Abstract
The therapeutic efficacy of liposomal clofazimine (L-CLF) was studied in mice infected with Mycobacterium tuberculosis Erdman. Groups of mice were treated with either free clofazimine (F-CLF), L-CLF, or empty liposomes twice a week for five treatments beginning on day 1 (acute), day 21 (established), or day 90 (chronic) postinfection. One day after the last treatment, the numbers of CFU of M. tuberculosis in the spleen, liver, and lungs were determined. F-CLF at the maximum tolerated dose of 5 mg/kg of body weight was ineffective; however, 10-fold-higher doses of L-CLF demonstrated a dose response with significant CFU reduction in all tissues without any toxic effects. In acutely infected mice, 50 mg of L-CLF/kg reduced CFU 2 to 3 log units in all three organs. In established or chronic infection, treated mice showed no detectable CFU in the spleen or liver and 1- to 2-log-unit reduction in the lungs. A second series of L-CLF treatments cleared M. tuberculosis in all three tissues. L-CLF appears to be bactericidal in the liver and spleen, which remained negative for M. tuberculosis growth for 2 months. Thus, L-CLF could be useful in the treatment of tuberculosis.
In recent years there has been a resurgence in the incidence of tuberculosis, in part due to the AIDS epidemic (25). It is especially disturbing because a significant number of new cases of the disease are caused by strains of Mycobacterium tuberculosis resistant to the standard first-line tuberculosis treatments, including isoniazid and rifampin (24). Thus, the development of improved antimycobacterial drugs and drug regimens is warranted.
There are a number of obstacles that must be overcome by potential candidate antituberculosis drugs. Their use, in most cases, is limited by problems such as low solubility, low levels of retention or stability in the cells after uptake, or degradation before they reach target tissues. Alternatively, there may be difficulty in achieving high concentrations of a drug at the site of infection due to its poor absorption properties or low penetration into cells. In addition, a potential drug may be too toxic, leading to a maximum tolerated dose well below what is necessary for efficient eradication of the infection.
Encapsulation of drugs into liposomes alleviates many of these obstacles (26). Liposome-encapsulated drugs often exhibit reduced toxicity, allowing for parenteral administration of much higher doses of the drug than could be tolerated with the free form. Liposome encapsulation has also been shown to enhance retention of drugs in the tissues. Thus, encapsulation of drugs in liposomes has often resulted in an improved overall therapeutic efficacy. Liposomes have also been used as drug carriers to improve the delivery of antimicrobial agents to macrophages for treatment of intracellular pathogens such as mycobacteria (5, 15, 17, 20, 21, 28).
Clofazimine (CLF) has a long history in the treatment of mycobacterial diseases, especially in the treatment of leprosy (14) but also occasionally in the treatment of drug-resistant tuberculosis (25). It has recently been shown that CLF can be effectively encapsulated in liposomes with an efficiency of 95 to 100% (21). In vitro and in vivo studies have demonstrated that liposome-encapsulated CLF (L-CLF) is much less toxic than free CLF (F-CLF) (15, 20). L-CLF could be delivered parenterally at doses not possible with F-CLF due to the lipophilic nature and insolubility of the free drug. Furthermore, encapsulation of CLF maintains its antimycobacterial properties, as L-CLF and F-CLF had similar MICs and minimum bactericidal concentrations against M. tuberculosis (21).
The purpose of this study was to evaluate the therapeutic efficacy of L-CLF in murine models of acute and chronic tuberculosis.
(Part of this work was presented at the 98th General Meeting of the American Society for Microbiology [May 1998] in Atlanta, Ga. [abstr. U-9].)
MATERIALS AND METHODS
Mice.
BALB/c mice (18 to 22 g), originally obtained from Jackson Laboratories (Bar Harbor, Maine), were bred locally and housed under standard laboratory animal housing conditions. They received water and food ad libitum.
Drugs, lipids, and reagents.
CLF was obtained as a generous gift from Ciba-Geigy (Basel, Switzerland). l-α-Dimyristoylphosphatidyl choline (DMPC) and l-α-dimyristoylphosphatidyl glycerol (DMPG) were obtained from Avanti-Polar Lipids Inc., Alabaster, Ala. All other chemicals and reagents were of analytical grade.
Preparation of drug formulations. (i) F-CLF.
F-CLF was prepared by dissolving 10 mg of CLF in 1 ml of acidified dimethyl sulfoxide as described earlier (20). Just before the injections, the stock solution of drug in dimethyl sulfoxide was diluted with sterile water to achieve the desired concentration.
