Abstract
Glucuronoyl esterases (GEs) are α/β serine hydrolases and a relatively new addition in the toolbox to reduce the recalcitrance of lignocellulose, the biggest obstacle in cost-effective utilization of this important renewable resource. While biochemical and structural characterization of GEs have progressed greatly recently, there have yet been no mechanistic studies shedding light onto the rate-limiting steps relevant for biomass conversion. The bacterial GE OtCE15A possesses a classical yet distinctive catalytic machinery, with easily identifiable catalytic Ser/His completed by two acidic residues (Glu and Asp) rather than one as in the classical triad, and an Arg side chain participating in the oxyanion hole. By QM/MM calculations, we identified deacylation as the decisive step in catalysis, and quantified the role of Asp, Glu and Arg, showing the latter to be particularly important. The results agree well with experimental and structural data. We further calculated the free-energy barrier of post-catalysis dissociation from a complex natural substrate, suggesting that in industrial settings non-catalytic processes may constitute the rate-limiting step, and pointing to future directions for enzyme engineering in biomass utilization.
Subject terms: Enzyme mechanisms, Hydrolases, X-ray crystallography, Biocatalysis, Computational chemistry
Zong and coworkers combine computational and experimental methods to decipher in detail the mechanism of action of glucuronoyl esterases, enzymes with significant biotechnological potential for decoupling lignin from polysaccharides in biomass.
Introduction
Lignocellulose is an important resource, for instance as feedstock for production of clean and renewable energy carriers, such as bioethanol, and furthermore both lignin and polysaccharides are potential sources of building blocks for materials and fine chemicals, alternative to the ones derived from fossil resources1,2. Lignocellulose is, however, an extremely complex and heterogeneous substrate, and is highly recalcitrant to enzymatic and chemical deconstruction3. A substantial obstacle is the chemical linkages between lignin and carbohydrates, the so-called lignin carbohydrate complex (LCC)4. Lignin is often an obstacle in lignocellulose processing and its removal has been regarded as the most expensive step for producing ethanol from plant biomass in industry, suggesting that recalcitrance is the uppermost cost factor5,6. Decreasing lignocellulosic recalcitrance and enhancing its degradability are, therefore, of paramount importance to lower the cost of bioconversion. Furthermore, lignin itself is a valuable biomass component with many possible applications. Thus, improving separation of polysaccharide and lignin fractions will additionally favor lignin valorization2.
Glucuronoyl esterases (GEs, EC number 3.1.1.117 at https://www.brenda-enzymes.org/) from carbohydrate esterase family 15 (CE15) in the carbohydrate-active enzymes database (CAZy; www.cazy.org)7 have been reported to attack small model substrates since their first discovery8 and are believed to attack lignin–carbohydrate ester bonds in LCCs9. To date, several fungal and bacterial CE15 enzyme structures have been determined9–15. Their biochemical features have been characterized using model substrates, such as alkyl and aryl alcohols of varying complexity ester linked to glucuronic acid (GlcA) or 4-O-methylated GlcA9–11,16–28, and in some cases with more natural ones, such as extracted LCC fractions29–32. Native LCC ester bonds are found between GlcA moieties branching from xylan polysaccharides and lignin moieties in the plant cell wall. Their exact nature has been heavily debated, with much discussion as to whether benzyl (α) esters and/or γ-esters are represented in native lignin, or if different species can be produced during extraction and pre-treatment33–35. However, it has been clearly shown that plant cell wall recalcitrance is greatly reduced in Arabidopsis mutants devoid of GlcA, further compounding the importance of ester linkages to GlcA as a main interface between xylan and lignin36.
Regardless of the nature of native esters in the plant LCC, synergy between GEs and depolymerizing enzymes in biomass degradation is well documented. In 2016, the first example for GE–cellulase synergy in degrading lignocellulosic biomass was reported by d’Errico et al.19. More sugars were found to be released from corn fiber in the presence of the fungal GE from Cerrena unicolor, compared to using commercial cellulases alone. In the study of Arnling Bååth et al., direct evidence of cleavage of native lignin–carbohydrate ester bonds was provided using the GE originating from Acremonium alcalophilum29. Furthermore, the bioconversion of ball-milled corncob by the commercial enzyme cocktail Ultraflo® was substantially increased when supplemented with different GE enzymes indicating an important role for the enzymes in synergistically enhancing biomass degradation9. Recent work has also shown that GEs can synergistically act together with α-glucuronidases to boost release of 4-O-methylated GlcA from native LCC fractions32.
CE15 GEs belong to the α/β hydrolase (ABH) superfamily and possess a Ser–His–Glu/Asp catalytic triad like many well-studied serine-type hydrolases9,10,37–39. In several fungal GEs, which were the first characterized members of the family12,13, the position of the catalytic acid is at the end of β-strand 6 (β6), rather than at the end of β7 as in classical ABHs37 (here referred to as canonical position). The canonical position of the acid has however been identified in several bacterial GEs studied since11,14. ABHs, comprising several industrially and biomedically important enzymes, have been extensively investigated. However, detailed mechanistic studies on GEs have not yet been carried out. Furthermore, the GE from Opitutus terrae (OtCE15A) is unusual, possessing an acidic residue at the end of β6 in addition to the classical triad, in other words acidic residues both at the end of β6 (non-canonical position) and β7 (canonical position). In addition to the fundamental interest for the function of this important and widespread enzyme class, a better understanding of the GE mechanism is also of high societal importance. Further studies on variations of the classical triad are important in inspiring the engineering of enzymes and synthetic enzyme mimics in a variety of contexts40. A deeper understanding of the possible rate-determining step for GEs degrading their complex natural substrate will also be extremely valuable in protein engineering efforts devoted to improving their industrial potential.
As a classical ABH, the reaction catalyzed by OtCE15A was assumed to proceed through a covalent intermediate preceded by an acylation and followed by a deacylation step, both going through a tetrahedral transition state. We then explored this canonical carboxyl esterase mechanism41 through quantum mechanics/molecular mechanics (QM/MM) simulations. A free energy method was additionally employed to investigate dissociation to infer the rate-limiting step for GE degradation of lignocellulose. To support the in silico results, we also performed additional biochemical and structural studies. The present work provides new fundamental understanding of catalytic triad variations in serine hydrolases and will be of great use for further development of these industrially important enzymes.
Results
Initial choice of a minimal active site for mechanistic studies
The catalytic site of ABHs, including GEs, is commonly constituted by residues Ser–His–Glu/Asp in a catalytic triad — the essential catalytic functional unit9,10,12,42. As for other ABHs, the nucleophilic Ser and general acid–base His are expected to be directly involved in the catalytic reaction (Fig. 1)43,44, and accordingly the S267A variant was severely impaired with a large decrease in turnover rate (kcat)10. The reaction is presumed to proceed through a covalent intermediate (CI), which has been experimentally demonstrated for the OtCE15A H408A variant.
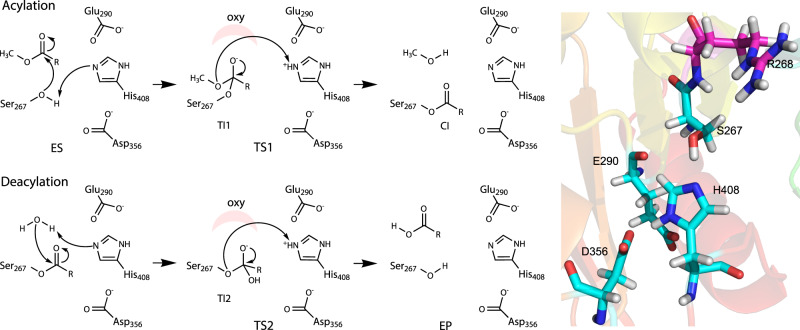
Fig. 1. Catalytic mechanism corresponding to the hydrolysis of a glucuronic acid methyl ester (with the sugar moiety shown as R) by OtCE15A.
