Abstract
There is a need to identify brain connectivity alterations predictive of transdiagnostic processes that may confer vulnerability for affective symptomology. Here, we tested whether amygdala resting-state functional connectivity (rsFC) mediated the relationship between catastrophizing (negative threat appraisals; predicting poorer functioning) and altered threat-safety discrimination learning (critical to flexibly adapt to new and changing environments) in adolescents with persistent pain. We examined amygdala rsFC in 46 youth with chronic pain and 29 healthy peers (age M=15.8, SD=2.9; 64 females), and its relationship with catastrophizing and threat-safety learning. We used a developmentally appropriate threat-safety learning paradigm and performed amygdala seed-based rsFC and whole-brain mediation analyses. Patients exhibited enhanced connectivity between left amygdala and right supramarginal gyrus (cluster-level p-FDR < .05), while right amygdala rsFC showed no group differences. Only in patients, elevated catastrophizing was associated with facilitated threat-safety learning (CS+>CS−; rp = .49, p = .001). Furthermore, in patients, elevated catastrophizing was associated with reduced left amygdala connectivity with supramarginal gyrus/parietal operculum, and increased left amygdala connectivity with hippocampus, dorsal striatum, paracingulate and motor regions (p < .001). And, blunted left amygdala rsFC with right supramarginal gyrus/parietal operculum mediated the association between catastrophizing and threat-safety learning (p < .001). To conclude, rsFC between left amygdala (a core emotion hub) and inferior parietal lobe (involved in appraisal and integration of bodily signals, and attentional reorienting) explains associations between daily-life relevant catastrophizing and threat-safety learning. Findings provide a putative model for understanding pathophysiology involved in core psychological processes that cut across diagnoses, including disabling pain, and are relevant for their etiology.
Introduction
Many interrelated and co-occurring disorders such as anxiety, depression, and chronic pain are characterized as complex network-based disorders [40,51]. In chronic pain, both sensory and affective neural network alterations are implicated in the etiology and maintenance of chronic pain [9,31,48]. Transdiagnostic affective processes such as fear, catastrophizing and threat-safety discrimination learning, also contribute to individual differences in pain persistence and disability [30,36,45]. However, integration and segregation of relative contributions of neural circuits to specific psychological processes remains largely elusive. And, neural correlates of these transdiagnostic mechanisms have yet to be identified in chronic pain.
Chronic pain is common and can have a major impact across life domains [7,13,54]. One factor shaping the way an individual processes pain, which may explain chronic pain vulnerability, is catastrophizing [44,73,74], also referred to as pain-related distress or worry, or repetitive negative thinking [15,22]. Broadly speaking, catastrophizing is conceived as a tendency to exaggerate a perceived threat, to overestimate the seriousness of its potential consequences [12,19], and to feel helpless and ruminate about it [62]. Elevated levels of catastrophizing are consistently associated with poorer functioning [18,24,26,28,53]. Catastrophizing may further influence threat-safety discrimination learning by affecting coping with threat. Learning to discriminate threat from safety signals guides our behavior and is fundamental to survival. Typically, threat-safety discrimination is studied using a classical conditioning paradigm, in which a neutral stimulus is paired with an aversive stimulus (i.e., turning into a threat signal/CS+), while other stimuli are never paired with the aversive stimulus (i.e., safety signals/CS−). Alterations in threat-safety learning have been reported across clinical samples [8,29,30,72]. Recently, we reported altered neural signatures in youth with chronic pain (e.g., amygdala, hippocampus, medial prefrontal cortex) during extinction of a learned threat [34], specifically in patients with elevated catastrophizing.
The amygdala forms an emotional hub, critical for processing and regulation of affective, social and stress-related information [39,60,63,64,80]. As pain inherently is an emotionally-salient threat, the amygdala is also implicated in acute pain processing [66] and is linked to individual differences in acute pain regulation [25,41]. In adults and adolescents with chronic pain, alterations in amygdala rsFC are observed [6,31,37] and associated with individual differences in pain-related distress and treatment responses [55,67]. In parallel, research has shown that amygdala circuitry serves a key role in threat-safety learning processes [20,42,60], including through its connectivity with prefrontal cortex [38,59]. Yet, no studies have modelled the specific relationship between these psychological and brain circuit factors.
Here, we sought to evaluate the role of amygdala circuitry in chronic pain as it relates to catastrophizing and threat-safety learning - two transdiagnostic processes conferring vulnerability to affective symptomology. We focused on youth (i.e., aged 10–24) to extend on our previous findings in this sample, and because adolescents offer unique insights into these processes as they pose risk for persistence into adulthood. We hypothesized that amygdala rsFC would (a) be altered in patients (in particular left amygdala rsFC, given its importance for clinical pain [66]), (b) mediate the association between catastrophizing and threat-safety learning, potentially in connectivity with the prefrontal cortex.
Methods and Materials
Participants
Ninety-seven adolescents participated in this study. 61 adolescent patients with chronic pain (5 males, 56 females) were recruited when they presented to the Pain Treatment Service - Chronic Pain Clinic at Boston Children’s Hospital for a multi-disciplinary evaluation. Inclusion criteria were age 10–24, pain duration > three months, confirmed diagnosis of chronic non-disease related pain. Patient exclusion criteria were significant cognitive impairment, significant medical or psychiatric disorder, pregnancy, claustrophobia and magnetic implants. The other 36 adolescents were pain-free peers (9 males, 27 females), recruited from the community. For controls, the same inclusion and exclusion criteria were used, plus one additional exclusion criterion: current or history of chronic pain (symptoms for more than 3 months). 11 participants could not be included in the analyses described here (n=6 terminated study prior to scan, n=2 scanner malfunctions, n=3 excluded due to chronic pain history/incidental finding), and another 11 participants were excluded due to extensive motion (see MRI data analysis). Thus, in the main analyses, 75 participants were included (age M=15.8, SD=2.9; 64 females); 46 patients and 29 controls. For additional information, see Table S1.
Study procedure
The data presented here is part of a larger study, examining fear acquisition and extinction in youth with chronic pain in comparison to healthy controls. This study was approved by the Boston Children’s Hospital Institutional Review Board (#P00013786). Participants and legal guardians provided written assent/consent. Figure 1 gives an overview of the study procedure. One paper has been published before describing the fear acquisition and extinction paradigm findings [34]. Resting-state data has not been described before.
Figure 1. Overview of the study visit and the data acquired, with an emphasis on the fear acquisition paradigm and related ratings.
