Abstract
Mechanisms of resistance to azoles in Candida albicans, the main etiologic agent of oropharyngeal candidiasis (OPC), include alterations in the target enzyme (lanosterol demethylase) and increased efflux of drug. Previous studies on mechanisms of resistance have been limited by the fact that only a single isolate from each OPC episode was available for study. Multiple isolates from each OPC episode were evaluated with oral samples plated in CHROMagar Candida with and without fluconazole to maximize detection of resistant yeasts. A total of 101 isolates from each of three serial episodes of OPC from four different patients were evaluated. Decreasing geometric means of fluconazole MICs with serial episodes of infection were detected in the four patients. However, 8-fold or larger (up to 32-fold) differences in fluconazole MICs were detected within isolates recovered at the same time point in 7 of 12 episodes. Strain identity was analyzed by DNA typing techniques and indicated that isolates from each patient represented mainly isogenic strains, but differed among patients. A Northern blot technique was used to monitor expression of ERG11 (encoding lanosterol demethylase) and genes coding for efflux pumps. This analysis revealed that clinical isolates obtained from the same patient and episode were phenotypically heterogeneous in their patterns of expression of these genes involved in fluconazole resistance. These results demonstrate the complexity of the distribution of the molecular mechanisms of antifungal drug resistance and indicate that different subpopulations of yeasts may coexist at a given time in the same patient and may develop resistance through different mechanisms.
Oropharyngeal candidiasis (OPC) occurs in as many as 90% of patients with human immunodeficiency virus (HIV) or AIDS (6, 12). OPC can be an early indicator of HIV infection, but oral candidiasis is associated with worsening immune function and may predict progressive immunodeficiency independently of CD4 lymphocyte counts (2, 17). Azole antifungal drugs, particularly fluconazole, have proven effective in treating mucosal candidiasis even in individuals with advanced immunodeficiency (20, 21). However, development of resistance, especially in patients with extensive prior azole use, is common (7, 18, 23). Recently the National Committee for Clinical Laboratory Standards (NCCLS) has approved standardized methods for susceptibility testing (13). Also, extensive clinical data have been used to establish the correlation between high in vitro MICs (mycological resistance) and clinical outcome (22). Mycological resistance may not always be predictive of a poor outcome, but increased failure rates occur against resistant yeasts (fluconazole MICs, >64 μg/ml). Strains for which fluconazole MICs were 16 to 32 μg/ml demonstrate dose-dependent susceptibility and may respond to higher doses of drug (20, 22).
At the cellular level, development of fluconazole resistance may emerge as a result of replacement of a susceptible strain by another, intrinsically resistant strain or species (reviewed in reference 37). At the molecular level, two major mechanisms appear to be responsible for development of fluconazole resistance in strains of Candida albicans. The first mechanism involves an altered target site, the cytochrome P-450 lanosterol 14α-demethylase, either by overproduction of the enzyme or due to point mutations in its encoding gene (ERG11) leading to amino acid substitutions resulting in decreased affinity of the enzyme for azole derivatives (9, 25, 32, 33, 36). A second major mechanism is through increased efflux of drug, mediated by two types of multidrug efflux pumps, the major facilitators and the ABC transporters (1, 10, 28, 34, 35). The MDR1 gene encodes a major facilitator implicated in resistance (3), and its overexpression leads to fluconazole resistance exclusively among azole drugs (10, 26, 28). The genes coding for several ABC transporters in C. albicans have been identified, including several CDR genes (19, 26). CDR1 and CDR2 were the first two members of this family identified in C. albicans, and both CDR1 and CDR2 have been described as playing a role in fluconazole resistance (10, 27, 28). Other azole drugs are also substrates for ABC transporters, and, thus, overexpression of CDR genes results in cross-resistance to other azole derivatives (10, 26, 28).
In general, description of these molecular mechanisms of resistance has been performed by analyzing their role in serial isolates with increasing resistance to the drug recovered from the same patient, as detected by antifungal susceptibility testing (1, 4, 10, 28, 35). However, most studies evaluating resistance have been limited due to the fact that only single isolates from each time point were available for study. Methods that increase detection of subpopulations of yeasts at the time of initial isolation, such as our novel agar dilution screening technique (14, 15), may be very useful to provide a more comprehensive assessment of the mechanisms of resistance.
In the present study, a total of 101 isolates from three serial OPC episodes from four different patients were included for analysis of resistance to azoles. Evidence is provided for (i) the heterogeneity of the susceptibility to fluconazole between isolates recovered during the same episode of OPC, (ii) the complexity of expression of genes implicated in development of fluconazole resistance, and (iii) the presence at the same time point of different subpopulations of yeast exhibiting different resistance phenotypes.
MATERIALS AND METHODS
Clinical samples and isolates.
Yeast isolates were obtained by direct swab or by oral saline rinses from four HIV-infected patients with recurrent OPC enrolled in a longitudinal study to assess significance of fluconazole resistance. At the time of initial isolation, oral samples were plated on CHROMagar Candida (CHROMagar, Paris, France) with and without fluconazole to maximize detection of resistant yeasts as previously described by our group (14, 15). Briefly, dilutions of oral samples were added to plates containing solid medium with and without fluconazole from which representative colonies were recovered. Patients were treated initially with fluconazole at 100 mg/day, and doses were increased to up to 800 mg/day if necessary for clinical resolution in an effort to achieve therapeutic response after development of clinical resistance (20). In all four patients, therapeutic response was achieved by increasing the dose of fluconazole to a range of 200 to 800 mg/day. The identity of these clinical isolates as C. albicans was confirmed by standard biochemical and microbiological procedures, including carbohydrate assimilation patterns (API 20C; Analytab Products, BioMerieux, France), germ tube formation in serum-containing medium, and color of colonies in chromogenic medium (CHROMagar Candida). Only patient A had Candida species other than albicans at the time of the second and third episodes, but the predominant isolates were C. albicans. All other patients had C. albicans only. Isolates were stored at room temperature as suspensions in sterile deionized water.
