Abstract
Variations in the cross-sectional properties of long bones are used to reconstruct the activity of human groups and differences in their respective habitual behaviors. Knowledge of what factors influence bone structure in Homo sapiens and Neandertals is still insufficient thus, this study investigated which biological and environmental variables influence variations in the femoral robusticity indicator of these two species. The sample consisted of 13 adult Neandertals from the Middle Paleolithic and 1959 adult individuals of H. sapiens ranging chronologically from the Upper Paleolithic to recent times. The femoral biomechanical properties were derived from the European data set, the subject literature, and new CT scans. The material was tested using a Mantel test and statistical models. In the models, the polar moment of area (J) was the dependent variable; sex, age, chronological period, type of lifestyle, percentage of the cortical area (%CA), the ratio of second moment areas of inertia about the X and Y axes (Ix/Iy), and maximum slope of the terrain were independent covariates. The Mantel tests revealed spatial autocorrelation of the femoral index in H. sapiens but not in Neandertals. A generalized additive mixed model showed that sex, %CA, Ix/Iy, chronological period, and terrain significantly influenced variation in the robusticity indicator of H. sapiens femora. A linear mixed model revealed that none of the analyzed variables correlated with the femoral robusticity indicator of Neandertals. We did not confirm that the gradual decline in the femoral robusticity indicator of H. sapiens from the Middle Paleolithic to recent times is related to the type of lifestyle; however, it may be associated with lower levels of mechanical loading during adolescence. The lack of correlation between the analysed variables and the indicator of femoral robusticity in Neandertals may suggest that they needed a different level of mechanical stimulus to produce a morphological response in the long bone than H. sapiens.
Subject terms: Anthropology, Anatomy
Introduction
The structure of a bone is optimized in accordance with altered loading regimes, as manifested in the specific morphology, the orientation of the trabecular networks, and the size and distribution of the osteons1. However, the precise mechanism of mechanical signal detection is still investigated1–3 especially given that each bone differs in terms of sensitivity to mechanical stimuli4. Recently, most research has assessed limb bone organization using cross-sectional geometry, which is typically calculated from periosteal and endosteal contours of specific cross-sections5–8. Due to the broader availability of CT and the development of semiautomated techniques, interpopulation comparisons on larger scales can now be carried out. Thus, cross-sectional geometry is being used to measure diaphyseal variables which characterize bending and torsional rigidity9.
According to Macintosh and Stock 10, minimizing stress in cortical bone renders the postcranial skeleton (especially the lower limbs) highly reactive to changes in types and levels of mobility and activity patterns. This variability in bone morphology can be applied in paleontological and bioarchaeological research to reconstruct the lifestyles of past populations. Differences in the biomechanical properties of the lower limbs between preagricultural and agricultural populations suggest that sedentary societies may be characterized by greater size and rigidity in the long bones than highly mobile societies, possibly due to a greater variety of physical activity types in agricultural group11. However, despite a great deal of research analyzing variation in cross-sections, the effect of mobility levels on the quantity and distribution of cortical tissues in the femur exhibits discrepancies in terms of results12. For example, Carlson et al 13. showed that less mobile populations exhibited greater robusticity in the femoral midshaft than Australian aborigines. In contrast, research carried out on populations from the forest biome, and the fynbos of southern Africa revealed significant differences only in the femoral midshaft indices (i.e., ratio of maximum to minimum second moments of area) and between women14. Sládek et al. 15 claimed that a transition in mechanical loading between Late Eneolithic and Early Bronze age groups from Central Europe was present only in females and exhibited a mosaic pattern. In contrast, Friedl et al16. found no robusticity trend in lower limbs but rather a fluctuating pattern in Holocene populations. Interpretation of biomechanical changes may also be hampered, as terrain type (e.g., flat, hilly, mountainous) may influence variation in biomechanical properties of the lower limbs17–21. These results may indicate that geometric properties and loading patterns are not straightforwardly related and that should also consider additional biological and environmental factors in biomechanical analysis.
Some issues can be resolved by studying variations in cross-sectional properties of lower limbs in large samples22. Such analyses usually focus on populations from western Europe19,22–24, North America12, and Mesoamerica25. Studies on past human populations from eastern Europe, the Middle East, and Australia are very scarce13,26. The overrepresentation of certain geographical regions in biological studies entails two consequences. First, geographically proximate populations tend to be biologically similar; thus, certain variables such as overall body and craniofacial size may be spatially correlated27. In cases where samples are not equally numerous and evenly distributed in space, the results may be biased in favor of regions with more numerous samples. Therefore biomechanical properties of long bones should be checked for spatial dependence and, if necessary, analyses should be carried out following control for the effect of autocorrelation between populations. Secondly, new biomechanical data from Eastern Europe or the Middle East might clarify the local patterns of cross-sectional variables, which could be helpful in understanding the process of changes in global trends in postcranial morphology.
Studies on the cross-sectional geometry of the long bones are also used to analyze the differences between Neandertals and H. sapiens6,28. Previous research has shown greater diaphyseal robusticity in Neandertal lower limbs than in H. sapiens29–32. Variation in biomechanical properties of the upper and lower limbs is explained as the result of different levels of mechanical loading experienced throughout life33,8 or combinations of activities differing between Neandertals and H. sapiens29. However, the relationship between mechanical loads and level of robusticity is not straightforward due to the factors influencing the transmission of mechanical stimuli to bone structure, such as loading intensity, repetitiveness, number of loading cycles, or loading directions (principal or atypical)34. Research on differences in cross-sectional properties between H. sapiens and Neandertals assumes that the same level of mechanical loading causes a similar response of the biological mechanisms (i.e., growth, modeling, and remodeling of long bones)35; however, this aspect has not been analyzed yet. Therefore, we checked what factors can potentially play a significant role in shaping the femoral robusticity indicators, separately for Neandertals and H. sapiens. Since our research is not an experimental study, we will not investigate the cause–effect relationships between the femoral rigidity and variables. Nevertheless, we believe that the results will help understand the causes of variation in long bone cross-sectional geometry. Without understanding the nature of changes in bone rigidity and various factors, we will not be able to interpret the differences between those two hominin species correctly.
Previous studies analyzing differences in long bone geometry have usually focused on simple statistical analysis examining only a few variables at a time36,37. We decided to create a model that would take into account the chronological context and influence of biological (sex, age), environmental (terrain), and cultural (type of lifestyle) variables on the polar moment of area (J). The J index is considered a marker of femoral robusticity, as it characterizes resistance to the torsional and bending rigidity loads of the lower limb38. Based on previous research showing the variability in the femoral cross-section in hominins38–42, we predict that each factor will similarly correlate with the standardized index of femoral robusticity in Neandertals and H. sapiens. This analysis was carried out on an extensive database representing Neandertals and H. sapiens that have lived in Europe, Asia, and Australia from Paleolithic to modern times.
Results
Descriptive statistics indicate that %CA is the least varied variable in H. sapiens and Neandertals. In turn, the standardized index of femoral robusticity (size-adjusted J), Ix/Iy, and age show a large variability in both hominin species (Tables S3, S4). Females and males of H. sapiens from the Middle Paleolithic are characterized by the highest means of size-adjusted J. The mean values of Neandertal females are slightly higher, while Neandertal males show slightly lower and similar means of size-adjusted J to Homo sapiens individuals from the Upper Paleolithic and Mesolithic. The mean relative amount of cortical area (%CA) in the femoral cross-sections of H. sapiens and Neandertals varied from 70.39 to 85.27%. The highest means of Ix/Iy were observed in H. sapiens from the Middle Paleolithic. The ratio of the second moment area of inertia about the X and Y axes (Ix/Iy) of Neandertals was close to 1. The differences in mean age between sexes, though not pronounced, varied between chronological periods.