(ii) L-CLF.
Multilamellar liposomes containing L-CLF were prepared as described previously (15). Briefly, lipids (DMPC-DMPG; 7:3 molar ratio) and CLF (lipid/drug ratio, 10:1) were dissolved in 80% tertiary butanol (Fisher Scientific). The drug-lipid solution was then sonicated, frozen with a dry ice-acetone mixture, and lyophilized for 2 days with a freeze dryer (Labconco Co., Kansas City, Mo.). The preliposomal powder was stored at −20°C until use. The liposome suspension was prepared by reconstituting the lyophilized powder in an appropriate volume of sterile saline for the required doses. The encapsulation efficiency of CLF was more than 95% as determined spectrophotometrically at 287 nm.
Empty liposomes were prepared, without addition of the drug to the lipid mixture, by the above procedure.
Culture of M. tuberculosis.
M. tuberculosis Erdman (ATCC 35801) was grown in batch culture in Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 0.2% glycerol (Sigma Chemical Co., St. Louis, Mo.), 0.05% Tween 80 (Sigma), and 10% oleic acid–albumin–dextrose–catalase solution (Sigma) at 37°C. Log-phase cultures were pelleted, washed in phosphate-buffered saline containing 0.05% Tween 80, filtered through an 8-μm-pore-size filter to minimize clumping, and stored in 0.5-ml aliquots at −80°C, as described elsewhere (13).
Infection of mice.
The course of M. tuberculosis infection in mice was monitored as previously described (1, 2). Briefly, bacilli were thawed, sonicated at 50% power for 15 s to disperse any clumps, and diluted in phosphate-buffered saline, and 106 organisms were injected into a lateral tail vein of each mouse.
Treatment of M. tuberculosis-infected mice.
On day 1 (acute), day 21 (established), or day 90 (chronic) postinfection, L-CLF was administered intravenously (i.v.) every 3 to 4 days over a 2-week period (total, five treatments). Control preparations of F-CLF and empty liposomes were also administered on the same schedule. One day after the last drug treatment, the spleens, livers, and lungs of groups of mice were homogenized, serially diluted, and plated on 7H11 agar (Difco) plates for the determination of the number of CFU of M. tuberculosis per organ. In the last set of experiments, a second round of L-CLF treatments was administered, and the number of CFU in the tissues was determined up to 2 months post-drug treatment. In addition, samples of tissues were fixed in buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin or Fite’s acid-fast stain.
Statistical analyses.
Both the original (raw) data and the natural-log-transformed data from all experiments were analyzed. Data from the acute, established, and chronic experiments were analyzed in a one-way analysis of variance with drug as the main effect by using the SAS statistical package (GLM procedure). Dunnett’s post hoc test was used to compare all treatment levels to controls. For the recovery experiments, the data were analyzed in a two-way analysis of variance with drug and time as the main effects and a drug-time interaction. For main-effect comparisons, Dunnett’s test was used to compare levels of drug to controls and Scheffes test was used with regard to time. Interaction effects were examined by using pairwise t tests of least-square means. The probability was considered significant at a P value of <0.01.
RESULTS
Effect of L-CLF treatment on acute murine tuberculosis.
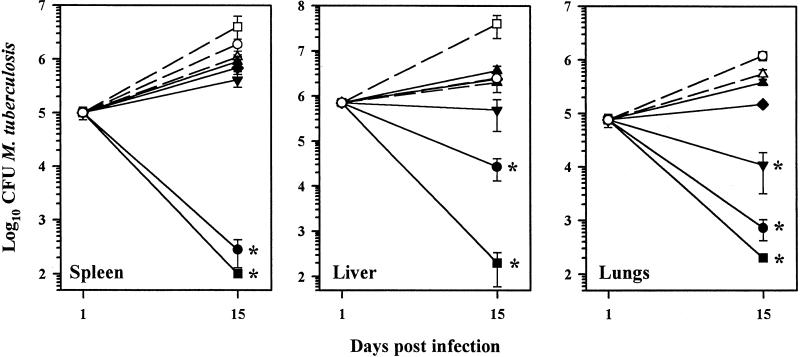
In order to determine the overall effectiveness of L-CLF in the treatment of tuberculosis, it was first evaluated in the acute phase of infection (i.e., the first 2 weeks). Mice were inoculated intravenously (i.v.) with M. tuberculosis Erdman and then treated with L-CLF on days 1, 5, 8, 11, and 14 postinfection. As shown in Fig. 1, control mice showed growth of M. tuberculosis in the spleen, liver, and lungs over the 15-day period. F-CLF at the maximum tolerated dose of 5 mg/kg of body weight (20) resulted in little inhibition in growth of the bacilli. Administration of L-CLF resulted in a dose response in all three tissues, especially in the liver and lungs. At a dose of 100 mg/kg, L-CLF reduced the number of CFU of M. tuberculosis by 4 log units in the spleen and >3 log units in the liver and lungs (P < 0.01). A dose of 50 mg of L-CLF/kg decreased the number of CFU by >3 log units in the spleen and lungs and 2 log units in the liver (P < 0.01). Empty liposomes, while showing no effect on the growth of M. tuberculosis in the lungs, enhanced growth slightly in the spleen and liver.