Strain identification.
Strain identity was investigated by karyotyping, restriction fragment length polymorphism (RFLP), and DNA fingerprinting with the moderately repetitive probe Ca3 (provided as a gift from D. Soll, University of Iowa) (29). Briefly, chromosomes from the different isolates were prepared in agarose plugs, separated by pulsed-field gel electrophoresis (Bio-Rad, Hercules, Calif.), stained with ethidium bromide, and photographed under UV light. RFLP patterns were generated by digestion of genomic DNA with SfiI (Boehringer Mannheim, Indianapolis, Ind.). After separation by pulsed-field gel electrophoresis, gels were stained with ethidium bromide and photographed. Following documentation, the materials present in the RFLP gels were transferred to nylon membranes (Nytran; Schleicher & Schuell, Keene, N.H.) and hybridized with a Ca3 probe radioactively labeled by random priming (Random Primers DNA Labeling System; GibcoBRL, Gaithersburg, Md.). The membranes were then washed and exposed to autoradiography film (Du Pont, Wilmington, Del.). Pictures of the gels or films were scanned with the Adobe Photo Shop program (Adobe Systems, Inc., Mountain View, Calif.). For preparation of figures, digital images were processed by using the Adobe Photo Shop program.
Drug susceptibility testing and MIC determinations.
Antifungal susceptibilities to fluconazole were determined by NCCLS method M-27A with broth macrodilution techniques and reading of the endpoints at 48 h (13). Isolates for which fluconazole MICs were ≤8 μg/ml are considered susceptible. MICs of 16 to 32 μg/ml indicate susceptible but dose-dependent isolates. Isolates for which MICs were ≥64 μg/ml are considered resistant to the drug (22). Additional susceptibility testing of selected isolates with itraconazole (Janssen Pharmaceutica, Beerse, Belgium), ketoconazole (Janssen Pharmaceutica), voriconazole (Pfizer Inc., Sandwich, United Kingdom), SCH 56592 (Schering Plough, Kenilworth, N.J.), amphotericin B (Bristol-Myers Squibb, Princeton, N.J.), and terbinafine (Novartis, Vienna, Austria) was determined according to NCCLS method M-27A by using a broth microdilution procedure and reading of the endpoints at 48 h (13).
Northern (RNA) blot analysis.
Isolates from the stocks in water were subcultured onto plates containing Sabouraud dextrose agar 48 h prior to propagation in YEPD medium (2% yeast extract, 1% peptone, 2% glucose). Total RNA from the different isolates grown to mid-logarithmic phase in YEPD medium was obtained with the RNAeasy mini kit (Qiagen, Inc., Santa Clarita, Calif.) according to the manufacturer’s instructions. Equal amounts (approximately 5 μg) of RNA as determined by A260 measurements were separated by electrophoresis (24). The gels were photographed and subsequently transferred to nylon membranes (Nytran; Schleicher & Schuell) by using the Turboblotter apparatus (Schleicher & Schuell). Probes for ERG11, MDR1, and CDR genes were prepared as described before (10). The resulting CDR probe is based on the whole sequence of CDR1 and has been shown to cross-hybridize with other members of this gene family (10, 27, 28, 34). Probes specific for the CDR1 and CDR2 genes were prepared by PCR amplification as described before (10, 27). All probes were labelled by random priming (Random Primers DNA Labeling System; GibcoBRL), and hybridizations were performed with Rapid-hyb buffer (Amersham Life Science, Inc., Arlington Heights, Ill.) according to the manufacturer’s instructions. After hybridization, blots were washed and exposed to autoradiography film (Du Pont). Nylon membranes were probed sequentially with the different probes following stripping of the previously bound probe (10). Autoradiograms were scanned by using the Adobe Photo Shop program (Adobe Systems, Inc.). Samples of 18S rRNA in the gels were used as a control for loading and subsequent normalization of signals in the autoradiograms (10, 24). For preparation of figures, digital images were processed with the Adobe Photo Shop program.
RESULTS
Analysis of isolates from patient A.
Table 1 shows results of fluconazole susceptibility testing for isolates recovered from patient A. Testing of 10 C. albicans isolates recovered during the first episode of OPC indicated the presence of highly susceptible isolates only (fluconazole MICs, 0.5 to 1 μg/ml; geometric mean, 0.6 μg/ml). Decreased susceptibility was observed for all five isolates recovered during the second episode (geometric mean of fluconazole MICs, 12.1), including the presence of a highly resistant isolate (isolate A.2.1; fluconazole MIC, 128 μg/ml), together with isolates with elevated in vitro susceptibilities but still in the susceptible range (fluconazole MICs, 4 to 8 μg/ml). A 32-fold difference in fluconazole MICs for isolates recovered at the same time point suggested the presence in the oral cavity of a heterogeneous yeast population. Increasing resistance was detected in representative isolates recovered during the third episode, with all three isolates demonstrating resistance (isolate A.3.1, fluconazole MIC, 128 μg/ml) or dose-dependent susceptibility (isolates A.3.2 and A.3.3, fluconazole MICs, 32 and 16 μg/ml, respectively) and an overall geometric mean of fluconazole MICs of 40.3 μg/ml.
TABLE 1.
