Highlights
-
•
Habenular hyper connectivity characterizes treatment-resistant depression.
-
•
An interplay between reward and default mode networks is linked to suicidality.
-
•
Abnormal habenula connectivity is a possible mechanism for anhedonia.
Keywords: Treatment-resistant depression, Treatment-sensitive depression, Functional connectivity, resting-state fMRI, Habenula, Anhedonia
Abstract
Background
A significant proportion of patients with major depressive disorder are resistant to antidepressant medication and psychological treatments. A core symptom of treatment-resistant depression (TRD) is anhedonia, or the inability to feel pleasure, which has been attributed to disrupted habenula function – a component of the reward network. This study aimed to map detailed neural circuitry architecture related to the habenula to identify neural mechanisms of TRD.
Methods
35 TRD patients, 35 patients with treatment-sensitive depression (TSD), and 38 healthy controls (HC) underwent resting-state functional magnetic resonance imaging. Functional connectivity analyses were performed using the left and right habenula as seed regions of interest, and the three groups were compared using whole-brain voxel-wise comparisons.
Results
The TRD group demonstrated hyperconnectivity of the left habenula to the left precuneus cortex and the right precentral gyrus, compared to the TSD group, and to the right precuneus cortex, compared to the TSD and HC groups. In contrast, TSD demonstrated hypoconnectivity than HC for both connectivity measures. These connectivity values were significantly higher in patients with a history of suicidal ideation.
Conclusions
This study provides evidence that, unlike TSD, TRD is characterized by hyperconnectivity of the left habenula particularly with regions of the default mode network. An increased interplay between reward and default mode networks is linked to suicidality and could be a possible mechanism for anhedonia in hard to treat depression.
1. Introduction
First line treatments for major depressive disorder (MDD) consist of focussed psychological interventions and antidepressant medications (ADMs) (Malhi et al., 2021). Unfortunately, more than 60% of patients when first prescribed ADMs fail to achieve remission and a further 50% of these do not respond to second and subsequent rounds of ADMs (Taylor et al., 2019). Patients who fail to produce a significant clinical and functional improvement with at least 2 trials of antidepressants from different pharmacologic classes (adequate in terms of dosage, duration, and compliance) are considered to suffer from treatment-resistant depression (TRD) (Gaynes et al., 2020). This definition has also recently been reframed to include patients who fail to respond to neuromodulation and psychotherapies (Malhi et al., 2019). Months or years of trying various treatment regimens without finding relief results in increased emotional and financial burden to the individual, family and caregivers. TRD also causes the highest direct and indirect medical costs among MDD patients. Individuals with TRD are twice as likely to be hospitalized, when compared to patients with treatment-sensitive depression (TSD) and the cost of this hospitalization for TRD is more than six times the mean total cost for TSD (Greenberg et al., 2015). This stresses the importance of understanding the neural mechanisms underlying this disorder to not only achieve timely, effective treatment by identifying these individuals earlier but to also identify novel neural treatment targets.
TRD patients exhibit the same diversity of symptoms, course, history and co-occurring conditions as for TSD patients (Akil et al., 2018). These symptoms heavily interfere with the individual’s life, and include impairments in cognition, sad mood, concentration difficulties, fatigue, and anhedonia (Kennedy & Ceniti, 2018). Anhedonia is broadly defined as lack of interest or the inability to experience pleasure (Delfino et al., 2021). It appears to be one of the main symptoms of TRD, with significant impact on course of treatment (Slupski et al., 2020). It has been considered as an independent somatic symptom in TRD and defined as a target for next generation treatments for these patients, including pharmacological and non-pharmacological interventions (Slupski et al., 2020). Identifying treatment-resistant forms of depression early in the course of the disease could potentially enable clinicians to manage more appropriate treatment in a timely manner.
Neuroimaging-based biomarkers offer potential to identify which depression profile will respond to particular treatments and predict treatment outcomes (Akil et al., 2018, Korgaonkar et al., 2020). In recent years, resting-state functional magnetic resonance imaging (rs-fMRI) has become widely applied in studying brain functional changes as it is more easily replicated and independent of task-related confounds. This approach measures temporal correlations of levels of blood-oxygen dependency signal in multiple brain regions to estimate their interactions and can be used to identify atypical intrinsic brain function in psychiatric disorders. Research suggests the functional architecture of the brain is made up from a collection of interacting but functionally dependent networks (Schaefer et al., 2017).
Previous fMRI studies indicate that abnormal circuitry underlying TRD may involve the affective, salience, auditory, visual networks, and the language processing cortex (He et al., 2016). Recent studies have shown, more specifically, abnormal functional connectivity (FC) in the habenular nucleus (Amiri et al., 2021), especially with the default mode network (DMN) (Luan et al., 2019) in TRD patients. This is very interesting given the putative functions of the habenula.
