Abstract
Due to cancer survivors’ increased vulnerability to complications from COVID-19, addressing vaccine hesitancy and improving vaccine uptake among this population is a public health priority. However, several factors may complicate efforts to increase vaccine confidence in this population, including the underrepresentation of cancer patients in COVID-19 vaccine trials and distinct recommendations for vaccine administration and timing for certain subgroups of survivors. Evidence suggests vaccine communication efforts targeting survivors could benefit from strategies that consider factors such as social norms, risk perceptions, and trust. However, additional behavioral research is needed to help the clinical and public health community better understand, and more effectively respond to, drivers of vaccine hesitancy among survivors and ensure optimal protection against COVID-19 for this at-risk population. Knowledge generated by this research could also have an impact beyond the current COVID-19 pandemic by informing future vaccination efforts and communication with cancer survivors more broadly.
Keywords: COVID-19 vaccines, cancer survivors, Vaccine hesitancy, Behavioral research, Health
The World Health Organization defines vaccine hesitancy as a delay in acceptance or refusal of vaccination despite the availability of vaccination services (MacDonald & the SAGE Working Group on Vaccine Hesitancy, 2015) and has identified vaccine hesitancy as one of the greatest public health threats of our time (World Health Organization, 2019). Improving vaccination uptake among healthy children and adults is an important priority for achieving population-level protection from vaccine-preventable diseases, including COVID-19. However, COVID-19-related vaccine hesitancy among cancer survivors1 warrants special attention due to the unique clinical and behavioral challenges affecting this population. Therefore, the aim of this commentary is to elucidate factors that may contribute to COVID-19 vaccine hesitancy and vaccination uptake among cancer survivors. The commentary will also highlight promising communication strategies for addressing COVID-19 vaccine hesitancy among survivors and outline behavioral research priorities for ensuring optimal protection of this at-risk population.
Unique considerations for COVID-19 vaccination among cancer survivors
Research suggests that individuals with a history of cancer are at increased risk for mortality and severe complications from COVID-19 (Fillmore et al., 2021; Ganatra et al., 2020; Korde et al., 2021; Wang et al., 2021). Survivors may also encounter disruptions in their cancer treatment and survivorship care due to COVID-19-related illness or precautions (Korde et al., 2021; Potter et al., 2021). Although it seems logical that these increased health risks and possible delays in cancer therapy would result in increased uptake of COVID-19 vaccines among survivors, several studies indicate substantial vaccine hesitancy among this population in the United States (U.S.). For example, Waters et al. surveyed 342 adolescent and young adult cancer survivors between October 2020 and January 2021, finding that over one-third of respondents (37.1%) reported COVID-19 vaccine hesitancy and that hesitancy was higher among female survivors and survivors with lower educational attainment (Waters, Mann et al., 2021). Additionally, in an online national survey of over 6500 blood cancer patients fielded in December 2020 by the Leukemia & Lymphoma Society (LLS), almost 20% of respondents indicated that they would be unlikely or very unlikely to accept a vaccine if it were offered to them for free – with several demographic factors, including younger age, non-White race, female gender, and rural or suburban residence, predicting vaccine hesitancy (Conti et al., 2021). A Kaiser Family Foundation COVID-19 Vaccine Monitor survey that was also conducted in December 2020 indicated that approximately one-quarter of the public was vaccine hesitant at that point in time (Kaiser Family Foundation, 2020), suggesting cancer survivors’ vaccine concerns may be similar, if not more heightened, than those of the broader public. As of February 2022, 16% of adults continue to say they definitely will not get the vaccine, and an additional 3% say they would only get vaccinated if it is required (Kaiser Family Foundation, 2022). The fact that only 65% of the U.S. population was fully vaccinated against COVID-19 in March 2022 (Centers for Disease Control and Prevention, 2021a) means that cancer survivors are still not protected by herd immunity at the population-level, making it even more vital to address vaccine hesitancy and increase uptake in this vulnerable population.
There are several intersecting clinical and behavioral considerations that may affect COVID-19 vaccination decisions and uptake among cancer survivors. First, there is a dearth of specific information and data available on COVID-19 vaccine safety and efficacy in this population given that cancer patients were underrepresented in COVID-19 vaccine trials (American Society of Clinical Oncology, 2021; Desai et al., 2021). This information gap may result in greater safety and efficacy concerns and consequently vaccine hesitancy among cancer survivors. For example, in a study evaluating a COVID-19 educational webinar targeted to cancer patients and caregivers, a lack of trust that the vaccine is safe for cancer patients was cited as a reason for vaccine hesitancy by some participants (Kelkar et al., 2021). Additionally, emerging research suggests a potential for reduced immune response to COVID-19 vaccines among cancer survivors (Palich et al., 2021), particularly those with hematological cancers (Agha et al., 2021; Greenberger et al., 2021; Monin et al., 2021). This may complicate attempts to encourage vaccination among survivors, as it may influence their perceptions of vaccine efficacy and have a negative impact on their decisional analysis regarding the vaccine.
There are also several special considerations for the recommendation and administration of COVID-19 vaccination in this population, which may complicate vaccine uptake. For example, the National Comprehensive Cancer Network (NCCN) COVID-19 Vaccination Advisory Committee’s guidelines recommend that vaccination be delayed for at least three months following certain cancer treatments, including hematopoietic cell transplantation and CAR-T cell therapy (National Comprehensive Cancer Network, 2021). The NCCN guidelines are meant to optimize immune response to the vaccine among cancer patients, but if not communicated carefully, could be misinterpreted as suggesting that COVID-19 vaccines can interfere with the effectiveness of cancer therapy. Further, Centers for Disease Control and Prevention (CDC) guidance released in August 2021 recommended that an additional dose of an mRNA COVID-19 vaccine should be considered for people with moderate to severe immune compromise due to a medical condition such as cancer or receipt of immunosuppressive medications or treatments (including certain cancer chemotherapeutic drugs) (Centers for Disease Control and Prevention, 2021c). Having different vaccine recommendations for cancer survivors may contribute to confusion and complicate message diffusion, especially in the absence of vaccine trials with adequate survivor representation. Additionally, it is possible that guidance around additional doses might reduce perceptions of vaccine efficacy, further highlighting the need for coordinated outreach to communicate specific recommendations for cancer survivors.