Fluconazole MICs for multiple C. albicans isolates recovered from three serial episodes of OPC from patient A
| Isolate | Fluconazole MIC (μg/ml) |
|---|---|
| Episode 1 | |
| A.1.1 | 0.5 |
| A.1.2 | 0.5 |
| A.1.3 | 0.5 |
| A.1.4 | 0.5 |
| A.1.5 | 1 |
| A.1.6 | 1 |
| A.1.7 | 0.5 |
| A.1.8 | 0.5 |
| A.1.9 | 0.5 |
| A.1.10 | 0.5 |
| Geometric mean | 0.6 |
| Episode 2 | |
| A.2.1 | 128 |
| A.2.2 | 8 |
| A.2.3 | 8 |
| A.2.4 | 4 |
| A.2.5 | 8 |
| Geometric mean | 12.1 |
| Episode 3 | |
| A.3.1 | 128 |
| A.3.2 | 32 |
| A.3.3 | 16 |
| Geometric mean | 40.3 |
DNA typing techniques indicated development of resistance in a persistent strain (Fig. 1). Minor differences in the karyotyping patterns across the different isolates indicated a certain degree of instability of their chromosomal organization. Also, isolate A.2.1 showed markedly different karyotyping and RFLP patterns, but the same Ca3 fingerprinting pattern, suggesting this isolate may constitute a different substrain or variant of the same persistent strain rather than an unrelated strain. Interestingly, this isolate was the only resistant isolate (among other susceptible isolates) detected during the second episode of OPC, but analysis of representative isolates of the third OPC episode revealed that this substrain did not persist, but rather development of resistance occurred in the same strain, representing the majority of yeasts present in the oral cavity during the first two episodes.
FIG. 1.
Karyotype (A), RFLP analysis generated by digestion with SfiI of genomic DNA (B), and fingerprinting analysis with the moderately repetitive probe Ca3 (C) of representative C. albicans clinical isolates recovered from three OPC episodes from patient A. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
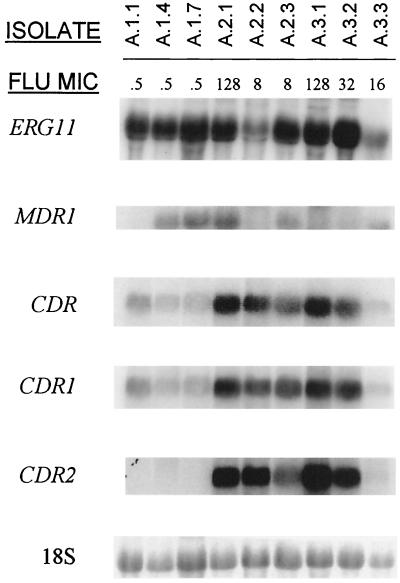
Further demonstration of phenotypic heterogeneity within isolates recovered at the same time point was evidenced by the analysis of expression of genes implicated in the development of fluconazole resistance, as shown in Fig. 2. Three isolates from the first OPC episode and for which the fluconazole MIC was the same (0.5 μg/ml) exhibited different patterns of gene expression. While isolates A.1.4 and A.1.7 showed expression of MDR1 and low constitutive levels of expression of CDR1, isolate A.1.1 exhibited increased levels of CDR1 but negligible levels of MDR1. Analysis of gene expression for isolate A.2.1 revealed moderate overexpression of MDR1 and strong expression of CDR1 and CDR2 genes, which were correlated with its high in vitro resistance to the antifungal drug (MIC, 128 μg/ml). Two other isolates recovered at the same time (isolates A.2.2. and A.2.3) showed distinct patterns of gene expression despite displaying the same in vitro fluconazole MIC (8 μg/ml). Negligible levels of MDR1 but increased message for CDR genes (especially CDR2) was detected in isolate A.2.2. On the other hand, constitutive MDR1 expression accompanied by moderate expression of CDR genes (mainly CDR1) was detected for isolate A.2.3. Strong expression of CDR genes together with a more moderate overexpression of ERG11 was detected in isolates A.3.1 and A.3.2 (fluconazole MICs, 128 and 32 μg/ml, respectively) recovered during the third OPC episode, but not in isolate A.3.3 (fluconazole MIC, 16 μg/ml) isolated at the same time point, which showed moderate expression of MDR1 only. Overall, development of resistance in this patient correlated with increased message for CDR genes (both CDR1 and CDR2), but overexpression of either MDR1 or ERG11 was detected in some isolates and was usually detected in conjunction with overexpression of CDR.
FIG. 2.
Northern-blot analysis of total RNA obtained from multiple C. albicans clinical isolates recovered from each of three serial episodes of OPC in patient A. The membranes were probed with ERG11, MDR1, a nonspecific probe for CDR genes, and probes specific for CDR1 and CDR2. The bottom panel shows amounts of 18S rRNA used to standardize signal levels according to lane loading parameters. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Analysis of isolates from patient B.
Fluconazole MICs for C. albicans isolates recovered from three serial episodes of OPC from patient B are shown in Table 2. Susceptibility testing of seven representative isolates recovered during the first OPC episode demonstrated fluconazole susceptibility (geometric mean of fluconazole MICs, 0.8 μg/ml), although susceptibility results were distributed among a wide range of MICs (0.25 to 4 μg/ml). Decreased susceptibility was detected for all six isolates recovered during the second OPC episode (geometric mean of fluconazole MICs, 5.0 μg/ml; range, 4 to 8 μg/ml) and the seven isolates evaluated from the third OPC episode (geometric mean of fluconazole MICs, 5.9 μg/ml; range, 4 to 16 μg/ml).
TABLE 2.