The habenula is a small midbrain structure in the pineal region divided into two nuclear complexes, the medial and the lateral habenula. Of those two compartments, the lateral habenula is thought to play a major role in the encoding of aversive stimuli and is strongly connected to both the reward system and motor regions (Hu et al., 2020, Metzger et al., 2019). There are a growing number of neuroimaging studies that suggest that abnormal lateral habenula function could be involved in the pathogenesis of psychiatric disorders, including MDD (Gosnell et al., 2019, Skandalakis et al., 2018, Zhu et al., 2019, Ambrosi et al., 2019, Wu et al., 2020). More specifically, evidence suggests that the lateral habenula is highly involved in the induction of depression-like symptoms, as the processing of negative-valence information (Yang et al., 2018) and anhedonia (Coccurello, 2019). Abnormalities in the lateral habenula are shown to be particularly associated with reward processing underlying anhedonia, or diminished sensitivity to rewarding stimuli, in depression (Coccurello, 2019). Circuitry wise, it may influence neurotransmission between dopaminergic neurons in the ventral tegmental area and the medial prefrontal cortex (mPFC) (Browne et al., 2018, Aizawa and Zhu, 2019).
Given that anhedonia is a core symptom of TRD, and that the habenula plays a crucial role in the development of anhedonia in patients with depression, it is important to understand if abnormalities in the habenula function are a distinguishing feature of TRD. Indeed, aberrant habenular functional connectivity has been found in patients with TRD. TRD patients were shown to have increased FC between the right habenula with medial superior frontal gyrus, anterior cingulate cortex and medial orbitofrontal gyrus, decreased FC of right habenula with corpus callosum (Luan et al., 2019) and with median raphe (Gosnell et al., 2019), increased FC in left habenula with the locus coerulus (Gosnell et al., 2019) and the inferior temporal gyrus, and decreased FC in left habenula with insula (Luan et al., 2019). There is also preliminary evidence linking abnormal habenular nucleus activity and the DMN (von Hohenberg et al., 2018), particularly in TRD patients (Luan et al., 2019). These findings indicate that dysfunction in habenular -related circuitry could be a key feature/marker for TRD. However, the literature is incomplete. Most previously reported findings cannot be generalized to all TRD patients, as most studies report results comparing TRD with healthy individuals rather than to TSD patients (Ge et al., 2019). Other studies limit their analyses to the responsiveness to a particular treatment being trialed e.g. psilocybin (Carhart-Harris et al., 2017), ketamine (Rivas-Grajales et al., 2021, Chen et al., 2019), transcranial magnetic stimulation (TMS) (Avissar et al., 2017), electroconvulsive therapy (ECT) (Waarde et al., 2015), making it difficult to generalize and to identify reliable diagnostic biomarkers for TRD.
The aim of this work is to investigate differences in habenular resting-state functional connectivity between TSD patients and TRD patients, across multiple treatment-types.
2. Materials and Methods
2.1. Participants
Thirty-nine TRD and thirty-five TSD patients were recruited through general practitioner referrals and clinics. Thirty-eight healthy individuals (HC) participants were recruited through community advertisements. Data collection was conducted at Westmead Hospital, Department of Radiology and at the Brain Dynamics Centre, The Westmead Institute for Medical Research, in Sydney, Australia.
TRD and TSD patients met DSM-5 criteria for primary diagnosis of MDD, according to the Structured Clinical Interview for the DSM-5 (SCID-5) (American Psychiatric Association, 2013). TRD was defined as no remission of symptoms with at least two adequate trials (in terms of dosage, duration – 6 weeks for each trial) of antidepressant of different pharmacologic classes, as well as the presence of moderate to severe symptoms. Severity of the symptoms was characterized by a 17 item Hamilton Depression Rating Scale (HAMD-17) (Hamilton, 1960) score greater or equal to 16. TSD patients were defined as symptom-remitted patients for at least two weeks, a HAMD-17 score of or less than or equal to 9. HC were healthy individuals with no psychiatric illnesses, assessed using the SCID-5. All participants were aged between 18 and 65 years old.
Exclusion criteria included a) inability to provide consent, b) insufficient English proficiency, c) current primary diagnosis of eating disorder, psychosis, personality disorder or primary post-traumatic stress disorder, d) substance dependence for the past 3 months, e) pregnancy, f) history or current neurological disorder or prior brain injury, g) ECT or TMS in the last 6 months, h) contraindication to MRI.
For both patient groups, indices of illness severity and chronicity were assessed. These indices included age of onset, number of inpatient hospitalizations, length of remission period since last episode, number of previous depressive episodes, history of suicidal ideation and behavior, and history of suicide attempt. Information on past and current medication and other forms of treatment (e.g. ECT, or TMS) was also collected. Level of functioning was assessed by the Social and Occupational Functioning Assessment Scale (SOFAS) (Goldman et al., 1992).
The research protocol was approved by the Western Sydney Local Health District Human Research Ethics Committee and all participants provided written consent.
2.2. Imaging acquisition
MRI acquisitions were performed in a 3 Tesla MRI Scanner (Prisma, Siemens Medical Solutions, Germany), with a 64-channel array head coil.
Participants underwent a scanning protocol that included an 8-minutes resting-state protocol in which the participants were instructed to remain still, to relax and let their mind wander while looking at a fixation cross projected onto the screen. Functional T2*-weighted echo-planar images were acquired (repetition time (TR) = 1500 ms, echo-time (TE) = 33.0 ms, field of view = 255 mm; flip angle = 90°, phase encoding direction = A ≫ P, excitation = standard, 60 axial slices resulting in isotropic voxels of 2.5 mm3 encompassing the whole brain). A three-dimensional T1-weighted structural images (TR/TE = 2400 ms/2.21 ms, field of view = 256 mm, flip angle = 8°, inversion time = 900 ms, phase encoding direction = A ≫ P, 192 sagittal slices resulting in isotropic voxels of 0.89 mm3 encompassing the whole brain).