Finally, the potential impact of health misinformation on cancer survivors must be taken into account. In a study conducted in 2020, Guidry et al. found that compared to individuals with no cancer history, cancer survivors undergoing active treatment were significantly more likely to believe misinformation related to COVID-19, but survivors who were no longer in treatment were less likely to endorse COVID-19 misinformation (Guidry, Miller et al., 2021). The authors suggest that individuals currently undergoing cancer treatment may have greater concern about the impact of the pandemic on their health, leading them to seek more information (including online), thereby increasing their risk of exposure to misinformation (Guidry, Miller et al., 2021). In a separate study, the same research team also found that parents of children with cancer were more likely to endorse COVID-19 misinformation than parents of children without cancer, similarly suggesting that parents of childhood cancer survivors may be at risk of higher exposure to misinformation due to greater health information seeking (Guidry, Miller et al., 2021). These findings are concerning, as research suggests that belief in misinformation about COVID-19 can contribute to COVID-19 vaccine hesitancy, potentially because misinformation increases confusion, distress, and mistrust (Lockyer et al., 2021).
The intersecting behavioral and clinical factors described above may negatively affect cancer survivors’ vaccine confidence and hinder efforts to protect this vulnerable population from COVID-19. Careful and timely attention to the vaccine information needs of survivors is critical for both healthcare providers and broader health behavior and health communication efforts. In response, several promising communication approaches for addressing vaccine hesitancy in this population are outlined below. However, as discussed in greater detail in the latter part of the commentary, additional behavioral research will be needed to better understand vaccine hesitancy among cancer survivors and guide efforts to address this public health concern.
Communication strategies for addressing COVID-19 vaccine hesitancy in cancer survivors
Based on current evidence, there are several recommended communication strategies that can be readily implemented to address vaccine hesitancy and bolster vaccine confidence among the broader population (Chou, Burgdorf, et al., 2020), which in turn can be purposively tailored for use in the cancer survivor population (Table 1).
Table 1.
Communication strategies to address COVID-19 vaccine hesitancy in cancer survivors
| Communication strategies for addressing COVID-19 vaccine hesitancy among cancer survivors | |
|---|---|
| Assess and communicate risk |
• Assess cancer survivors’ risk perceptions to understand likelihood of COVID-19 vaccination uptake. • Pair messages about increased risk of COVID-19 complications with messages about the effectiveness and availability of vaccines. |
| Establish positive social norms and utilize peer models |
• Recruit cancer survivors to serve as vaccine advocates and promote vaccination among their peers. • Create messages that (1) highlight the large number of cancer survivors who have already received the vaccine and (2) showcase the stories of survivors who have made the decision to vaccinate. |
| Apply patient-centered communication approaches |
• Improve the trustworthiness, quality, and consistency of provider communication regarding COVID-19 vaccines. • Use patient-centered communication approaches, such as motivational interviewing, to understand survivors’ vaccination concerns and increase their engagement in care. • Communicate availability of onsite vaccination or facilitate vaccine appointments at other vaccine locations. • Encourage all providers who see individuals with a cancer history to assess their vaccine status and promote vaccination for both cancer survivors and their caregivers. |
| Leverage technology | • Use digital tools, such as web-based decision aids, to help individuals understand the benefits and risks of vaccination. |
|
Address health misinformation |
• Help cancer survivors and their caregivers more easily assess the credibility of health information they find online and avoid unreliable sources through digital literacy efforts and “information prescriptions” to high quality resources. |
Risk assessment and communication
Assessment of survivors’ risk perceptions (i.e., risk likelihood, susceptibility, and severity) provides foundational information on whether or not they’re likely to vaccinate against COVID-19 and may inform related health messaging for survivors, as higher risk perceptions are positively associated with vaccination behavior (Brewer et al., 2007). Specifically, perceived threat from infection is a potentially important factor in decision making around COVID-19 preventive behaviors (Van Bavel et al., 2020), suggesting that efforts to inform cancer survivors of their increased risk of complications could be an effective way to promote vaccine acceptance. However, research has also shown that messages that increase threat perceptions (e.g., fear appeals) can be counterproductive and lead to defensive reactions when individuals do not feel they have the capacity to deal with the threat (Van Bavel et al., 2020). Taken together, pairing messaging about survivors’ heightened susceptibility to COVID-19 complications and the potential negative impact of infection on cancer care with messages regarding the widespread availability and effectiveness of vaccination may be an impactful way to increase vaccine confidence.
Social norms and peer models
Health behavior is also heavily influenced by perceived social norms, particularly norms that are held by members of a person’s in-group (or individuals with whom they share an identity) (Van Bavel et al., 2020). Prior behavioral interventions have successfully leveraged peer cancer survivors to increase physical activity (Pinto et al., 2015), improve stress management (Nápoles et al., 2020), and enhance well-being (Giese-Davis et al., 2016) among cancer survivors, suggesting that peer influence might be especially effective in encouraging the adoption of health-promoting actions. Although vaccination behaviors may be unique in certain respects (e.g., vaccination is a discrete rather than long-term behavior and may be more affected by misinformation), the use of peer models could still be a promising strategy, and research suggests that survivors may be willing to serve as vaccine advocates, especially when they believe vaccines are safe (Shelal et al., 2020). Moreover, the literature on cancer support groups demonstrates the value patients derive from sharing their experiences and learning from others in similar circumstances (Cipolletta et al., 2019; Öster et al., 2013). The sense of shared identity among cancer survivors and desire to hear from fellow survivors suggests that messages specifically highlighting how many cancer survivors have already received the vaccine, as well as messaging that showcases narratives from survivors about their decision to vaccinate, may be effective in establishing positive social norms and motivating desired vaccination behavior.