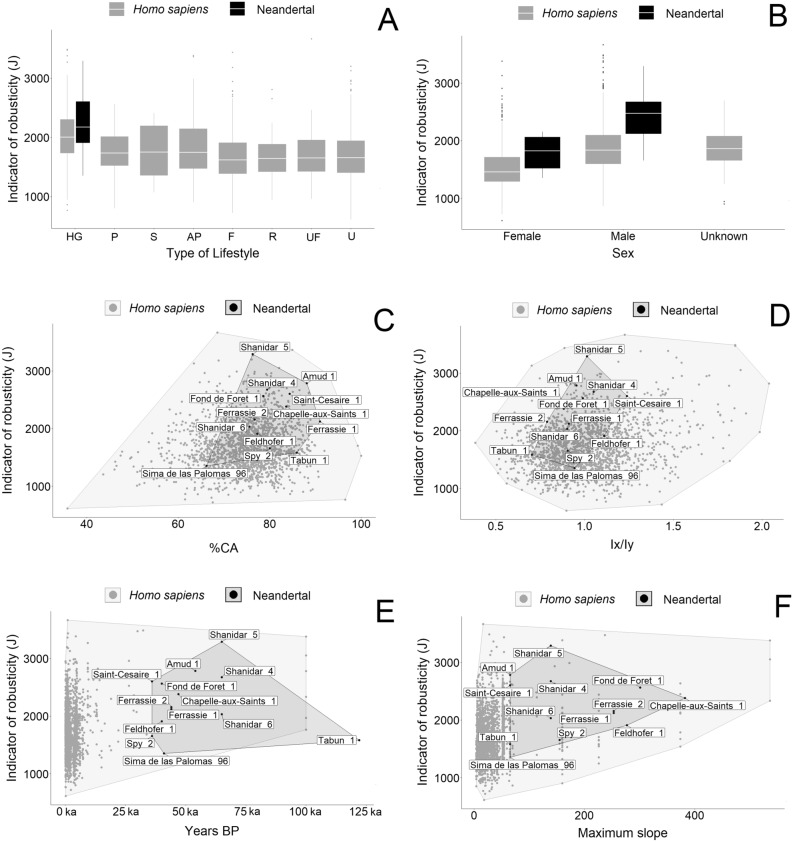
The Mantel test revealed that the index of femoral robusticity (size-adjusted J) is spatially dependent in H. sapiens but not in Neandertals. Therefore, a generalized additive mixed model (GAMM) that removed the autocorrelation effect was used to investigate the variation in the H. sapiens sample. The GAMM showed that variables such as the chronological period in years (BP), sex, %CA, Ix/Iy, and maximum slope of the terrain significantly correlated with the standardized index of femoral robusticity (size-adjusted J) in H. sapiens (Table 1, S5). In the case of a linear mixed model (LMM), none of the analyzed predictors (years, maximum slope, sex, %CA, or Ix/Iy) significantly influenced the standardized indicator of femoral robusticity (size-adjusted J) in Neandertals (Table 2, S5). Figure 1A shows that different types of lifestyle observed in the H. sapiens sample do not differ in the level of the femoral robusticity indicator (size-adjusted J). Males of H. sapiens show higher dependent variable levels (size-adjusted J) than females, but with values close to the individuals of unknown sex (Fig. 1B). In the case of Neandertals, the differences in the femoral robusticity indicator (size-adjusted J) between sexes are visible but not statistically significant (Fig. 1B). According to the GAMM model and Fig. 1C,D, the femoral robusticity indicator (size-adjusted J) in H. sapiens increases simultaneously with two predictors (i.e., %CA and Ix/Iy). In the case of the H. sapiens sample, there is a decline in the robusticity indicator (size-adjusted J) over time, while no trend appears for the Neandertals (Fig. 1E). The maximum slope of terrain also increases with the femoral robusticity indicator but is only visible in the H. sapiens sample and not in the Neandertals (1F).
Table 1.
Results of the generalized additive mixed model (GAMM) calculated for Homo sapiensa.
| Effect | Estimate | SE | T | p |
|---|---|---|---|---|
| Intercept | 2079.19 | 33.66 | 61.75 | < 0.001 |
| Years (BP) | 12.71 | 13.41 | 3.49 | 0.020 |
| Lifestyle | 2.07 | 3.81 | 0.54 | 0.582 |
| Sex | − 262.25 | 16.25 | − 16.13 | < 0.001 |
| Age | 35.67 | 19.79 | 1.80 | 0.071 |
| %CA | 36.80 | 32.83 | 10.25 | < 0.001 |
| Ix/Iy | 125.99 | 28.68 | 4.39 | < 0.001 |
| Maximum slope | − 4.61 | 17.44 | 3.05 | 0.041 |
SE standard error, T value of the t test, Age mean age of each individual, %CA percent of cortical area in a cross section, Ix/Iy ratio of Ix and Iy, R2 = 0.229.
aSide of the body was used as a random effect with SD = 13.54. Bold indicates statistically significant at p < 0.05.
Table 2.
Results of the linear mixed model (LMM) calculated for Neandertalsa.
| Effect | Value | SE | T | p |
|---|---|---|---|---|
| Intercept | 1123.98 | 4437.10 | 0.25 | 0.800 |
| Years (BP) | − 0.0012 | 0.012 | -0.10 | 0.920 |
| Sex | − 395.26 | 463.85 | -0.85 | 0.420 |
| %CA | 12.41 | 29.25 | 0.42 | 0.680 |
| Ix/Iy | 804.46 | 1945.94 | 0.41 | 0.690 |
| Maximum slope | − 0.534 | 2.24 | − 0.23 | 0.820 |
SE standard error, T value of the t test, %CA percent of cortical area in a cross section, Ix/Iy ratio of Ix and Iy, R2 = 0.0054.
aSide of the body was used as a random effect with SD = 143.97. Bold indicates statistically significant p < 0.05.
Figure 1.
A graphic representation of the association between the standardized indicator of robusticity (size-adjusted J) and analyzed predictors in a cross-section of the femur. (A) Standardized indicator of robusticity (size-adjusted J) according to type of lifestyle. (B) Standardized indicator of robusticity (size-adjusted J) according to sex. (C) Association between standardized indicator of robusticity (size-adjusted J) and %CA. (D) Association between standardized indicator of robusticity (size-adjusted J) and Ix/Iy. (E) Distribution of standardized indicator of robusticity (size-adjusted J) during the chronological periods. (F) Association between standardized indicator of robusticity (size-adjusted J) and maximum slope of the terrain. HG hunting-gathering, P pastoralism, S seminomadic, AP agropastoralism, F farming, R rural, UF urban/farming, U urban.
Discussion
Spatial autocorrelation
The populations of H. sapiens are not evenly distributed in space; therefore, some geographical regions are over-represented by human groups. Our results do not clarify which factors among gene flow43, migration leading to a patchy distribution of a given variable44,45, a spatiotemporal pattern in the distribution of food, or various biotic and abiotic factors46 contributed the most to the spatial autocorrelations of femoral robusticity indicator (size-adjusted J) in H. sapiens. Most of the analyzed populations belonged to hunter-gatherer or nomadic societies and were from periods in which significant changes occurred in the structure and economy of populations. Therefore, it can be assumed that this spatial autocorrelation may result from two factors: migration events causing gene flow and the shifts in the amount of physical activity between the analyzed societies.
The Neandertal samples were not spatially dependent. It is difficult to characterize the chronospatial diversity of Neandertals due to an insufficiently large sample; however, the northern regions of Eurasia were settled by heavier and more robust individuals than those in South Europe37,47,48. Moreover, the fossils from Asia are dated from 122 to 54 ka; in turn, the European samples range from 47 to 36 ka. The observed lack of spatial autocorrelation can be explained by the overlapping of two variables: the large differences in chronology and the non-uniform distances between the sites47.
Variation in the index of femoral robusticity
The high degree of variation in cross-sectional properties may be evidence of diverse ecology and subsistence demands in the analyzed H. sapiens groups. According to previous research12,14,25,48–51, the most important factors shaping hominin robusticity of the lower limbs are levels of mobility related to subsistence strategies, lifestyle, terrain, and climatic factors. Another study indicated a gradual decline in the cross-sectional properties of the lower limb during the Holocene due to intensification in food production22. Our study shows a slightly different relationship between the analyzed properties, factors such as years (BP), sex, two cross-sectional properties (%CA and Ix/Iy), and terrain have the greatest impact on the index of femoral robusticity (size-adjusted J).
Differences between females and males are more pronounced in the upper than in lower limbs12,13,26,36,52. Highly mobile groups are more sexually dimorphic than less mobile horticultural and industrial societies due to the greater role of the male in hunting activities compared to females26,36. However, Wescott12 emphasized that this kind of conclusion should be drawn based on the analysis carried out within subsistence strategies. The GAMM did not analyze whether mobile populations were more sexually dimorphic than sedentary groups; however, our results confirmed the previous research25,26,15,53 that sex was an important factor correlating with the level of femoral robusticity indicator (size-adjusted J). Apart from the amount of physical activity, other factors associated with the endocrine system, osteoporosis, genes, and development of the long bones may also be responsible for sex differences in the femoral rigidity54–56. Differences in the expansion period of the periosteal and endosteal surfaces cause the cortical bone mass in males to be placed farther from the neutral axis of the long bone cross-sections than it is in females56, thus explaining the greater bending rigidity in men than in women shown in our research.
Individuals of H. sapiens with greater bending rigidity in the anteroposterior plane (Ix/Iy > 1.0) were characterized by higher indexes of femoral robusticity (size-adjusted J) than samples with greater bending rigidity in the mediolateral plane (Ix/Iy < 1.0). The Ix/Iy ratio level reflects the variation in physical activity, as changes in levels of mobility may correlate with anteroposterior but not necessarily mediolateral bending41. If this assumption is accurate, we can expect that individuals of H. sapiens exposed to high mechanical loads (high index of size-adjusted J) and bending rigidity in the anteroposterior plane (Ix/Iy > 1.0) were also characterized by a wide variety of physical activity types of the lower limbs. Also, according to our analysis, the more robust the femur (size-adjusted J), the higher the content of cortical surface (%CA), which means that femurs more resistant to bending and with higher torsional rigidity, are better adapted to axial loads.
Populations occupying hilly and mountainous regions showed greater standardized indicators of femoral robusticity than populations from flat terrain20,41. We also found that H. sapiens inhabiting mountainous terrain exhibited more robust femora than individuals from lowlands. This association may partially explain why populations with the same lifestyle but inhabiting various terrain are characterized by different femoral biomechanical properties. It has also been suggested that rough terrain should exert the strongest influence on anteroposterior bending loads near the knee joint18,19,41. Perhaps that is why our model showed that greater degrees of femoral robusticity (size-adjusted J) were connected with increases in anteroposterior bending (values of Ix/Iy greater than 1.0).