FIG. 1.
L-CLF treatment in acutely infected mice. BALB/c mice (n = 4 per group) were infected i.v. with 106 M. tuberculosis Erdman organisms and left untreated (○) or treated i.v. with L-CLF (■, 100 mg/kg; ●, 50 mg/kg; ▾, 25 mg/kg; ⧫, 10 mg/kg; and ▴, 5 mg/kg), F-CLF (▵, 5 mg/kg), or empty liposomes (□, lipid content equivalent to 100-mg/kg dose) on days 1, 5, 8, 11, and 14 postinfection. The mice were sacrificed 1 day after the last drug injection (day 15), and their spleens, livers, and lungs were homogenized and plated for CFU. The results are shown as means ± standard deviations. ✻, P < 0.01.
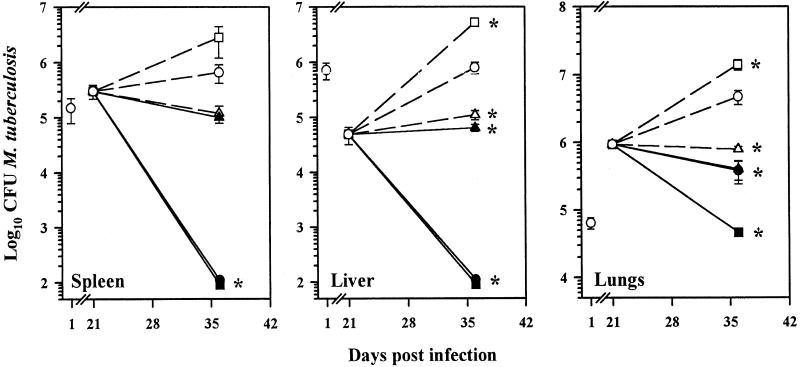
Effect of L-CLF treatment on established murine tuberculosis.
The therapeutic efficacy of L-CLF in the treatment of tuberculosis was studied subsequently in mice with established M. tuberculosis infection (i.e., weeks 4 and 5). As depicted in Fig. 2, administration of L-CLF at 100 and 50 mg/kg appeared to clear the organisms from both the spleen and liver (limits of detection for the CFU assay, 100 bacilli per organ) (P < 0.01), and there was a 1-log-unit decrease in M. tuberculosis growth in the lungs with 50 mg of L-CLF/kg (P < 0.01). A dose of 100 mg of L-CLF/kg, however, reduced the number of CFU in the lungs by 2 log units. Again, in comparison to untreated controls, empty liposomes caused a slight enhancement of growth in all three tissues. Note that empty liposomes were administered at a lipid content equivalent to the highest dose of L-CLF.
FIG. 2.
L-CLF treatment in mice with established infection. BALB/c mice (n = 4 per group) were infected i.v. with 106 M. tuberculosis Erdman organisms. Beginning on day 21 postinfection, the mice were left untreated (○) or treated i.v. every 3 to 4 days over a 2-week period (total, five injections) with L-CLF (■, 100 mg/kg; ●, 50 mg/kg; ▴, 5 mg/kg) F-CLF (▵, 5 mg/kg), or empty liposomes (□, equivalent to 100-mg/kg dose). The mice were sacrificed 1 day after the last drug injection (day 36), and the spleens, livers, and lungs were homogenized and plated for CFU. The results are shown as means ± standard deviations. ✻, P < 0.01.