Patient-centered communication
Trust in those who recommend and administer vaccinations is also widely recognized as an important driver of vaccine decisions. As health providers remain the most trusted source of information on vaccination for most patients (Dubé et al., 2013), they have an important role to play in addressing vaccine hesitancy (Potter et al., 2021). Prior research has not only demonstrated the importance of a provider recommendation on vaccine uptake – including among cancer patients (Kasting et al., 2019; Klosky et al., 2015; Lu et al., 2018; Warner et al., 2020), but has also shown that recommendation strength is an important factor in vaccination outcomes (Gilkey et al., 2016; Rosenthal et al., 2011). Improving the trustworthiness, quality, and consistency of provider communication regarding vaccines could therefore be an important strategy for building trust and addressing COVID-19 vaccine hesitancy among cancer survivors.
Similarly, approaches that are rooted in patient-centered communication, that is to say, those that consider patients’ needs, perspectives, and individual experiences; provide opportunities for patients to participate in their care; and enhance the patient-clinician relationship (Epstein & Street, 2007), are a promising avenue for improving patient-provider discussions about the COVID-19 vaccine. For example, motivational interviewing (MI) is recommended by the CDC as an evidence-based practice for healthcare professionals to consider using when discussing COVID-19 vaccination with their patients (Centers for Disease Control and Prevention, 2021d) and empirical evidence has shown it to be effective in reducing vaccine hesitancy in other contexts (Verger & Dubé, 2020). MI is a person-centered counseling approach based on empathetic listening that supports self-efficacy, emphasizes autonomy in decision making, acknowledges and works with resistance, and seeks to resolve ambivalence about a behavior (Britt et al., 2004; Copeland et al., 2015), which could make it particularly useful in the context of vaccine discussions with cancer survivors. In the absence of providers having dedicated time to engage in MI, additional self-affirmation strategies that involve asking cancer survivors to reflect on important values, attributes, or social relations in response to COVID-19 vaccine information could also be used in patient-centered communication approaches to address vaccine hesitancy given evidence suggesting self-affirmation has positive effects on message acceptance, intentions to change, and resultant behavior (Epton et al., 2015).
In addition to effective patient-provider communication approaches, provision of onsite vaccination or facilitation of appointments at other vaccine locations should be implemented in all settings where cancer survivors may receive healthcare services, including primary care offices, pediatric clinics, community health centers, cancer centers, and oncology practices (Potter et al., 2021). Vaccination messages can be delivered by a range of providers including nurses, doctors, and pharmacists and via electronic patient communication portals during both active cancer care and long-term survivorship care, which is important as survivors at different phases of treatment may have varying levels of contact with the health system and obtain care from different types of providers (Institute of Medicine and National Research Council, 2006; Mayo et al., 2021). Furthermore, different providers might be able to leverage different strengths in recommending vaccines – for example, primary care providers might be able to take advantage of established long-term relationships with survivors and a focus on preventive care (Nekhlyudov, 2021), while oncologists may be well-positioned to discuss survivors’ unique vulnerability due to their cancer history, why it is especially important for them to be vaccinated, and any related treatment considerations (Potter et al., 2021; Waters, Mann, et al., 2021). Consequently, all providers who see individuals with a cancer history should prioritize documenting their vaccine status and encouraging hesitant survivors to consider vaccination. When possible, these vaccine-focused conversations should also include the survivor’s caregiver(s) – especially if the caregivers are also vaccine hesitant – both because having their household vaccinated can further protect survivors if they do not mount a robust immune response to the vaccine (Woodfield et al., 2021), and because in some cases vaccination is a familial decision making process (Klosky et al., 2009), such that strategies targeting both the survivor and their caregiver(s) or family members may increase likelihood of ultimate uptake.
Leveraging technology
Technology could also serve as another tool to support communication about COVID-19 vaccination for cancer survivors who are hesitant, while placing fewer demands on clinicians’ time. For example, web-based patient decision aids, which are commonly used in both cancer and vaccination contexts, may help individuals understand their clinical options and the related benefits and risks. Vaccination decision aids in particular have been shown to be effective in reducing decisional conflict, improving attitudes toward vaccines, and increasing intentions to vaccinate (Shourie et al., 2013; Witteman et al., 2015). Additionally, a recent study from France demonstrated that an interactive web tool providing information on risks and benefits of COVID-19 vaccination was able to increase intention to receive COVID-19 vaccines among patients with chronic conditions who were initially hesitant (Tran et al., 2021).
Addressing health misinformation
Lastly, in addition to providing accurate information about vaccines, communication efforts should address survivors’ vulnerability to COVID-19 vaccine misinformation. For example, digital literacy efforts to help cancer survivors and their caregivers more easily assess the credibility of information they find online and avoid unreliable sources may help reduce susceptibility to health misinformation. As one approach, providers could offer patients “information prescriptions” to high quality resources (Chou & Miller, 2021) on COVID-19 vaccines that are specifically written for cancer patients (e.g., American Cancer Society, LUNGEVITY, and LLS webpages on COVID-19 vaccines and frequently asked questions for patients and caregivers). Beyond health literacy efforts, social media platforms have a responsibility to curb the spread of false COVID-19 vaccine content by changing their algorithms and content moderation practices so that vaccine misinformation does not get circulated unchecked and such misinformation may be corrected/debunked (Chou, Gaysynsky, et al., 2020).