Our statistical analysis also showed a decrease in the polar moment of area (size-adjusted J) in H. sapiens over the duration of chronological periods. Other research exhibited a fluctuating pattern rather than consistently observing decreasing or increasing trends6,22,16. Due to a lack of temporal decline in femoral head size and articular surfaces, Ruff et al.38 claimed that a decrease in mechanical loading on the postcranial skeleton, as opposed to genetic factors, was responsible for changes in femoral properties. In turn, Pearson37 suggested that fluctuations in bone rigidity were also a result of climate change. However, skeletal proportions are correlated with climate, and since the cross-sectional properties used here were standardized to the biomechanical length of the femur and body mass, we can expect that climate will not directly infleunce variation in femoral rigidity. On the other hand, climate influences environmental conditions, which can favor certain physical activity types (such as foraging) that require substantial mechanical power37,57. This means that climate may indirectly and partially influence their levels despite the standardization of the cross-sectional properties.
Lower levels of mechanical stimuli can be attributed to changes in food production associated with increased sedentism in European H. sapiens from the Upper Paleolithic to recent times22,49. Our results do not confirm the relationship between a more sedentary lifestyle and a decline in the femoral robusticity indicator of H. sapiens. Our assessment of the type of lifestyle may be prone to error since information on some human societies is still scarce, and foraging behavior is flexible due to short-term or local changes in the environment and food abundance. Other research on femoral rigidity and type of lifestyle has also failed to indicate any morphological pattern12,22,49,58,59. Stock and Pfeiffer14 drew different conclusions, as they found stronger lower limbs in a Later Stone Age African population than in a mobile marine group from the Andaman Islands. However, this research compared two populations from different chronological periods and geographical regions, influencing the results. The pattern between the index of femoral robusticity and type of lifestyle has not been revealed in this study, as the variation in physical activity levels among populations with the same type of lifestyle is large. Perhaps, the use of generalized categories typically for lifestyle is too broad even when analyzing large datasets.
Changes in diaphyseal rigidity begin to develop at an early stage of ontogeny as children improve their locomotor skills33,60 and may persist after a prolonged decline in physical activity levels61. In young females, the time of long-term loading before or after menarche is another crucial factor that regulates the reaction of the periosteal surface of the long bones to mechanical stimuli later in life62. Therefore, a gradual decline in the femoral rigidity observed in H. sapiens between the Middle Paleolithic to the present time may result from a different time of occurrence and lower physical activity levels before maturity. In addition, the increase in nutritional stress during the transition to agriculture may also contribute to a decline in postcranial robusticity33.
An increase in bone rigidity is observed until the fourth decade16 after which females show a decrease and males an increase in values of the biomechanical properties63. In our research, the variable age did not significantly correlate with the femoral robusticity indicator (size-adjusted J) of H. sapiens, which may be due to different age distribution and classes. Some individuals were assigned an average age of 38 years since the samples could not be classified more precisely in this regard. Perhaps, age is a relevant factor affecting changes in bone rigidity; however, for material comprising past populations, where the number of older adults (> 50 years old) is low, the aging trend may be ambiguous.
Values of size-adjusted J of the Neandertals fall within the range of the H. sapiens from the Mesolithic and Upper Paleolithic. However, it is difficult to indicate the group of H. sapiens to which two other Neandertal femoral properties (%CA and Ix/Iy) are most similar, as the relative cortical area and cross-sectional rigidity index showed no clear trend of changes in the former. The descriptive statistics showed that each Neandertal individual was characterized by a different level of torsional and bending rigidity loads of the lower limb. This variation may reflect an adaptation to physical activity experienced by Neandertal individuals during development64; however, it is still unclear how the level and type of physical activity may have differentially correlate with their femoral rigidity. Our results revealed that the variability in the standardized indicator of femoral robusticity in Neandertals was not caused by any of the investigated variables (i.e., chronological period, %CA, Ix/Iy, sex, or terrain). The coefficients of variation (CVs) of Neandertal variables are within the range of variation in human groups (Tables S3, S4), so we believe that the structure of their long bones was susceptible to changes to minimize the environmental impact pressure65,66.
Some research has revealed greater cross-sectional variables in Neandertals than in H. sapiens8,50; other research has found no differences in the level of robusticity between these two hominin species29,32. Nevertheless, differences in the femoral index and rigidity between Neandertals and H. sapiens are usually interpreted as differences in climatically-influenced types of habitual behaviors, hip mechanics, mechanical stress, or activity levels and patterning32,16,64,67. We showed that, whereas biological and environmental factors did not correlate with the variation in the femoral robusticity indicator (size-adjusted J) of Neandertals, some of them correlated with the femoral structures of H. sapiens. Perhaps those differences were not the level of environmental factors but the developmental plasticity of the postcranial skeleton.
The dental growth of Neandertals was accelerated relative to the dental development of H. sapiens68,69 (but see70,71). Still, the growth trajectory of Neandertal femora may appear delayed due to the accelerated dental development in Neandertals72. This slow femoral development was probably not directly affected by climate factors such as temperature or nutritional stress, given the lack of similarities in growth trajectory between Neandertals and the cold-adapted Inuit population72. The differences in skeletal morphology between Neandertals and H. sapiens at the beginning stage of postnatal development were probably caused by various genetic components72–74. The research on Neandertal locomotor efficiency shows that despite their shorter total lower limb length, no selective disadvantage in terms of locomotion on sloped terrain was found75. Moreover, an increase in the overall bending rigidity of the tibial midshaft is observed in Neandertals from non-flat terrain21. Therefore we suggest that the lower limbs of Neandertals are susceptible to bone modeling affected by mechanical loading but may need stronger stimuli than H. sapiens to cause changes in biomechanical properties. This can be supported by the concept of bone functional adaptation, assuming that bone tissue responds to mechanical stimuli, adjusting changes in its size, shape, and distribution76. According to Frost35,77, each bone has a threshold range at which bone strains above this threshold activate mechanically-controlled modeling as, e.g., bone resorption and deposition on the endosteal and periosteal surface. Perhaps, the level of mechanical loading was not high enough to generate modeling responses in the biomechanical properties of Neandertal long bones because the minimum effective signal may differ from that of H. sapiens.
Although all known Neandertal femurs for which body mass and biomechanical length were assessed, the sample size is small (but see other research6,16,78). Therefore, the ‘n effect’ was controlled in the analysis. Nevertheless, the possibility that non-significant predictors may be due to sample size effects should be taken into account so that new findings may better explain the changes in the biomechanical properties of Neandertal femora.
The research shows that factors such as terrain slope, level and type of physical activity, and mobility may have played an important role in shaping cross-sectional properties21,34,41,15,60. Statistical analysis on a large sample showed that variation in the femoral robusticity indicator of H. sapiens changed over time and was influenced by sex, terrain, and two biomechanical properties (%CA, Ix/Iy). In the case of the Neandertal samples, variation in femoral was not influenced by any of the analyzed factors. Since our research is not an experimental study that allows testing cause–effect relationships, it is difficult to identify precisely the reasons for the differences in the results between the samples of H. sapiens and Neandertals. Moreover, this cross-sectional study analyzes each individual at only a one-time point, not longitudinally. Nevertheless, based on the differences in the growth trajectories between H. sapiens and Neandertals69–72, we suggest that depending on what stage of postnatal development the mechanical loading begins at, the response of long bone will vary in these two hominin species. Second, H. sapiens and Neandertals may differ in the set point for minimum effective strain stimulus that causes bone changes35 as measured by biomechanical properties. The midshaft of the tibia was more responsive to loading than the femur among living women10; while Neandertals and Upper Paleolithic H. sapiens from mountainous terrain exhibited greater overall anteroposterior and mediolateral relative bending rigidity in the tibial midshaft than individuals from mixed and flat terrain21. This indicates regional variability in the distribution of strain in the lower limb and within a particular bone10 (but see79), proving that a more accurate interpretation of behavior will be possible when analysis of the plasticity levels of long bones has been conducted. Therefore, future research should focus not only on other elements of the postcranial skeleton but also on examining the level of bone response to different degrees of stimuli. The results may help us understand the differences in diaphyseal rigidity between hominin species and explain the causes of variability in robusticity within an individual.
Summary
Our research shows that a lot of data on biological variables needs to be tested for spatial autocorrelation to avoid possible bias. Statistical analysis of the biomechanical properties of the lower limbs confirms previous studies, which showed a significant effect of variables such as sex, chronological periods, and terrain slope on femoral robusticity indicator. The decline in the diaphyseal rigidity of H. sapiens over certain chronological periods was not related to the type of lifestyle, as no difference in the indicator of robusticity was found between farmers, hunter-gatherers, pastoralists, seminomads, rural and urban societies. Perhaps this decline in postcranial robusticity was caused by a lower level of long-term loading during adolescence and an increase in nutritional stress during the transition to agriculture. Our analysis also clarified a positive correlation between the femoral robusticity indicator and two other biomechanical properties (%CA and Ix/Iy). Increases in %CA and anteroposterior rigidity (Ix/Iy > 1.0) may have helped the femur adjust to high mechanical loads during the life of an H. sapiens individual. The LMM showed that biological and environmental factors did not correlate with variation in the femoral robusticity indicator of Neandertals. This finding is surprising and may suggest the differences in the femoral response to mechanical loading between these two hominin species. As this research is not an experimental study, it is not possible to precisely indicate what levels of the analyzed variables are needed to obtain the effect of changes in biomechanical properties. However, based on the results, we suggest that the Neandertal femur required a different level of mechanical stimulus to produce a morphological response in the long bone than H. sapiens. The development of the Neandertal femur was delayed compared to that of H. sapiens, and possibly the level of mechanical stimuli was too low to produce a morphological response in Neandertal long bones. Assuming the lower limbs of H. sapiens were more susceptible to external factors than Neandertals, conclusions based on a comparative analysis between these two hominin species should be drawn with caution. The use of models to investigate the biomechanical properties of long bones enables the consideration of more variables and enhances the study of biological variability.