2.3. Imaging data analyses
Data processing and analyses were performed using Matlab R2018b (The Mathworks inc, Natick, Massachusetts), SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) and CONN functional connectivity toolbox v16b (http://www.nitrc.org/projects/conn/). Anatomical images were segmented into grey matter, white matter, and cerebrospinal fluid. Pre-processing of the functional images included realignment, unwrapping, motion correction, co-registration to native space structural data, smoothing with a 6-mm FWHM Gaussian kernel, and normalization to Montreal Neurological Institute (MNI) space. To eliminate the influence of residual noise components in the blood-oxygen-level-dependent (BOLD) signal, the data was also subject to a denoising process, using the default pipeline for denoising (including anatomical component-based noise correction procedure and default bandpass filtering [0.01, 0.1] Hz) andfunctional outlier detection tools in CONN (ART-based scrubbing). Scrubbing correction outputs were analysed to detect datasets with high-motion volumes of BOLD data, and subjects with a volume-to-volume index of head motion (head displacement from previous frame) higher than 1 mm were considered outliers. Four TRD participant datasets were excluded for excessive head motion during the scan.
Seed-based - a priori selection of regions of interest (ROIs) - FC analyses on the rs-fMRI data were performed, using the left and the right habenula as seeds. Due to the small size of the habenular nucleus, the left and right habenula seed ROIs were manually created for every subject (Luan et al., 2019). The T1-weighted structural images were used to visually identify the left and right habenula, using SPM12. In T1-W images, the habenula is clearly visible as two small triangular structures, hyperintense to the surrounding cerebrospinal fluid and grey matter, pointing into the third ventricle, on the epithalamus. Each functional ROI was a 3 × 3 × 3 mm cube placed around the central MNI coordinate in the habenula, identified individually for each participant.
For each seed, whole-brain voxelwise FC was quantified. In the first-level FC analyses, CONN calculated the functional connectivity values as bivariate Fisher’s z-transformed correlation coefficients for the association between each seed BOLD timeseries and each voxel of the whole brain BOLD timeseries, using generalized linear model (GLM). The correlation coefficients were then used in subsequent second-level statistical analysis, and compared between the TRD, TSD, and HC groups through ANOVA tests of variance (for three groups). The statistical parametric maps were thresholded at the cluster-level false discovery rate (FDR)-corrected for multiple comparisons p < 0.05, using an initial voxel-wise p < 0.001.
Secondly, mean beta resting-state FC values were extracted from significant clusters found in the seed-to-voxel whole brain analyses to explore post-hoc paired contrasts.
2.4. Analyses of demographic and clinical factors
Statistical analyses were performed using SPSS software version 21 (IBM Corp, 2012).
All three groups were compared for age and gender (demographic variables), using a one-way ANOVA and chi square test, respectively. The TRD and TSD groups were compared for age of onset, age of first episode, depression severity (HAMD-17 score), functionality (SOFAS score), severity of worst depressive episode, number of previous depressive episodes, using student t-tests. The groups were also compared for history of hospitalizations, suicidal attempts and suicidal ideation, using chi square tests of independence.
We further explored the effect of group, age, age at first episode, length of time on ADMs, HAMD-17 score, and SOFAS score, and suicidal ideation in explaining the variance in the significant neural measures using a univariate GLM ANOVA, with group as the fixed factor and the demographic and clinical variables as co-variants in the model.
FC values were tested for correlations with clinical and demographic variables in the TRD group. The effect of history of suicidal ideation, history of suicidal attempt, and ECT treatment in explaining the variance of the neural measures were also explored, using independent samples t-tests to compare differences in the FC measures between those with and without history of suicidal ideation, suicidal attempt and ECT treatment. Histograms were generated to visualize the distribution of frequencies for the variables with significant differences between the groups.
All statistical tests were corrected for multiple comparisons, and all effects considered significant at the p < 0.05 significance level.
3. Results
3.1. Demographic and clinical characteristics
Demographic and clinical data for the final sample are summarized in Table 1. All three groups were comparable for age and gender. The TRD group was significantly worse in their depressive profile when compared with the TSD group, with a greater severity of depressive symptoms (severity of worst episode), poorer functioning (SOFAS) and more hospitalizations and suicide attempts. There were no significant differences between groups for motion during the scan, after the exclusion of the four TRD participants for excessive motion (Supplementary Section).
Table 1.