Fluconazole MICs for multiple C. albicans isolates recovered from three serial episodes of OPC from patient B
| Isolate | Fluconazole MIC (μg/ml) |
|---|---|
| Episode 1 | |
| B.1.1 | 0.25 |
| B.1.2 | 0.5 |
| B.1.3 | 0.5 |
| B.1.4 | 4 |
| B.1.5 | 4 |
| B.1.6 | 0.5 |
| B.1.7 | 0.5 |
| Geometric mean | 0.8 |
| Episode 2 | |
| B.2.1 | 4 |
| B.2.2 | 8 |
| B.2.3 | 4 |
| B.2.4 | 4 |
| B.2.5 | 8 |
| B.2.6 | 4 |
| Geometric mean | 5.0 |
| Episode 3 | |
| B.3.1 | 16 |
| B.3.2 | 4 |
| B.3.3 | 4 |
| B.3.4 | 4 |
| B.3.5 | 16 |
| B.3.6 | 4 |
| B.3.7 | 4 |
| Geometric mean | 5.9 |
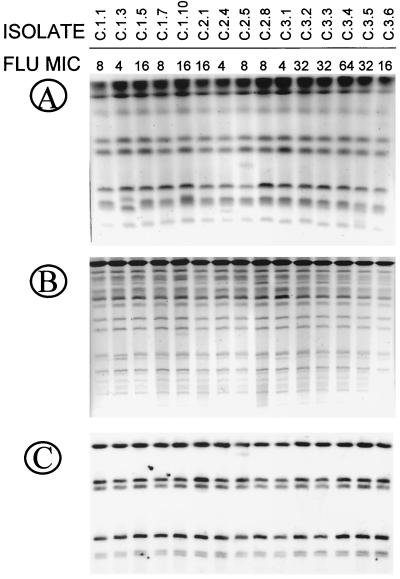
Investigation of strain identity by using DNA typing techniques revealed the presence of three different strains during the first OPC episode, as detected by differences in karyotyping, RFLP, and Ca3 fingerprinting patterns (Fig. 3). However, isolates recovered during the second and third episodes showed a single pattern for each technique used, which was the same as the one displayed by three of five isolates in the first episode. Again, these results suggest the development of decreasing susceptibility to the azole agent in the same persistent strain, which was already present during the first OPC episode.
FIG. 3.
Karyotype (A), RFLP analysis generated by digestion with SfiI of genomic DNA (B), and fingerprinting analysis with the moderately repetitive probe Ca3 (C) of representative C. albicans clinical isolates recovered from three OPC episodes from patient B. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Northern blot analysis in this series of isolates (Fig. 4) revealed a correlation between overexpression of CDR genes (both CDR1 and CDR2) and isolates with decreased fluconazole susceptibility in all three episodes. Levels of ERG11 remained constant throughout the series. MDR1 levels were below the detection limit for all isolates from this patient.
FIG. 4.
Northern blot analysis of total RNA from obtained from multiple C. albicans clinical isolates recovered from each of three serial episodes of OPC in patient B. The membranes were probed with ERG11, MDR1, a nonspecific probe for CDR genes, and probes specific for CDR1 and CDR2. The bottom panel shows amounts of 18S rRNA used to standardize signal levels according to lane loading parameters. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Analysis of isolates from patient C.
Table 3 shows results of fluconazole susceptibility testing for isolates recovered from patient C during three serial OPC episodes. Fifteen isolates recovered from each of the first two episodes showed a decreased fluconazole susceptibility (geometric mean of fluconazole MICs, 9.2 and 8.8 μg/ml, respectively, for isolates recovered from the first and second episodes), including the presence of isolates exhibiting dose-dependent susceptibility (fluconazole MIC, 16 μg/ml). Isolates recovered from the third episode showed a further decrease in fluconazole susceptibility (geometric mean of fluconazole MICs, 27.4 μg/ml) and included mostly resistant (isolate C.3.4; fluconazole MIC, 64 μg/ml) and dose-dependent susceptible (MICs, 16 to 32 μg/ml) isolates. However, a susceptible isolate was also present (isolate C.3.1; fluconazole MIC, 4 μg/ml).
TABLE 3.
Fluconazole MICs for multiple C. albicans isolates recovered from three serial episodes of OPC from patient C
| Isolate | Fluconazole MIC (μg/ml) |
|---|---|
| Episode 1 | |
| C.1.1 | 8 |
| C.1.2 | 8 |
| C.1.3 | 4 |
| C.1.4 | 8 |
| C.1.5 | 16 |
| C.1.6 | 8 |
| C.1.7 | 8 |
| C.1.8 | 8 |
| C.1.9 | 8 |
| C.1.10 | 16 |
| C.1.11 | 16 |
| C.1.12 | 4 |
| C.1.13 | 16 |
| C.1.14 | 16 |
| C.1.15 | 8 |
| Geometric mean | 9.2 |
| Episode 2 | |
| C.2.1 | 16 |
| C.2.2 | 16 |
| C.2.3 | 16 |
| C.2.4 | 4 |
| C.2.5 | 8 |
| C.2.6 | 16 |
| C.2.7 | 8 |
| C.2.8 | 8 |
| C.2.9 | 4 |
| C.2.10 | 4 |
| C.2.11 | 16 |
| C.2.12 | 8 |
| C.2.13 | 8 |
| C.2.14 | 8 |
| C.2.15 | 8 |
| Geometric mean | 8.8 |
| Episode 3 | |
| C.3.1 | 4 |
| C.3.2 | 32 |
| C.3.3 | 32 |
| C.3.4 | 64 |
| C.3.5 | 32 |
| C.3.6 | 16 |
| C.3.7 | 32 |
| C.3.8 | 32 |
| C.3.9 | 64 |
| Geometric mean | 27.4 |
Investigation of strain identity by DNA typing techniques demonstrated that all isolates were highly related, as determined by similar karyotype (although slight differences in mobility were detected in several isolates in this series), RFLP, and Ca3 fingerprinting patterns (Fig. 5). Thus, development of resistance in this series of isolates also occurred in a persistent strain.
FIG. 5.