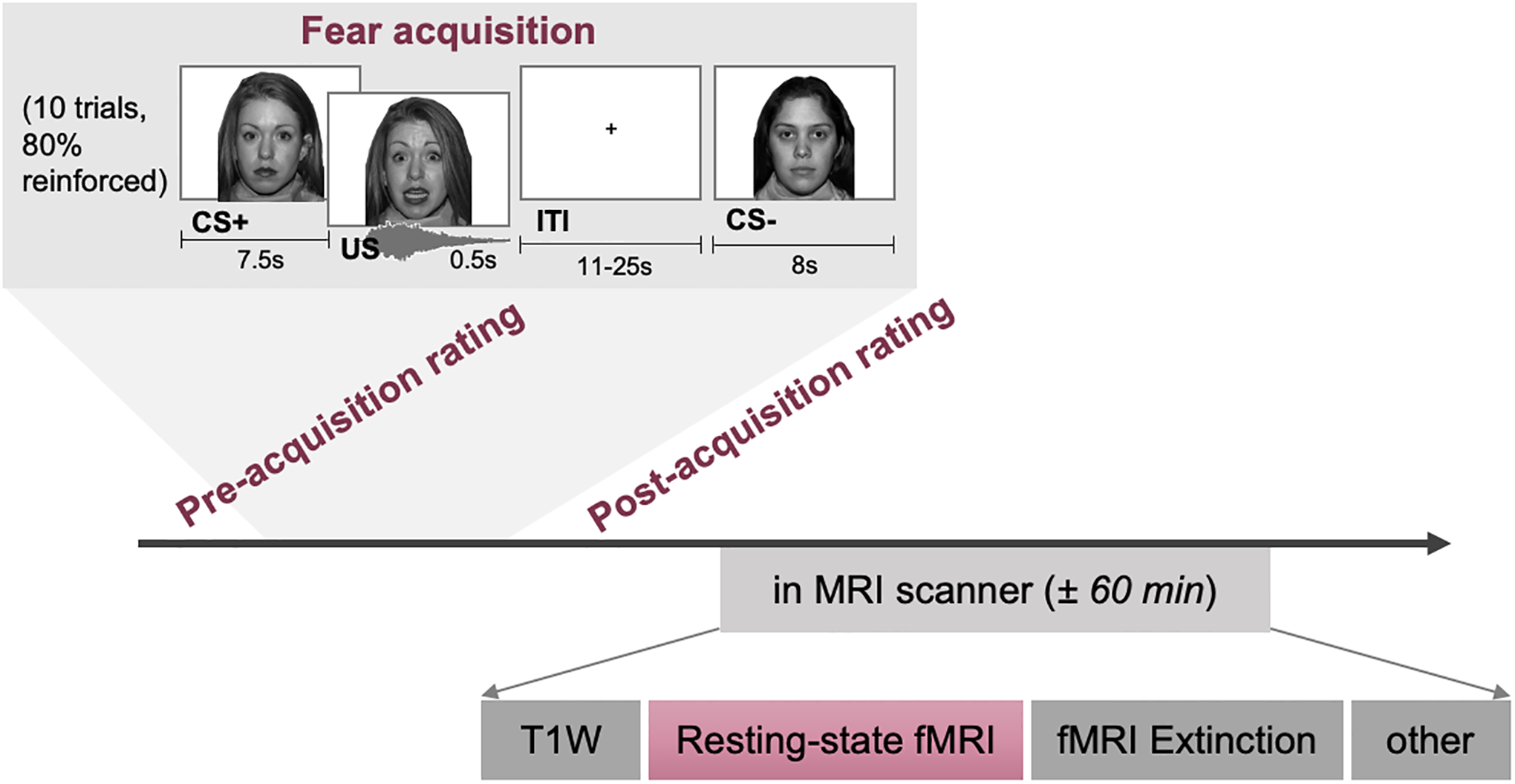
CS+/CS− = conditioned stimulus, US = conditioning stimulus, ITI = intertrial interval, T1W = T1-weighted (anatomical MR scan), fMRI = functional magnetic resonance imaging
Fear conditioning paradigm and fear assessment
The Screaming Lady Paradigm was used in which during the fear acquisition phase a neutral stimulus (neutral face; CS+) was paired with an aversive stimulus (95 dB scream and scared face; US), while another neutral stimulus was never paired with the US (CS−; Figure 1) [8,43]. In the subsequent fear extinction phase, both stimuli (CS+ and CS−) are presented in absence of the US. At several time points, we assessed how anxious the participants were of each of the faces on a numerical rating scale (NRS) ranging from 1 (not anxious) to 10 (extremely anxious), as well as how unpleasant the faces were ranging from 1 (not unpleasant) to 10 (extremely unpleasant), and combined these into one ‘fear’ composite rating (in line with [34]). Note that the resting-state fMRI was acquired following acquisition, prior to extinction. Additional details regarding the fear conditioning paradigm can be found in Heathcote et al. [34].
Other self-reported assessments
Participants filled out questionnaires using REDCap electronic data capture tools hosted at Boston Children’s hospital. Here, pain catastrophizing is of greatest interest, and was assessed using the Pain Catastrophizing Scale for Children (PCS-C), which is a 13-item questionnaire assessing catastrophic thinking about pain, including rumination, helplessness and magnification [14]. Participants respond to each item using a 5-point scale, ranging from 0 (not at all true) to 4 (very true). This measure is a direct adaptation of the adult version, is widely used in youth, and shows high validity and reliability [14,21]. In the current sample, internal consistency was good to excellent (Cronbach’s alpha = .92 for the entire sample; α = .89 for the patient sample; α = .84 for the controls). Scores were roughly normally distributed in both groups. The range of scores for patients was 4–40 with the majority in the moderate (45%) and high (31%) range, while the range for controls was 0–24 with the majority in the low range (86%) (see Table S1) [61].
Other assessments included demographics (e.g., age, sex) and information about their pain (e.g., when it started, what type of pain). In addition, average pain intensity was reported using a Visual Analogue Scale (VAS) ranging from 0 (no pain) – 10 (worst possible pain). Pain-related functioning was assessed using the Functional Disability Inventory (FDI), which is a 15-item self-report measure [77]. Trait anxiety was assessed using the trait part of the State-Trait Anxiety Inventory for Children (STAI-C), a 20-item questionnaire assessing anxiety symptoms [71]. Finally, alertness levels before and after the resting state scan were assessed using a VAS ranging from 0–10. See Table S1 for more information on these variables across the groups.
MRI acquisition
MRI data were collected using a 3 Tesla whole body MRI scanner (Siemens Magnetom TrioTim syngo MR B17) using a 12-channel head coil. For the resting-state functional images, a T2*-weighted standard echo-planar imaging (EPI) sequence was used to acquire 51 axial slices (3mm isotropic) covering the entire cortical volume, using the following parameters: repetition time (TR) = 1110 ms, echo time (TE) = 30 ms, flip angle = 70°, field of view (FOV) = 228 × 228 mm, slice acceleration factor = 3. In total, 425 functional volumes were collected with eyes open looking at a black screen. In addition, 8 functional volumes using the same parameters, but reverse phase encoding direction, were acquired.
T1-weighted anatomical images were acquired using a 3D multi-echo magnetization-prepared rapid gradient-echo (ME-MPRAGE) sequence with the following parameters: 176 slices, 1 mm isotropic, TR = 2520 ms, TE1 = 1.74 ms, TE2 = 3.6 ms, TE3 = 5.46 ms, TE4 = 7.32 ms, flip angle = 7°, FOV = 240 × 240, GRAPPA acceleration factor = 2.
MRI data analysis
MRI data - pre-processing.
Pre-processing of resting-state functional data was initiated with estimation and correction of geometric distortions. From the pairs of EPI images that were acquired using reversed phase-encoding directions (i.e., with distortions facing opposing directions), the susceptibility-induced off-resonance field was estimated using a method similar to the one described in Andersson et al. [1] (topup of FMRIB Software Library/FSL; [70]. In a next step, these distortions were corrected in the full resting-state dataset (FSL’s applytopup). Undistorted images were uploaded to CONN [79] where pre-processing continued, including 3D head motion correction, segmentation into white matter, grey matter and cerebral spin fluid (DARTEL), normalization to MNI space and spatial smoothing (6 mm using a full-width at half-maximum Gaussian kernel [FWHM]).
MRI data – denoising and first-level analysis.
Denoising procedures included regression of motion parameters and their first derivatives (12 parameters) as well as WM/CSF noise components (estimated using anatomical component-based noise correction, aCompCor [5]; 2×5 parameters), linear trend removal, and simultaneous band pass filtering (.005 – .1 Hz). Quality assurance steps were performed to identify outliers. Datasets with motion exceeding 3 mm/degrees were excluded from the analysis (i.e., 11 participants were excluded: 9 patients, 2 healthy controls). First-level analysis then estimated bivariate correlation coefficients from the defined ROIs or seeds with the rest of the brain.
MRI data – seeds.
The a priori defined seed was the amygdala and was based on the Harvard-Oxford atlas, using probabilistic maps with a threshold of 50% [16,23,27,47]. As left and right amygdala showed different rsFC patterns, we assessed these homologues separately (Supplementary Results).
MRI data – second-level statistical analysis.