Demographic and clinical characteristics of participants from TRD, TSD and HC groups.
| TRD (35) | TSD (35) | HC (38) | F/t/X^2 | sig | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, Mean ± SD [Min-Max] | 42.3 ± 14.1 [18.1–64.3] | 37.2 ± 11.0 [20.0–57.4] | 47.1 ± 14.3 [18.9–66.0] | n.s. | n.s. |
| Gender (M), N (%) | 14 (40) | 17 (48.6) | 17 (44.7) | n.s. | n.s. |
| Clinical Profile | |||||
| Age of onset, Mean ± SD [Min-Max] | 26.97 ± 13.13 [8–53] | 21.66 ± 9.26 [8–50] | n.a. | n.s. | n.s. |
| Number of previous MDE, Mean ± SD [Min-Max] | 8 ± 11 [1–40] | 6 ± 6 [1–30] | n.a. | n.s. | n.s. |
| CGI-S - Severity of worst MDE, Mean ± SD [Min-Max] | 6.77 ± 0.49 [5–7] | 5.21 ± 1.36 [3–7] | n.a. | 6.346 | <0.001 |
| Length of current episode, days, Mean ± SD [Min-Max] | 117.1 ± 117.2 [14–365] | n.a. | n.a. | n.a. | n.a. |
| HAMD-21 score, Mean ± SD [Min-Max] | 25.23 ± 6.46 [16–41] | 4.18 ± 3.10 [0–9] | n.a. | 15.819 | <0.001 |
| SOFAS score, Mean ± SD [Min-Max] | 73.91 ± 16.18 [40–100] | 89.58 ± 5.47 [78–95] | n.a. | −5.275 | <0.001 |
| History of Hospitalizations, N (%) | 25 (71.4) | 6 (17.1) | n.a. | 20.902 | <0.001 |
| History of ECT, N (%) | 10 (28.6) | 0 (0) | n.a. | 11.667 | 0.001 |
| History of TMS, N (%) | 3 (8.6) | 0 (0) | n.a. | n.a. | n.a. |
| History of Suicidal Ideation, N (%) | 28 (80) | 26 (74.3) | n.a. | n.s. | n.s. |
| History of Suicidal Attempt, N (%) | 19 (54.3) | 5 (14.3) | n.a. | 13.938 | <0.001 |
| Remission time (days), Mean ± SD [Min-Max] | n.a. | 409 ± 1062 [14 – 6205] | n.a. | n.a. | n.a. |
n.a. – not applicable, n.s. – not significant; SD – Standard Deviation; M – male; MDE – Major Depressive Episode; HAMD-21 – Hamilton Depression Rating Scale, 21 items; SOFAS – Social and Occupational Functioning Assessment Scale; CGI-S – Clinical Global Impression, Severity; ECT – Electroconvulsive Therapy; TMS – Transcranial Magnetic Stimulation; N – total number.
3.2. Functional connectivity analyses
The 3-way ANOVA identified significant connectivity differences between the three groups for the left habenula. There were significant connectivity group differences for the left habenula with the precentral gyrus and bilateral precuneus cortical regions (Table 2, Fig. 1). More details on supplementary FC results comparing only the TRD and TSD groups are presented on the Supplementary Section. Next, we extracted mean FC beta values for the voxels from the three significant clusters identified in the whole-brain analyses and compared them between the groups.
Table 2.
Summary of the main findings from the seed-based functional connectivity analyses.
| Source seeds | Brain regions | Side | Cluster size (voxels) | F value | Post-hoc | Peak MNI coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Habenula | L | |||||||
| Precentral Gyrus | R | 102 | 12.757 | TRD, HC > TSD | 62 | 2 | 42 | |
| Precuneus Cortex | L | 122 | 14.714 | TRD, HC > TSD | −12 | −76 | 48 | |
| Precuneus Cortex | R | 69 | 13.542 | TRD > HC > TSD | 18 | −72 | 38 | |
R – right, L – left; TRD – treatment-resistant depressive patients, TSD – treatment-sensitive depressive patients, HC – healthy controls.
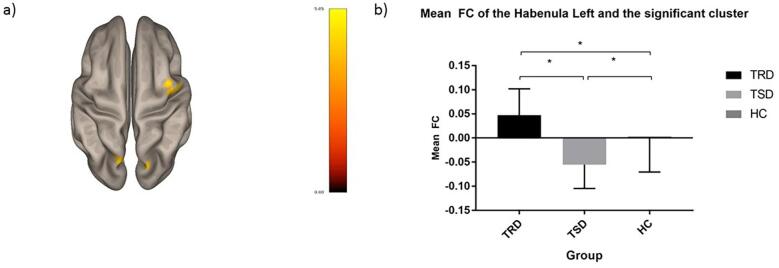
Fig. 1.
Functional connectivity differences between the three groups. Illustration of the differences functional connectivity (FC) patterns of the left habenula between the three groups: (a) the left and the right precuneus cortex and right precentral gyrus FC with the left habenula showed differences between the treatment-resistant depression (TRD) group and the treatment-sensitive depression (TSD) group (TRD > TSD) (superior view); (b) means of FC of the left habenula ot the combined clusters described at (a) for TRD, TSD and healthy controls (HC). The color scale bar on (a) represents the strength of the t statistic. The “mean FC” (mean functional connectivity) on (b) represents beta values of functional connectivity between the two regions.
Post-hoc tests revealed hyperconnectivity of the TRD group relative to TSD and HC groups in the right precuneus cortex cluster, and relative to the TSD group in the left precuneus cortex and the right precentral gyrus clusters.
The TSD group on the other hand demonstrated significant hypoconnectivity than TRDs in the left precuneus and right precentral gyrus clusters, and relative to the HC group in all the three clusters.