Karyotype (A), RFLP analysis generated by digestion with SfiI of genomic DNA (B), and fingerprinting analysis with the moderately repetitive probe Ca3 (C) of representative C. albicans clinical isolates recovered from three OPC episodes from patient C. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
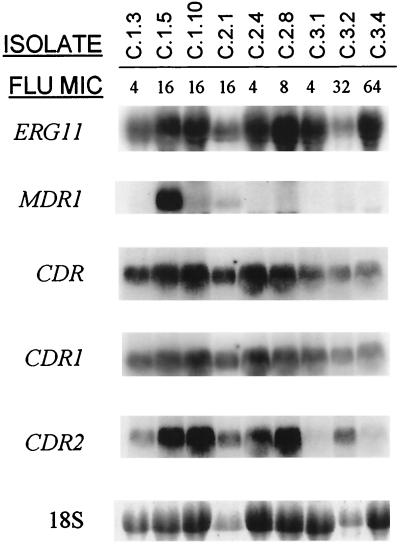
Analysis of expression of genes implicated in the development of fluconazole resistance is shown in Fig. 6. Dose-dependent susceptible isolates C.1.5 and C.1.10 (fluconazole MICs, 16 μg/ml) showed increased expression of CDR genes (especially CDR2) compared to that of the susceptible isolate C.1.3 (fluconazole MIC, 4 μg/ml) recovered at the same time during the first OPC episode. In the case of isolate C.1.5, but not C.1.10, this overexpression was accompanied by a strong expression of MDR1. In vitro MIC data for isolates recovered during the second episode closely mirrored expression of CDR genes, particularly CDR2. However, while levels of ERG11 expression remained quite constant along the series, isolate C.2.8 showed elevated levels of this gene along with overexpression of CDR. A great degree of heterogeneity was detected for isolates from the third OPC episode. As expected, the susceptible isolate C.3.1 (fluconazole MIC, 4 μg/ml) showed no elevated message for any of these genes. Isolate C.3.2 (fluconazole MIC, 32 μg/ml) showed overexpression of CDR2. However, the resistant isolate C.3.4 (fluconazole MIC, 64 μg/ml) showed no apparent overexpression of any of these genes, suggesting that an alternate mechanism or mechanisms may be responsible for its increased fluconazole resistance.
FIG. 6.
Northern blot analysis of total RNA from obtained from multiple C. albicans clinical isolates recovered from each of three serial episodes of OPC in patient C. The membranes were probed with ERG11, MDR1, a nonspecific probe for CDR genes, and probes specific for CDR1 and CDR2. The bottom panel shows amounts of 18S rRNA used to standardize signal levels according to lane loading parameters. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Analysis of isolates from patient D.
Eightfold or larger differences in fluconazole MIC values were detected within C. albicans isolates recovered at the same time in each of three episodes of OPC in patient D (Table 4). These results indicate coexistence of subpopulations of yeast with different sensitivities to azole derivatives. Overall, susceptibility tests showed decreasing fluconazole susceptibility with repetitive episodes, as revealed by the increase in the geometric mean of fluconazole MICs for isolates recovered from serial episodes (0.9 μg/ml for the initial episode in which all six isolates were susceptible to the drug versus 2.1 and 5.7 μg/ml for 12 and 6 isolates recovered during the second and third episodes, respectively).
TABLE 4.
Fluconazole MICs for multiple C. albicans isolates recovered from three serial episodes of OPC from patient D
| Isolate | Fluconazole MIC (μg/ml) |
|---|---|
| Episode 1 | |
| D.1.1 | 2 |
| D.1.2 | 0.5 |
| D.1.3 | 2 |
| D.1.4 | 2 |
| D.1.5 | 0.5 |
| D.1.6 | 0.25 |
| Geometric mean | 0.9 |
| Episode 2 | |
| D.2.1 | 16 |
| D.2.2 | 1 |
| D.2.3 | 8 |
| D.2.4 | 2 |
| D.2.5 | 2 |
| D.2.6 | 0.5 |
| D.2.7 | 8 |
| D.2.8 | 1 |
| D.2.9 | 2 |
| D.2.10 | 1 |
| D.2.11 | 1 |
| D.2.12 | 2 |
| Geometric mean | 2.1 |
| Episode 3 | |
| D.3.1 | 2 |
| D.3.2 | 8 |
| D.3.3 | 16 |
| D.3.4 | 16 |
| D.3.5 | 1 |
| D.3.6 | 8 |
| Geometric mean | 5.7 |
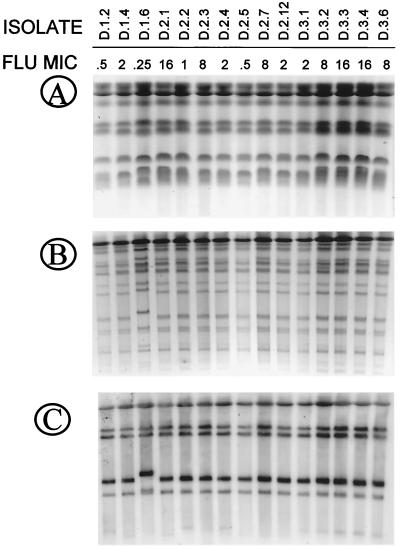
Once more, DNA strain typing techniques confirmed the high degree of relatedness among all isolates recovered from this patient (Fig. 7) and development of decreased susceptibility to fluconazole in a persistent strain. Minor differences in electrophoretic mobility were detected between different isolates. Also, differences were detected by RFLP and Ca3 probe fingerprinting for isolate D.1.6 that may represent a substrain or variant of the same strain.
FIG. 7.