Behavioral research priorities to address vaccine hesitancy among cancer survivors
Although the existing evidence base suggests several promising communication approaches for mitigating COVID-19 vaccine hesitancy and building vaccine confidence among cancer survivors, there are still knowledge gaps unique to vaccine hesitancy in the cancer context that can be addressed through additional behavioral research (Table 2). Below we outline several research priorities.
Table 2.
Behavioral research priorities to address COVID-19 vaccine hesitancy
| Behavioral research needed to address COVID-19 vaccine hesitancy in cancer survivors | |
|---|---|
| Surveillance |
• Ongoing assessment of vaccine intentions and uptake among cancer survivors using surveys with large, representative samples of survivors. • Studies to identify disparities by sociodemographic characteristics, cancer diagnosis and treatment variables, healthcare access barriers, and other factors. |
| Drivers of vaccine hesitancy | • Studies on the drivers of hesitancy among cancer survivors, including analyses to identify unique factors/concerns in this population. |
| Health misinformation |
• Studies to assess vaccine misinformation exposure among cancer survivors and identify subgroups of survivors that may be more vulnerable to health misinformation. • Research examining the impact of health misinformation exposure on cancer survivors’ knowledge, attitudes, risk perceptions, and subsequent vaccine hesitancy. • Work to identify measures that can help reduce the spread and impact of health misinformation and improve the communication environment. |
| Intervention development and implementation |
• Research to optimize vaccine hesitancy interventions for survivors, including studies to identify the best approach for patient-provider communication in the context of COVID-19 vaccination and cancer survivorship. • Development, implementation, and evaluation of communication interventions that can be delivered at multiple levels and by multiple stakeholders. • Additional work to explore the benefits and limitations of technology-based interventions. |
Surveillance
As a first step, similar to efforts that monitor the general public’s evolving COVID-19 vaccine attitudes and behaviors (Centers for Disease Control and Prevention, 2021b), comprehensive and up-to-date assessments of vaccine intentions and uptake among cancer survivors are needed. The few studies conducted to date have been small-scale, convenience sample-based surveys with specific subpopulations (e.g., (Conti et al., 2021; Waters, Mann et al., 2021) that were administered prior to widespread vaccine availability and the Food and Drug Administration’s approval of the Pfizer-BioNTech COVID-19 vaccine (Food and Drug Administration, 2021), which may have increased vaccine acceptance among survivors. Larger, more representative surveys are needed to obtain a clearer picture of the current prevalence of vaccine hesitancy among cancer survivors and identify disparities by sociodemographic factors, cancer diagnosis and treatment variables, or healthcare access barriers. Ongoing monitoring of survivors’ vaccination behaviors and related attitudes could serve as an early warning system, alerting healthcare providers and public health practitioners to changes that may require intervention, and could also be a source of data for evaluating the impact of intervention efforts.
Drivers of vaccine hesitancy
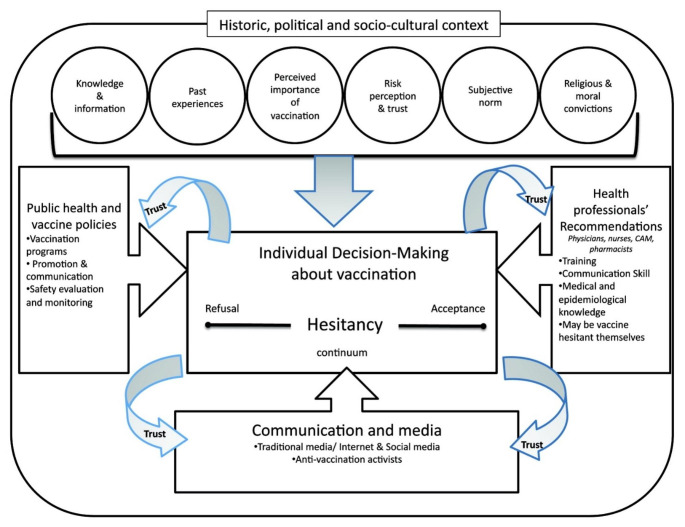
A better understanding of key drivers of hesitancy in this population is needed given that cancer survivors’ vaccination decisions are complicated by their cancer diagnosis, immunocompromised status, and associated uncertainties. A conceptual model of vaccine hesitancy developed by Dubé and colleagues (Fig. 1) highlights the importance of knowledge, past experience, subjective norms, risk perceptions, and trust in vaccination decision making (Dubé et al., 2013). However, it is unclear whether these factors are all relevant in the survivorship context, or if there may be additional factors or unique concerns driving survivors’ vaccine hesitancy (such as concerns about vaccine interference with cancer treatment (Kelkar et al., 2021)). Additional research to identify the most salient factors driving cancer survivors’ COVID-19 vaccination decisions is therefore needed.
Fig. 1.
Conceptual Model of Vaccine Hesitancy
Reprinted from Dubé, E., Laberge, C., Guay, M., Bramadat, P., Roy, R., & Bettinger, J. A. (2013). Vaccine hesitancy: an overview. Human vaccines & immunotherapeutics, 9(8), page 1764 by permission of the publisher (Taylor & Francis Ltd - https://www.tandfonline.com)
Health misinformation
The model from Dubé and colleagues also highlights the important influence of the communication environment (e.g., social media) on vaccine hesitancy (Dubé et al., 2013). Research is needed to estimate exposure to vaccine misinformation among cancer survivors, especially specific subgroups of survivors that may be at higher risk for exposure to misinformation (e.g., those in active treatment and those with lower health literacy). There is also a knowledge gap related to the impact of health misinformation exposure on cancer survivors’ knowledge, attitudes, risk perceptions, and subsequent vaccine hesitancy. Moreover, additional research is needed about not only the impact of COVID-19 vaccine misinformation among cancer survivors, but also the best ways to address it. The U.S. Surgeon General’s 2021 advisory called for a whole-of-society effort to address misinformation, including product design and policy changes on social media platforms, initiatives to build resilience against health misinformation (e.g., through literacy programs), and efforts to improve public health communication (Office of the Surgeon General, 2021). However, the advisory also recognized that in order to mount an effective response to health misinformation, more research to identify the most effective interventions for reducing the spread and impact of misinformation would be needed (Office of the Surgeon General, 2021). It will be important for these research efforts to consider and prioritize vulnerable populations, including cancer survivors and their caregivers, who are at risk for significant harm from health misinformation, including misinformation about COVID-19 vaccines.