3.3. Correlations between FC and demographic and clinical measures
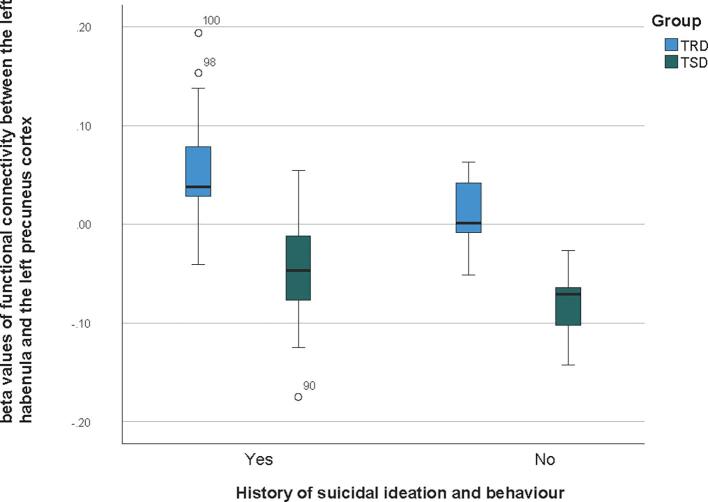
TRD patients with a history of suicidal ideation were shown to have higher FC in the left habenula – left and right precuneus cortex – right precentral gyrus clusters, when compared to patients without history of suicidal ideation (t = 2.407, p = 0.038) (see Fig. 2). As suicidal ideation was found to be significantly associated with the FC measures, a follow-up analyses as conducted to evaluate if it was a contributing factor for the groups differences found, by running a GLM ANOVA on the two clinical groups, controlling for suicidal ideation. The results (F(2,68) = 40.02, p < 0.001) indicate that there is an effect of group in the neural measure, controlling for suicidal ideation – so, differences between the clinical groups in the FC between the left habenula and the precuneus cortex remain beyond the effect of suicidal ideation.
Fig. 2.
Frequency distributions for functional connectivity between the left habenula and the precuneus cortex, in individuals with and without a history of suicidal ideation, in the TRD group and TSD group. Boxplots illustrating of the distrubition of frequencies for functional connectivity (FC) beta values between the left habenula and the left precuneus cortex, in patients with (Yes) and without (No) a history of suicidal ideation and behaviour. Frequency distributions are represented separately for the treatment-resistant depression (TRD) group and the treatment-sensitive depression (TSD) group.
There were no other significant effects (more details on Supplementary Section).
4. Discussion
This study identified a pattern of hyperconnectivity in TRD, especially between the left habenula and the bilateral precuneus cortex and the right precentral gyrus, which is not only abnormal (different from HC) but also greater than TSD. These results suggest that abnormal resting state connectivity in the habenular circuitry might be a distinguishing feature of TRD, as compared to those patients who respond to treatment.
The precuneus is known to be part of the posterior default mode network, the functional neural system that controls internal rumination and switching between external and internal cognition (Cavanna and Trimble, 2006, Fransson and Marrelec, 2008). Our findings confirm that abnormal functional connectivity in the DMN distinguishes TRD from TSDs. Dysfunction of this network has been previously implicated in the maintenance of depressive states (Liu et al., 2021). Recent functional neuroimaging studies in healthy subjects indicate that the precuneus plays a central role in a broad range of highly integrated functions, episodic memory retrieval, and self-processing operations, namely first-person perspective taking and an experience of agency (or the ability to control external events through our own actions) (Cavanna & Trimble, 2006).
The reward circuit is critical for motivated behavior and the capacity to feel pleasure in response to an event so it is conceivable that abnormalities in this circuitry may underpin what is clinically known as anhedonia (Yang et al., 2021). Habenula function is thought to integrate dopaminergic and serotonergic inputs in to this, encoding reward value, probability and magnitude. As a critical node of the reward-related circuit, habenula hyperconnectivity might be associated with a higher influx of internal negative thoughts which could then feedback into the reward system leading to increased anhedonia and/or that sensory information is being overly processed as negative stimuli. It has also been reported that reduced engagement of the precuneus cortex is associated with difficulties in positive future scene simulation in individuals with anhedonia (Yang et al., 2021). Further, there is evidence of TRD patients characterized by impaired connectivity of the DLPFC and precuneus component of the attention and default mode networks (Williams et al., 2021). This indicates there may be abnormalities in the interaction between DMN and sensory information in the encoding of negative reward by the habenula, in patients with TRD. However it is important to note that our study used resting state fMRI and future work should explore these functional relationships using task based fMRI and cognitive behavioral data.
Abnormalities in connectivity between the reward network and the precuneus cortex are also thought to be related to other clinical symptoms of depression, such as suicidality (Zhang et al., 2016). Although we confirmed that, for this sample, suicidal ideation is not a contributing factor for group differences in FC, the higher levels of suicidal ideation in the TRD group compared to the TSD group may be associated with the hyperconnectivity of the habenular circuit involving regions of the DMN in the TRD group. The higher habenular FC found in people with a history of suicidal behaviour may also mediate a dysfunction in the mechanism that links the habenula with motor activity and contextual associative processing. This could be linked to its hyperconnectivity with the right precentral gyrus, as it is the location of the primary motor cortex. Furthermore, knowing that the habenula is closely linked to the function of reward processing, particularly with regards to encoding negative feedback on negative reward (Baker et al., 2016), our findings suggest that TRD patients exhibit alterations in the brain circuits mediating reward (interrelated with the default mode network) that may affect their proclivity for suicide. This is not necessarily due to decreased motivation, but rather an inability to engage in alternative strategies and actions, as the habenula acts a regulator for behaviour flexibility (Baker et al., 2016). Thus, chronic dysregulation of the habenula circuit seems to be associated with long-term changes in the dopaminergic, serotoninergic and norepinephrinergic activity that are in the background of dysfunctional coping strategies related to suicide-related behavior (Ambrosi et al., 2019).