ES and EP denote non-covalent enzyme–substrate and enzyme–product complexes, respectively. TS1 and TS2 denote transition states in acylation and deacylation, respectively, and CI represents the covalent intermediate. TI1 and TI2 denote the tetrahedral intermediates in acylation and deacylation, respectively. Oxy represents the oxyanion hole. Glu290 and Asp356 correspond to the non-canonical and canonical positions, respectively, for the acidic residue in the ABH triad, both present in OtCE15A. A 3D view of the active site is presented on the right (PDB 6SYR).
Although the acidic residues in ABH catalytic triads are not involved in the catalytic reaction directly, they are reported to stabilize the transition states, for example in acetylcholine esterase, by stabilizing the histidine charge during the reaction43,44. As described above, in contrast to other studied GEs, OtCE15A features acidic residues at both the canonical and non-canonical positions, i.e., Glu290 and Asp35610,15. The formation of the CI (acylation step) and breakdown of the CI to release the final product (deacylation) both go through formation of a tetrahedral transition state with a charge on the oxygen stabilized by the so-called oxyanion hole42. In many ABHs, this is formed solely by backbone nitrogen atoms from the enzyme, but for bacterial GEs, the side chain guanidinium moiety of the Arg immediately following the Ser nucleophile (Arg268 in OtCE15A) has been proposed to play this role10,11. As a starting point in this investigation, we focus on Ser267, His408 and Glu290 (the non-canonical position and the position found in the first characterized GEs) as the minimal set of active site residues needed to investigate the catalytic mechanism of OtCE15A, and, as such, the side chains of these residues and a small model substrate were included in the QM region considered (SHE).
Acylation
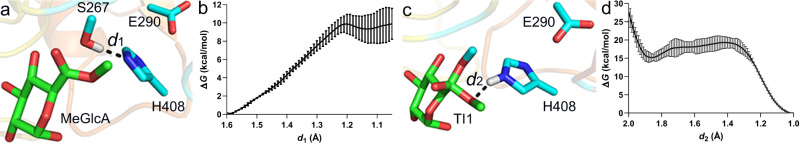
As shown in Fig. 2a, a GlcA methyl ester (MeGlcA) was constructed based on the complex of OtCE15A and GlcA obtained in our previous work (PDB 6SYR)10, and used as the initial structure to investigate the acylation reaction. The transition coordinates (TCs) characterizing the reaction pathways in acetylcholine esterases have been investigated by Zhang et al.43 and Nemukhin et al.44 in detail, using a combined coordinates approach. A single TC has been adopted by Yan et al.45 and Zong et al.46 to investigate the mechanism of proton transfer in the glycosylation reaction of the Trichoderma reesei Cel7A cellulase successfully. In the present work, on the assumption that the catalytic reaction starts from proton transfer, we employed two individual distances as the TCs to investigate the two proton transfer processes involved in the acylation and deacylation steps. Two factors were taken into account. Firstly, although a single TC, such as d1 in Fig. 2a, was used to describe the first proton transfer process, another important process—nucleophilic attack, which is highly coupled with the first process, was found to be well sampled in our well-tempered variant of meta-eABF (WTM-eABF) simulation (as discussed below), indicating that using the individual distance as the TC is justified. Secondly, the free-energy barrier of each process in acylation and deacylation can be obtained by this approach, helping us find out the rate-limiting steps in the whole reaction and in each step.
Fig. 2. Processes of acylation with the minimal active site assembly.
a Initial structure of OtCE15A and MeGlcA constructed based on in silico esterification of GlcA complex of OtCE15A (based on PDB 6SYR). c Relevant TI1 state to describe the reaction in acylation. b, d Corresponding relevant free-energy profiles generated from QM/MM simulations. Carbon atoms of MeGlcA and the catalytic triad, i.e., Ser267, Glu290, and His408 in the QM area are shown in green and cyan, respectively. For clarity, some hydrogen atoms in the QM area, the counterions and water molecules are not shown, here and in the forthcoming figures. There are a total of 48 atoms in the QM area, and the charge is −1. d1 denotes the distance between the hydroxyl proton of Ser267 and Nε of His408. d2 denotes the distance between the proton of the Nε of His408 and O of the ester in MeGlcA. Data are presented as mean values ± the standard error inferred from three independent runs. Source data for b, d are provided as a Source Data file.
Here, the distance between the hydroxyl proton of Ser267 and Nε in His408, d1, was used as the TC to describe the first proton transfer process, along which the potential of mean force (PMF) describing this process was determined over three independent runs. In our simulations, the proton transfer was found to be accompanied by the nucleophilic attack (Supplementary Movie 1 and Supplementary Fig. 1a). As depicted in Fig. 2b, the free-energy barrier against this first process is ~10 kcal/mol. Furthermore, three distances, i.e., d1, Ser267 nucleophilic O—carbonyl C in MeGlcA, and methanol group O—carbonyl C in MeGlcA were analyzed to monitor the processes of proton transfer and nucleophilic attack (Supplementary Fig. 1a). The result indicates that proton transfer and nucleophilic attack occur simultaneously to form the TI1. In the next step of acylation, the proton on Nε His408 donated by Ser267 will finally transfer to the O atom of the methanol group in MeGlcA. The distance between His408 Nε and O in MeGlcA, d2, was employed to describe this process (Fig. 2c). As depicted in Fig. 2d, there is no more than 4 kcal/mol barrier for the proton transferring to create CI and leaving group (LG) (Supplementary Movie 2). Corresponding distances were analyzed (Supplementary Fig. 1b), showing that the LG and CI form successfully. Overall, the first process, i.e., the proton transfer from Ser267 to His408 is the key process for acylation in energetic terms.
Priming for deacylation
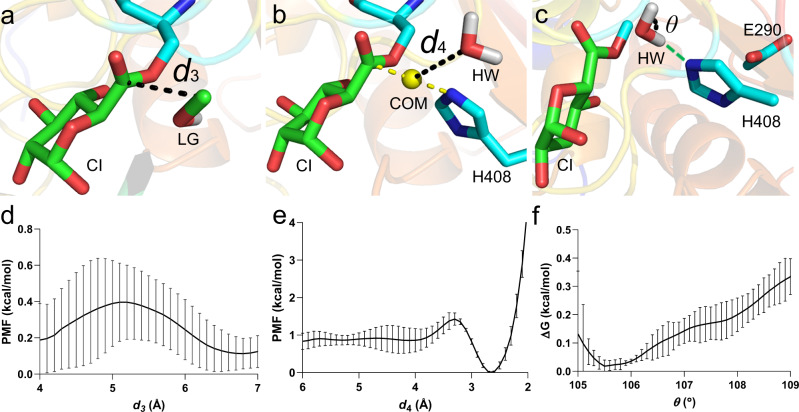
As illustrated in Fig. 3a, a CI complex in silico was obtained from the trajectory of the third process in acylation. We have previously obtained an experimental CI structure for a H408A variant of OtCE15A (PDB 6SZ4)10, aligning well with our computationally obtained structure (Supplementary Fig. 2). After the acylation reaction, the LG will leave the active site, creating sufficient space for a hydrolytic water (HW) molecule to attack the CI. The d3 coordinate, which measures the distance between LG C and CI C atoms, was, therefore, employed to describe this process of methanol group leaving the active site (Fig. 3a), along which the PMF was determined. Figure 3d indicates that the LG leaves the catalytic site with a negligible free energy transfer barrier.