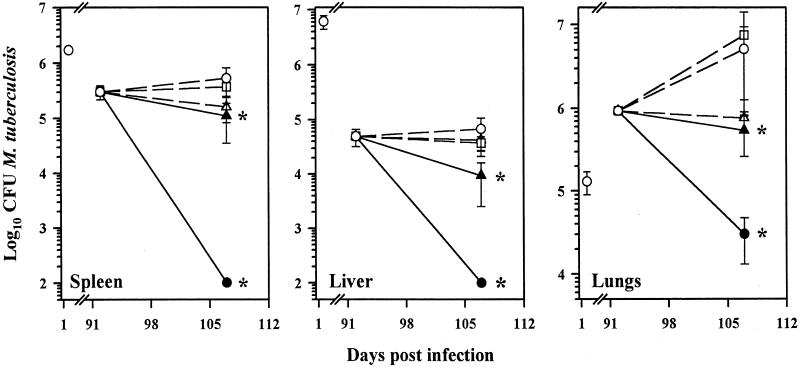
Effect of L-CLF treatment on chronic murine tuberculosis.
In the next experiment, mice chronically infected with M. tuberculosis were treated 3 months after infection (i.e., weeks 13 and 14) with L-CLF. As shown in Fig. 3, a dose of 50 mg of L-CLF/kg resulted in no bacilli recovered from the spleen and liver, and there was a 2-log-unit reduction in the number of bacilli in the lungs (P < 0.01). Interestingly, the efficacy of L-CLF improved as the infection progressed, implying an enhancement of L-CLF efficacy with acquired immunity and granuloma formation.
FIG. 3.
L-CLF treatment in chronically infected mice. BALB/c mice (n = 4 per group) were infected i.v. with 106 M. tuberculosis Erdman organisms. Beginning on day 92 postinfection, the mice were left untreated (○) or treated i.v. every 3 to 4 days over a 2-week period (total, five injections) with L-CLF (●, 50 mg/kg; ▴, 5 mg/kg), F-CLF (▵, 5 mg/kg), or empty liposomes (□, lipid content equivalent to 50-mg/kg dose). The mice were sacrificed 1 day after the last drug injection (day 107), and the spleens, livers, and lungs were homogenized and plated for CFU. The results are shown as means ± standard deviations. ✻, P < 0.01.
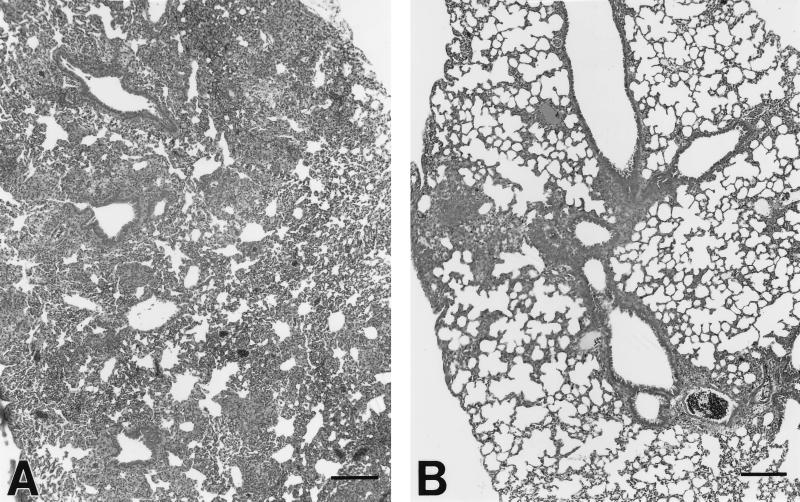
At 5 weeks after infection with M. tuberculosis, there was an intense infiltration of inflammatory cells into the lungs of the control mice (Fig. 4A), with loose granulomas containing epithelioid macrophages. An early granulomatous response with aggregates of epithelioid macrophages interspersed with lymphocytes was observed. In contrast, similarly infected mice treated with 50 mg of L-CLF/kg during weeks 4 and 5 (Fig. 4B) exhibited much less mononuclear cell infiltration, suggesting the anti-inflammatory properties of CLF. Treated mice also exhibited perivascular and peribronchiolar cuffing and more-localized granuloma formation without the extensive involvement of lung parenchyma observed in the untreated mice.
FIG. 4.
Effect of L-CLF administration on early lung granuloma formation in M. tuberculosis-infected mice. (A) In control mice, there was an intense mononuclear cell infiltration into the lung parenchyma. An early granulomatous response with aggregates of epithelioid macrophages interspersed with lymphocytes was observed. (B) In contrast, mice treated with L-CLF exhibited perivascular and peribronchiolar cuffing and more localized granuloma formation without extensive involvement of lung parenchyma. The tissues were stained with hematoxylin-eosin. Bars = 150 μm.
