsgai E.R., Peterson E.L., Rodino-Klapac L.R. Presented at the American Society of Gene and Cell Therapy (ASGCT ) 22nd Annual Meeting, April 29-May 2, 2019, Washington, DC. 2019. Adeno-associated virus serotype rh74 prevalence in muscular dystrophy population. [Google Scholar]
- 50.Goedeker N.L., Griffin D., Dharia S., Santra S., Coy J., Zaidman C.M. Presented at the 2021 World Muscle Society Virtual Congress, September 20–24. 2021. Evaluation of total binding antibodies against rAAVrh74 in patients with Duchenne muscular dystrophy. [Google Scholar]
- 51.Denard J., Rouillon J., Leger T., Garcia C., Lambert M.P., Griffith G., Jenny C., Camadro J.M., Garcia L., Svinartchouk F. AAV-8 and AAV-9 vectors cooperate with serum proteins differently than AAV-1 and AAV-6. Mol. Ther. Methods Clin. Dev. 2018;10:291–302. doi: 10.1016/j.omtm.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendell J.R., Rodino-Klapac L., Sahenk Z., Malik V., Kaspar B.K., Walker C.M., Clark K.R. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci. Lett. 2012;527:90–99. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mingozzi F., High K.A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 55.Ito T., Yamamoto S., Hayashi T., Kodera M., Mizukami H., Ozawa K., Muramatsu S. A convenient enzyme-linked immunosorbent assay for rapid screening of anti-adeno-associated virus neutralizing antibodies. Ann. Clin. Biochem. 2009;46:508–510. doi: 10.1258/acb.2009.009077. [DOI] [PubMed] [Google Scholar]
- 56.Masat E., Pavani G., Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov. Med. 2013;15:379–389. [PubMed] [Google Scholar]
- 57.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falese L., Sandza K., Yates B., Triffault S., Gangar S., Long B., Tsuruda L., Carter B., Vettermann C., Zoog S.J., Fong S. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krotova K., Aslanidi G. Modifiers of adeno-associated virus-mediated gene expression in implication for serotype-universal neutralizing antibody assay. Hum. Gene Ther. 2020;31:1124–1131. doi: 10.1089/hum.2020.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denard J., Beley C., Kotin R., Lai-Kuen R., Blot S., Leh H., Asokan A., Samulski R.J., Moullier P., Voit T., et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J. Virol. 2012;86:6620–6631. doi: 10.1128/JVI.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denard J., Marolleau B., Jenny C., Rao T.N., Fehling H.J., Voit T., Svinartchouk F. C-reactive protein (CRP) is essential for efficient systemic transduction of recombinant adeno-associated virus vector 1 (rAAV-1) and rAAV-6 in mice. J. Virol. 2013;87:10784–10791. doi: 10.1128/JVI.01813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang M., Sun J., Crosby A., Woodard K., Hirsch M.L., Samulski R.J., Li C. Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications. Gene Ther. 2017;24:49–59. doi: 10.1038/gt.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang D., Zhong L., Li M., Li J., Tran K., Ren L., He R., Xie J., Moser R.P., Fraser C., et al. Adeno-associated virus neutralizing antibodies in large animals and their impact on brain intraparenchymal gene transfer. Mol. Ther. Methods Clin. Dev. 2018;11:65–72. doi: 10.1016/j.omtm.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meliani A., Boisgerault F., Hardet R., Marmier S., Collaud F., Ronzitti G., Leborgne C., Costa Verdera H., Simon Sola M., Charles S., et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 2018;9:4098. doi: 10.12688/f1000research.11243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau C.H., Suh Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res. 2017;6:2153. doi: 10.12688/f1000research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse L.V., Klinc K.A., Madigan V.J., Castellanos Rivera R.M., Wells L.F., Havlik L.P., Smith J.K., Agbandje-McKenna M., Asokan A. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl. Acad. Sci. U S A. 2017;114:E4812–E4821. doi: 10.1073/pnas.1704766114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giles A.R., Govindasamy L., Somanathan S., Wilson J.M. Mapping an adeno-associated virus 9-specific neutralizing epitope to develop next-generation gene delivery vectors. J. Virol. 2018;92 doi: 10.1128/JVI.01011-18. e01011–01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elmore Z.C., Oh D.K., Simon K.E., Fanous M.M., Asokan A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight. 2020;5:e139881. doi: 10.1172/jci.insight.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leborgne C., Barbon E., Alexander J.M., Hanby H., Delignat S., Cohen D.M., Collaud F., Muraleetharan S., Lupo D., Silverberg J., et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020;26:1096–1101. doi: 10.1038/s41591-020-0911-7. [DOI] [PubMed] [Google Scholar]
- 70.Bertin B., Veron P., Leborgne C., Deschamps J.Y., Moullec S., Fromes Y., Collaud F., Boutin S., Latournerie V., van Wittenberghe L., et al. Capsid-specific removal of circulating antibodies to adeno-associated virus vectors. Sci. Rep. 2020;10:864. doi: 10.1038/s41598-020-57893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]