Fig. 3. Processes before deacylation with the minimal active site assembly.
a–c Initial structure of CI with a the LG in the catalytic site, b the water molecule (HW) near the catalytic site, and c HW now moved into the catalytic site (hydrogen bond formed with Nε of His408 is shown in green dotted line). d–f Corresponding PMFs calculated along reaction coordinates d3, d4, and θ, respectively. d3 denotes the distance between the C of CI and C in LG. d4 denotes the distance between the HW O atom and the yellow sphere, i.e., the center of mass (COM) of C in CI and Nε in His408. θ denotes the bond angle in HW. There are a total 45 atoms in the QM area for the angle-change calculation, and the charge is −1. Data are presented as mean values ± the standard error inferred from three independent runs. Source data for d–f are provided as a Source Data file.
After the methanol group leaves, the HW molecule will undergo two processes: (i) approach, i.e., the HW enters the active site region from the LG side, arrives at the catalytic position and forms a hydrogen bond with Nε of His408; (ii) angle change, i.e., its bond angle changes from approximately 105° to 109°42. In the crystal structure of CI (PDB 6SZ4), a structural water (Wat 704 in the PDB file) was located at the LG side (Supplementary Fig. 2). In our computational simulations, the water molecule nearest to the one in the crystal structure was thus selected and used for the following calculation. The PMF describing the approach process by the TC d4, i.e., the distance between the water O atom and the center of mass (COM) of CI C and Nε in His408 (Fig. 3b), was determined. As shown in Fig. 3e, the HW molecule was found to approach the catalytic region at a 0.6 kcal/mol energetic cost, and stabilizes the complex, mainly owing to the hydrogen bond formed with Nε of His408 (Fig. 3c).
After analyzing the simulation trajectory for the approach step, the structure corresponding to the minimum of the PMF in Fig. 3e was used as the starting model in the following QM/MM calculations for the angle change process (Fig. 3c). Figure 3f indicates that the HW molecule surmounts ~0.3 kcal/mol barrier to complete its angle change to 109°. In view of the marginal free-energy costs against the three processes of priming the active site for deacylation, we suggest that once the CI is formed, the enzyme will expel the LG and prepare the HW molecule quickly, getting ready for the next step—deacylation.
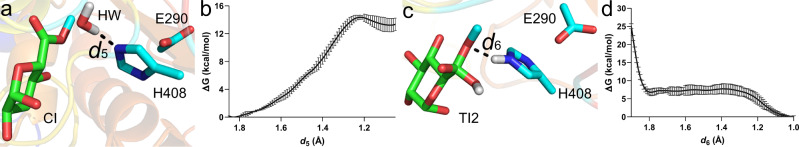
Deacylation reaction
As depicted in Fig. 4a, the complex between the CI and HW, obtained from the aforementioned trajectory of QM/MM simulation, was employed as the initial structure to investigate the deacylation reaction. Here, d5, namely the distance between the HW proton–Nε in His408, was employed to describe the initial proton transfer, along which the PMF was determined. In our simulations, the processes of initial proton transfer from the water molecule and the nucleophilic attack of HW on CI were found to be concerted (Supplementary Movie 3). As shown in Fig. 4b, the barrier against the first proton transfer is 14.4 kcal/mol, which is higher than for acylation and indicates that deacylation is the rate-limiting step, as previously indicated by similar kcat of OtCE15A against a variety of substrates with different LGs10. The TC d6, denoting the distance between the proton of Nε His408 and O in CI was used to reveal the process of the final proton transfer (Fig. 4c). As illustrated in Fig. 4d, the determined PMF indicates that the energy is very favorable for this process (Supplementary Movie 4). In deacylation, the initial proton transfer from HW to His408 was thus considered as the dominant process.
Fig. 4. Processes of deacylation with the minimal active site assembly.
a, c Initial structure of CI with (a) the HW and (c) the relevant TI2 state to describe the deacylation reaction; b, d Relevant free-energy profiles generated from QM/MM simulations. There are 45 atoms in the QM area, and the total charge is −1. d5 denotes the distance between the proton of HW molecule and Nε in His408. d6 denotes the distance between the proton of Nε His408 and O in CI. Data are presented as mean values ± the standard error inferred from three independent runs. Source data for b, d are provided as a Source Data file.
Finding the optimal QM region by including additional residues
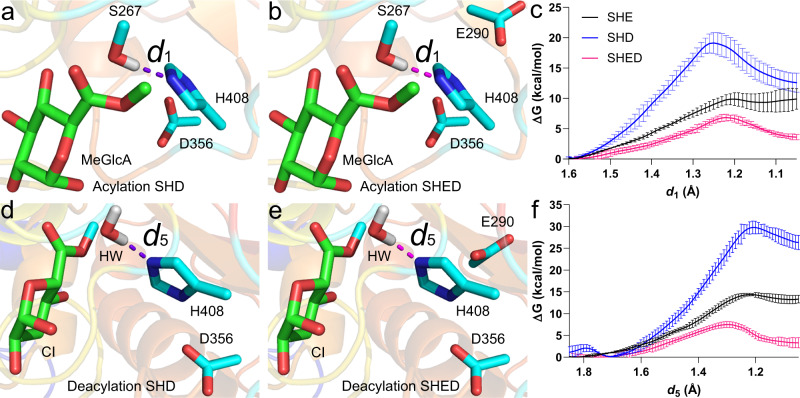
Since OtCE15A has two acidic residues in the active site, we also investigated Ser–His–Asp (SHD) and Ser–His–Glu–Asp (SHED) in addition to the Ser–His–Glu catalytic assembly (investigated above, SHE), to determine how important their inclusion in the QM region is to the calculated barriers. As shown in Fig. 5a, d, each QM area of SHD/SHE includes only one acidic residue, while SHED includes both (Fig. 5b, e). As depicted in Fig. 5c, f, the barriers of SHD are higher than that of SHE and SHED both in acylation and deacylation, while the barriers of SHED are the lowest (see Table 1) and quite close to each other for each step. Deacylation remains in all cases the rate-limiting step, as suggested experimentally by the similar kcat of WT with different substrates10. Interestingly, during acylation (Supplementary Fig. 3) the H408 Nδ1-H temporarily transfers to D356 Oδ1, aiding proton abstraction from S267.
Fig. 5. Initial proton transfers in the acylation and deacylation steps with the optimal SHED QM region.
a–c Acylation step. d–f Deacylation step. In (a, d), QM areas of SHD include only one acidic residue for the calculations of acylation and deacylation, respectively. In (b) and (e), QM areas of SHED comprise two acidic residues for the calculations of acylation and deacylation, respectively. (c, f) show the relevant free-energy profiles generated from QM/MM simulations. The corresponding results of SHE (black lines) are shown for reference. Data are presented as mean values ± the standard error inferred from three independent runs. Source data for c, f are provided as a Source Data file.
Table 1.
ΔG (kcal/mol) against initial proton transfers in acylation and deacylation of WT OtCE15A and OtCE15A–D356A, –E290A, and –R268A variants with the MeGlcA substrate using units of SHE, SHD, and SHED, respectively.
| WT-SHE | WT-SHD | WT-SHED | D356A-SHE | E290A-SHD | R268A-SHED | |
|---|---|---|---|---|---|---|
| Acylation | 10.0 ± 1.1 | 19.1 ± 1.7 | 6.8 ± 0.6 | 9.3 ± 0.3 | 19.2 ± 1.7 | 9.6 ± 0.8 |
| Deacylation | 14.4 ± 0.3 | 29.8 ± 1.5 | 7.5 ± 0.7 | 18.4 ± 0.4 | 32.0 ± 0.7 | >110 |
The given error is the standard error inferred from three independent runs (see Figs. 2, 4 and 5). Source data are provided as a Source Data file (see individual figures)*.
*QM area of WT/D356A-SHE and WT/E290A-SHD includes one acidic residue, i.e., Glu290 and Asp356, respectively (total charge: −1), while SHED consists of two acidic residues—Glu290 and Asp356 (total charge: −2). The QM atoms for WT/D356A-SHE, WT/E290A-SHD, and SHED are 48, 48, and 54 in acylation, and 45, 45, and 51 in deacylation, respectively.