Clearance and recovery of M. tuberculosis after L-CLF treatment in chronic tuberculosis.
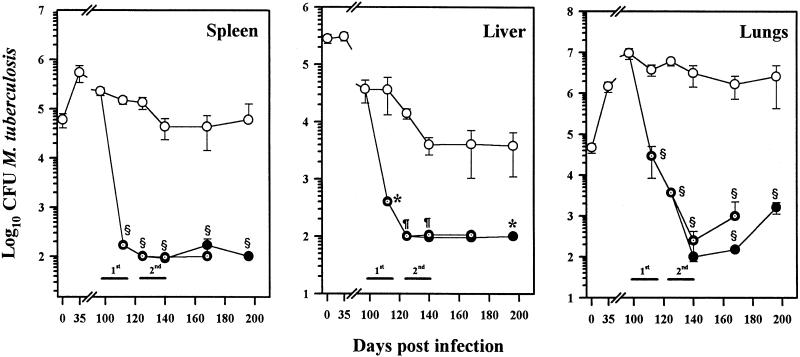
Our next experiment was designed to determine if there was actual clearance of M. tuberculosis from the tissues and if the animals remained free of infection after treatment with L-CLF. Chronically infected mice were monitored for 2 months after treatment with L-CLF. As shown in Fig. 5, mice treated with one series of L-CLF treatments essentially cleared M. tuberculosis from the spleen and liver (P < 0.01) and there was no recovery of bacilli from these tissues up to 2 months posttreatment. In the lungs, CFU counts of M. tuberculosis were reduced 2 log units at the end of the first series of L-CLF treatments (P = 0.0001). Interestingly, at 1 month posttreatment, the CFU count of M. tuberculosis was further reduced to 4 log units below that of the control (P = 0.0001), indicating a prolonged release of drug from multilamellar liposomes and/or the sustained effect of the CLF accumulated in the tissues. By 2 months posttreatment, there was some regrowth of bacilli in the lungs, although the numbers of CFU in the L-CLF-treated group were still 3 log units below those in control mice (P = 0.0001).
FIG. 5.
Clearance and recovery of M. tuberculosis in tissues of mice after treatment with L-CLF. BALB/c mice (n = 4 per group) were infected i.v. with 106 M. tuberculosis Erdman organisms. Beginning on day 97 postinfection, the mice were left untreated (○) or were treated i.v. every 3 to 4 days over a 2-week period (1st) (total, five injections) with 50 mg of L-CLF/kg (⊙). Groups of mice were sacrificed 1 day after the last drug injection (day 112), 2 weeks posttreatment, 1 month posttreatment, and 2 months posttreatment. Another set of mice (●) were administered a second round (2nd) of L-CLF treatments (50 mg/kg) beginning 2 weeks after the completion of the first round (day 125). Groups of mice were sacrificed 1 day after the last drug injection (day 140), 1 month posttreatment, and 2 months posttreatment. The spleens, livers, and lungs were homogenized and plated for CFU. The results are shown as means ± standard deviations. ✻, P < 0.01; ¶, P < 0.001; §, P < 0.0001.
In an effort to obtain clearance of M. tuberculosis from the lungs, a second series of L-CLF treatments was administered (Fig. 5). At the end of the second L-CLF treatment, no bacilli were recovered from the lungs (P = 0.0001) or from the spleen (P = 0.0001) and liver (P = 0.0002). However, again by 2 months posttreatment, approximately a 1-log-unit regrowth of the bacilli in the lungs (P = 0.0001) was apparent, suggesting a weaker defense system in alveolar macrophages or a protective environment for M. tuberculosis in the lungs.
DISCUSSION
Our results demonstrate that L-CLF was highly effective in treatment of M. tuberculosis infection in an acute as well as a chronic mouse model of the disease. Earlier, we showed that liposome encapsulation of CLF reduced in vitro and in vivo the toxic effects associated with administration of free drug and enhanced its therapeutic activity against murine disseminated Mycobacterium avium-M. intracellulare complex (MAC) infection. Liposome encapsulation also allowed parenteral administration of the drug not otherwise possible because of its insolubility and lipophilic character. We have also shown that L-CLF is more effective in treatment of MAC infection than F-CLF injected i.v. or administered orally in beige mice (15, 20).