Karyotype (A), RFLP analysis generated by digestion with SfiI of genomic DNA (B), and fingerprinting analysis with the moderately repetitive probe Ca3 (C) of representative C. albicans clinical isolates recovered from three OPC episodes from patient D. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Northern blot analysis of isolates from this patient (Fig. 8) revealed strong baseline expression of MDR1 and, to a lesser extent, CDR1 for isolate D.1.2, susceptible to fluconazole (MIC, 0.5 μg/ml). Isolate D.1.4 (fluconazole MIC, 2 μg/ml) showed a low level of expression of MDR1 and levels of CDR genes below the detection limits. In contrast, increased message for CDR1 only was detected in isolate D.1.6 (fluconazole MIC, 0.25 μg/ml). All isolates recovered during the second OPC episode (D.2.1, D.2.2, and D.2.3; fluconazole MICs, 16, 1, and 8 μg/ml, respectively) expressed MDR1, with increased expression correlating with increasing MICs. For these three isolates, levels of expression of CDR genes were below the detection limit. On the other hand, both dose-dependent susceptible isolates recovered during the third episodes (isolates D.3.3 and D.3.4; fluconazole MICs, 16 μg/ml) showed similar levels of overexpression of CDR genes, including CDR1 and CDR2, but negligible levels of MDR1. Analysis of isolate D.3.1 (fluconazole MIC, 2 μg/ml) revealed moderate levels of expression of MDR1 but negligible message for CDR genes. Levels of expression of ERG11 varied slightly throughout the series. Of notice, ERG11 mRNA was almost undetectable for isolate D.1.6 representing a strain variant. Overall, isolates from this patient showed dominance of MDR1 overexpression during initial episodes followed by higher levels of expression of CDR genes in the final episode.
FIG. 8.
Northern-blot analysis of total RNA from obtained from multiple C. albicans clinical isolates recovered from each of three serial episodes of OPC in patient D. The membranes were probed with ERG11, MDR1, a nonspecific probe for CDR genes, and probes specific for CDR1 and CDR2. The bottom panel shows amounts of 18S rRNA used to standardize signal levels according to lane loading parameters. Fluconazole (FLU) MICs are shown at the top (micrograms per milliliter).
Antifungal susceptibility testing against a panel of antifungal drugs.
Antifungal susceptibility testing against amphotericin B, itraconazole, ketoconazole, voriconazole, SCH 56592, and terbinafine was performed according to NCCLS method M-27A by using a broth microdilution technique and reading of endpoints at 48 h. Table 5 shows MICs of the different antifungal agents for the 36 isolates (3 isolates per episode from the four different patients) included in the study of gene expression. Two laboratory strains were used as controls. Isolates were all susceptible to amphotericin B (48-h MIC range, 0.125 to 0.25 μg/ml). Levels of susceptibility to other azole derivatives varied among the different isolates and often paralleled increases in MICs of fluconazole, especially in those isolates demonstrating overexpression of CDR genes. Although several isolates displayed somewhat decreased susceptibilities to a given azole derivative, the MICs obtained were not high enough to be considered resistant according to currently accepted interpretative breakpoints (13, 22). Interpretative criteria have not been defined for the investigational azole derivatives voriconazole and SCH 56592. The ranges of MICs of terbinafine were 0.5 to >2 μg/ml.
TABLE 5.
Antifungal susceptibilities of multiple C. albicans isolates from each of three episodes of OPC from four HIV-infected patientsa
| Patient isolate | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Fluconazoleb | Itraconazole | Ketoconazole | Voriconazole | SCH 56592 | Amphotericin B | Terbinafine | |
| A | |||||||
| A.1.1 | 0.5 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| A.1.4 | 0.5 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| A.1.7 | 0.5 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| A.2.1 | 128 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| A.2.2 | 8 | 0.125 | 0.25 | <0.125 | 0.125 | 0.25 | >2 |
| A.2.3 | 8 | 0.125 | 0.25 | 0.25 | 0.125 | 0.25 | >2 |
| A.3.1 | 128 | 0.125 | 0.25 | <0.125 | 0.125 | 0.25 | >2 |
| A.3.2 | 32 | 0.125 | 0.25 | 0.25 | 0.125 | 0.25 | >2 |
| A.3.3 | 16 | <0.015 | 0.06 | <0.125 | <0.015 | 0.125 | >2 |
| B | |||||||
| B.1.1 | 0.25 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| B.1.2 | 0.5 | <0.015 | 0.06 | <0.125 | <0.015 | 0.125 | >2 |
| B.1.5 | 4 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| B.2.2 | 8 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| B.2.4 | 4 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| B.2.5 | 8 | 0.06 | 0.125 | <0.125 | 0.06 | 0.25 | >2 |
| B.3.1 | 16 | 0.06 | 0.125 | <0.125 | 0.06 | 0.25 | >2 |
| B.3.4 | 4 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | >2 |
| B.3.5 | 16 | 0.06 | 0.25 | <0.125 | 0.06 | 0.25 | >2 |
| C | |||||||
| C.1.3 | 4 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| C.1.5 | 16 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 | >2 |
| C.1.10 | 16 | 0.125 | 0.25 | <0.125 | 0.06 | 0.25 | >2 |
| C.2.1 | 16 | 0.06 | 0.25 | <0.125 | 0.06 | 0.25 | >2 |
| C.2.4 | 4 | <0.015 | 0.25 | <0.125 | 0.06 | 0.25 | >2 |
| C.2.8 | 8 | 0.125 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| C.3.1 | 4 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| C.3.2 | 32 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | >2 |
| C.3.4 | 64 | <0.015 | 0.25 | <0.125 | 0.06 | 0.25 | >2 |
| D | |||||||
| D.1.2 | 0.5 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | 2 |
| D.1.4 | 2 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | 0.5 |
| D.1.6 | 0.25 | <0.015 | 0.06 | <0.125 | <0.015 | 0.25 | 1 |
| D.2.1 | 16 | <0.015 | 0.25 | 0.25 | 0.03 | 0.25 | 1 |
| D.2.2 | 1 | <0.015 | 0.06 | <0.125 | <0.015 | 0.125 | 2 |
| D.2.3 | 8 | <0.015 | 0.25 | <0.125 | <0.015 | 0.25 | 1 |
| D.3.1 | 2 | <0.015 | 0.25 | <0.125 | <0.015 | 0.125 | >2 |
| D.3.3 | 16 | 0.125 | 0.25 | 0.25 | 0.125 | 0.125 | >2 |
| D.3.4 | 16 | 0.125 | 0.25 | 0.25 | 0.125 | 0.125 | >2 |
Susceptibility was tested with the panel of antifungal drugs shown by a broth microdilution method and by reading of endpoints at 48 h, except as noted.