We also attempted to introduce the R268 side chain, the proposed oxyanion hole residue, in the QM region. In this simulation, we found that the proton in Arg268 transferred to the carbonyl group of the substrate (Supplementary Fig. 4a). However, the expected back-transfer of this proton does not occur during simulation time, obstructing further progress of the reaction, with sharp free-energy increase (Supplementary Fig. 4b). We conclude that a reaction itinerary involving at least five potentially key players such as in the SHEDR assembly cannot be adequately handled with the given methodology. Full description of complex, multidimensional reaction itineraries, without presumption of reaction coordinates, would require prohibitively costly computational strategies. Thus, we continued our investigations with the SHED assembly as best realistic choice.
Determination of the experimental Ea by Arrhenius analysis
In order to obtain an additional term of comparison for our calculations, we determined the experimental activation energy of the overall reaction with the small substrate benzyl GlcA (BnzGlcA) by determining kcat at different temperatures. Ea was determined as 4.03 ± 0.5 kcal/mol (Fig. 6). Considering the differences in the experimental and computational set ups, we find it encouraging that this is within 2-fold of the calculated value of 7.5 ± 0.7 kcal/mol barrier for deacetylation of MeGlcA by the WT-SHED assembly, also highlighting that this is the most appropriate assembly among the tested ones.
Fig. 6. Arrhenius activation energy determination of the OtCE15A BnzGlcA cleavage reaction.
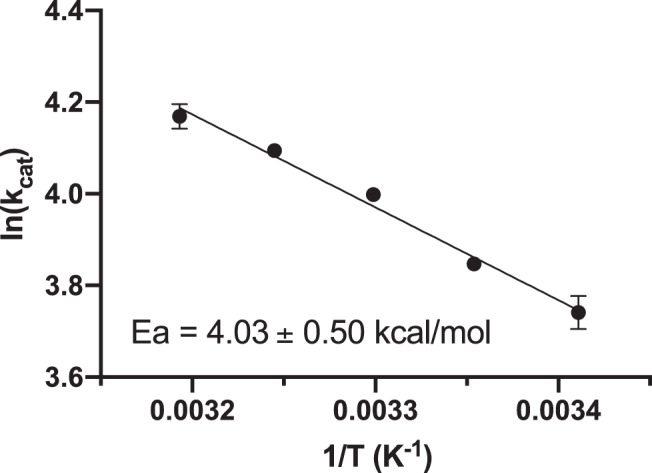
Assays for the determination of Michaelis constants were carried out in duplicate at 5 °C intervals from 20 to 40 °C from a single batch of purified enzyme as described in the “Methods” section. Errors represent the standard error of the mean for kcat values determined by non-linear regression.
Investigating the role of the acidic residues by in silico mutation
In our simulations, the residues in the MM area can interact electrostatically with the ones in the QM area. The acidic residues can accordingly play roles in catalysis by electrostatic interactions when they were included in MM area. Two assemblies of OtCE15A were constructed to further probe the roles of the acidic residues using catalytic units SHE and SHD with D356A and E290A variants, respectively. As shown in Table 1 and Supplementary Fig. 5, the barriers of D356A-SHE and E290A-SHD against the first proton transfer in acylation are similar to that of the corresponding wild types (WTs). However, the barriers of the variants are 4 and 2.2 kcal/mol higher than that of their WTs in deacylation, quantifying the role of the acidic residues in stabilizing the reaction electrostatically. These results are qualitatively in line with our previously published results showing decreases in kcat both with E290A and D356A substitutions using BnzGlcA as substrate10. However, the experimentally measured effects for single substitutions were at most a ~3-fold decrease in kcat for D356A (see reference values in Table 2) while a more substantial decrease would be expected from the calculations. Experimental substitution of both residues does cause a much larger decrease of in kcat (~85-fold). The discrepancies between experiment and calculations suggest that additional compensatory mechanisms in the enzyme are not taken into account by the calculations, such as changes in the surrounding residues’ pKa caused by removal of negative charges.
Table 2.
| Enzyme | Km (mM) | kcat (s−1) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| BnzGlcA | |||
| Wild Type | 3.57 ± 0.091 | 16.6 ± 0.110 | 4.65E+03 ± 1.2E+02 |
| S267A | 2.83 ± 0.660 | 0.000983 ± 0.000059 | 3.47E−01 ± 8.3E−02 |
| H408A | 0.184 ± 0.035 | 0.00999 ± 0.000400 | 5.43E+01 ± 1.1E+01 |
| E290A | 2.03 ± 0.110 | 10.3 ± 0.110 | 5.07E+03 ± 2.8E+02 |
| D356A | 1.86 ± 0.110 | 5.21 ± 0.073 | 2.80E+03 ± 1.7E+02 |
| E290A/D356A | 0.502 ± 0.034 | 0.196 ± 0.003 | 3.90E+02 ± 2.7E+01 |
| R268A* | 0.408 ± 0.051 | 0.204 ± 0.007 | 5.01E+02 ± 6.5E+01 |
| MeGlcA | |||
| Wild Type* | 2.77 ± 0.150 | 19.0 ± 0.310 | 6.85E+03 ± 3.9E+02 |
| R268A* | 0.386 ± 0.060 | 0.214 ± 0.009 | 5.54E+02 ± 9.3E+01 |
The error on the kinetic parameters was calculated by non-linear regression based on duplicate measurements at 8 substrate concentrations.
Proposed role of conserved active-site arginine as oxyanion hole
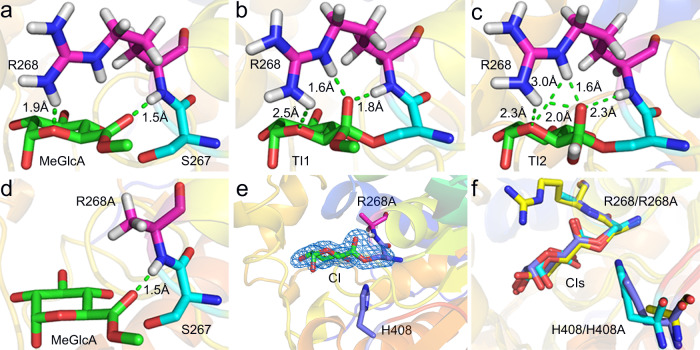
In many ABHs, the oxyanion hole is solely contributed by main chain atoms47, including the main chain amide of the residue immediately following the nucleophile. In contrast, in GEs the side chain (in addition to main chain) of the conserved Arg (Arg268 in OtCE15A) following the nucleophilic Ser, is thought to contribute significantly to stabilization of the transition state10. There is at least one additional example of an ABH where an Arg side chain has been implicated in the oxyanion hole stabilization48, but in this case the Arg is contributed from a helix facing the active site. Indeed, during the simulations with the SHED catalytic assembly, compared with the starting point structure (Fig. 7a), more hydrogen bonds were found to be formed between Arg268 and the carboxylate oxygen in transient states of acylation (Fig. 7b) and deacylation (Fig. 7c), especially the latter. As described above, we did not obtain fully meaningful simulation results in the given time scale using SHEDR unit as the QM area. To further probe the role of R268, the R268A variant was constructed in silico (Fig. 7d) in the SHED catalytic assembly as the QM region, which can in our simulations interact electrostatically with the MM area. The barrier against initial proton transfer accompanied by the nucleophilic attack in acylation of OtCE15A-R268A with catalytic unit SHED was 9.6 kcal/mol (see Table 1 and Supplementary Fig. 6).