Since mycobacteria invade and reside within phagocytic cells, such as macrophages, adequate concentrations of antimycobacterials need to be achieved within the cellular compartments where the bacilli are located. Liposomes containing antibiotics naturally deliver high concentrations of antimycobacterials into infected macrophages, thus improving treatment outcomes for intracellular infections.
Oral administration of CLF has been the route of choice, but, in addition to causing many side effects (8, 10, 11), it has not been therapeutically beneficial for tuberculosis. CLF has been reported to accumulate within macrophages, where mycobacteria multiply; however, it is doubtful whether oral administration can lead to intracellular concentrations high enough to kill the bacteria. Also, CLF in its free form interacts with membranes and produces toxic effects whereas liposome encapsulation sequesters the drug from cell membranes, protecting the cells from the toxic effects of the free drug. Therefore, a parenteral formulation of CLF would be a better choice for treatment of intracellular pathogens such as mycobacteria. Even though liposome-encapsulated drug may be present in higher amounts inside the macrophages, it is far less toxic, is retained inside the cells for a longer time, and is released slowly, thereby producing longer-lasting effects. Thus, liposomes can deliver high concentrations of antimicrobials into cells, making inhibitory or bactericidal cellular concentrations of active antibiotics achievable. Furthermore, because liposome-encapsulated antimicrobials make it possible to achieve high concentrations within phagocytic cells, the chances of emergence of resistance may be reduced.
As observed earlier (20), the maximum tolerated dose of F-CLF was 5 mg/kg of body weight, which was not enough to cause a significant reduction in the number of viable M. tuberculosis bacilli. Even this concentration of free drug cannot be administered i.v. to patients because of its lipophilicity, the presence of organic solvents, and crystallization in the aqueous phase. An equivalent concentration of L-CLF did not improve the treatment outcome; however, encapsulation of CLF in liposomes reduced toxicity and allowed i.v. administration as well as administration of higher doses, enhancing therapeutic efficacy as observed in our studies with MAC (15, 20). Studies with some other antimicrobials have shown similar results (4, 16, 19, 22, 23).
The therapeutic efficacy of L-CLF increased with increasing doses. At a dose of 50 mg/kg, a statistically significant (P < 0.01 to 0.0001) response was observed in the different M. tuberculosis models used in this study. However, the effect was more pronounced in the chronic-infection model. The enhanced antibacterial activity of CLF with progression of infection in the established and chronic models can be attributed to the combined effect of the drug, the acquired specific immunity, and granuloma formation. We have observed similar results in our study of MAC treatment after different periods postinfection (15).
Another important observation in this study was that the treatment was more effective in the liver and spleen than in the lungs, similar to our previous studies with MAC (15, 20). These differences could be due to the differences in distribution of the drug and localization of bacteria in various organs. We also noted earlier that the lungs responded poorly to L-CLF therapy when the animals were infected with greater numbers of bacteria. Results of the studies with MAC led us to conclude that the drug concentration in the lungs was enough to kill only a small number of bacteria, and as the number of infecting bacteria increased, the drug was not able to reduce the bacterial load. In the present study, one series of L-CLF treatments resulted in the clearance of M. tuberculosis from the liver and spleen, and the lungs showed 2- to 3-log-unit reduction. It was interesting to note that there was no recurrence of bacterial growth in the liver and spleen for up to 2 months postinfection, whereas the lungs showed recovery of M. tuberculosis growth even after two series of L-CLF treatments. A closer look at the results indicated that the growth of the bacilli in control (untreated) mice increased steadily in the lungs, unlike the liver and spleen, which showed no increase in M. tuberculosis growth 3 months postinfection (Fig. 3 and 4). These findings suggest the presence of a more favorable environment for the organisms in the lungs than in the liver and spleen. The induction of various stimulatory and suppressive cytokines in response to M. tuberculosis infection in the lungs may be different from that in the liver and spleen; this indirect effect might also contribute to the above-mentioned differences.
Similar to our observations, earlier studies with other drugs (6, 7, 9, 12, 18, 29) also could not demonstrate a significant reduction in the number of bacteria in the lungs. The difficulty in the treatment of lung infection can be overcome either by increasing the uptake of liposomes in the lungs (3, 27) or by direct delivery of drugs by using aerosols. We have already standardized an aerosolized formulation of L-CLF for use in future studies (unpublished). The use of an aerosolized challenge model will further confirm the therapeutic efficacy of L-CLF in naturally acquired pulmonary disease. However, aerosolized administration of L-CLF will be the most important treatment regimen for pulmonary infections and will help delineate the role of lung pathology in treatment of these infections. In addition, studies of localization of L-CLF in specific areas of the lungs may also help in designing improved regimens for treatment with L-CLF.