A seed-to-voxel functional connectivity analysis was performed in CONN [79]. To investigate patients’ amygdala rsFC in comparison to the control group, seed-based FC analyses were performed having Group (Controls, Patients) as between-subject factor. Main effects of group were evaluated at a cluster-defining threshold of p < .001, and subsequent cluster-level p-FDR < .05. In case of significant effects, estimated bivariate correlation coefficients were extracted and transformed using Fisher z for further exploration. Age and staiT-C [71] were used as regressors of no interest [34]. In addition, in case of significant group differences, the mediation toolbox (CANlab; github.com/canlab/MediationToolbox; [75,76] was used to examine rsFC brain mediators of the relation between pain catastrophizing and threat-safety learning in the patient group. Pain catastrophizing was assessed using the Pain Catastrophizing Scale for Children (PCS-C) (see Other self-reported assessments) [14]. The mediation model including all paths is visually depicted in Figure 2: pain catastrophizing was modelled as the independent variable (X), threat-safety learning (i.e., differential fear, CS+>CS−, at post-acquisition) as the dependent variable (Y) and the rsFC maps (i.e., from the seed to rest of the brain) were inputted as mediator (M). The paths were evaluated separately and were thresholded using p < .001, as well as more liberally at p < .005, p < .01 and p < .05 for anatomical reference. For significant brain mediators, the paths were visualized by repeating an offline mediation analysis using Hayes mediation macro for SPSS (PROCESS, version 3.0), which estimates indirect effects using bias‐corrected bootstrapped confidence intervals (with N = 5,000 bootstrap resamples, estimating a 99% confidence interval) [32].
Figure 2. Schematic of the total path model (A) and the mediation model with its separate paths (B).
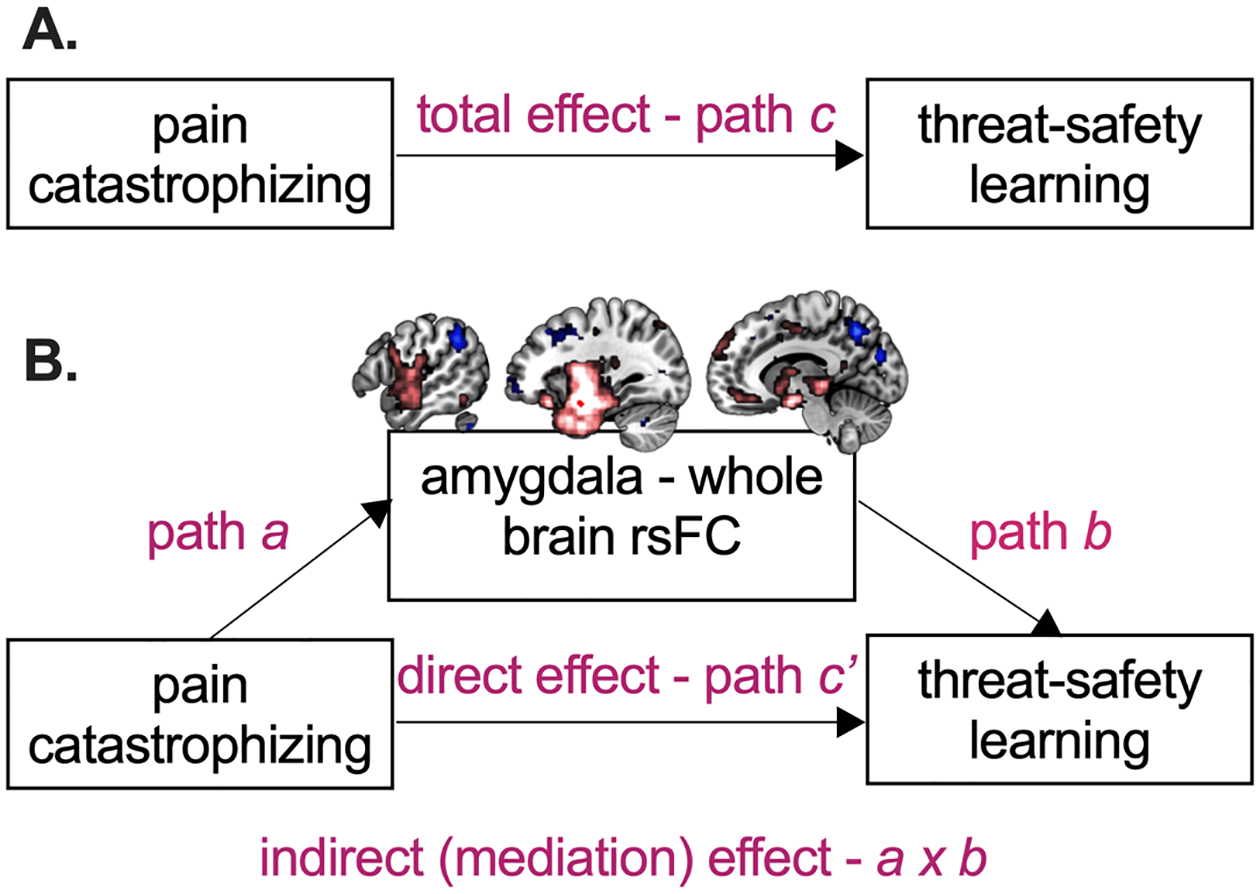
rsFC = resting state functional connectivity. Pain catastrophizing is the independent variable (X), threat-safety learning (i.e., differential fear, CS+ > CS−, post-acquisition) the dependent variable (Y), and amygdala rsFC is the proposed mediator (M).
Results
Contrasting amygdala rsFC across patients and controls
To learn whether and how amygdala rsFC was altered in this patient group compared to controls, we conduced seed-to-whole-brain connectivity analyses and group comparisons. Figure 3A and B show the connectivity maps of the left amygdala per group, showing robust bilateral positive coupling with the amygdalae, hippocampus, insular region, medial and lateral prefrontal cortex, temporal fusiform gyrus, thalamus, putamen and caudate as well as negative coupling with precuneus, supramarginal gyrus (SMG), angular gyrus and lateral frontal cortex in both groups (Table S2–S3). The right amygdala showed very similar patterns overall (Figure 3A–3B, Table S4–S5, but see Supplementary Results too). Group contrasts show that rsFC between left amygdala and right anterior SMG was enhanced in patients compared to controls (MNI x = 64, y = −28, z = 48, cluster-level p-uncorr = .002, cluster-level p-FDR = .035; Figure 3C, Table S6). In patients, this rsFC was furthermore correlated with pain catastrophizing (r = −.33, p = .03), such that enhanced amygdala-SMG rsFC was associated with lower levels of catastrophizing, but not with current pain intensity levels (r = −.09, p = .57). In controls, the relation with pain catastrophizing was not present (r = .03, p = .89). There were no group differences in rsFC of the right amygdala, and there was no interaction between group and hemisphere (i.e., no differences across left and right amygdala that were dependent on group) (also not when lowering the threshold to cluster-level p-uncorr < .05).
Figure 3. Amygdala rsFC across groups.