Fig. 7. Role of active-site Arg268.
a Starting structure of OtCE15A (PDB 6SYR) with MeGlcA. b, c Snapshots of TI1 and TI2 in our simulations with Arg268. d Starting structure of OtCE15A-R268A variant in silico. e Difference omit electron density of CI in crystal structure of OtCE15A-R268A (PDB 7B7H) generated experimentally here (contoured at 4σ level). f Comparison of CI in OtCE15A-R268A generated from our simulation (cyan) with the two experimental ones, i.e., OtCE15A-R268A (blue) and OtCE15A-H408A (PDB 6SZ4, yellow)9 variants. In a–e, C atoms of Arg268 and R268A are highlighted in pink. C atoms of MeGlcA are shown in green. C atoms of catalytic residues generated from simulation and from crystal structure are shown in cyan and blue, respectively. In f, the C atoms in the structure of the R268A simulation, and crystal structures of the two variants—R268A and H408A - are colored in cyan, blue and yellow, respectively.
However, in the deacylation step of the R268A variant, the proton transfer was not accompanied by nucleophilic attack. Furthermore, in the specified time scale, the proton cannot complete its transfer process to His408, as the corresponding barrier is exceedingly large (see Table 1). This suggests that the CI would easily accumulate in this variant. The OtCE15A-R268A variant was constructed experimentally and kinetic parameters determined with both BnzGlcA and MeGlcA (Table 2). With both substrates the kcat is reduced >80-fold compared to the WT enzyme, and there is also reduction in Km as observed previously with the H408A variant, which would be consistent with accumulation of the CI. Indeed, crystallographic determination of the OtCE15A-R268A structure (Supplementary Table 1) after soaking with the BnzGlcA substrate clearly shows the formation of a CI (Fig. 7e), mainly arising from the significantly damaged interactions in TI2 (Fig. 7c). Thus, the experimental results are in qualitative agreement with the calculations. The CI in the crystal structure of OtCE15A-R268A was compared with the one generated from our simulation and the one of OtCE15A-H408A variant (Fig. 7f), showing little differences.
Dissociation action
On a natural substrate, after a lignin fragment has been expelled from the active site, GEs will undergo a dissociation process of the EP (enzyme-product) complex to complete the hydrolysis cycle. This process, unlike the catalytic event itself, is very likely affected by the properties of the saccharide portion of the substrate. In some biomass degrading enzymes, the presence of carbohydrate-binding modules (CBMs) will also affect this process, but OtCE15A is naturally devoid of a CBM. In the construct used for both structural and biochemical characterization, only 15 N-terminal residues from the original sequence were removed, and these were predicted to be unstructured and shown experimentally not to alter the enzyme kinetics on model substrates.
As depicted in Fig. 8a, we previously obtained a complex of OtCE15A with the largest model ligand to date in a CE15 protein structure—the tetrasaccharide 23-(4-O-methyl-α-d-glucuronyl)-xylotriose (XUX)10. This crystal complex was used as the starting structure to investigate the process of dissociation between enzyme and model ligand. In the present work, a natural substrate complex (NSC), consisting of an 18-chain Iβ cellulose fibril with a 2-3-4-4-3-2 habit49 and a hemicellulose chain adsorbed at the (110) face was constructed in silico. Six cellulose chains at the bottom of the fibril were removed for simplicity. The adsorbed xylan chain is decorated with α-(1,2)-MeGlcA branches every sixth xylosyl unit, and the entire chain adopts a 2-fold helical screw conformation, based on the findings of Dupree and co-workers (Fig. 8c)10,50–52. Referring to the binding model between OtCE15A and XUX in the crystal structure, the complex of enzyme with NSC was constructed and used as the starting model for the following calculation of dissociation. Fifteen residues in OtCE15A, found in the interaction interface and including the active site residues, interact with NSC by hydrogen bonds (Supplementary Fig. 7). The processes of dissociation between enzyme and substrates were then investigated in silico.
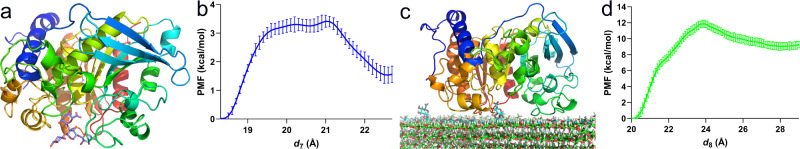
Fig. 8. Enzymatic dissociation processes from model and complex substrates.
a, c GE with a XUX (PDB 6T0I) and c NSC. b, d Corresponding free-energy profiles characterizing the dissociation processes. C atoms of XUX are highlighted in blue. In NSC, cellulose and xylan with GlcA are colored in green and cyan, respectively. d7 and d8 denote the distance of COMs between enzyme and substrate. Data are presented as mean values ± the standard error inferred from three independent runs. Source data for b, d are provided as a Source Data file.
As shown in Fig. 8b, d, the barriers for assemblies of XUX and NSC are approximately 3.3 and 12 kcal/mol, respectively, indicating that the enzymatic dissociation rate from NSC will be much slower than that from the smaller and soluble XUX molecule. We suggest that, depending on the exact LCC structure being attacked, different steps of the catalytic cycle can be rate limiting for GEs, including dissociation of the EP complex. Thus, the rate-limiting step needs to be carefully considered in protein engineering programs on GEs, and generally in the analysis of variants. In particular, improvement of activity on smaller model substrates commonly used, may not necessarily result in improvement on real biomass, where the nature of the LG or non-catalytic processes of dissociation (and perhaps association) may alter the rate-limiting step.
Discussion
Based on our previously obtained considerable biochemical and structural information, in the present work we shed light on the catalytic mechanism and biomass association of OtCE15A, which has a distinctive variation on the classical serine hydrolase catalytic triad. The roles of the two putative acidic residues which were shown to be important for catalysis experimentally10, were quantified in silico by using different QM regions and relevant Ala mutations as MM areas. Furthermore, our theoretical results indicated that the conserved active-site arginine, whose side chain is proposed to contribute to forming the oxyanion hole, plays a prominent role in both acylation and deacylation steps, especially in the latter. This is reflected in a large reduction in activity of the R268A mutant as produced experimentally here and accumulation of the CI in the variant demonstrated in the determined X-ray structure. The clear roles of these key residues in catalysis expand and deepen our knowledge on the classical catalytic triad of serine hydrolases, and can contribute to the improvement of designed biomimetics based on it. In addition, the QM/MM calculations based on the assumption of a classic ABH mechanism, confirm deacylation to be the rate-determining step in all the six different assemblies, with the initial proton transfer being the energetically dominant process for both the two catalytic steps. The lowest and probably most reliable energy barriers for the WT OtCE15A are obtained with a QM region including catalytic Ser, His and two acidic residues. The barrier of the deacylation step as calculated for this WT-SHED assembly is also the closest to our experimentally determined Ea. Finally, the calculations suggest that with bigger substrates, non-catalytic events, such as dissociation between the enzyme and its final product can become rate-limiting, and this needs to be carefully considered in engineering GEs for biomass applications. This work, at the confluence of computation and experiment, is expected to help drive the use of GEs as important and perhaps decisive accessory enzymes to help reduce the inherently high recalcitrance of lignocellulose in a more cost-effective and environment friendly way.
Methods
QM/MM free-energy calculations
The PMFs of acylation, deacylation and angle change of the HW were determined for OtCE15A in aqueous solution by QM/MM simulations. The starting structure for the simulations is the complex of OtCE15A with MeGlcA. The QM region for acylation of WT-SHE, which has an overall charge of −1, includes Ser267, Glu290, His408, as well as the MeGlcA ligand (48 atoms). To investigate the roles of acidic residues and R268 in acylation, catalytic assemblies of WT-SHD, WT-SHED, WT-SHEDR, D356A-SHE and E290A-SHD and R268A-SHED were used. Their QM areas consist of 48 (charge: −1), 54 (charge: −2), 66 (charge: −1), 48 (charge: −1), 48 (charge: −1) and 54 (charge: −2) atoms, respectively. The QM areas of WT-SHE for deacylation and the angle change of HW, which have the overall charge of −1, contain the atoms of WT-SHE for acylation (except for the LG) and a HW (45 atoms). Catalytic assemblies of WT-SHD, WT-SHED, WT-SHEDR, D356A-SHE and E290A-SHD and R268A-SHED in deacylation consist of 45 (charge: −1), 51 (charge: −2), 63 (charge: −1), 45 (charge: −1), 45 (charge: −1) and 51 atoms (charge: −2), respectively.