Material and methods
The biomechanical properties of the femur used in this study were derived from three different sources: (1) the European data set, (2) new CT images of various osteological materials, and (3) the subject literature. Each type of material was described separately to clarify our methodological approach. The final database contained cross-sectional properties of 1972 individuals, of which 1750, 196, and 26 were derived from the European data set, new scans, and literature, respectively.
Material from the European data set
The European data set was created as part of a project financed by the US National Science Foundation and carried out between 2007 and 2015. Within this project, a large amount of representative material, including many European populations from several major environmental transitions, was collected41. The database thus created contains the following variables for 2177 individuals: date range, geographic region, culture, site, geographical coordinates of the site, characteristics of the relevant terrain, sex, age range, linear dimensions of the postcranial skeleton, estimated anatomical stature and body mass, and cross-sectional dimensions of humeri, femora, and tibiae (for a detailed description of variables, see41). Biomechanical properties and other variables of all samples included in the European data set were collected using the same methodological approach41.
Cross-sectional properties in the European data set were calculated for long bones such as the humerus (1578 individuals), femur (1830 individuals), and tibia (1652 individuals). In the presented study, only femoral biomechanical properties from the European data set were analyzed; thus, data for further analysis contained 1830 individuals. Due to the low level of directional asymmetry of the lower limbs80, femoral biomechanical properties were selected from each individual from only one side of the body. Information about the side of the body was coded and used in the statistical models as a random factor. Since long bone diaphyseal rigidity is closely related to body size, some of the biomechanical properties of the weight-bearing lower limbs (e.g., J) had to be standardized to body mass and biomechanical length81. Therefore, individuals without estimated body mass and measured biomechanical femoral length were removed from the database. Of 1830 individuals, the amended data set consisted of 1750 individuals from the Early Upper Paleolithic (32,000–26,000 BP) to very recent times (after 1900 AD), aged between 15 and 87 years and from almost all of Europe41.
Furthermore, additional variables, such as the type of subsistence economy, were added to the amended data. Each individual or population was assigned one of the following types of lifestyle: hunting-gathering, farming, agropastoralism, pastoralism, seminomadic, rural, urban, urban/farming. This type of variable was added because each type of lifestyle is associated with a slightly different pattern of activity that influences bone development and formation during life11,13. In our opinion, biomechanical analysis in the context of lifestyle will expand knowledge of past populations and provide insights into behavioral diversity. While lifestyle assessment for some populations may be problematic, this was done based on archaeological and ethnographic literature, which allowed a complex view. Human societies that relied on hunting wild animals and foraging plants (e.g., fruits, vegetables, nuts) were classified as hunter-gatherers. The farming category was assigned to settled populations where domesticated crops (especially cereals and pulses) was the primary economic activity, while partially occupied human groups that relied on plant cultivation and livestock raising were classified as agropastoralists. Populations whose main economic activity was based on domesticated livestock such as goats, chickens, yaks, camels, sheep, and cattle were assigned to pastoralism. Seminomadic groups were defined as those for which evidence of seasonal migration and crop cultivation during periods of settlement was found. The rural category was assigned to communities living in nonurban areas where was a low ratio of inhabitants and the most important economic activities were usually agriculture and production of raw materials, but unlike farming societies, their settlements were close to urban centers. The urban category included heterogeneous groups in terms of socioeconomic status, living in densely populated settlements, where the most inhabitants have nonagricultural activities such as handicrafts and trade. The last lifestyle category, urban/farming, was assigned to populations growing crops (and sometimes also livestock) in towns and other urban areas. Table S1 contains the detailed list of human populations divided into archaeological/historical periods and geographical regions.
Scanned material
To expand the database, femora from Neolithic populations from Poland (Bronocice, Kazimierza Mała, Koniusza, Samborzec, Smroków, Złota), modern Australian aborigines, and a mediaeval society from Poland (Ostrów Lednicki) were scanned in the cranio-caudal direction using cone beam computed tomography (CBCT) technique with the standard protocol (0.625 × 0.625 × 0.625 mm3) at a power level of 120 kV. All femurs were scanned using Fidex-FS CT system at the University Center of Veterinary Medicine (Poznań University of Life Sciences). The lower limbs of individuals from three Iranian populations (Dinkha Tepe, Hasanlu, and Tepe Hissar) were scanned by The Open Research Scan Archive using a CT with the same standard protocol (0.625 × 0.625 × 0.625 mm3). Based on the subject literature, for each new scanned individual, the same variables were collected as in the European data set: date range, geographic region, culture, site, and geographical coordinates of the site. Characteristics of the terrain were calculated within a 10 km radius of each archaeological site using QGIS software. According to Holt et al.19, a 10 km radius covers the terrain of a single-day foraging trip.
The material consisted of femora dated from 4950 to 3581 BP, derived from Tepe Hissar, located in the north-eastern Central Iranian Plateau. Excavations conducted by the University of Pennsylvania Museum show this settlement was occupied constantly from the fifth to the second millennium BC. The archaeological data suggest that the subsistence economy was based mainly on agriculture; thus, the investigated population was assessed as having been farmers82. Individuals from the Złota population originated in a Neolithic cemetery, dated from 4450 to 4180 BP, and located in a small Polish village near the river Żyć83. The instability and small size of the settlements, the chronological sequence of their development, and the great importance of animal husbandry in the economy suggest that this population was partially nomadic but also participated in agriculture and raising livestock84,85. Individuals from these human groups were assessed as agropastoralists. The remaining Polish Neolithic populations (Bronocice, Kazimierza Mała, Koniusza, Samborzec, and Smroków), dated to the period from 4210 to 3910 BP, inhabited the Little Poland Upland. Anthropological and archaeological literature indicates a partly nomadic lifestyle, including plant cultivation and breeding of livestock84,86; therefore, they were also classified as agropastoralists.
Moreover, the osteological material of two populations from West Iran was investigated. Dinkha Tepe (3252–3096 BP) and Hasanlu (3550–2350 BP) were settlements based on farming, located in fertile valley areas86. Another group derived from a mediaeval cemetery located in Ostrów Lednicki (Western Poland) dated from the mid-11th to the end of the fourth century AD87. This was a homogenous population in terms of socioeconomic structure; it relied mainly on agriculture but occasionally bred domestic animals (see Kubicka et al.87). Therefore, it was classified as a group of farmers. In the research, the femoral bones of Australian aborigines were also scanned. These individuals belonged to a modern hunter-gatherer society dated to the end of the nineteenth century AD, from West and South Australia88.
Skeletal remains accepted for the study were free of observable pathological changes (e.g., fractures, osteophytes, porosity) and free of visible wear on the periosteal surface of the shaft. The intact surface of the shaft is important because accurate modeling of the periosteal surface enables a high level of accuracy in the calculation of cross-sectional geometric properties9, especially since the total area is closely correlated with the polar moment of area, which is used to estimated diaphyseal rigidity9. The sex and age of the human populations from Australia, Ostrów Lednicki, Bronocice, Kazimierza Mała, Koniusza, Samborzec, Smroków, and Złota were assessed based on the standards of Buikstra and Ubelaker89 and White and Folkens90, using pelvis and skull (morphological traits and cranial suture closure). The individuals from Iran were classified as unknown sex due to the lack of bones other than femora that might have been used to assess them. The age status of these individuals was defined based on the maturation stages of the distal femoral end, femoral head, greater trochanter and lesser trochanter, using the standards of Buikstra and Ubelaker89 for the timing of the fusion of various postcranial elements. Individuals with a fully fused greater trochanter, lesser trochanter and a united (i.e. almost fused) femoral head and distal end were assessed as adults and included in the research. A more precise classification in terms of age was not possible, so that all individuals from the Iranian populations were assigned a mean age of 38 years. A similar solution was applied by Ruff41, who assigned all individuals lacking diagnostic elements an age of 38 years, which was the mean age for the entire European data set.