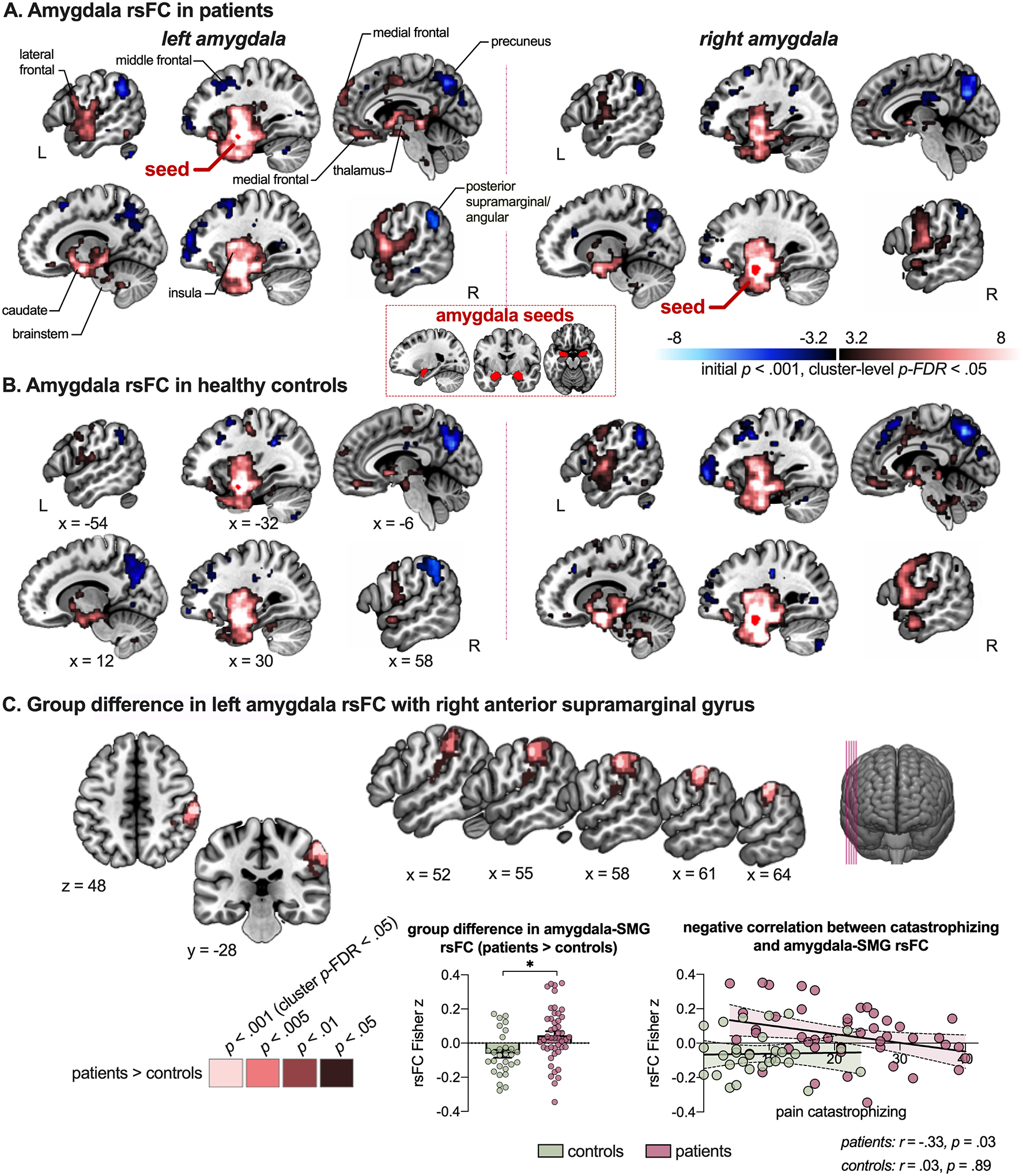
Maps depict left and right amygdala coupling with the rest of the brain, with the seed region overlaid (red) for patients (A) and controls (B). In C, the group difference (patients > controls) in left amygdala – right anterior supramarginal gyrus coupling is presented, including the anatomical reference, the connectivity values per group (showing enhanced rsFC in patients compared to controls) and the association with pain catastrophizing (showing negative correlation between catastrophizing and amygdala rsFC with supramarginal gyrus in the patient group). Note that labels mostly refer to peak coordinates, even though clusters may extend into other regions (see also Tables S2–S5). rsFC = resting-state functional connectivity; SMG = supramarginal gyrus. * depicts significant difference
Relation between pain catastrophizing and threat-safety learning (total effect - path c)
In patients, there was a moderate, positive correlation between pain catastrophizing and self-reported differential fear (CS+ > CS−; rp = .49, p = .001) (Figure 4), with elevated pain catastrophizing being associated with increased differential fear (i.e., the total effect, path c). In controls, this correlation was not present (rp = .15, p = .44; see Figure S1).
Figure 4. Association between pain catastrophizing and threat-safety learning (path c) in patients with chronic pain.
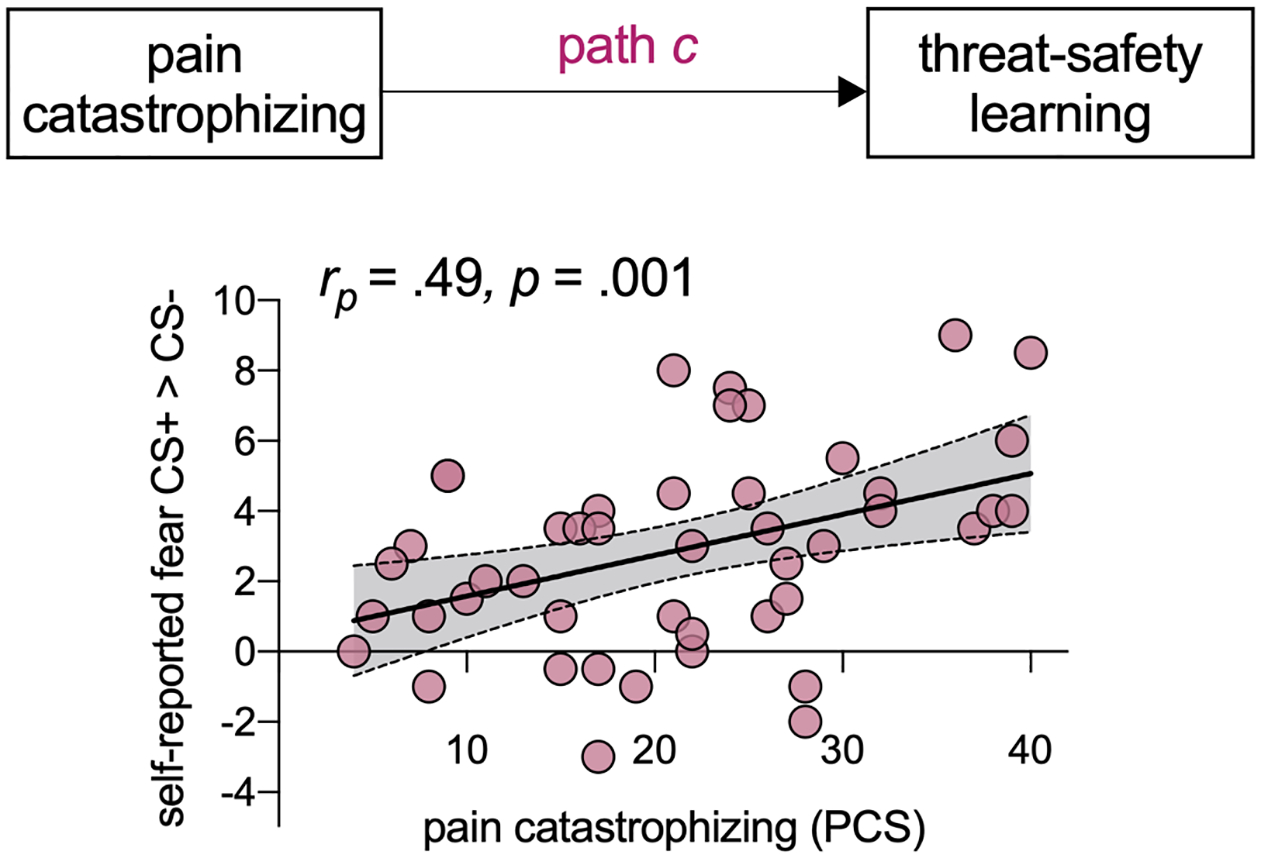
There is a positive, moderate relation between pain catastrophizing and threat-safety learning.
Relation between pain catastrophizing and amygdala rsFC (path a)
To investigate left amygdala rsFC associations with pain catastrophizing, we inspected path a of the mediation analysis (Figure 5, Table S7). Higher levels of pain catastrophizing were associated with greater connectivity from left amygdala to several regions, including hippocampus, caudate, putamen, precentral gyrus, and angular gyrus (positive correlations). And, higher levels of pain catastrophizing were related to weaker connectivity between left amygdala and regions including bilateral SMG / parietal operculum (PO), paracingulate gyrus, occipital cortex and cerebellum (negative correlations).
Figure 5. Relation between pain catastrophizing and left amygdala rsFC.