All the assemblies were solvated in an equilibrated box of water, and the overall charge neutrality was achieved by adding Na+ ions to the solution. The total molecular numbers for each assembly were about 63,250 atoms. Each complex was optimized by 5000 steps minimization and 100 ps molecular dynamics (MD) simulation with gradually relaxing restraints: (a) protein and substrate were restrained; (b) only substrate and catalytic residues were restrained; and (c) 1000 steps minimization was performed before each run without restraint. Then three independent runs of the QM/MM simulation for each molecular assembly were carried out. Furthermore, the transition pathways were stratified for increasing the efficiency of the calculations (see Supplementary Table 2). A recently developed importance-sampling algorithm, WTM-eABF53–56, implemented in the Colvars57 module of the NAMD58 molecular dynamics program, was employed in all the free-energy calculations. MOPAC3559 and NAMD 2.13 were used as the QM and MD engines, respectively. PM760 was utilized in the QM calculations, wherein electrical embedding was used to treat Coulombic interactions at the QM/MM interface61,62. The PM7 semi-empirical method provides a good balance between accuracy and efficiency in QM/MM calculations. Apart from benchmarking63, the reliability of PM7 for modeling the catalytic mechanism in enzymes has been assessed previously64. In a previous study, we have successfully combined PM7 with the WTM-eABF method to study the proton transfer in the glycosylation of the catalytic cycle of glycoside hydrolase46. Therefore, PM7 is suitable for systems that are not particularly complex. All the simulations for these assemblies were carried out using NAMD with the CHARMM36 force field65–67, and the TIP3P water model68. VMD 1.9.4 program69 and PyMOL molecular graphics system (version 2.4 Schrödinger, LLC) were employed to visualize and analyze results.
Free-energy calculations
The assemblies for the investigation of the methanol group leaving the active site, HW approach and dissociation action from XUX and NSC were treated as described above. The free-energy profiles underlying these processes were calculated via WTM-eABF. The details for each molecular assembly are provided in Supplementary Table 3. All the free-energy profiles reported in this study were obtained over three independent WTM-eABF simulations.
Enzyme production and characterization
The OtCE15A R268A variant was created by site-specific mutagenesis via QuikChange by the primers listed in Supplementary Table 4. The variant was recombinantly produced in E. coli BL21 (λDE3) and purified by immobilized metal affinity chromatography and subsequently by size-exclusion chromatography as described previously9,10. Glucuronoyl esterase activity was assayed with BnzGlcA or MeGlcA (Carbosynth) and monitored continuously with the K-URONIC kit (Megazyme) as reported previously9,10. Initial velocity measurements at defined substrate concentrations were carried out in duplicate from a single batch of recombinantly purified enzyme and kinetic parameters were determined from at least eight different substrate concentrations ranging from 0.1 to 10 times the Km value. Determination of the Arrhenius activation energy (Ea) for the BnzGlcA cleavage reaction was carried out similarly as described above at temperatures ranging from 20 to 40 °C. Reaction conditions containing all the components, except substrate, were pre-equilibrated at the defined temperature for 5 min before starting the assays. Substrate was kept at room temperature and added in volumes ≤5% of the total volume to ensure minimal temperature disruption. Reactions were monitored continuously in a SPECTRO star nano spectrophotometer (BMG Labtech) equipped with controlled thermal incubation9,10.
Crystal structure determination
The OtCE15A R268A variant was crystallized using a Morpheus screen (Molecular Dimensions Limited, Sheffield, UK). Sitting drops of 0.3 μL total volume were set up in MRC two-drop crystallization plates (Molecular Dimensions) with protein at concentration of 7.1 mg/mL in a standard chromatography buffer (50 mM Tris–HCl pH 8.0, 100 mM NaCl) mixed with reservoir solutions in either a 3:1 or 1:1 ratio with an Oryx8 robot (Douglas Instruments, East Garston, UK). Crystals used for data collection were obtained in condition 2-38 of the Morpheus screen (https://www.moleculardimensions.com/products/morpheusv3) consisting of 0.1 M buffer system 1 (imidazole and MES, pH 6.5), 30% precipitant mix 2 (ethylene glycol and PEG 8000) and amino acid additives (l-glutamate, alanine (racemic); glycine; lysine HCl (racemic); serine (racemic)). Soaking was carried out in a saturated solution of BnzGlcA in 10% DMSO and 90% mother liquor for 30 s before being flash frozen in liquid N2. Diffraction data was collected at the BioMAX70 beamline at MAX IV (February 23, 2020). The dataset was processed with XDS (version 20200131)71 and the structure solved by molecular replacement with Phaser 2.172 using the WT OtCE15A (PDB code 6GS0) as a template. Molecular replacement was utilized as rigid body refinement in Phenix Refine73 failed to correctly determine phases which was likely attributable to differences in the cell dimensions between the WT and OtCE15A-R268A dataset. Coot 0.8.9.274 and Phenix Refine73 (Phenix 1.19.2-4158-000) were used in iterative cycles of real space and reciprocal space refinement. Two stretches of residues (154–175 and 385–388) and the residues from the protein expression His6-tag were not modeled due to a lack of electron density likely owing to soaking in the presence of DMSO as has been observed in crystal soaks in our previous study10. The data collection, processing, and refinement statistics can be found in Supplementary Table 1. Data was collected on BioMAX beamline at the MAXIV facility in Sweden on February 23, 2020.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Prof. Paul Dupree, University of Cambridge, for useful discussion of the biomass model and Jens-Christian N. Poulsen and Mohannad Aloula for technical help with crystallization. This study was supported by Novo Nordisk Foundation grants NNF17OC0027698 and NNF21OC0071611 to L.L.L. and J.L. We acknowledge MAX IV Laboratory for time on Beamline Biomax under Proposal 20190334 to L.L.L. Research conducted at MAX IV, a Swedish national user facility, is supported by the Swedish Research council under contract 2018-07152, the Swedish Governmental Agency for Innovation Systems under contract 2018-04969, and Formas under contract 2019-02496. L.L.L. and Z.Z. are members of ISBUC, Integrative Structural Biology at the University of Copenhagen (www.isbuc.ku.dk). Travel to synchrotrons was supported by the Danish Ministry of Higher Education and Science through the Instrument Center DANSCATT. Computations were mainly carried out on Computerome 2.0 (Danish National Supercomputer for Life Sciences installed at the DTU National Lifescience Center at the Technical University of Denmark (DTU), Lyngby, Danmark, with access funded by the University of Copenhagen. Furthermore, partial computations in this study were supported by National Natural Science Foundation of China (22005157 to Z.Z.). M.S.S. and C.S.P. thank the support from FAPESP (grant # 2013/08293-7 and 15/25031-1).
Source data
Author contributions
L.L.L., J.L. and Z.Z. designed the research. Z.Z. carried out the QM/MM and free-energy calculations with input from C.S.P., H.F., W.C., X.S. and M.S.S. C.S.P. and M.S.S. constructed the NSC. S.M. produced, characterized and solved the structure of the enzyme variant. Z.Z. and L.L.L. drafted the manuscript with the other author’s contributions. All authors participated in editing the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The data that support this study are available from the corresponding author upon reasonable request. Crystallographic data for the OtCE15A R268A variant have been deposited at the Protein Data Bank with accession code 7B7H. Previously released structural data used in the course of this study: 6SYR, 6SZ4, 6GS0, and 6T0I. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28938-w.