According to Auerbach and Ruff91, body mass was estimated with the use of mean values calculated from three formulas92–94, using femoral head superoinferior breadth. The biomechanical length of the femur was measured on CT images as the distance between a line tangential to the distal surfaces of the medial and lateral condyles and the deepest point on the superior surface of the femoral neck95. Next, an additional variable, such as lifestyle type, was added for each analyzed population, based on anthropological and archaeological literature. The newly scanned data consisted of 196 right femora of adult individuals (over 20 years old) from 10 different human populations from eastern Europe, western Asia, and Australia (see details in Table S1, S2). In cases where the right femur was poorly preserved, the left was included in the study. The femurs have been used with permission from museums where the osteological material is stored (the Museum of the First Piasts at Lednica, the University of Pennsylvania Museum of Archaeology and Anthropology, the Open Research Scan Archive at Penn). No administrative and ethical permissions were obtained because (1) according to Polish law there is no need to obtain permission from a biological commission when research is carried out on historical osteological collections, (2) no new archaeological excavations were carried out to gain the osteological material, (3) the research protocol used in this study was already tested by other researchers. The authors confirm that all stages of the research (collection, CT scanning and analysis) were performed in accordance with the regulations on the analysis of human remains.
Material from the literature
According to the bioarchaeological literature, the femoral biomechanical properties of 13 adult Neandertals belonging to presumably 4 females (La Ferrassie 2, Sima de las Palomas 96, Shanidar 6, Tabun 1), and 9 males (Saint-Cesaire 1, Spy 2, La Ferrassie 1, Feldhofer 1, Fond-de-Forêt 1, Chapelle-aux-Saints 1, Amud 1, Shanidar 4 and 5) were collected50,96–100. Similar data from the literature were found for H. sapiens from the Upper and Middle Paleolithic. Tables S1 and S2 contain detailed lists of individuals. According to the methodology described by Ruff41 for the European data set, the following variables were collected for each individual: chronological date range, geographic region, coordinates of the site, archaeological site, sex, age range, biomechanical length of the femur, estimated anatomical stature and body mass, and raw cross-sectional dimensions of the femur. The characteristics of the terrain were calculated using QGIS software based on the geographical coordinates of the archaeological sites.
Femoral biomechanical properties
The biomechanical geometric properties of the femora being scanned for the first time were calculated using the methodology proposed by Ruff41. The long bones were positioned according to a procedure developed by Ruff and Hayes101. A cross-sectional image was obtained using OsiriX software, for which the biomechanical geometric properties were then calculated using NIH ImageJ software with the Moment Macro developed by Ruff102. For each CT scan of the femur, a cross-section was created in the transverse plane, at 35% of biomechanical length as measured from the distal end of the bone. We focused only on the 35% cross-section, since this region is one of the most frequently analyzed (based on the European Data Set and paleoanthropological literature), and due to the massiveness of the distal femoral epiphysis, the preservation of this part is usually very good with no damage to the outer surface. For each 35% cross-section, five variables were determined: cortical area (CA), total area (TA), polar moment of area (J), and second moment areas of inertia about the X and Y axes (Ix and Iy, respectively, Table 3). Cortical and total areas were used to calculate the relative cortical area in a cross-section (Table 3). Ix and Iy were used to obtain the ratio of second moment areas of inertia about the X and Y axes, describing in which plane (anteroposterior or mediolateral) bending rigidity was greater (Table 3). We chose biomechanical geometric properties that are reported routinely in the subject literature in order to compare our findings with other research. Moreover, the calculated properties enable characterization of the axial, torsional, and bending rigidities of the lower limb. Various methods of standardization of biomechanical properties are still being debated; however, we used the procedure described in Ruff41, where the polar moment of area (J) was standardized to the estimated body mass and biomechanical length of the femur. Previous studies22,40,16,103 show that this standardized procedure of cross-sectional geometric properties is prone to errors. This aspect is important, especially in the Neandertal samples that are in a different state of preservation. Moreover, this standardization method is widely used and accepted by other paleoanthropologists; therefore, the new calculated values can be compared with previous results in further research. Further statistical analyses and interpretation of the results were carried out for the standardized indicator of femoral robusticity (size-adjusted J). The descriptive statictis are available in the supplementary material (Tables S3, S4).
Table 3.
Description of variables used in the models.
| Variable | Description |
|---|---|
| Dependent variable | |
| J (mm4) | Polar moment of area; describes resistance to torsional and bending rigidity loads. The variable is used as an indicator of robusticity; therefore, this variable was accepted in this research as an equivalent of femoral robusticity |
| Independent variables | |
| %CA | Relative amount of cortical area in a cross section, reflecting distribution of cortical area vs subperiosteal area as well as the resistance of the shaft to axial loadings. Calculated as: %CA = (CA/TA) × 100%, where CA is cortical area and TA is total area |
| Ix/Iy | Ratio of Ix (second moment area of inertia about the X axis) and Iy (second moment area of inertia about the Y axis), describing relative and bending rigidity in the anteroposterior plane relative to the mediolateral plane. Values close to 1 indicate equivalent rigidity in these two planes |
| Years (BP) | Age, calculated as the average of the period range |
| Lifestyle | Each population was classified as fitting one of the following types of economy: hunting-gathering, pastoralism, seminomadism, agropastoralism, farming, rural, urban/farming, urban |
| Maximum slope | Maximum slope of the terrain calculated within a 10 km radius of each archaeological site |
| Sex | Each individual was assigned to one of three categories: female, male, or unknown |
| Age | The mean age calculated for each individual as the average of the age range |
Statistical analysis
The observed variable of a population may be spatially dependent, meaning that its value depends on the variable of a neighboring group. Many analyzed variables are spatially correlated for various reasons, such as gene flow or natural selection resulting from similar environments27,43,104. For example, spatial autocorrelation analysis has revealed variation in human cranial variables44. Notwithstanding, studies of the relationship between biological and geographical distances have rarely been applied in biological anthropology27. In anthropological research, a component of spatial pattern should be an important factor, especially in considerations where many populations are examined, and some geographical regions are underrepresented.
Therefore, firstly, spatial autocorrelation for the dependent variable, i.e. the standardized indicator of robusticity (size-adjusted J), was tested separately for H. sapiens (n = 1959) and Neandertals (n = 13). For this purpose, we used Mantel tests105, which indicated whether the data were spatially dependent or not. To test the statistical significance of the Mantel statistics, Monte Carlo permutations with 999 randomizations were calculated106. According to this procedure, close spatial autocorrelation was found for the standardized indicator of femoral robusticity in H. sapiens (Mantel test: rM = 0.062, p = 0.001), whereas for the femoral variable representing Neandertals, the Mantel test was not significant (rM = 0.105, p = 0.105).
Before calculating the models for H. sapiens and Neandertals, two variables (age and years in BP) were calculated for each individual as the average of their range (Table 3). Next, a generalized additive mixed model (GAMM) was used in this study to analyze the effect of several variables on the standardized indicator of femoral robusticity (size-adjusted J) of H. sapiens. The GAMM was an extension of a general additive model enabling the inclusion of random factor and correction in spatial autocorrelation in the model. Therefore, as a response variable, we used the standardized indicator of robusticity (size-adjusted J), whereas years (in BP), type of lifestyle, maximum slope, sex, age, %CA, and Ix/Iy were used as predictors. Due to changes in relative bone rigidity over lifespans16, we decided to add age as a predictor to the GAMM. Differences in hormone levels between females and males also influence bone rigidity and the amount of cortical bone; accordingly, sex was also added to the model107,108. As far as we know, no other study has analyzed the relationship between J and Ix/Iy, and only one study has focused on the possible relationship between J and %CA in femurs109, which means that femoral cross-sectional properties have usually been investigated using separate statistical analyzes. According to Stock and Pfeiffer14, the shafts and epiphyses differ in plasticity. Moreover, the cross-sectional properties (e.g., %CA or Ix/Iy) indicate differences in developmental patterns among femoral shafts during successive locomotor stages and maturation60. Therefore, in our opinion, investigation of how other cross-sectional properties influence femoral robusticity based on a large dataset can provide valuable information about femur biomechanics. Furthermore, the side of the body was used as a random factor, along with the longitude and latitude of the archaeological sites as a spherical spatial correlation (Table 3).
Since the standardized indicator of the femoral robusticity of Neandertals was not spatially dependent, we used a linear mixed model (LMM) to analyze which factors influenced this parameter. The LMM was an extension of simple linear models, enabling the addition of random effects (e.g., side of the body). In this statistical analysis, we decided to use factors similar to those in the model with H. sapiens samples, except that we did not include two variables. The first excluded factor was the type of lifestyle since Neandertals represent exclusively a society of hunter-gatherers. Individual age was also excluded from the LMM due to the uncertain age assessment of some Neandertal specimens. In the LMM, the standardized indicator of femoral robusticity (size-adjusted J) was used as a response variable; years (in BP), maximum slope, sex, %CA, and Ix/Iy were used as predictors; side of the body was used as a random factor.
To allow some degree of complexity in the functions while avoiding overfitting of the data, we defined the basic dimension, i.e., k = 4110. The Gaussian distribution of errors and the identity link function were applied. As a measure of deviance reduction, we used R2111. The most parsimonious model was selected using the Akaike Information Criterion (Table S5, mgcv library in R112). Moreover, descriptive statistics such as group mean, median, standard deviation, variation, maximum, and minimum were calculated for all biomechanical properties (size-adjusted J, %CA, Ix/Iy) and mean age according to temporal period and sex. All statistical analyses were calculated using R software (R Core Team, 2017).