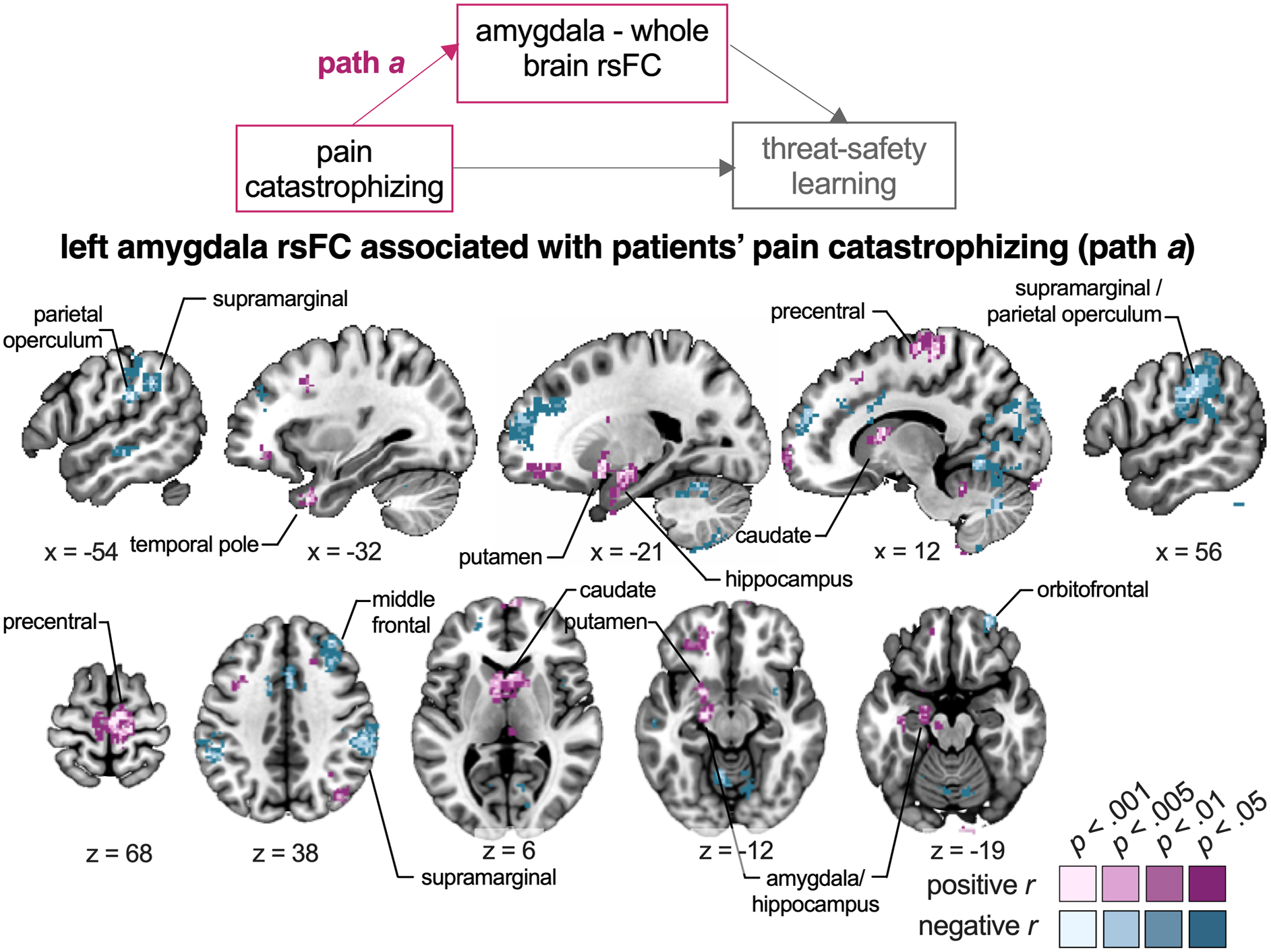
The seed-to-whole-brain group-level mediation model is presented, highlighting the path of focus (path a). Regions in purple show positive correlations, i.e., where higher levels of catastrophizing across patients are related to greater amygdala connectivity. Regions in blue show negative correlations, i.e., where higher levels of catastrophizing across patients are related to weaker amygdala connectivity. Note that only clusters surviving p < .001 are presented, along with their surrounding subthreshold voxels, for a more complete illustration of the functional anatomy of significant clusters. Labels refer to peak coordinates, even though clusters may extend into other regions (see Table S7 for more details).
Relation between amygdala rsFC and threat-safety learning (path b)
To evaluate the relation between left amygdala rsFC and threat-safety learning while controlling for the effect of catastrophizing, we inspected path b (Figure 6, Table S8). Greater differential self-reported fear was associated with weaker rsFC between left amygdala and regions including the SMG/PO, anterior and posterior cingulate cortex, precuneus, fusiform, angular, postcentral gyrus, and occipital cortex (negative correlations). In contrast, greater differential self-reported fear was associated with stronger rsFC between left amygdala and regions including right inferior frontal, middle temporal gyrus, and lateral occipital cortex (positive correlations).
Figure 6. Relation between left amygdala rsFC and threat-safety learning while controlling for pain catastrophizing.
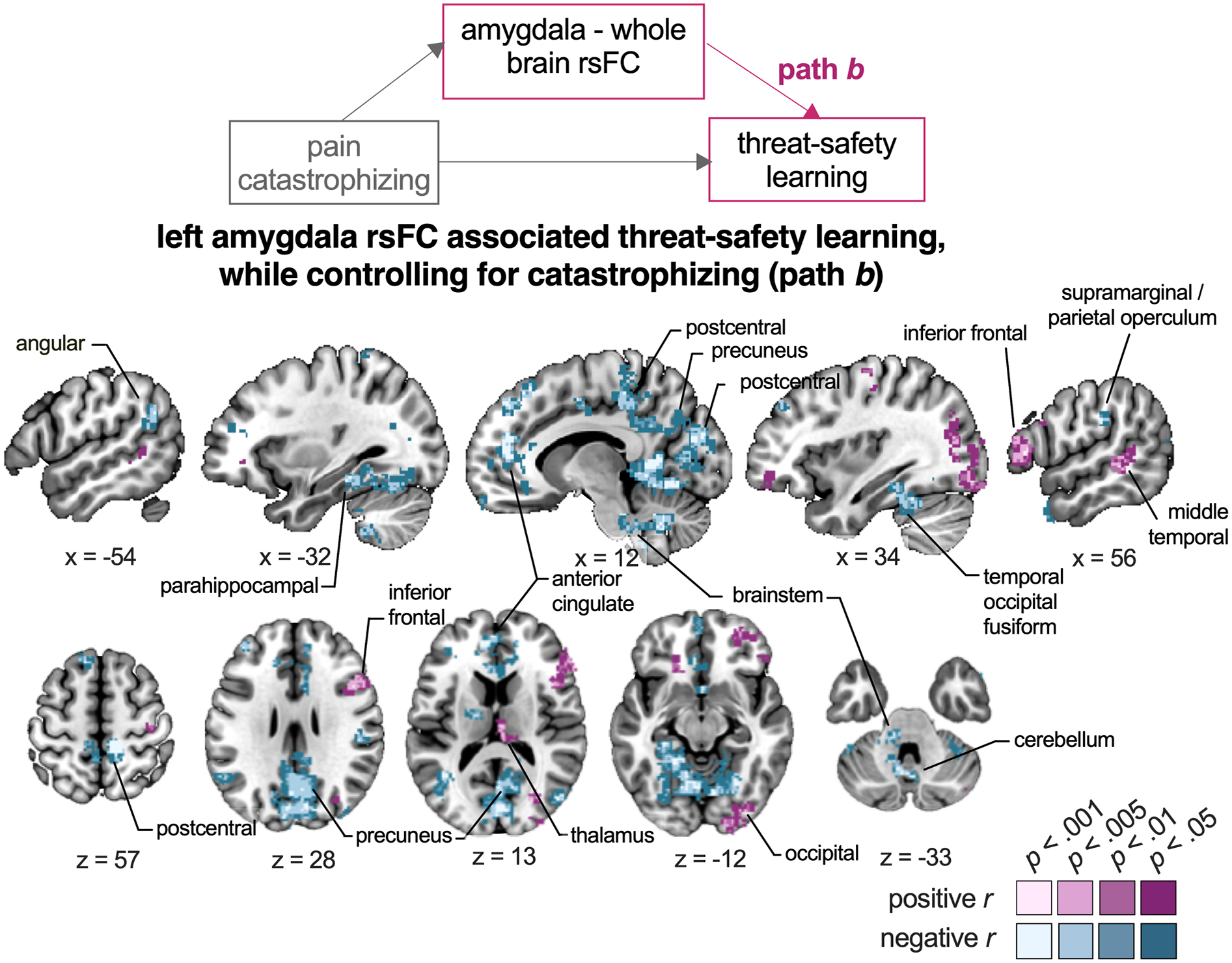
The seed-to-whole-brain group-level mediation model is presented, as well as the brain regions that -in coupling with the amygdala- are associated with threat-safety discrimination, after controlling for the influence of pain catastrophizing. Regions in purple show positive correlations, i.e., where greater amygdala connectivity is related to increased differential threat-safety learning. Regions in blue show negative correlations, i.e., where weaker amygdala connectivity is related to increased differential threat-safety learning. Note that only clusters surviving p < .001 are presented, along with their surrounding subthreshold voxels, for a more complete illustration of the functional anatomy of significant clusters. Labels refer to peak coordinates, even though clusters may extend into other regions (see Table S8 for more details).