References
- 1.Xia QN, et al. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016;7:11162. doi: 10.1038/ncomms11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hämäläinen V, et al. Enzymatic processes to unlock the lignin value. Front. Bioeng. Biotechnol. 2018;6:20. doi: 10.3389/fbioe.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himmel ME, et al. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T. Structural studies on the covalent bonds between lignin and carbohydrate in lignin–carbohydrate complexes by selective oxidation of the lignin with 2, 3-dichloro-5, 6-dicyano-l, 4-benzoquinone. Wood Res. 1989;76:59–123. [Google Scholar]
- 5.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007;25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 6.Couturier M, et al. Lytic xylan oxidases from wood-decay fungi unlock biomass degradation. Nat. Chem. Biol. 2018;14:306–310. doi: 10.1038/nchembio.2558. [DOI] [PubMed] [Google Scholar]
- 7.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spániková S, Biely P. Glucuronoyl esterase—novel carbohydrate esterase produced by Schizophyllum commune. FEBS Lett. 2006;580:4597–4601. doi: 10.1016/j.febslet.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Arnling Bååth J, et al. Biochemical and structural features of diverse bacterial glucuronoyl esterases facilitating recalcitrant biomass conversion. Biotechnol. Biofuels. 2018;11:213. doi: 10.1186/s13068-018-1213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurkewich S, Poulsen JCN, Lo Leggio L, Larsbrink J. Structural and biochemical studies of the glucuronoyl esterase OtCE15A illuminate its interaction with lignocellulosic components. J. Biol. Chem. 2019;294:19978–19987. doi: 10.1074/jbc.RA119.011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnling Bååth J, et al. Structure-function analyses reveal that a glucuronoyl esterase from Teredinibacter turnerae interacts with carbohydrates and aromatic compounds. J. Biol. Chem. 2019;294:6635–6644. doi: 10.1074/jbc.RA119.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokkuluri PR, et al. Structure of the catalytic domain of glucuronoyl esterase Cip2 from Hypocrea jecorina. Proteins. 2011;79:2588–2592. doi: 10.1002/prot.23088. [DOI] [PubMed] [Google Scholar]
- 13.Charavgi MD, Dimarogona M, Topakas E, Christakopoulos P, Chrysina ED. The structure of a novel glucuronoyl esterase from Myceliophthora thermophila gives new insights into its role as a potential biocatalyst. Acta Crystallogr. 2013;69:63–73. doi: 10.1107/S0907444912042400. [DOI] [PubMed] [Google Scholar]
- 14.De Santi C, Gani OA, Helland R, Williamson A. Structural insight into a CE15 esterase from the marine bacterial metagenome. Sci. Rep. 2017;7:17278. doi: 10.1038/s41598-017-17677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst HA, et al. The structural basis of fungal glucuronoyl esterase activity on natural substrates. Nat. Commun. 2020;11:1026. doi: 10.1038/s41467-020-14833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong, D. W., Chan, V. J., McCormack, A. A., Hirsch, J. & Biely, P. Functional cloning and expression of the Schizophyllum commune glucuronoyl esterase gene and characterization of the recombinant enzyme. Biotechnol. Res. Int. 2012, 951267 (2012). [DOI] [PMC free article] [PubMed]
- 17.Tsai AY, et al. Constitutive expression of a fungal glucuronoyl esterase in Arabidopsis reveals altered cell wall composition and structure. Plant Biotechnol. J. 2012;10:1077–1087. doi: 10.1111/j.1467-7652.2012.00735.x. [DOI] [PubMed] [Google Scholar]
- 18.Duranova M, Hirsch J, Kolenova K, Biely P. Fungal glucuronoyl esterases and substrate uronic acid recognition. Biosci. Biotechnol. Biochem. 2009;73:2483–2487. doi: 10.1271/bbb.90486. [DOI] [PubMed] [Google Scholar]
- 19.d’Errico C, et al. Improved biomass degradation using fungal glucuronoyl—esterases—hydrolysis of natural corn fiber substrate. J. Biotechnol. 2016;219:117–123. doi: 10.1016/j.jbiotec.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Katsimpouras C, et al. Enzymatic synthesis of model substrates recognized by glucuronoyl esterases from Podospora anserina and Myceliophthora thermophila. Appl. Microbiol. Biotechnol. 2014;98:5507–5516. doi: 10.1007/s00253-014-5542-9. [DOI] [PubMed] [Google Scholar]
- 21.Vafadi C, Topakas E, Biely P, Christakopoulos P. Purifcation, characterization and mass spectrometric sequencing of a thermophilic glucuronoyl esterase from Sporotrichum thermophile. FEMS Microbiol. Lett. 2009;296:178–184. doi: 10.1111/j.1574-6968.2009.01631.x. [DOI] [PubMed] [Google Scholar]
- 22.Huynh HH, Arioka M. Functional expression and characterization of a glucuronoyl esterase from the fungus Neurospora crassa: identification of novel consensus sequences containing the catalytic triad. J. Gen. Appl. Microbiol. 2016;62:217–224. doi: 10.2323/jgam.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Hüttner S, Klaubauf S, de Vries RP, Olsson L. Characterisation of three fungal glucuronoyl esterases on glucuronic acid ester model compounds. Appl. Microbiol. Biotechnol. 2017;101:5301–5311. doi: 10.1007/s00253-017-8266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topakas E, Moukouli M, Dimarogona M, Vafadi C, Christakopoulos P. Functional expression of a thermophilic glucuronoyl esterase from Sporotrichum thermophile: identifcation of the nucleophilic serine. Appl. Microbiol. Biotechnol. 2010;87:1765–1772. doi: 10.1007/s00253-010-2655-7. [DOI] [PubMed] [Google Scholar]
- 25.Huynh HH, Ishii N, Matsuo I, Arioka MA. Novel glucuronoyl esterase from Aspergillus fumigatus—the role of conserved Lys residue in the preference for 4-O-methyl glucuronoyl esters. Appl. Microbiol. Biotechnol. 2018;102:2191–2201. doi: 10.1007/s00253-018-8739-5. [DOI] [PubMed] [Google Scholar]
- 26.Biely P, Malovikova A, Uhliarikova I, Li XL, Wong DW. Glucuronoyl esterases are active on the polymeric substrate methyl esterifed glucuronoxylan. FEBS Lett. 2015;589:2334–2339. doi: 10.1016/j.febslet.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 27.De Santi C, Willassen NP, Williamson A. Biochemical characterization of a family 15 carbohydrate esterase from a bacterial marine arctic metagenome. PLoS ONE. 2016;11:e0159345. doi: 10.1371/journal.pone.0159345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krska D, Larsbrink J. Investigation of a thermostable multi-domain xylanase-glucuronoyl esterase enzyme from Caldicellulosiruptor kristjanssonii incorporating multiple carbohydrate-binding modules. Biotechnol. Biofuels. 2020;13:68. doi: 10.1186/s13068-020-01709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bååth JA, Giummarella N, Klaubauf S, Lawoko M, Olsson LA. A glucuronoyl esterase from Acremonium alcalophilum cleaves native lignin–carbohydrate ester bonds. FEBS Lett. 2016;590:2611–2618. doi: 10.1002/1873-3468.12290. [DOI] [PubMed] [Google Scholar]
- 30.Mosbech C, Holck J, Meyer AS, Agger JW. The natural catalytic function of CuGE glucuronoyl esterase in hydrolysis of genuine lignin-carbohydrate complexes from birch. Biotechnol. Biofuels. 2018;11:71. doi: 10.1186/s13068-018-1075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosbech C, Holck J, Meyer A, Agger JW. Enzyme kinetics of fungal glucuronoyl esterases on natural lignin–carbohydrate complexes. Appl. Microbiol. Biotechnol. 2019;103:4065–4075. doi: 10.1007/s00253-019-09797-w. [DOI] [PubMed] [Google Scholar]
- 32.Raji, O. et al. The coordinated action of glucuronoyl esterase and a-glucuronidase promotes the disassembly of lignin–carbohydrate complexes. FEBS Lett. 591 (2020). [DOI] [PMC free article] [PubMed]