In conclusion, we demonstrate a highly effective therapeutic response of L-CLF alone against M. tuberculosis infection in acute, established, and chronic murine models; the absence of recurrence of M. tuberculosis growth suggested a bactericidal effect of L-CLF in the liver and spleen. We therefore believe that L-CLF can be used as an effective therapeutic agent for the treatment of M. tuberculosis infections.
ACKNOWLEDGMENTS
This study was supported, in part, by grants from the Texas Higher Education Coordinating Board, ATP-D 000015084 and ATP 000015091, to R.T.M.; by NIH-NCI Cancer Center (Core) Support Grant CA-16672 to the Department of Veterinary Medicine and Surgery for animal care and maintenance; and by Intra-agency agreement Y1-AT-5016 to S.G.F.
We thank Julie Loesch, Nashone Soileau, Cheryl Lewis, Rhea Fajardo, and Joe Allen for technical assistance and Michael Kearney of Veterinary Statistical Services at the Louisiana State University School of Veterinary Medicine for statistical analyses.
REFERENCES
- 1.Adams L B, Dinauer M C, Morgenstern D E, Krahenbuhl J L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis. 1997;78:237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 2.Adams L B, Mason C M, Kolls J K, Scollard D, Krahenbuhl J L, Nelson S. Exacerbation of acute and chronic murine tuberculosis by administration of a TNF receptor-expressing adenovirus. J Infect Dis. 1995;171:400–405. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Kandpal H, Gupta H P, Singh N B, Gupta C M. Tuftsin-bearing liposomes as rifampin vehicles in treatment of tuberculosis in mice. Antimicrob Agents Chemother. 1994;38:588–593. doi: 10.1128/aac.38.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E. Use of liposomal preparations to treat mycobacterial infections. Immunobiology. 1994;191:578–583. doi: 10.1016/S0171-2985(11)80465-1. [DOI] [PubMed] [Google Scholar]
- 5.Bermudez L E M, Wu M, Young L S. Intracellular killing of Mycobacterium avium complex by rifapentine and liposome-encapsulated amikacin. J Infect Dis. 1987;156:510–513. doi: 10.1093/infdis/156.3.510. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez L E M, Yau-Young A O, Lin J P, Cogger J, Young L S. Treatment of disseminated Mycobacterium avium complex infection of beige mice with liposome encapsulated aminoglycosides. J Infect Dis. 1990;161:1262–1268. doi: 10.1093/infdis/161.6.1262. [DOI] [PubMed] [Google Scholar]
- 7.Cynamon M H, Swenson C E, Palmer G S, Ginsberg R S. Liposome-encapsulated amikacin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1989;33:1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayan Sandler E, Ng V L, Hadley W K. Clofazimine crystals in alveolar macrophages from a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1992;116:541–543. [PubMed] [Google Scholar]
- 9.Duzgunes N, Ashtekar D R, Flasher D L, Ghori N, Jacobs R J, Friends D S, Gangadharam P R. Treatment of Mycobacterium avium-intracellulare complex infection in beige mice with free and liposomal encapsulated streptomycin: role of liposome type and duration of treatment. J Infect Dis. 1991;164:143–151. doi: 10.1093/infdis/164.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Forster D J, Causey D M, Rao N A. Bull’s eye retinopathy and clofazimine. Ann Intern Med. 1992;116:876–877. doi: 10.7326/0003-4819-116-10-876_2. [DOI] [PubMed] [Google Scholar]
- 11.Gallets J C. Clofazimine: a review of its use in leprosy and Mycobacterium avium complex infection. Ann Pharmacother. 1991;25:525–531. doi: 10.1177/106002809102500513. [DOI] [PubMed] [Google Scholar]
- 12.Gangadharam P R, Ashtekar D A, Ghori N, Goldstein J A, Debs R J, Duzgunes N. Chemotherapeutic potential of free and liposome encapsulated streptomycin against experimental Mycobacterium avium complex infections in beige mice. J Antimicrob Chemother. 1991;28:425–435. doi: 10.1093/jac/28.3.425. [DOI] [PubMed] [Google Scholar]