Fluconazole results are from a broth macrodilution method.
DISCUSSION
Recent studies have demonstrated the multifactorial nature of resistance. Antifungal resistance may result from replacement of a susceptible strain by a more resistant isolate or by the development of resistance in the original strain mediated by multiple mechanisms at the molecular level. The use of a novel agar dilution screening technique developed by our group (14, 15) increases detection of subpopulations of yeasts at the time of initial isolation based on the different susceptibilities of individual colonies to the antifungal agent, thus allowing a comprehensive assessment of the epidemiology of resistance. By using this technique for initial sampling of oral rinses and swabs, it was obvious that different colonies present in the plates differed in their susceptibilities to the antifungal agent, confirming previous results by our group and others (14, 15, 30, 31). These differences were further confirmed by susceptibility testing by the NCCLS standard methodology (Tables 1 to 4). Susceptibility tests revealed 8-fold or larger (up to 32-fold) differences in fluconazole MICs for isolates recovered at the same time point in 7 of 12 OPC episodes in four patients. In general, discrepancies up to two tube dilutions are considered “acceptable” when performing standardized antifungal susceptibility testing according to NCCLS methodologies (16). Thus, eightfold or larger differences should be considered highly significant.
Investigation of strain identity by a combination of DNA typing techniques revealed that in all four patients studied, development of resistance occurred in a single, persistent strain. Of note, in patient A, a highly resistant isolate recovered during the second episode (isolate A.2.1) showed karyotyping and RFLP patterns different from those of all other isolates from the same patient (Fig. 1A and B). However, the fact that this isolate displayed exactly the same Ca3 fingerprinting pattern as those of all other isolates (Fig. 1C) suggests that it may represent a substrain or a strain variant rather than a completely different strain of C. albicans. One would expect that this resistant substrain should be selected over the more prevalent but less resistant population of yeasts, as has been described previously (38). However, representatives of this substrain were not further recovered, but rather development of high levels of resistance occurred in the more prevalent (but more susceptible) strain. This could be an indication that development of resistance may not always confer an ecological advantage, as suggested by the fact that some highly resistant isolates demonstrate decreased virulence in vivo (5).
We have previously demonstrated a high degree of complexity in the molecular mechanisms responsible for the development of fluconazole resistance with five distinct patterns of gene expression associated with the development of fluconazole resistance in serial C. albicans isolates from five different HIV-infected patients with OPC (10). The present study provides evidence for an additional level of complexity in the molecular mechanisms of fluconazole resistance, as demonstrated by differences in expression of genes implicated in the development of fluconazole resistance (ERG11, MDR1, CDR1, and CDR2) within isolates recovered at the same time point from the same OPC episode and from the same patient. This analysis revealed that isolates obtained from the same patient and episode were heterogeneous in their patterns of expression of these genes. As shown in Table 5, in general, high levels of mRNA for CDR genes correlated with decreased susceptibilities to other azole derivatives (i.e., isolates A.3.1 and A.3.2 from patient A). Isolates D.2.1 and D.2.3 showed overexpression of MDR1 only, and although their susceptibilities to itraconazole and SCH 56592 were unchanged (as expected, since fluconazole is the only substrate for Mdr1p), the MICs of ketoconazole (both isolates) and voriconazole (isolate D.2.1 only) for them were slightly elevated. Thus, since the present study is limited to the study of expression of these set of genes implicated in the development of fluconazole resistance, and since levels of gene expression did not always parallel the MICs, it should be noted that other mechanisms (changes in the ergosterol biosynthesis pathway, point mutations in the gene coding for the target enzyme for azole derivatives, and other yet uncharacterized resistance mechanisms) may be operational in these series of isolates, which may contribute to the overall decrease in susceptibility.
It is not clear how this high degree of phenotypic heterogeneity originates in an apparently homogeneous population of yeasts (as indicated by typing techniques). In the case of genotypic microheterogeneities described by some authors, the most likely explanation was that these microheterogeneities arise due to physical or functional separation of two populations (8). In this regard, although the oral cavity is many times considered a uniform environment, in truth, its great anatomical diversity results in the presence of several habitats, each of them characterized by different physicochemical properties (11). Thus, subpopulations of yeast in each of these microniches may evolve differently not only genotypically, but also phenotypically in trying to adapt to a particular microhabitat. Also unknown are the factors (related to the organism, the host, and the environment) affecting patterns of gene expression associated with resistance. For example, levels of antifungal drug attained in each of these diverse microniches in the oral cavity may be different and may result in different degrees of antifungal pressure and ultimately lead to microheterogeneity in patterns of expression of genes associated with development of resistance, as observed in this study. Other factors that could influence gene expression include host defense mechanisms and the microbiota occupying the same ecological niche (11).
Overall, this report shows that C. albicans isolates obtained from the same patient and episode were phenotypically heterogeneous in their susceptibilities to fluconazole and in their patterns of expression of certain genes involved in resistance to this antifungal agent. These results further demonstrate the complexity of the distribution of the molecular mechanisms of antifungal drug resistance and indicate that different subpopulations of yeasts may coexist at a given time in the oral cavity of the same patient and may develop resistance through different mechanisms.