- 33.Giummarella N, Balakshin M, Koutaniemi S, Kärkönen A, Lawoko M. Nativity of lignin carbohydrate bonds substantiated by biomimetic synthesis. J. Exp. Bot. 2019;70:5591–5601. doi: 10.1093/jxb/erz324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giummarella N, Pu Y, Ragauskas AJ, Lawoko M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019;7:1573–1595. [Google Scholar]
- 35.Sapounaa I, Lawoko M. Deciphering lignin heterogeneity in ball milled softwood: unravelling the synergy between the supramolecular cell wall structure and molecular events. Green Chem. 2021;23:3348–3364. [Google Scholar]
- 36.Lyczakowski JJ, et al. Removal of glucuronic acid from xylan is a strategy to improve the conversion of plant biomass to sugars for bioenergy. Biotechnol. Biofuels. 2017;10:224. doi: 10.1186/s13068-017-0902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ollis DL, et al. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 38.Denesyuk A, Dimitriou PS, Johnson MS, Nakayama T, Denessiouk K. The acid–base–nucleophile catalytic triad in ABH-fold enzymes is coordinated by a set of structural elements. PLoS ONE. 2020;15:e0229376. doi: 10.1371/journal.pone.0229376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silveira, R. L. et al. Transition path sampling study of the feruloyl esterase mechanism. J. Phys. Chem. B125, 2019–2030 (2021). [DOI] [PubMed]
- 40.Nothling MD, et al. Simple design of an enzyme-inspired supported catalyst based on a catalytic triad. Chem. 2017;2:732–745. [Google Scholar]
- 41.Rauwerdink A, Kazlauskas RJ. How the same core catalytic machinery catalyzes 17 different reactions: the serine-histidine-aspartate catalytic triad of α/β-hydrolase fold enzymes. ACS Catal. 2015;5:6153–6176. doi: 10.1021/acscatal.5b01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedstrom L. Serine protease mechanism and specificity. Chem. Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Kua J, McCammon JA. Role of the catalytic triad and oxyanion hole in acetylcholinesterase catalysis: an ab initio QM/MM Study. J. Am. Chem. Soc. 2002;124:10572–10577. doi: 10.1021/ja020243m. [DOI] [PubMed] [Google Scholar]
- 44.Nemukhin AV, Lushchekina SV, Bochenkova AV, Golubeva AA, Varfolomeev SD. Characterization of a complete cycle of acetylcholinesterase catalysis by ab initio QM/MM modeling. J. Mol. Model. 2008;14:409–416. doi: 10.1007/s00894-008-0287-y. [DOI] [PubMed] [Google Scholar]
- 45.Yan SH, Li T, Yao LS. Mutational effects on the catalytic mechanism of cellobiohydrolase I from Trichoderma reesei. J. Phys. Chem. B. 2011;115:4982–4989. doi: 10.1021/jp200384m. [DOI] [PubMed] [Google Scholar]
- 46.Zong Z, et al. Lysine mutation of the claw-arm-like loop accelerates catalysis by cellobiohydrolases. J. Am. Chem. Soc. 2019;141:14451–14459. doi: 10.1021/jacs.9b08477. [DOI] [PubMed] [Google Scholar]
- 47.Dimitriou PS, Denesyuk AI, Nakayama T, Johnson MS, Denessiouk K. Distinctive structural motifs co-ordinate the catalytic nucleophile and the residues of the oxyanion hole in the alpha/beta-hydrolase fold enzymes. Protein Sci. 2019;28:344–364. doi: 10.1002/pro.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen NA, Lin H, Wei R, Fischbach MA, Walsh CT. Structural characterization of enterobactin hydrolase IroE. Biochemistry. 2006;45:10184–10190. doi: 10.1021/bi060950i. [DOI] [PubMed] [Google Scholar]
- 49.Terrett OM, et al. Molecular architecture of softwood revealed by solid-state NMR. Nat. Commun. 2019;10:4978. doi: 10.1038/s41467-019-12979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Busse-Wicher M, et al. Evolution of xylan substitution patterns in gymnosperms and angiosperms: implications for xylan interaction with cellulose. Plant Physiol. 2016;171:2418–2431. doi: 10.1104/pp.16.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons TJ, et al. Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun. 2016;7:13902. doi: 10.1038/ncomms13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cresswell, R. et al. Importance of water in maintaining softwood secondary cell wall nanostructure. Biomacromolecules22, 4669–4680 (2021). [DOI] [PMC free article] [PubMed]
- 53.Fu H, Shao X, Chipot C, Cai W. Extended adaptive biasing force algorithm. An on-the-fly implementation for accurate free-energy calculations. J. Chem. Theory Comput. 2016;12:3506–3513. doi: 10.1021/acs.jctc.6b00447. [DOI] [PubMed] [Google Scholar]
- 54.Fu H, et al. Zooming across the free-energy landscape: shaving barriers & flooding valleys. J. Phys. Chem. Lett. 2018;9:4738–4745. doi: 10.1021/acs.jpclett.8b01994. [DOI] [PubMed] [Google Scholar]
- 55.Fu H, Shao X, Cai W, Chipot C. Taming rugged free energy landscapes using an average force. Acc. Chem. Res. 2019;52:3254–3264. doi: 10.1021/acs.accounts.9b00473. [DOI] [PubMed] [Google Scholar]
- 56.Fu H, et al. Finding an optimal pathway on a multidimensional free-energy landscape. J. Chem. Inf. Model. 2020;60:5366–5374. doi: 10.1021/acs.jcim.0c00279. [DOI] [PubMed] [Google Scholar]
- 57.Fiorin G, Klein ML, Hénin J. Using collective variables to drive molecular dynamics simulations. Mol. Phys. 2013;111:3345–3362. [Google Scholar]
- 58.Phillips JC, et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart JJP. MOPAC: a semiempirical molecular orbital program. J. Comput.-Aided Mol. Des. 1990;4:1–105. doi: 10.1007/BF00128336. [DOI] [PubMed] [Google Scholar]
- 60.Stewart JJP. Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013;19:1–32. doi: 10.1007/s00894-012-1667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melo MCR, et al. NAMD goes quantum: an integrative suite for hybrid simulations. Nat. Methods. 2018;15:351–354. doi: 10.1038/nmeth.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin H, Truhlar DG. QM/MM: what have we learned, where are we & where do we go from here? Theor. Chem. Acc. 2007;117:185. [Google Scholar]
- 63.Dral PO, Wu X, Spörkel L, Koslowski A, Thiel W. Semiempirical quantum-chemical orthogonalization-corrected methods: benchmarks for ground-state properties. J. Chem. Theory Comput. 2016;12:1097–1120. doi: 10.1021/acs.jctc.5b01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart JJP. An investigation into the applicability of the semiempirical method PM7 for modeling the catalytic mechanism in the enzyme chymotrypsin. J. Mol. Model. 2017;23:154. doi: 10.1007/s00894-017-3326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanommeslaeghe K, et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2009;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacKerell AD, Jr., et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 67.MacKerell AD, Jr., Feig M, Brooks CL. Improved treatment of the protein backbone in empirical force fields. J. Am. Chem. Soc. 2004;126:698–699. doi: 10.1021/ja036959e. [DOI] [PubMed] [Google Scholar]
- 68.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 69.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 70.Ursby T, et al. BioMAX—the first macromolecular crystallography beamline at MAX IV laboratory. J. Synchrotron Radiat. 2020;27:1415–1429. doi: 10.1107/S1600577520008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liebschner D, et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in phenix. Acta Crystallogr. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request. Crystallographic data for the OtCE15A R268A variant have been deposited at the Protein Data Bank with accession code 7B7H. Previously released structural data used in the course of this study: 6SYR, 6SZ4, 6GS0, and 6T0I. Source data are provided with this paper.