Treatment studies also point to the importance of the habenula in TRD (von Hohenberg et al., 2018). The antidepressant effects of SSRIs may result from down-regulation of pre-treatment serotonin activity in terminal regions receiving serotonergic projection, such as the habenula. These findings further indicate an important role for the habenula in regulating serotonin levels that are relevant to the symptoms of depression and suggest the habenula as a potential target for antidepressant treatments (Zhao et al., 2015).
5. Limitations
Functional MRI studies on the habenula have several limitations. From an imaging perspective, the habenula volume is very small, ranging from approximately 29 to 36 mm3 in each hemisphere. This may be smaller than the voxel size of a standard fMRI, making its identification challenging. Ideally, lateral and medial habenula should be identified and segregated, as they are known to have differential functions (Coccurello, 2019) – however, given the resolution limitation of the acquisition protocol used in this study, this was not feasible.We acknowledge that the habenula is a tiny structure and there is risk of signal bleeding from adjacent structures due to the limited resolution. Calculating the absolute volume of the left and right habenular nucleus for each participant would have also been a more accurate way of segmenting this region, and future work should use individual-specific habenula masks based on anatomic manual segmentation.
Secondly, it is likely that the habenular signal is contaminated by activity in adjacent structures, such as the medial dorsal thalamus or the epithalamic paraventricular nucleus. This work needs to replicated using more high-resolution fMRI scanning at possibly higher field strengths. A further limitation is that the sample size is relatively small and that the participants had been trialled on many different antidepressant medications. The response may also have been different in participants who were managed with non-pharmacological treatments. Although we collected information about current and past medication, it was based on patients’ self-report, and it is likely that we may have not captured the medication history accurately. With an increased sample size, it may be possible to differentiate these subtypes, and we strongly suggest this to be done in future work. The generalizability of our findings also needs to be validated in independent cohorts.
6. Conclusions
These findings indicate that different responsiveness profiles in depression are associated with distinct pathophysiological mechanisms. Unlike TSD, TRD is characterized by hyperconnectivity of the habenula with regions of the default mode network and sensorimotor networks, which may be associated with the capacity to encode negative feedback, and the mechanisms of suicidal ideation. This may suggest that this connectivity feature could be a potential treatment target for hard to treat depression. Future research should also consider assessing this functional connectivity feature in depression patients prior to treatment resistance is determined and exploring if this feature characterizes non-remission to one type of treatment (i.e. ADMs) or if it is a general mechanism to resistance across multiple treatments. This may potentially enable clinicians to identify treatment-resistant forms of depression and initiate appropriate treatment options earlier.
CRediT authorship contribution statement
Ana Rita Barreiros: Data curation, Investigation, Resources, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Isabella Breukelaar: Formal analysis, Writing – review & editing. Prashanth Mayur: Resources, Writing – review & editing. Jagadeesh Andepalli: Resources, Writing – review & editing. Yoshiro Tomimatsu: Conceptualization, Methodology, Writing – review & editing. Kenta Funayama: Conceptualization, Methodology, Writing – review & editing. Sheryl Foster: Resources, Writing – review & editing. Philip Boyce: Writing – review & editing. Gin S. Malhi: Writing – review & editing. Anthony Harris: Conceptualization, Project administration, Data curation, Resources, Writing – review & editing. Mayuresh S. Korgaonkar: Conceptualization, Project administration, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Anthony Harris received funding from Takeda Pharmaceutical Company for this project. There are no other financial disclosures related to the work. Mayuresh Korgaonkar received funding from Takeda Pharmaceutical Company for this project. There are no other financial disclosures related to the work. And all other authors has declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Dr. Atsushi Suzuki (Takeda Pharmaceutical Company Ltd) for his support in the conceptualization of this study.
Funding
This work was supported by Takeda Pharmaceutical Company Limited COCKPI-T (Co-Create Knowledge for Pharma Innovation with Takeda) Research Grant [to Mayuresh Korgaonkar]), the National Health and Medical Research Council (NHMRC) (Grant No. APP1087560 [to Mayuresh Korgaonkar]), and The Stephen and Barbara Penfold PhD Scholarship (to Ana Rita Barreiros). None of the Takeda members had any specific role in the design and execution of the clinical study itself, but supported the conceptualization, data analysis and its interpretation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102990.
Contributor Information
Ana Rita Barreiros, Email: ana.barreiros@sydney.edu.au.