Supplementary Information
Acknowledgements
We thank Anna Wrzesińska from the Museum of the First Piasts at Lednica, for giving the possibility to use skeletal material from Ostrów Lednicki. We would like to thank the University of Pennsylvania Museum of Archaeology and Anthropology, the Open Research Scan Archive at Penn, Janet Monge and P. Thomas Schoenemann. We also thank Szymon Stelting from Poznań Universty of Life Sciences for technical support in scanning the osteological material. A.M.K thanks the Polish National Agency for Academic Exchange for the scholarship at the Musee de l’Homme, which allowed to conduct this research (PPB/BEK/2018/1/00390/U/00001/02). Last but not least, we thank the reviewers for very valuable comments that improved the quality of this work. This research has been supported by the National Science Centre (NCN 2015/19/N/NZ8/00177). Publication was co-financed within the framework of the Polish Ministry of Science and Higher Education’s program: “Regional Initiative Excellence” in the years 2019–2022 (No. 005/RID/2018/19)”, financing amount 12 000 00 PLN.
Author contributions
M.K. carried out research, acquired financial support and resources, collected and curated data, made cross-sections, conducted the data analysis as well as wrote the manuscript. A.B. conceptualized the research idea, acquired resources, provided material, managed and advised on the project, conducted the data analysis, wrote the manuscript initial draft. J.K. conceptualized the research idea, conducted the data analysis as well as wrote the manuscript initial draft, W.N. conceptualized the research idea, provided material, managed and advised on the project, wrote the manuscript initial draft. E.H. provided material, reviewed the manuscript. A.S. provided material, reviewed the manuscript. J.P. provided material, reviewed the manuscript.
Data availability
The data is available on an external repository (Mendeley Data: 10.17632/zsnydxhhnf.1).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the link in the Data availability section where, “The data is available on an external repository (Mendeley Data: 10.17632/fdpxmn5pjx.1).” now reads: “The data is available on an external repository (Mendeley Data: 10.17632/zsnydxhhnf.1).”
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/13/2022
A Correction to this paper has been published: 10.1038/s41598-022-10446-y
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08405-8.
References
- 1.Judex S, Carlson KJ. Is bone’s response to mechanical signals dominated by gravitational loading? Med. Sci. Sports Exerc. 2009;41:2037–2043. doi: 10.1249/MSS.0b013e3181a8c6e5. [DOI] [PubMed] [Google Scholar]
- 2.Meakin LB, Price JS, Lanyon LE. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front. Endocrinol. (Lausanne) 2014;5:1–13. doi: 10.3389/fendo.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banes AJ, et al. Mechanical forces and signaling in connective tissue cells: Cellular mechanisms of detection, transduction, and responses to mechanical deformation. Curr. Opin. Orthop. 2001;12:389–396. [Google Scholar]
- 4.Kubicka AM, Myszka A. Are entheseal changes and cross-sectional properties associated with the shape of the upper limb? Am. J. Phys. Anthropol. 2020;173:293–306. doi: 10.1002/ajpa.24096. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes JA, Knüsel CJ. Activity-related skeletal change in medieval humeri: Cross-sectional and architectural alterations. Am. J. Phys. Anthropol. 2005;128:536–546. doi: 10.1002/ajpa.20147. [DOI] [PubMed] [Google Scholar]
- 6.Trinkaus E, Ruff CB. Femoral and tibial diaphyseal cross-sectional geometry in Pleistocene Homo. PaleoAnthropology. 2012;2012:13–62. [Google Scholar]
- 7.MacIntosh AA, Davies TG, Ryan TM, Shaw CN, Stock JT. Periosteal versus true cross-sectional geometry: A comparison along humeral, femoral, and tibial diaphyses. Am. J. Phys. Anthropol. 2013;150:442–452. doi: 10.1002/ajpa.22218. [DOI] [PubMed] [Google Scholar]
- 8.Kubicka AM, Nowaczewska W, Balzeau A, Piontek J. Bilateral asymmetry of the humerus in Neandertals, Australian aborigines and medieval humans. Am. J. Phys. Anthropol. 2018;167:46–60. doi: 10.1002/ajpa.23601. [DOI] [PubMed] [Google Scholar]
- 9.Stock JT, Shaw CN. Which measures of diaphyseal robusticity are robust? A comparison of external methods of quantifying the strength of long bone diaphyses to cross-sectional geometric properties. Am. J. Phys. Anthropol. 2007;134:412–423. doi: 10.1002/ajpa.20686. [DOI] [PubMed] [Google Scholar]
- 10.Macintosh AA, Stock JT. Intensive terrestrial or marine locomotor strategies are associated with inter- and intra-limb bone functional adaptation in living female athletes. Am. J. Phys. Anthropol. 2019;168:566–581. doi: 10.1002/ajpa.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges P. Changes in activities with the shift to agriculture in the southeastern united states. Curr. Anthropol. 1989;30:385–394. [Google Scholar]
- 12.Wescott DJ. Effect of mobility on femur midshaft external shape and robusticity. Am. J. Phys. Anthropol. 2006;130:201–213. doi: 10.1002/ajpa.20316. [DOI] [PubMed] [Google Scholar]
- 13.Carlson KJ, Grine FE, Pearson OM. Robusticity and sexual dimorphism in the postcranium of modern hunter-gatherers from Australia. Am. J. Phys. Anthropol. 2007;134:9–23. doi: 10.1002/ajpa.20617. [DOI] [PubMed] [Google Scholar]
- 14.Stock J, Pfeiffer S. Linking structural variability in long bone daiphyses to habitual behaviors: Forager from the Sothern African Later Stone Age and the Andaman Islands. Am. J. Phys. Anthropol. 2001;115:337–348. doi: 10.1002/ajpa.1090. [DOI] [PubMed] [Google Scholar]
- 15.Sládek V, Berner M, Sailer R. Mobility in Central European Late Eneolithic and Early Bronze Age: Femoral cross-sectional geometry. Am. J. Phys. Anthropol. 2006;130:320–332. doi: 10.1002/ajpa.20372. [DOI] [PubMed] [Google Scholar]
- 16.Friedl L, Eisová S, Holliday TW. Re-evaluation of Pleistocene and Holocene long bone robusticity trends with regards to age-at-death estimates and size standardization procedures. J. Hum. Evol. 2016;97:109–122. doi: 10.1016/j.jhevol.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Ruff CB. Skeletal structure and behavioral patterns of prehistoric Great Basin populations. In: Hemphill B, Larsen C, editors. Prehistoric Lifeways in the Great Basin Wetlands: Bioarchaeological Reconstruction and Interpretation. University of Utah Press; 1999. pp. 71–102. [Google Scholar]
- 18.Ruff CB. Mechanical determinants of bone form: Insights from skeletal remains. J. Musculoskelet. Neuronal Interact. 2005;5:202–212. [PubMed] [Google Scholar]
- 19.Holt B, et al. Temporal and geographic variation in robusticity. In: Ruff CB, et al., editors. Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century. Wiley; 2017. pp. 91–132. [Google Scholar]
- 20.Pfeiffer S, Harrington L. Regional Continuity and Local Challenges to Resilience among Holocene Hunter-Gatherers of the Greater Cape Floristic Region, South Africa. In: Temple DH, Harrington L, editors. Hunter-Gatherer Adaptation and Resilience. A Bioarchaeological Perspective. Cambridge University Press; 2018. pp. 26–46. [Google Scholar]
- 21.Higgins RW. Reconstructing mobility. Environmental, behavioral, and morphological determinants. In: Carlson KJ, Marchi D, editors. The Effects of Terrain on Long Bone Robusticity and Cross-Sectional Shape in Lower Limb Bones of Bovids, Neandertals, and Upper Paleolithic Modern Humans. Springer; 2014. pp. 227–252. [Google Scholar]
- 22.Ruff CB, et al. Gradual decline in mobility with the adoption of food production in Europe. Proc. Natl. Acad. Sci. 2015;112:201502932. doi: 10.1073/pnas.1502932112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinkaus E, Ruff CB. Diaphyseal cross-sectional geometry of Near Eastern Middle Palaeolithic humans: The humerus. J. Archaeol. Sci. 1999;26:1289–1300. [Google Scholar]
- 24.Niskanen M, Ruff CB, Holt B, Sládek V, Berner M. Temporal and geographic variation in body size and shape of Europeans from the Late Pleistocene to recent times. In: Ruff CB, editor. Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century. Wiley; 2017. pp. 49–89. [Google Scholar]