Intervention development and implementation
Behavioral research is also needed to optimize vaccine communication for cancer survivors and develop tailored messaging to address their specific concerns. Behavioral studies could help identify the best approach for patient-provider communication in the context of COVID-19 vaccination and cancer survivorship, as the most effective communication approach may vary based on the specific vaccine and population in question. For example, some studies on human papillomavirus (HPV) vaccine suggest that a presumptive approach (i.e., using brief statements that assume parents are ready to vaccinate) may be more effective than a participatory approach (i.e., engaging parents in open-ended discussions) for increasing vaccine uptake (Brewer et al., 2017). However, it is unclear whether an approach that works for established vaccines offered to healthy children and adolescents would work equally well for a novel vaccine offered to medically vulnerable individuals.
In addition to improving patient-provider conversations in healthcare settings, there is also a need to develop and test communication interventions that can be delivered at multiple levels (e.g., individual, organizational, community, policy) through cancer research and patient advocacy groups, the National Cancer Institute’s Cancer Information Service, NCI-designated Cancer Centers, CDC’s Division of Cancer Prevention and Control, oncology professional societies, state and local public health agencies, healthcare systems, and community organizations, among other key stakeholders. Potter et al. note that healthcare organizations in particular have boots-on-the-ground in the communities they serve and are therefore well positioned to encourage vaccinations among vulnerable populations, for example, by holding public forums, giving media interviews, distributing information in their clinics, and creating vaccination patient advocate programs (Potter et al., 2021). A recent study showed that an educational webinar targeting cancer survivors and caregivers created by a cancer center, in collaboration with a regional cancer collaborative and a state cancer council, was able to shift COVID-19 vaccine attitudes and intentions among participants (Kelkar et al., 2021). These types of communication interventions should be co-developed with cancer survivors to ensure salience and appropriateness and should also be made accessible (e.g., offered in multiple languages, available on credible websites, developed using health literacy best practices).
How technology-based interventions can be leveraged to address vaccine hesitancy among cancer survivors is also an area that could benefit from additional behavioral research. In particular, web-based interventions have the potential for significant reach, meaning that even small effect sizes can have considerable impact on the population level. Such interventions can be available on-demand and therefore can overcome barriers related to time constraints and geographic distance. An additional advantage of web-based interventions is that they can be personalized to match salient user characteristics (including demographics, cancer history, values, vaccine concerns, etc.), which may help increase effectiveness (Dempsey et al., 2020; Gowda et al., 2013; Panozzo et al., 2020). However, given that technology access and high-speed internet availability are not universal and communication inequalities are driven by structural determinants (like socioeconomic status and geography) (Viswanath et al., 2007), the potential that certain groups of survivors may have limited access to quality COVID-19 vaccine information is an important consideration. Therefore, it is vital to ensure that deployment of technology-based interventions does not exacerbate existing disparities and that interventions are developed with survivors’ communication preferences and available resources in mind.
Conclusions
Because cancer survivors are particularly vulnerable to complications from COVID-19, addressing vaccine hesitancy and improving uptake among this population should be a public health priority. Therefore, specific efforts should be developed and implemented for this unique and growing population. Existing behavioral research, for example, regarding social norms, risk perceptions, trust, and use of technology can help guide communication efforts with this population. There have been calls for greater inclusion of cancer survivors in COVID-19 vaccine clinical trials in order to generate more information about vaccine safety and efficacy among this patient population (American Society of Clinical Oncology, 2021), and additional behavioral research will be needed to complement vaccine response research to ensure that emerging evidence is communicated effectively, thereby increasing vaccine confidence among cancer survivors. Collectively, surveillance, communication research, psychosocial science, and intervention research is needed to better understand, and effectively respond to, the drivers of vaccine hesitancy in this population. These studies can help to identify specific characteristics of cancer survivors that make them more or less likely to experience vaccine hesitancy and inform efforts to adapt, target, and tailor interventions to their needs. For example, if surveillance research shows that rates of vaccine hesitancy are higher among rural cancer survivors, qualitative methods could be used to ascertain drivers of hesitancy in this population (e.g., lower perceptions of risk, lack of trust, lack of vaccine access), and interventions could then be designed to address the most salient factors for this group. Similarly, if vaccine hesitancy continues to be high among female survivors (Conti et al., 2021; Waters, Mann et al., 2021), unique decisional factors should be examined (e.g., concerns about fertility, greater fear of side effects), and tailored strategies to address those concerns should be explored – for example, stories about the experiences of other female survivors.
Lessons learned from behavioral research targeting COVID-19 vaccination among cancer survivors can also have an impact beyond the current pandemic, as hesitancy and limited uptake among cancer survivors and their caregivers has been documented for other vaccines, including HPV (Hoffman et al., 2012; Kirchhoff et al., 2019) and influenza vaccines (Chang et al., 2019). Robust and timely behavioral research on vaccine hesitancy in survivors may help future communication and implementation efforts to improve uptake of other recommended vaccines in this vulnerable population and could also advance broader cancer communication science efforts for survivors and their caregivers.
Acknowledgements
The authors would like to thank Douglas R. Lowy, MD, Principal Deputy Director, National Cancer Institute, for his insightful comments on the manuscript.