ACKNOWLEDGMENTS
This work was supported by a grant from Pfizer, Inc., and by Public Health Service grants 1 R29 AI42401 (to J.L.L.-R.), 1 R01 DE11381 (to T.F.P.), and M01-RR-01346 for the Frederic C. Bartter General Clinical Research Center.
Chromogenic medium was provided by CHROMagar Company (Paris, France). We thank the Fungus Testing Laboratory at The University of Texas Health Science Center at San Antonio for performing antifungal susceptibility testing.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challacombe S J. Immunological aspects of candidiasis. Med Oral Pathol. 1994;78:202–210. doi: 10.1016/0030-4220(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 3.Fling M E, Kopf J, Tamrkin A, Gorman J A, Smith H A, Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991;227:318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- 4.Franz R, Kelly S L, Lamb D C, Kelly D E, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybill J R, Montalbo E, Kirkpatrick W R, Luther M F, Revankar S G, Patterson T F. Fluconazole versus Candida albicans: a complex relationship. Antimicrob Agents Chemother. 1998;42:2938–2942. doi: 10.1128/aac.42.11.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R S, Carol A H, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–3588. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 7.Law D, Moore C B, Wardle H M, Ganguli L A, Keaney M G L, Denning D W. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J Antimicrob Chemother. 1994;34:659–668. doi: 10.1093/jac/34.5.659. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart S R, Fritch J J, Meier A S, Schröppel K, Srikantha T, Galask R, Soll D R. Colonizing populations of Candida albicans are clonal in origin but undergo microevolution through C1 fragment reorganization as demonstrated by DNA fingerprinting and C1 sequencing. J Clin Microbiol. 1995;33:1501–1509. doi: 10.1128/jcm.33.6.1501-1509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löffler J, Kelly S L, Hebart H, Schumacher U, Lass-Flörl C, Einsele H. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol Lett. 1997;151:263–268. doi: 10.1111/j.1574-6968.1997.tb12580.x. [DOI] [PubMed] [Google Scholar]
- 10.López-Ribot J L, McAtee R K, Lee L N, Kirkpatrick W R, White T C, Sanglard D, Patterson T F. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcotte H, Lavoie M C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meunier F. Candidiasis. Eur J Clin Microbiol Infect Dis. 1989;8:438–447. doi: 10.1007/BF01964058. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Patterson T F, Kirkpatrick W R, Revankar S G, McAtee R K, Fothergill A W, McCarthy D I, Rinaldi M G. Comparative evaluation of macrodilution and chromogenic agar screening for determining fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1996;34:3237–3239. doi: 10.1128/jcm.34.12.3237-3239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson T F, Revankar S G, Kirkpatrick W R, Dib O, Fothergill A W, Redding S W, Sutton D A, Rinaldi M G. Simple method for detecting fluconazole-resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–1797. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical implications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 17.Plettenberg A, Reisinger E, Lenzner U, Listemann H, Ernst M, Kern P, Dietrich M, Meigel W. Oral candidosis in HIV-infected patients. Prognostic value and correlation with immunological parameters. Mycoses. 1990;33:421–425. doi: 10.1111/myc.1990.33.9-10.421. [DOI] [PubMed] [Google Scholar]
- 18.Powderly W G. Resistant candidiasis. AIDS Res Hum Retroviruses. 1994;10:925–929. doi: 10.1089/aid.1994.10.925. [DOI] [PubMed] [Google Scholar]
- 19.Prasad R, De Wergifosse W P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 20.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, McGough D A, Rinaldi M G, Patterson T F. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in HIV-infected patients. J Infect Dis. 1996;174:821–827. doi: 10.1093/infdis/174.4.821. [DOI] [PubMed] [Google Scholar]
- 21.Revankar S G, Kirkpatrick W R, McAtee R K, Dib O P, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in human immunodeficiency virus-infected patients: clinical outcomes and development of fluconazole resistance. Am J Med. 1998;105:7–10. doi: 10.1016/s0002-9343(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 22.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Berry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J F, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 28.Sanglard D, Kuchler K, Ischer F, Pagani J-L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid J, Voss E, Soll D R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990;28:1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoofs A, Odds F C, Colebunders R, Ieven M, Wouters L, Goossens H. Isolation of Candida species on media with and without added fluconazole reveals high variability in relative growth susceptibility phenotypes. Antimicrob Agents Chemother. 1997;41:1625–1635. doi: 10.1128/aac.41.8.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takasuka T, Baily G G, Birch M, Anderson M J, Law D, Denning D W. Variation in morphotype, karyotype and DNA type of fluconazole resistant Candida albicans from an AIDS patient. J Infect. 1998;36:57–62. doi: 10.1016/s0163-4453(98)93162-0. [DOI] [PubMed] [Google Scholar]
- 32.Vanden Bossche H, Marichal P, Odds F C. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 1994;2:393–400. doi: 10.1016/0966-842x(94)90618-1. [DOI] [PubMed] [Google Scholar]
- 33.Vanden Bossche H, Marichal P, Gorrens J, Bellens D, Moereels H, Janssen P A J. Mutation in cytocrome P450-dependent 14α demethylase results in decreased affinity for azole antifungals. Biochem Soc Trans. 1990;18:56–59. doi: 10.1042/bst0180056. [DOI] [PubMed] [Google Scholar]
- 34.Walsh T J, Kasai M, Francesconi A, Landsman D, Chanock J. New evidence that Candida albicans possesses additional ATP-binding cassette MDR-like genes: implications for antifungal azole resistance. J Med Vet Mycol. 1997;35:133–137. [PubMed] [Google Scholar]
- 35.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White T C, Pfaller M A, Rinaldi R G, Smith J, Redding S W. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 1997;3:S102–S109. doi: 10.1111/j.1601-0825.1997.tb00336.x. [DOI] [PubMed] [Google Scholar]