Mayuresh S. Korgaonkar, Email: m.korgaonkar@sydney.edu.au.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aizawa H., Zhu M. Towards an understanding of the habenula’s various roles in human depression. Psychiatry and Clinical Neuroscience. 2019;73:607–612. doi: 10.1111/pcn.12892. [DOI] [PubMed] [Google Scholar]
- Akil H., Gordon J., Hen R., Javitch J., Mayberg H., McEwen B., Meaney M.J., Nestler E.J. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–288. doi: 10.1016/j.neubiorev.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi E., Arciniegas D.B., Curtis K.N., Patriquin M.A., Spalletta G., Sani G., Frueh B.C., Fowler J.C., Madan A., Salas R. Resting-state functional connectivity of the habenula in mood disorder patients with and without suicide-related behaviors. J Neuropsychiatry Clin Neurosci. 2019;31(1):49–56. doi: 10.1176/appi.neuropsych.17120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013): Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: Author.
- Amiri S., Arbabi M., Kazemi K., Parvaresh-Rizi M., Mirbagheri M.M. Characterization of brain functional connectivity in treatment-resistant depression. Progress in Neuropsychopharmacology & Biological Psychiatry. 2021;111:110346. doi: 10.1016/j.pnpbp.2021.110346. [DOI] [PubMed] [Google Scholar]
- Avissar M., Powell F., Ilieva I., Respino M., Gunning F.M., Liston C., Dubin M.J. Functional Connectivity of the Left DLPFC to Striatum Predicts Treatment Response of Depression to TMS. Brain Stimul. 2017;10(5):919–925. doi: 10.1016/j.brs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P.M., Jhou T., Li B.o., Matsumoto M., Mizumori S.J.Y., Stephenson-Jones M., Vicentic A. The lateral habenula circuitry: reward processing and cognitive control. J Neurosci. 2016;36(45):11482–11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C.A., Hammack R., Lucki I. Dysregulation of the Lateral Habenula in Major Depressive Disorder. Front Synaptic Neurosci. 2018;10:46. doi: 10.3389/fnsyn.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R.L., Roseman L., Bolstridge M., Demetriou L., Pannekoek J.N., Wall M.B., Tanner M., Kaelen M., McGonigle J., Murphy K., Leech R., Curran H.V., Nutt D.J. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-13282-710.21203/rs.2.15119/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.E. Cavanna M.R. Trimble The precuneus: a review of its functional anatomy and behavioural correlates Brain 129 3 2006 2006 564 583. [DOI] [PubMed]
- Chen M.-H., Lin W.-C., Tu P.-C., Li C.-T., Bai Y.-M., Tsai S.-J., Su T.-P. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: A double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J. Affect. Disord. 2019;259:15–20. doi: 10.1016/j.jad.2019.08.022. [DOI] [PubMed] [Google Scholar]
- Coccurello R. Anhedonia in depression symptomatology: Appetite dysregulation and defective brain reward processing. Behav Brain Res. 2019;372 doi: 10.1016/j.bbr.2019.112041. Epub 2019 Jun 17 PMID: 31220485. [DOI] [PubMed] [Google Scholar]
- Delfino R.S., Del-Porto J.A., Surjan J., Magalhães E., Sant L.C.D., Lucchese A.C., Tuena M.A., Nakahira C., Fava V.A.R., Steglich M.S., Barbosa M.G., Sarin L.M., Lacerda A.L.T. Comparative effectiveness of esketamine in the treatment of anhedonia in bipolar and unipolar depression. J Affect Disord. 2021;278:515–518. doi: 10.1016/j.jad.2020.09.056. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gaynes B.N., Lux L., Gartlehner G., Asher G., Forman‐Hoffman V., Green J., Boland E., Weber R.P., Randolph C., Bann C., Coker‐Schwimmer E., Viswanathan M., Lohr K.N. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134–145. doi: 10.1002/da.22968. [DOI] [PubMed] [Google Scholar]
- Ge R., Torres I., Brown J.J., Gregory E., McLellan E., Downar J.H., et al. Functional dysconnectivity of the hippocampal network and neural correlates of memory impairment in treatment-resistant depression. J. Affect. Disord. 2019;253:248–256. doi: 10.1016/j.jad.2019.04.096. [DOI] [PubMed] [Google Scholar]
- Goldman H.H., Skodol A.E., Lave T.R. Revising Axis V for DSM-IV: A Review of Measures of Social Functioning. Am. J. Psychiatry. 1992;149:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- Gosnell S.N., Curtis K.N., Velasquez K., Fowler J.C., Madan A., Goodman W., Salas R. Habenular connectivity may predict treatment response in depressed psychiatric inpatients. J. Affect. Disord. 2019;242:211–219. doi: 10.1016/j.jad.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Greenberg P.R., Fournier A.A., Sisitsky T., Pike C.T., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2015 to 2010) J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Cui Q., Zheng J., Duan X., Pang Y., Gao Q., Han S., Long Z., Wang Y., Li J., Wang X., Zhao J., Chen H. Frequency-specific alterations in functional connectivity in treatment-resistant and -sensitive major depressive disorder. J. Psychiatr. Res. 2016;82:30–39. doi: 10.1016/j.jpsychires.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Hu H., Cui Y., Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020;21(5):277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- IBM Corp. Released . IBM Corp; Armonk, NY: 2012. IBM SPSS Statistics for Windows, Version 21.0. [Google Scholar]