Authors’ Contributions
All authors contributed to the commentary conception and execution. Material preparation was performed by Robin Vanderpool and Anna Gaysynsky with significant input from Wen-Ying Sylvia Chou and Emily Tonorezos. The first draft of the manuscript was written by Robin Vanderpool and Anna Gaysynsky and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Declarations
Conflicts of interest/competing interests
The authors have no relevant conflicts of interests or competing interests to disclose.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Disclaimer
The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
Footnotes
As defined by the National Cancer Institute, an individual is considered a cancer survivor from the time of
diagnosis through the balance of his or her life. There are many types of survivors, including those living with
cancer and those free of cancer (National Cancer Institute - Office of Cancer Survivorship, 2021).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infectious Diseases. 2021;8(7):ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Clinical Oncology (2021). Inclusion of Individuals with Cancer on COVID-19 Vaccine Trials: A Joint Position Statement from the American Society of Clinical Oncology and Friends of Cancer Research. Retrieved from https://www.asco.org/sites/new-www.asco.org/files/content-files/blog-release/pdf/2021-ASCO-Friends-Vaccine-Trials-Position-Statement.pdf
- Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health psychology. 2007;26(2):136. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- Brewer, N. T., Hall, M. E., Malo, T. L., Gilkey, M. B., Quinn, B., & Lathren, C. (2017). Announcements versus conversations to improve HPV vaccination coverage: a randomized trial.Pediatrics, 139(1) [DOI] [PMC free article] [PubMed]
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Education and Counseling. 2004;53(2):147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2021a). COVID Data Tracker - COVID-19 Vaccinations in the United States. Retrieved from https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- Centers for Disease Control and Prevention (2021b). COVID Data Tracker - Trends in COVID-19 Vaccine Confidence in the US. Retrieved from https://covid.cdc.gov/covid-data-tracker/#vaccine-confidence
- Centers for Disease Control and Prevention (2021c). Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Retrieved from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?ACSTrackingID=USCDC_9_13-DM63868&ACSTrackingLabel=DCPC%20Announcement%3A%20COVID-19%20Vaccine%20Third%20Dose%20for%20Cancer%20Patients&deliveryName=USCDC_9_13-DM63868#considerations-additional-vaccine-dose
- Centers for Disease Control and Prevention (2021d). Talking with Patients about COVID-19 Vaccination: An Introduction to Motivational Interviewing for Healthcare Professionals. Retrieved from https://www.cdc.gov/vaccines/covid-19/hcp/engaging-patients.html#:~:text=Motivational%20interviewing%20is%20an%20evidence,with%20their%20values%20and%20needs
- Chang A, Payne JB, Allen PB, Koff JL, Ahmed R, Flowers CR, Bednarczyk RA. Influenza vaccination documentation rates during the first year after diagnosis of diffuse large B cell lymphoma. Clinical Lymphoma Myeloma and Leukemia. 2019;19(4):239–243. doi: 10.1016/j.clml.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, W. Y. S., Burgdorf, C. E., Gaysynsky, A., & Hunter, C. M. (2020). COVID-19 Vaccination Communication: Applying Behavioral and Social Science to Address Vaccine Hesitancy and Foster Vaccine Confidence. Retrieved from https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Chou WYS, Gaysynsky A, Cappella JN. Where We Go From Here: Health Misinformation on Social Media. American journal of public health. 2020;110(S3):S273–S275. doi: 10.2105/ajph.2020.305905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, W. Y. S., & Miller, R. S. (2021). Cancer-Related Misinformation Online: How Bad Is It and What Should Oncologists Do About It? ASCO Daily News. Retrieved from https://dailynews.ascopubs.org/do/10.1200/ADN.21.200673/full/
- Cipolletta S, Simonato C, Faccio E. The effectiveness of psychoeducational support groups for women with breast cancer and their caregivers: a mixed methods study. Frontiers in psychology. 2019;10:288. doi: 10.3389/fpsyg.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, R., Akesson, J., Weiss, E., Sae-Hau, M., Lee, M., Gracia, G., & Metcalfe, R. (2021). COVID-19 vaccine hesitancy among blood cancer patients
- Copeland L, McNamara R, Kelson M, Simpson S. Mechanisms of change within motivational interviewing in relation to health behaviors outcomes: a systematic review. Patient Education and Counseling. 2015;98(4):401–411. doi: 10.1016/j.pec.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Dempsey, A., Kwan, B. M., Wagner, N. M., Pyrzanowski, J., Brewer, S. E., Sevick, C., & Glanz, J. (2020). A values-tailored web-based intervention for new mothers to increase infant vaccine uptake: development and qualitative study. Journal of medical Internet research, 22(3), 98–113 [DOI] [PMC free article] [PubMed]
- Desai, A., Gainor, J. F., Hegde, A., Schram, A. M., Curigiliano, G., Pal, S., & Grande, E. (2021). COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nature Reviews Clinical Oncology,1–7 [DOI] [PMC free article] [PubMed]
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy: an overview. Human vaccines & immunotherapeutics. 2013;9(8):1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, R. M., & Street, R. L. (2007). Patient-centered communication in cancer care: promoting healing and reducing suffering
- Epton T, Harris PR, Kane R, van Koningsbruggen GM, Sheeran P. The impact of self-affirmation on health-behavior change: A meta-analysis. Health Psychology. 2015;34(3):187. doi: 10.1037/hea0000116. [DOI] [PubMed] [Google Scholar]