- 25.Maggiano IS, et al. Cross-sectional analysis of long bones, occupational activities and long-distance trade of the Classic Maya from Xcambo—Archaeological and osteological evidence. Am. J. Phys. Anthropol. 2008;136:470–477. doi: 10.1002/ajpa.20830. [DOI] [PubMed] [Google Scholar]
- 26.Hill EC, et al. An examination of the cross-sectional geometrical properties of the long bone diaphyses of Holocene foragers from Roonka, South Australia. Am. J. Phys. Anthropol. 2020;172:682–697. doi: 10.1002/ajpa.24021. [DOI] [PubMed] [Google Scholar]
- 27.Relethford JH. Geostatistics and spatial analysis in biological anthropology. Am. J. Phys. Anthropol. 2008;136:1–10. doi: 10.1002/ajpa.20789. [DOI] [PubMed] [Google Scholar]
- 28.Trinkaus E, Ruff CB, Conroy GC. The anomalous archaic Homo femur from Berg Aukas, Namibia: A biomechanical assessment. Am. J. Phys. Anthropol. 1999;110:379–991. doi: 10.1002/(SICI)1096-8644(199911)110:3<379::AID-AJPA9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Trinkaus E, Ruff CB. Diaphyseal cross-sectional geometry of Near Eastern Middle Palaeolithic humans: The femur. J. Archaeol. Sci. 1999;26:1289–1300. [Google Scholar]
- 30.Lovejoy CO, Trinkaus E. Strength and robusticity of the Neandertal tibia. Am. J. Phys. Anthropol. 1980;53:465–470. [Google Scholar]
- 31.Trinkaus E, Ruff C. Diaphyseal cross-sectional morphology and biomechanics of the Fond-de-Forêt 1 femur and the Spy 2 femur and tibia. Bull. Soc. R. Belge Anthropol. Prehist. 1989;100:33–42. [Google Scholar]
- 32.Churchill SE. Cold adaptation, heterochrony, and Neandertals. Evol. Anthropol. 1998;7:46–60. [Google Scholar]
- 33.Cowgill LW. The ontogeny of Holocene and late Pleistocene human postcranial strength. Am. J. Phys. Anthropol. 2010;141:16–37. doi: 10.1002/ajpa.21107. [DOI] [PubMed] [Google Scholar]
- 34.Niinimäki S, Narra N, Härk L, Abe S. The relationship between loading history and proximal femoral diaphysis cross-sectional geometry. Am. J. Hum. Biol. 2017;29:e22965. doi: 10.1002/ajhb.22965. [DOI] [PubMed] [Google Scholar]
- 35.Frost HM. Bone, “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 36.Ruff C. Sexual dimorphism in human lower limb bone structure: Relationship to subsistence strategy and sexual division of labor. J. Hum. Evol. 1987;16:391–416. [Google Scholar]
- 37.Pearson OM. Activity, climate, and postcranial robusticity. Curr. Anthropol. 2000;41:569–607. [PubMed] [Google Scholar]
- 38.Ruff CB, Trinkaus E, Walker A, Larse CS. Postcranial robusticity in Homo. I: Temporal trends and mechanical interpretation. Am. J. Phys. Anthropol. 1993;91:21–53. doi: 10.1002/ajpa.1330910103. [DOI] [PubMed] [Google Scholar]
- 39.Trinkaus E, Ruff CB. Diaphyseal cross-sectional geometry of Near Eastern Middle Palaeolithic humans: The tibia. J. Archaeol. Sci. 1999;26:1289–1300. [Google Scholar]
- 40.Ruff CB. Body size, body shape, and long bone strength in modern humans. J. Hum. Evol. 2000;38:269–290. doi: 10.1006/jhev.1999.0322. [DOI] [PubMed] [Google Scholar]
- 41.Ruff CB. Skeletal Variation and Adaptation in Europeans : Upper Paleolithic to the Twentieth Century. Wiley; 2018. [Google Scholar]
- 42.Stock J, Pfeiffer S. Linking structural variability in long bone daiphyses to habitual behaviors: Foragers from the Sothern African Later Stone Age and the Andaman Islands. Am. J. Phys. Anthropol. 2001;115:337–348. doi: 10.1002/ajpa.1090. [DOI] [PubMed] [Google Scholar]
- 43.Sokal RR, Menozzi P. Spatial autocorrelations of HLA frequencies in Europe support demic diffusion of early farmers. Am. Nat. 1982;119:1–17. [Google Scholar]
- 44.Sokal RR, Uytterschaut H. Cranial variation in European populations: A spatial autocorrelation study at three time periods. Am. J. Phys. Anthropol. 1987;74:21–38. doi: 10.1002/ajpa.1330740103. [DOI] [PubMed] [Google Scholar]
- 45.Sokal RR, Oden NL. Spatial autocorrelation in biology. Some biological implications and four applications of evolutionary and ecological interest. Biol. J. Linn. Soc. 1978;10:229–249. [Google Scholar]
- 46.Koenig WD. Spatial autocorrelation of ecological phenomena. Trends Ecol. Evol. 1999;14:22–26. doi: 10.1016/s0169-5347(98)01533-x. [DOI] [PubMed] [Google Scholar]
- 47.Arsuaga JL, et al. Neandertal mtDNA from a Late Pleistocene Human Mandibule from the Cova del Gegant (Spain) In: Condemi S, Weniger G, et al., editors. Continuity and Discontinuity in the Peopling of Europe. Springer; 2011. pp. 213–217. [Google Scholar]
- 48.Churchill SE. Thin on the Ground: Neandertal Biology, Archeology and Ecology. Thin on the Ground: Neandertal Biology, Archeology and Ecology. Wiley; 2014. [Google Scholar]
- 49.Holt BM. Mobility in upper Paleolithic and Mesolithic Europe: Evidence from the lower limb. Am. J. Phys. Anthropol. 2003;122:200–215. doi: 10.1002/ajpa.10256. [DOI] [PubMed] [Google Scholar]
- 50.Shaw CN, Stock JT. Extreme mobility in the Late Pleistocene? Comparing limb biomechanics among fossil Homo, varsity athletes and Holocene foragers. J. Hum. Evol. 2013;64:242–249. doi: 10.1016/j.jhevol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Ruff CB, Larsen CS. Long bone structural analyses and the reconstruction of past mobility: A historical review. In: Carlson KJ, Marchi D, editors. Reconstructing Mobility: Environmental, Behavioral, and Morphological Determinants. Springer; 2014. pp. 13–29. [Google Scholar]
- 52.Stock JT, Pfeiffer SK. Long bone robusticity and subsistence behaviour among Later Stone Age foragers of the forest and fynbos biomes of South Africa. J. Archaeol. Sci. 2004;31:999–1013. [Google Scholar]
- 53.Sparacello V, Marchi D. Mobility and subsistence economy: A diachronic comparison between two groups settled in the same geographical area (Liguria, Italy) Am. J. Phys. Anthropol. 2008;136:485–495. doi: 10.1002/ajpa.20832. [DOI] [PubMed] [Google Scholar]
- 54.Martin RB, Atkinson PJ. Age and sex-related changes in the structure and strength of the human femoral shaft. J. Biomech. 1977;10:223–231. doi: 10.1016/0021-9290(77)90045-8. [DOI] [PubMed] [Google Scholar]
- 55.Kannus P, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann. Intern. Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 56.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J. Clin. Endocrinol. Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 57.Weaver TD, Steudel-Numbers K. Does climate or mobility explain the differences in body proportions between neandertals and their upper paleolithic successors? Evol. Anthropol. 2005;14:218–223. [Google Scholar]
- 58.Stock JT, et al. Body size, skeletal biomechanics, mobility and habitual activity from the Late Palaeolithic to the Mid-Dynastic Nile Valley. In: Pinhasi R, Stock JT, et al., editors. Human Bioarchaeology of the Transition to Agriculture. Wiley; 2011. pp. 347–367. [Google Scholar]
- 59.Bridges PS, Blitz JH, Solano MC. Changes in long bone diaphyseal strength with horticultural intensification in west-central Illinois. Am. J. Phys. Anthropol. 2000;112:217–238. doi: 10.1002/(SICI)1096-8644(2000)112:2<217::AID-AJPA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 60.Swan KR, Ives R, Wilson LAB, Humphrey LT. Ontogenetic changes in femoral cross-sectional geometry during childhood locomotor development. Am. J. Phys. Anthropol. 2020;173:80–95. doi: 10.1002/ajpa.24080. [DOI] [PubMed] [Google Scholar]
- 61.Kontulainen S, et al. Good maintenance of exercise-induced bone gain with decreased training of female tennis and squash players: A prospective 5-year follow-up study of young and old starters and controls. J. Bone Miner. Res. 2001;16:195–201. doi: 10.1359/jbmr.2001.16.2.195. [DOI] [PubMed] [Google Scholar]
- 62.Kontulainen S, Sievänen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J. Bone Miner. Res. 2003;18:352–359. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 63.Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J. Orthop. Res. 1988;6:886–896. doi: 10.1002/jor.1100060613. [DOI] [PubMed] [Google Scholar]
- 64.Volpato, V., Mazurier, A., Puymerail, L. & Macchiarelli, R. Lower limb. Spy I and II internal structure of the femurs and tibia. in Spy Cave—Human Remains 1–10 (2010).