- 13.Grover A A, Kim H K, Wiegeshaus E H, Smith D W. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J Bacteriol. 1967;94:832–840. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson R R. Treatment of leprosy. In: Hastings R C, editor. Leprosy. 2nd ed. Edinburgh, United Kingdom: Churchill Livingstone; 1997. p. 470. [Google Scholar]
- 15.Kansal R G, Gomez-Flores R, Sinha I, Mehta R T. Therapeutic efficacy of liposomal clofazimine against Mycobacterium avium complex in mice depends on size of initial inoculum and duration of infection. Antimicrob Agents Chemother. 1997;41:17–23. doi: 10.1128/aac.41.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlowsky J A, Zhanel G G. Concepts of the use of liposomal antimicrobial agents: applications for aminoglycosides. Clin Infect Dis. 1992;15:654–667. doi: 10.1093/clind/15.4.654. [DOI] [PubMed] [Google Scholar]
- 17.Kesavalu L, Goldstein J A, Debs R J, Duzgunes N, Gangadharam P R J. Differential effects of free and liposome-encapsulated amikacin on the survival of Mycobacterium avium complex in mouse peritoneal macrophages. Tuber Lung Dis. 1990;71:215–218. doi: 10.1016/0041-3879(90)90079-n. [DOI] [PubMed] [Google Scholar]
- 18.Le Conte P, LeGallou F, Potel G, Struillou L, Baron D, Drugeon H B. Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob Agents Chemother. 1994;38:2695–2701. doi: 10.1128/aac.38.12.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta R T. Liposomes as drug carriers for polyene antibiotics. Adv Drug Deliv Rev. 1989;3:283–306. [Google Scholar]
- 20.Mehta R T. Liposome encapsulation of clofazimine reduces toxicity in vitro and in vivo and improves therapeutic efficacy in the beige mouse model of disseminated Mycobacterium avium-M. intracellulare complex infection. Antimicrob Agents Chemother. 1996;40:1893–1902. doi: 10.1128/aac.40.8.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta R T, Keyhani A, McQueen T J, Rosenbaum B, Rolston K V, Tarrand J J. In vitro activities of free and liposomal drugs against Mycobacterium avium-M. intracellulare complex and M. tuberculosis. Antimicrob Agents Chemother. 1993;37:2584–2587. doi: 10.1128/aac.37.12.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta R T, McQueen T J, Keyhani A, Lopez-Berestein G. Liposomal hamycin: reduced toxicity and improved antifungal efficacy in vitro and in vivo. J Infect Dis. 1991;164:1003–1006. doi: 10.1093/infdis/164.5.1003. [DOI] [PubMed] [Google Scholar]
- 23.Mehta R T, Poddar S, Kalidas M, Gomez-Flores G, Dulski K. Role of macrophages in the candidacidal activity of liposomal amphotericin B. J Infect Dis. 1996;175:214–217. doi: 10.1093/infdis/175.1.214. [DOI] [PubMed] [Google Scholar]
- 24.Pablos-Mendez A, Raviglione M C, Laszlo A, Binkin N, Rieder H L, Bustreo F, Cohn D L, Lambregts-van Weezenbeek C S B, Kim S J, Chaulet P, Nunn P. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 25.Sepkowitz K A, Raffalli J, Riley L, Kiehn T E, Armstrong D. Tuberculosis in the AIDS era. Clin Microbiol Rev. 1995;8:180–199. doi: 10.1128/cmr.8.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swenson C R, Popescu M C, Ginsberg R S. Preparation and use of liposomes in the treatment of microbial infections. Crit Rev Microbiol. 1988;15:S1–S31. doi: 10.3109/10408418809104463. [DOI] [PubMed] [Google Scholar]
- 27.Takada M, Yuzuhira T, Katayama K, Iwamoto K, Sunamoto J. Increased lung uptake of liposomes coated with polysaccharides. Biochim Bioiphys Acta. 1984;802:237–244. doi: 10.1016/0304-4165(84)90167-3. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka H, Saito H, Sato K, Yoneyama T. Therapeutic efficacy of liposome-encapsulated kanamycin against Mycobacterium intracellulare infection induced in mice. Am Rev Respir Dis. 1991;144:575–579. doi: 10.1164/ajrccm/144.3_Pt_1.575. [DOI] [PubMed] [Google Scholar]
- 29.Vladimirsky M A, Ladigina G A. Antibacterial activity of liposome-entrapped streptomycin in mice infected with Mycobacterium tuberculosis. Biomedicine. 1982;36:375–377. [PubMed] [Google Scholar]