- Fillmore NR, La J, Szalat RE, Tuck DP, Nguyen V, Yildirim C, Munshi NC. Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study. JNCI: Journal of the National Cancer Institute. 2021;113(6):691–698. doi: 10.1093/jnci/djaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2021). FDA Approves First COVID-19 Vaccine [Press release]. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
- Ganatra S, Dani SS, Redd R, Rieger-Christ K, Patel R, Parikh R, Brar SS. Outcomes of COVID-19 in Patients With a History of Cancer and Comorbid Cardiovascular Disease. Journal of the National Comprehensive Cancer Network. 2020;1(aop):1–10. doi: 10.6004/jnccn.2020.7658. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Bliss‐Isberg C, Wittenberg L, White J, Star P, Zhong L, Spiegel D. Peer‐counseling for women newly diagnosed with breast cancer: A randomized community/research collaboration trial. Cancer. 2016;122(15):2408–2417. doi: 10.1002/cncr.30036. [DOI] [PubMed] [Google Scholar]
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine. 2016;34(9):1187–1192. doi: 10.1016/j.vaccine.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C, Schaffer SE, Kopec K, Markel A, Dempsey AF. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Human vaccines & immunotherapeutics. 2013;9(2):437–445. doi: 10.4161/hv.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger, L. M., Saltzman, L. A., Senefeld, J. W., Johnson, P. W., DeGennaro, L. J., & Nichols, G. L. (2021). Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies.Cancer Cell [DOI] [PMC free article] [PubMed]
- Guidry, J. P., Carlyle, K. E., Miller, C. A., Ksinan, A. J., Winn, R., Sheppard, V. B., & Fuemmeler, B. F. (2021). Endorsement of COVID-19 related misinformation among cancer survivors.Patient Education and Counseling [DOI] [PMC free article] [PubMed]
- Guidry JP, Miller CA, Ksinan AJ, Rohan JM, Winter MA, Carlyle KE, Fuemmeler BF. COVID-19–Related Misinformation among Parents of Patients with Pediatric Cancer. Emerging Infectious Diseases. 2021;27(2):650. doi: 10.3201/eid2702.203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Okcu MF, Dreyer ZE, Suzawa H, Bryant R, Middleman AB. Human papillomavirus vaccination in female pediatric cancer survivors. Journal of pediatric and adolescent gynecology. 2012;25(5):305–307. doi: 10.1016/j.jpag.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council . From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- Kaiser Family Foundation (2020). KFF COVID-19 Vaccine Monitor: December 2020. Retrieved from https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
- Kaiser Family Foundation (2022). KFF COVID-19 Vaccine MonitorFebruary 2022. Retrieved from https://files.kff.org/attachment/TOPLINE-KFF-COVID-19-Vaccine-Monitor-February-2022.pdf
- Kasting ML, Head KJ, Cox D, Cox AD, Zimet GD. The effects of message framing and healthcare provider recommendation on adult hepatitis B vaccination: A randomized controlled trial. Preventive medicine. 2019;127:105798. doi: 10.1016/j.ypmed.2019.105798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar AH, Blake JA, Cherabuddi K, Cornett H, McKee BL, Cogle CR. Vaccine Enthusiasm and Hesitancy in Cancer Patients and the Impact of a Webinar. Healthcare. 2021;9(3):351. doi: 10.3390/healthcare9030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff AC, Mann K, Warner EL, Kaddas HK, Fair D, Fluchel M, Kepka D. HPV vaccination knowledge, intentions, and practices among caregivers of childhood cancer survivors. Human vaccines & immunotherapeutics. 2019;15(7–8):1767–1775. doi: 10.1080/21645515.2019.1619407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosky JL, Gamble HL, Spunt SL, Randolph ME, Green DM, Hudson MM. Human papillomavirus vaccination in survivors of childhood cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009;115(24):5627–5636. doi: 10.1002/cncr.24669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosky JL, Russell KM, Simmons JL, Foster RH, Peck K, Green DM, Hudson MM. Medical and sociodemographic factors associated with human papillomavirus (HPV) vaccination adherence among female survivors of childhood cancer. Pediatric blood & cancer. 2015;62(9):1630–1636. doi: 10.1002/pbc.25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde LA, Best AF, Gnjatic S, Denicoff AM, Mishkin GE, Bowman M, Chanock SJ. Initial reporting from the prospective National Cancer Institute (NCI) COVID-19 in Cancer Patients Study (NCCAPS) Journal of Clinical Oncology. 2021;39(15_suppl):6565–6565. doi: 10.1200/JCO.2021.39.15_suppl.6565. [DOI] [Google Scholar]
- Lockyer, B., Islam, S., Rahman, A., Dickerson, J., Pickett, K., Sheldon, T., Wright, J., McEachan, R., Sheard, L. & the Bradford Institute for Health Research Covid-19 Scientific Advisory Group. (2021). Understanding COVID-19 misinformation and vaccine hesitancy in context: Findings from a qualitative study involving citizens in Bradford, UK. Health Expectations, 24(4), 1158–1167 [DOI] [PMC free article] [PubMed]
- Lu P, Srivastav A, Amaya A, Dever JA, Roycroft J, Kurtz MS, Williams WW. Association of provider recommendation and offer and influenza vaccination among adults aged ≥ 18 years–United States. Vaccine. 2018;36(6):890–898. doi: 10.1016/j.vaccine.2017.12.016. [DOI] [PubMed] [Google Scholar]
- MacDonald NE, the SAGE Working Group on Vaccine Hesitancy Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Mayo, S. J., Ajaj, R., & Drury, A. (2021). SURVIVORS’PREFERENCES FOR THE ORGANIZATION AND DELIVERY OF SUPPORTIVE CARE AFTER TREATMENT: AN INTEGRATIVE REVIEW.European Journal of Oncology Nursing,102040 [DOI] [PubMed]
- Monin, L., Laing, A. G., Muñoz-Ruiz, M., McKenzie, D. R., Barrio, D., Alaguthurai, I. D. M., & Seow, T. (2021). J. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. The Lancet Oncology [DOI] [PMC free article] [PubMed]