Brain mediators of the relation between pain catastrophizing and threat-safety learning (indirect effect - path a × b)
Then, we explored whether left amygdala coupling was a mediator for the relation between catastrophizing and threat-safety discrimination learning. We observed that left amygdala rsFC with two clusters served as a mediator: right SMG/PO and brainstem (SMG/PO: MNI x = 50, y = −26, z = 30, p-uncorr = .0004; brainstem: MNI x = 10, y = −48, z = −46, p-uncorr = 0.0006; Figure 7A, Table S9). Together, this indicates that stronger associations between pain catastrophizing and threat-safety discrimination learning are mediated by reduced left amygdala coupling with right SMG and brainstem. The separate paths and their coefficients are visualized in Figure 7B. Specifically, it shows that the higher the levels of pain catastrophizing the weaker the connectivity between amygdala and SMG (also shown in path a), and the weaker such connectivity, the greater the differential threat-safety learning (even controlling for path a, depicted in path b). In particular, the mediating result shows that if variability in amygdala rsFC with these clusters was kept constant across patients, the relationship between pain catastrophizing and threat-safety learning would no longer exist. In controls, these clusters did not serve as a mediator (i.e., none of the paths were significant).
Figure 7. Brain mediators of the relation between pain catastrophizing and threat-safety discrimination.
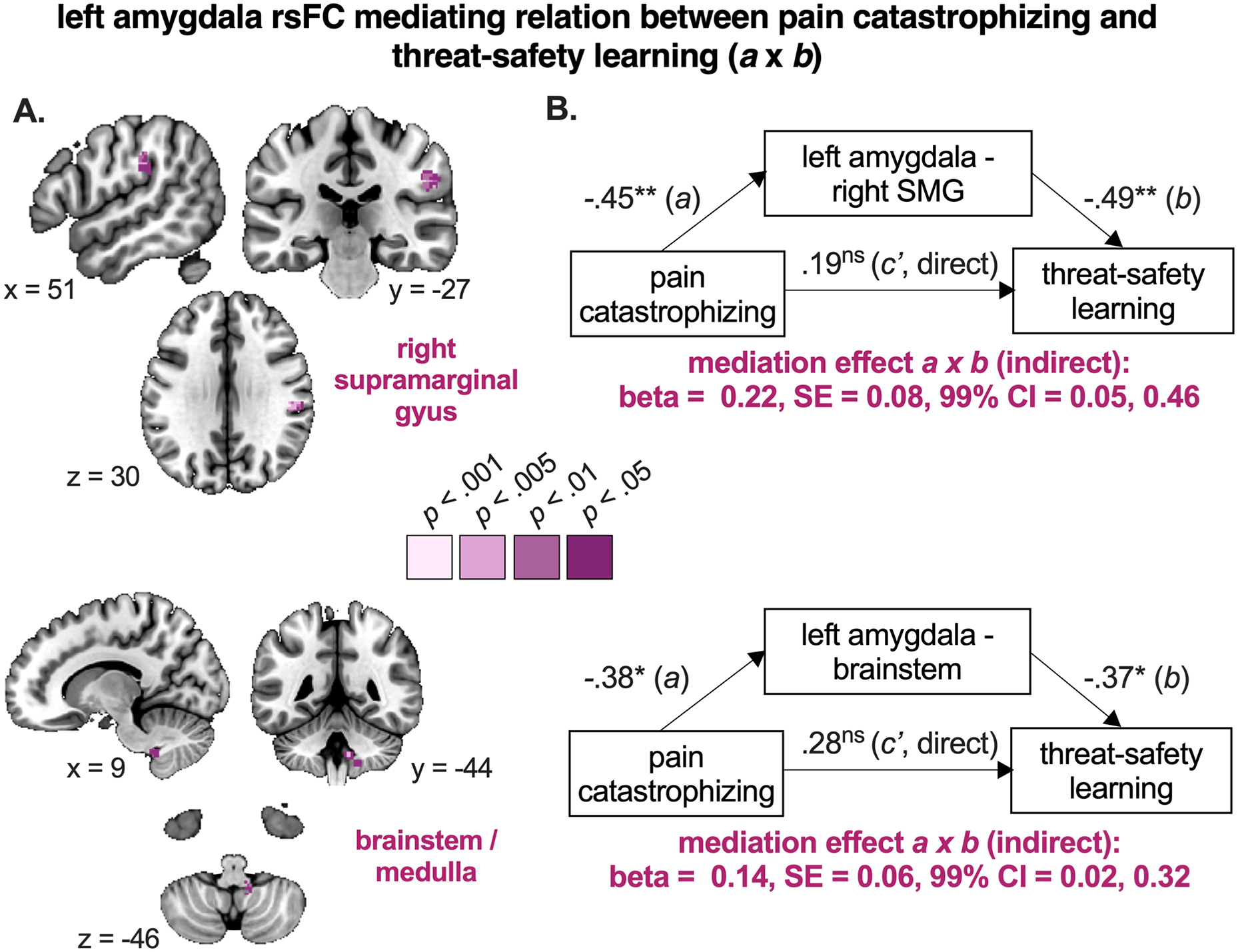
A. Brain regions in purple are significant mediators of the relationship between pain catastrophizing and threat-safety learning, meaning that stronger associations between pain catastrophizing and threat-safety discrimination learning are mediated by reduced left amygdala coupling with right supramarginal gyrus and brainstem. Note that only clusters surviving p < .001 are presented, along with their surrounding subthreshold data. B. The path diagrams and standardized coefficients are presented for descriptive purposes, as calculated offline (post-hoc). See Table S9 for more details. SE = standard error, CI = confidence interval. * p < .01, ** p < .005, *** p < .001, ns p > .05 (not significant)
Overview of findings implicating the inferior parietal lobe
The group contrast as well as all paths in the mediation analysis implicate amygdala coupling with the inferior parietal lobe (IPL). Figure 8 shows the close vicinity of the group difference and brain mediator clusters, yet it also shows that the center of mass of the brain mediator cluster as well as the IPL cluster in path a and path b are more inferior compared to the group difference cluster; see Figure 8A and B for a comparison. When overlaying on the Harvard-Oxford atlas (Figure 8C), the superior cluster overlaps with right SMG while the inferior one overlaps with SMG too, but also extends towards the PO. The Supplementary Information presents more comparative details, including the moderate correlation between amygdala rsFC with the two clusters (r = .48, p = .001), suggesting differentiation.
Figure 8. Overview of inferior parietal findings and atlas reference.