- 65.Skrzypek G, Winiewski A, Grierson PF. How cold was it for Neanderthals moving to Central Europe during warm phases of the last glaciation? Quatern. Sci. Rev. 2011;30:481–487. [Google Scholar]
- 66.Weyrich LS, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–361. doi: 10.1038/nature21674. [DOI] [PubMed] [Google Scholar]
- 67.Weaver TD. The shape of the Neandertal femur is primarily the consequence of a hyperpolar body form. Proc. Natl. Acad. Sci. 2003;100:6926–6929. doi: 10.1073/pnas.1232340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith TM, Toussaint M, Reid DJ, Olejniczak AJ, Hublin JJ. Rapid dental development in a Middle Paleolithic Belgian Neanderthal. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20220–20225. doi: 10.1073/pnas.0707051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith TM, et al. Brief communication: Dental development and enamel thickness in the lakonis neanderthal molar. Am. J. Phys. Anthropol. 2009;138:112–118. doi: 10.1002/ajpa.20898. [DOI] [PubMed] [Google Scholar]
- 70.Tompkins RL. Relative dental development of upper pleistocene hominids compared to human population variation. Am. J. Phys. Anthropol. 1996;99:103–118. doi: 10.1002/(SICI)1096-8644(199601)99:1<103::AID-AJPA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 71.Macchiarelli R, et al. How Neanderthal molar teeth grew. Nature. 2006;444:748–751. doi: 10.1038/nature05314. [DOI] [PubMed] [Google Scholar]
- 72.Thompson JL, Nelson AJ. The place of Neandertals in the evolution of hominid patterns of growth and development. J. Hum. Evol. 2000;38:475–495. doi: 10.1006/jhev.1999.0364. [DOI] [PubMed] [Google Scholar]
- 73.Gunz P, Neubauer S, Maureille B, Hublin JJ. Brain development after birth differs between Neanderthals and modern humans. Curr. Biol. 2010;20:921–922. doi: 10.1016/j.cub.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 74.De León MSP, Zollikofer CPE. Neanderthals and modern humans—chimps and bonobos: Similarities and differences in development and evolution. In: Hublin JJ, Harvati K, Harrison T, editors. Neanderthals Revisited: New Approaches and Perspectives. Springer; 2008. pp. 71–88. [Google Scholar]
- 75.Higgins RW, Ruff CB. The effects of distal limb segment shortening on locomotor efficiency in sloped terrain: Implications for Neandertal locomotor behavior. Am. J. Phys. Anthropol. 2011;146:336–345. doi: 10.1002/ajpa.21575. [DOI] [PubMed] [Google Scholar]
- 76.Barak MM. Bone modeling or bone remodeling: That is the question. Am. J. Phys. Anthropol. 2020;172:153–155. doi: 10.1002/ajpa.23966. [DOI] [PubMed] [Google Scholar]
- 77.Frost HM. Bone’s Mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 78.Ruff CB, Trinkaus E, Walker A, Larsen CS. Postcranial robusticity in Homo. I: Temporal trends and mechanical interpretation. Am. J. Phys. Anthropol. 1993;91:21–53. doi: 10.1002/ajpa.1330910103. [DOI] [PubMed] [Google Scholar]
- 79.Shaw CN, Stock JT, Davies TG, Ryan TM. Does the distribution and variation in cortical bone along lower limb diaphyses reflect selection for locomotor economy? In: Carlson KJ, Marchi D, editors. Reconstructing Mobility: Environmental, Behavioral, and Morphological Determinants. Springer; 2014. pp. 49–66. [Google Scholar]
- 80.Auerbach BM, Ruff CB. Limb bone bilateral asymmetry: Variability and commonality among modern humans. J. Hum. Evol. 2006;50:203–218. doi: 10.1016/j.jhevol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Ruff CB. Long bone articular and diaphyseal structure in old world monkeys and apes. II: Estimation of body mass. Am. J. Phys. Anthropol. 2003;120:16–37. doi: 10.1002/ajpa.10118. [DOI] [PubMed] [Google Scholar]
- 82.Afshar Z. Mobility and economic transition in the 5th to the 2nd millennium B.C. in the population of the Central Iranian Plateau, Tepe Hissar. Durham University; 2014. [Google Scholar]
- 83.Wilk S. A Złota Culture Cemetery at Książnice site 2, Świętokrzyskie Province. In: Kadrow S, editor. Sprawozdania Archeologiczne. Instytut Archeologii i Entologii Polskiej Akademii Nauk; 2013. pp. 311–362. [Google Scholar]
- 84.Włodarczak P. Cemetery of the Corded Ware Culture at Site 17 in Smroków, Słomniki Commune, District of Kraków. Spraw. Archeol. 2006;58:377–400. [Google Scholar]
- 85.Włodarczak P. The past societies 5500–2000BC. Polish lands from the first evidence of human presence to the early Middle Ages. Institute of Archaeology and Ethnology, Polish Academy of Sciences; 2017. [Google Scholar]
- 86.Dyson RH. The Hasanlu Project. Science. 1962;135:637–647. doi: 10.1126/science.135.3504.637. [DOI] [PubMed] [Google Scholar]
- 87.Kubicka AM, Lubiatowski P, Długosz JD, Romanowski L, Piontek J. Directional asymmetry of upper limbs in a medieval population from Poland: A combination of linear and geometric morphometrics. Am. J. Hum. Biol. 2016;28:817–824. doi: 10.1002/ajhb.22873. [DOI] [PubMed] [Google Scholar]
- 88.Kruczkiewicz E. Ossa Australica. Mater. i Pr. Antropol. 1962;58:1–93. [Google Scholar]
- 89.Buikstra, J. E. & Ubelaker, D. H. Standards for data collection from human skeletal remains (Arkansas Archeological Survey Research Series, 1994).
- 90.White T, Folkens P. The Human Bone Manual. Elsevier; 2005. [Google Scholar]
- 91.Auerbach BM, Ruff CB. Human body mass estimation: A comparison of ‘morphometric’ and ‘mechanical’ methods. Am. J. Phys. Anthropol. 2004;125:331–342. doi: 10.1002/ajpa.20032. [DOI] [PubMed] [Google Scholar]
- 92.Ruff CB, Scott WW, Liu AYC. Articular and diaphyseal remodeling of the proximal femur with changes in body mass in adults. Am. J. Phys. Anthropol. 1991;86:397–413. doi: 10.1002/ajpa.1330860306. [DOI] [PubMed] [Google Scholar]
- 93.McHenry HM. Body size and proportions in early hominids. Am. J. Phys. Anthropol. 1992;87:407–431. doi: 10.1002/ajpa.1330870404. [DOI] [PubMed] [Google Scholar]
- 94.Grine FE, Jungers WL, Tobias PV, Pearson OM. Fossil Homo femur from Berg Aukas, northern Namibia. Am. J. Phys. Anthropol. 1995;97:151–185. doi: 10.1002/ajpa.1330970207. [DOI] [PubMed] [Google Scholar]
- 95.Mongle C, Wallace IJ, Mongle CS, Wallace IJ, Grine FE. Cross-sectional structural variation relative to midshaft along hominine diaphysis. II. The hind limb. Am. J. Phys. Anthropol. 2015;158:398–407. doi: 10.1002/ajpa.22802. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes JA, Churchill SE. Throwing in the Middle and Upper Paleolithic: Inferences from an analysis of humeral retroversion. J. Hum. Evol. 2009;56:1–10. doi: 10.1016/j.jhevol.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 97.Plavcan JM, et al. The Regourdou 1 Neandertal body size. C.R. Palevol. 2014;13:747–754. [Google Scholar]
- 98.Bermudez De Castro JM, et al. Early pleistocene human humeri from the gran dolina-TD6 site (sierra de atapuerca, spain) Am. J. Phys. Anthropol. 2012;147:604–617. doi: 10.1002/ajpa.22020. [DOI] [PubMed] [Google Scholar]
- 99.Pomeroy E, et al. Newly discovered Neanderthal remains from Shanidar Cave, Iraqi Kurdistan, and their attribution to Shanidar 5. J. Hum. Evol. 2017;111:102–118. doi: 10.1016/j.jhevol.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 100.Trinkaus E. Sexual differences in Neanderthal limb bones. J. Hum. Evol. 1980;9:377–397. [Google Scholar]
- 101.Ruff CB, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae—A biomechanical investigation: I. Method and general patterns of variation. Am. J. Phys. Anthropol. 1983;60:359–381. doi: 10.1002/ajpa.1330600308. [DOI] [PubMed] [Google Scholar]
- 102.Ruff, C. B. Moment macro for NIH Image and Image J (2006).
- 103.Marchi D, et al. The locomotion of Babakotia radofilai inferred from epiphyseal and diaphyseal morphology of the humerus and femur. J. Morphol. 2016;277:1199–1218. doi: 10.1002/jmor.20569. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez PN, et al. Description and analysis of spatial patterns in geometric morphometric data. Evol. Biol. 2019;46:260–270. [Google Scholar]
- 105.Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- 106.Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial 1–43 (2010).
- 107.Feik SA, Thomas CDL, Bruns R, Clement JG. Regional variations in cortical modeling in the femoral mid-shaft: Sex and age differences. Am. J. Phys. Anthropol. 2000;112:191–205. doi: 10.1002/(SICI)1096-8644(2000)112:2<191::AID-AJPA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 108.Ahlborg HG, Johnell MD, Turner C, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N. Engl. J. Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 109.O’Neill MC, Ruff CB. Estimating human long bone cross-sectional geometric properties: A comparison of noninvasive methods. J. Hum. Evol. 2004;47:221–235. doi: 10.1016/j.jhevol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 110.Santana J, Porto M, Gordinho L, Reino L, Beja P. Long-term responses of Mediterranean birds to forest fuel management. J. Appl. Ecol. 2012;49:632–643. [Google Scholar]
- 111.Weisberg S. Some large-sample tests for nonnormality in the linear regression model: Comment. J. Am. Stat. Assoc. 1980;75:28–31. [Google Scholar]
- 112.Wood SN. A simple test for random effects in regression models. Biometrika. 2013;100:1005–1010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available on an external repository (Mendeley Data: 10.17632/zsnydxhhnf.1).