- Nápoles AM, Santoyo-Olsson J, Stewart AL, Ortiz C, Samayoa C, Torres‐Nguyen A, Gonzalez N. Nuevo Amanecer‐II: Results of a randomized controlled trial of a community‐based participatory, peer‐delivered stress management intervention for rural Latina breast cancer survivors. Psycho‐Oncology. 2020;29(11):1802–1814. doi: 10.1002/pon.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute - Office of Cancer Survivorship (2021). Statistics, Graphs and Definitions. Retrieved from https://cancercontrol.cancer.gov/ocs/statistics
- National Comprehensive Cancer Network (2021). Recommendations of the NCCN COVID-19 Vaccination Advisory Committee. Retrieved from https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v3-0.pdf?sfvrsn=b483da2b_60
- Nekhlyudov, L. (2021). How Your Primary Care Provider Can Help You Throughout Your Cancer Experience. Retrieved from https://www.cancer.net/blog/2021-03/how-your-primary-care-provider-can-help-you-throughout-your-cancer-experience
- Office of the Surgeon General (2021). Confronting Health Misinformation: The U.S. Surgeon General’s Advisory on Building a Healthy Information Environment. Retrieved from Washington (DC): https://www.hhs.gov/sites/default/files/surgeon-general-misinformation-advisory.pdf [PubMed]
- Öster I, Hedestig O, Johansson M, Klingstedt N, Lindh J. Sharing experiences in a support group: men’s talk during the radiotherapy period for prostate cancer. Palliative & supportive care. 2013;11(4):331–339. doi: 10.1017/S1478951512000661. [DOI] [PubMed] [Google Scholar]
- Palich, R., Veyri, M., Marot, S., Vozy, A., Gligorov, J., Maingon, P., & Spano, J. P. (2021). Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients.Annals of Oncology [DOI] [PMC free article] [PubMed]
- Panozzo CA, Head KJ, Kornides ML, Feemster KA, Zimet GD. Tailored messages addressing human papillomavirus vaccination concerns improves behavioral intent among mothers: a randomized controlled trial. Journal of Adolescent Health. 2020;67(2):253–261. doi: 10.1016/j.jadohealth.2020.01.024. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: A randomized controlled trial. Health Psychology. 2015;34(5):463. doi: 10.1037/hea0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, D. A., Thomas, A., & Rugo, H. S. (2021). A Neoadjuvant Chemotherapy Trial for Early Breast Cancer is Impacted by COVID-19: Addressing Vaccination and Cancer Trials Through Education, Equity, and Outcomes.Clinical Cancer Research [DOI] [PubMed]
- Rosenthal S, Weiss TW, Zimet GD, Ma L, Good M, Vichnin M. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29(5):890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Shelal Z, Cho D, Urbauer DL, Lu Q, Ma BY, Rohrer AM, Ramondetta LM. Knowledge matters and empowers: HPV vaccine advocacy among HPV-related cancer survivors. Supportive Care in Cancer. 2020;28(5):2407–2413. doi: 10.1007/s00520-019-05035-1. [DOI] [PubMed] [Google Scholar]
- Shourie S, Jackson C, Cheater FM, Bekker HL, Edlin R, Tubeuf S, Bleasby B. A cluster randomised controlled trial of a web based decision aid to support parents’ decisions about their child’s Measles Mumps and Rubella (MMR) vaccination. Vaccine. 2013;31(50):6003–6010. doi: 10.1016/j.vaccine.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VT, Sidorkiewicz S, Péan C, Ravaud P. Impact of an interactive web tool on patients’ intention to receive COVID-19 vaccination: a before-and-after impact study among patients with chronic conditions in France. BMC medical informatics and decision making. 2021;21(1):1–7. doi: 10.1186/s12911-021-01594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bavel JJ, Baicker K, Boggio PS, Capraro V, Cichocka A, Cikara M, Druckman JN. Using social and behavioural science to support COVID-19 pandemic response. Nature Human Behaviour. 2020;4(5):460–471. doi: 10.1038/s41562-020-0884-z. [DOI] [PubMed] [Google Scholar]
- Verger, P., & Dubé, E. (2020). Restoring confidence in vaccines in the COVID-19 era. In: Taylor & Francis [DOI] [PubMed]
- Viswanath K, Ramanadhan S, Kontos EZ. Mass media. In: Galea S, editor. Macrosocial determinants of population health. New York, New York: Springer; 2007. pp. 275–294. [Google Scholar]
- Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA oncology. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner EL, Lopez V, Kepka PL, Mann D, Kaddas K, Fair HK, Martel D. Influence of provider recommendations to restart vaccines after childhood cancer on caregiver intention to vaccinate. Journal of Cancer Survivorship. 2020;14:757–767. doi: 10.1007/s11764-020-00890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AR, Kepka D, Ramsay JM, Mann K, Lopez V, Anderson PL, Ray JS. COVID-19 Vaccine Hesitancy Among Adolescent and Young Adult Cancer Survivors. JNCI cancer spectrum. 2021;5(3):pkab049. doi: 10.1093/jncics/pkab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, A. R., Mann, K., Lopez, P. L. V., Kepka, D., Wu, Y. P., & Kirchhoff, A. C. (2021). HPV Vaccine Experiences and Preferences Among Young Adult Cancer Survivors and Caregivers of Childhood Cancer Survivors.Journal of Cancer Education, 1–6 [DOI] [PMC free article] [PubMed]
- Witteman HO, Chipenda Dansokho S, Exe N, Dupuis A, Provencher T, Zikmund-Fisher BJ. Risk communication, values clarification, and vaccination decisions. Risk Analysis. 2015;35(10):1801–1819. doi: 10.1111/risa.12418. [DOI] [PubMed] [Google Scholar]
- Woodfield, M. C., Pergam, S. A., & Shah, P. D. (2021). Cocooning against COVID-19: The argument for vaccinating caregivers of patients with cancer.Cancer [DOI] [PMC free article] [PubMed]
- World Health Organization (2019). Ten threats to global health in 2019. Retrieved from https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.