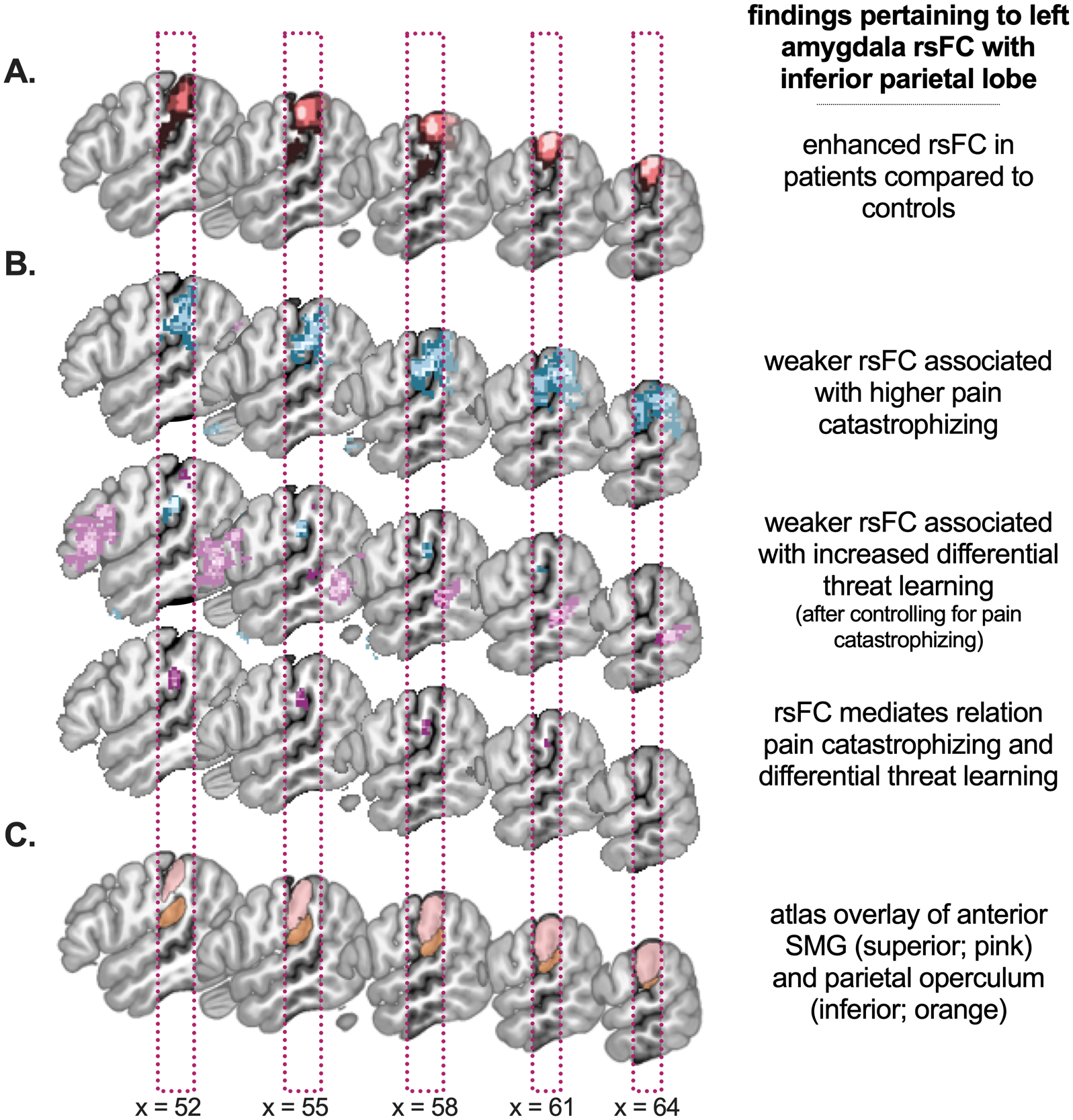
A. The group difference is presented, with patients showing enhanced left amygdala rsFC with right supramarginal gyrus. B. Findings from the different paths of the mediation analysis: path a, b and a × b (indirect effect), which all converge on a cluster in supramarginal gyrus / parietal operculum. C. For anatomical reference, the atlas regions supramarginal gyrus (anterior part; superior cluster) and parietal operculum (inferior cluster) are presented as defined in Harvard-Oxford cortical atlas (thresholded at .25). Note that the specific details of all findings can be found in Figures 3, 5, 6 and 7.
Discussion
We evaluated a role for amygdala rsFC in the association between catastrophizing and threat-safety learning. Our key findings are that 1) overall, youth with chronic pain show enhanced coupling between left amygdala and right SMG compared to controls, while right amygdala showed no group differences, and 2) in patients, reduced coupling between left amygdala and the right SMG/PO mediates the association between catastrophizing and threat-safety learning. Thus, findings converge on the right IPL, indicating that amygdala-IPL connectivity is aberrant in youth with chronic pain and that this circuitry plays a mechanistic role underlying how individual difference factors in appraisal of and learning about threats confer increased risk for affective disorders.
Enhanced left amygdala rsFC in youth with chronic pain
Our findings demonstrate that left amygdala-SMG connectivity was enhanced in youth with chronic pain compared to controls, while no differences were observed for right amygdala. This is generally in line with fMRI activation studies of pain, observing greater left amygdala activation in clinical compared to experimental studies [66]. Greater involvement of the left amygdala is also seen in other affective symptomologies (e.g., depression) [58,81]. Interestingly, the left amygdala matures earlier [65], and may therefore be more vulnerable to adversity. It is unclear, though, what the enhanced rsFC with SMG reflects. The SMG lies in the IPL and is part of the somatosensory association cortex, involved in integration and interpretation of somatosensory information. The peak coincided with the anterior IPL [10,11], mainly connecting with prefrontal and premotor cortex [78]. The right IPL is further involved in several higher-order cognitive processes, including attentional reorienting to relevant, salient stimuli [4].
Previous studies in youth with chronic pain have also identified altered IPL connectivity patterns. The IPL was among the clusters showing enhanced rsFC with left amygdala in youth with complex regional pain syndrome [67], and another study reported enhanced right SMG/IPL rsFC as part of the salience and sensorimotor network, and lower rsFC in an SMG cluster in the frontoparietal network [3]. In a recent review, though, the IPL was not identified as a region with robust alterations in pediatric chronic pain, although research is still scarce [6].
Interestingly, while amygdala-IPL rsFC was enhanced in patients on a group-level, across patients there was a negative correlation with catastrophizing. This is seemingly contradictory, as it indicates that patients with lower catastrophizing showed greater amygdala-IPL rsFC (bearing in mind that levels were predominantly moderate and high). In patients, amygdala-IPL engagement may therefore reflect a protective or compensatory factor, such that greater rsFC with IPL has regulatory effects on the amygdala. In controls, catastrophizing was not associated with amygdala-IPL rsFC. These findings suggest that amygdala engagement and its association with catastrophizing may be distinctive for individuals for whom catastrophizing is daily-life relevant.
Together, these findings indicate that communication between amygdala and IPL was altered in patients, and that across patients, individuals with relatively less negative threat appraisals show greater amygdala-IPL connectivity. This may promote altered -potentially compensatory- (attentional) responses to threat stimuli, which would fit with the proposed key role for vigilance and attention to bodily signals in chronic pain [33,49].
Amygdala rsFC as a brain mediator
The mediation analysis demonstrated that in patients the amygdala-IPL circuitry, peaking more ventrally (SMG/PO), was associated with catastrophizing and with threat-safety learning. Previously, we reported aberrant neural signatures of threat extinction learning in youth with elevated catastrophizing [34]. Here, we extend those findings by demonstrating that amygdala circuitry during rest also track with catastrophizing. Elevated catastrophizing was reflected in enhanced amygdala coupling with regions involved in stress regulation and memory (hippocampus), habitual learning and reward signaling (caudate, putamen; dorsal striatum) [2,57], and response selection and motor planning (dorsal striatum, motor cortex). This is roughly in line with another rs-fMRI study, in which enhanced amygdala connectivity with the central executive network was related to catastrophizing [37]. In contrast, elevated catastrophizing was reflected in blunted coupling with somatosensory regions that process somatosensory signals and integrate these with other senses (SMG/PO).