- Kennedy S.H., Ceniti A.K. Unpacking Major Depressive Disorder: From Classification to Treatment Selection. Can. J. PsychiatryRevue canadienne de psychiatrie. 2018;63(5):308–313. doi: 10.1177/0706743717748883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Goldstein-Piekarski A.N., Fornito A., Williams L.M. Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol. Psychiatry. 2020;25(7):1537–1549. doi: 10.1038/s41380-019-0574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wang Y., Chen X., Zhang Z., Xiao L.e., Zhou Y. Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage: Clinical. 2021;30:102599. doi: 10.1016/j.nicl.2021.102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S.-X., Zhang L., Wang R., Zhao H., Liu C. A resting-state study of volumetric and functional connectivity of the habenular nucleus in treatment-resistant depression patients. Brain and Behavior. 2019;9 doi: 10.1002/brb3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G.S., Bell E., Bassett D., Boyce P., Bryant R., Hazell P., Hopwood M., Lyndon B., Mulder R., Porter R., Singh A.B., Murray G. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust. N. Z. J. Psychiatry. 2021;55(1):7–117. doi: 10.1177/0004867420979353. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Das P., Mannie Z., Irwin L. Treatment-resistant depression: problematic illness or a problem in our approach? The British Journal of Psychiatry. 2019;214(1):1–3. doi: 10.1192/bjp.2018.246. [DOI] [PubMed] [Google Scholar]
- Metzger M., Rouza R., Lima L.B., Bueno D., Goncalves L., Sego C., et al. Habenular connection with the dpominergic and serotonergic system and their role in stress-related psychiatric disorders. Eur J Neurosci. 2019;00:1–24. doi: 10.1111/ejn.14647. [DOI] [PubMed] [Google Scholar]
- Rivas-Grajales A.M., Salas R., Robinson M.E., Qi K., Murrough J.W., Mathew S.J. Habenula connectivity and intravenous ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol. 2021;24(5):383–391. doi: 10.1093/ijnp/pyaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.-N., Holmes A.J., et al. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb. Cortex. 2017:1–20. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skandalakis G.P., Koutsarnakis C., Kalyvas A.V., Skandalakis P., Johnson E.O., Stranjalis G. The habenula in neurosurgery for depression: A convergence of functional neuroanatomy, psychiatry and imaging. Brain Res. 2018;1694:13–18. doi: 10.1016/j.brainres.2018.04.041. [DOI] [PubMed] [Google Scholar]
- Słupski J., Cubała W.J., Górska N., Słupska A., Gałuszko-Węgielnik M. Copper and anti-anhedonic effect of ketamine in treatment-resistant depression. Med. Hypotheses. 2020;144:110268. doi: 10.1016/j.mehy.2020.110268. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Marwood L., Greer B., Strawbridge R., Cleare A.J. Predictors of response to augmentation treatment in patients with treatment-resistant depression: a systematic review. J. Psychopharmacol. 2019;33(11):1323–1339. doi: 10.1177/0269881119872194. [DOI] [PubMed] [Google Scholar]
- Clemm von Hohenberg C., Weber-Fahr W., Lebhardt P., Ravi N., Braun U., Gass N., Becker R., Sack M., Cosa Linan A., Gerchen M.F., Reinwald J.R., Oettl L.-L., Meyer-Lindenberg A., Vollmayr B., Kelsch W., Sartorius A. Lateral habenula perturbation reduces default-mode network connectivity in a rat model of depression. Translation Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waarde J., Scholte H.S., van Oudheusden L.J.B., Verwey B., Denys D., van Wingen G.A. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol. Psychiatry. 2015;20(5):609–614. doi: 10.1038/mp.2014.78. [DOI] [PubMed] [Google Scholar]
- Williams L.M., Coman J.T., Stetz P.C., Walker N.C., Kozel F.A., George M.S., Yoon J., Hack L.M., Madore M.R., Lim K.O., Philip N.S., Holtzheimer P.E. Identifying response and predictive biomarkers for Transcranial magnetic stimulation outcomes: protocol and rationale for a mechanistic study of functional neuroimaging and behavioral biomarkers in veterans with Pharmacoresistant depression. BMC Psychiatry. 2021;21(1) doi: 10.1186/s12888-020-03030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Wang C., Ma Z., Pang M., Wu Y., Zhang N., Zhong Y. Abnormal functional connectivity of habenula in untreated patients with first-episode major depressive disorder. Psychiatry Res. 2020;285:112837. doi: 10.1016/j.psychres.2020.112837. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang H., Hu J.i., Hu H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang R., Wang Y., Huang J., Zhou H., Cheung E.F.C., Chan R.C.K. Altered activation and functional connectivity in individuals with social anhedonia when envisioning positive future episodes. Psychol. Med. 2021;1–9 doi: 10.1017/S0033291721000970. [DOI] [PubMed] [Google Scholar]
- Zhang S., Chen J.-M., Kuang L.i., Cao J., Zhang H., Ai M., Wang W.o., Zhang S.-D., Wang S.-y., Liu S.-J., Fang W.-D. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16(1) doi: 10.1186/s12888-016-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhang B.-L., Yang S.-J., Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain Res. 2015;277:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Qi S., Zhang B., He D., Teng Y., Hu J., Wei X. Connectome-based biomarkers predict subclinical depression and identify abnormal brain connections with the lateral habenula and thalamus. Front Psychiatry. 2019;10:371. doi: 10.3389/fpsyt.2019.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.