The amygdala circuitry that was associated with threat-safety learning is in line with previous studies. In adults, both positive and negative connectivity with the amygdala is typically observed in the context of threat-safety learning, with blunted connectivity related to increased differential learning indices [82]. Also in our adolescent sample, increased differential fear was associated with blunted amygdala connectivity with a circuit involving precuneus, IPL, cingulate, fusiform and occipital regions, but also with enhanced amygdala rsFC with inferior frontal and middle temporal regions. This supports the idea that amygdala connectivity is implicated in associative learning in adults and adolescents, particularly for threat-relevant stimuli [20,38].
Going beyond associations, our findings demonstrate that amygdala rsFC mediated the relation between catastrophizing and threat-safety learning in patients. This suggests that blunted amygdala connectivity is a core mechanism explaining how individuals with elevated catastrophizing tend to have facilitated threat-safety discrimination learning – both conferring increased vulnerability for affective disorders, and likely reinforcing each other. The peak of the IPL mediator cluster was also located more inferior/ventral compared to the IPL group-difference cluster and covered part of SMG, while extending into PO (secondary somatosensory cortex/SII). This implies that this mediator region may be more specifically involved in somatosensory processing compared to the IPL region that was enhanced in patients, although this remains speculative. It should be noted that amygdala-brainstem circuitry mediated this relation too, but we are more cautious in its interpretation, given that the cluster is at the edge of the field-of-view and the subthreshold data (i.e., up to p<.05) did not show the expected anatomical consistency. Thus, whether and how amygdala-brainstem rsFC is involved will have to be further investigated in studies with a clearer focus on brainstem circuitry.
Considerations and future perspectives
Catastrophizing is proposed as a core transdiagnostic mechanism [26], and elevated levels are robustly associated with poorer outcomes in other affective disorders too [28,50]. The absence of associations between catastrophizing, threat-safety learning and amygdala rsFC patterns in controls suggests that catastrophizing is particularly mechanistically pertinent when it is daily-life relevant. Fortunately, treatment approaches targeting catastrophizing (e.g., cognitive behavioral treatment/CBT) are successful in improving outcomes in these clinical samples [35,56,68,69]. In analogue, threat-safety learning is found to be impaired across these diagnoses [8,17,52], reflecting that individuals with affective symptomology more easily and more strongly associate certain cues with threat and/or have more difficulties with safety learning. Research has shown that the acquisition of such associations may be facilitated, may generalize more quickly and may be more resistant to subsequent extinction [46]. Our findings demonstrate robust brain-behavior interactions, and indicate that blunted communication between the core emotional hub (amygdala) and sensory regions involved in the integration and appraisal of bodily signals (IPL) may be crucial in explaining how worries and amplifications of threat result in facilitated threat-safety learning. Research using task-based fMRI could shed more light on this. As previous studies have found stronger engagement of the amygdala during threat-safety learning in adolescents compared to adults [42], it remains to be tested whether our findings extend to adults. It would also be interesting to examine whether this generalizes to other affective diagnoses with less involvement of the sensory system (e.g., anxiety). Our findings furthermore suggest that targeting catastrophizing may have beneficial effects on associative learning processes, potentially extending to extinction. Whether and how catastrophizing, extinction learning, and amygdala-IPL circuitry are associated and interact, remains to be investigated.
Findings need to be interpreted in light of some considerations. First, we did not differentiate across amygdala subnuclei, although they carry different functions. Second, although we used the same stringent statistical thresholding across all imaging analyses (p<.001), we only applied additional cluster-extent based thresholding for the group contrasts. The group-level whole-brain mediation analysis is quite strict and specific already and to exclude noise clusters we inspected the anatomical consistency using sub-threshold data. Third, we focused on resting-state data, which can arguably be considered as a more trait-like representation of brain connectivity but has inherent interpretation limitations. Last, our mediation analysis was performed on cross-sectional, group-level data, and hence we cannot infer on causality, but rather provide initial understanding on mechanistic involvement of amygdala rsFC warranting further testing (e.g., using multi-level brain mediation analyses allowing trial-by-trial estimations; [75]).
Conclusion
Chronic pain and affective disorders make up a significant part of individuals living with social, functional and school- or work-related impairments. Treatments that target catastrophizing as well as associative learning processes are deployed in these clinical samples, yet the specific relation and interactions between them, their neural correlates and shared mechanisms had not yet been identified. Here, we identified robust brain-behavior interactions showing that, in chronic pain, when catastrophizing is relevant to daily-life, left amygdala-IPL circuitry may be mechanistically involved in the association between catastrophizing and facilitated threat-safety learning, and hence should be a therapeutic target. Although the generalizability to adults and other disorders remains to be established, our findings provide a putative model for understanding the pathophysiology involved in core psychological processes that cut across affective diagnoses and are relevant for their etiology, including that in disabling pain.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (#R01HD083270 to LES), Stanford Maternal and Child Health Research Institute (IT, LCH), and European Union’s Horizon 2020 research and innovation programme (Marie Skłodowska-Curie Action, grant agreement N° 841426 to IT). We thank the participants (and their parents) for their time and effort, and Farah Mahmud, Corey Kronman and Maya Hernandez for their assistance in data collection.
References
- [1].Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20(2):870–888. [DOI] [PubMed] [Google Scholar]
- [2].Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007;27(31):8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Becerra L, Sava S, Simons LE, Drosos AM, Sethna N, Berde C, Lebel Aa, Borsook D. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. NeuroImage: Clinical 2014;6:347–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol 2004;14(2):212–217. [DOI] [PubMed] [Google Scholar]
- [5].Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhatt RR, Gupta A, Mayer EA, Zeltzer LK. Chronic pain in children: structural and resting-state functional brain imaging within a developmental perspective. Pediatr Res 2020. Dec;88(6):840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European journal of pain (London, England) 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- [8].Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, Pine DS. Response to learned threat: An FMRI study in adolescent and adult anxiety. Am J Psychiatry 2013;170(10):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 2008;212(6):481–495. [DOI] [PubMed] [Google Scholar]
- [11].Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 2006;33(2):430–448. [DOI] [PubMed] [Google Scholar]
- [12].Chaves JF, Brown JM. Spontaneous cognitive strategies for the control of clinical pain and stress. J Behav Med 1987;10(3):263–276. [DOI] [PubMed] [Google Scholar]
- [13].Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003;104(3):639–646. [DOI] [PubMed] [Google Scholar]
- [15].Crombez G, De Paepe AL, Veirman E, Eccleston C, Verleysen G, Van Ryckeghem DML. Let’s talk about pain catastrophizing measures: an item content analysis. PeerJ 2020;8:e8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- [17].Dibbets P, van den Broek A, Evers EA. Fear conditioning and extinction in anxiety- and depression-prone persons. Memory (Hove, England) 2015;23(3):350–364. [DOI] [PubMed] [Google Scholar]
- [18].Drost J, van der Does W, van Hemert AM, Penninx BW, Spinhoven P. Repetitive negative thinking as a transdiagnostic factor in depression and anxiety: A conceptual replication. Behav Res Ther 2014;63:177–183. [DOI] [PubMed] [Google Scholar]
- [19].Ellis A. Reason and emotion in psychotherapy. Oxford: Lyle Stuart, 1962. [Google Scholar]
- [20].Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol 2005;56:207–234. [DOI] [PubMed] [Google Scholar]
- [21].Fisher E, Heathcote LC, Eccleston C, Simons LE, Palermo TM. Assessment of Pain Anxiety, Pain Catastrophizing, and Fear of Pain in Children and Adolescents With Chronic Pain: A Systematic Review and Meta-Analysis. J Pediatr Psychol 2018;43(3):314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Flink IL, Boersma K, Linton SJ. Pain catastrophizing as repetitive negative thinking: a development of the conceptualization. Cogn Behav Ther 2013;42(3):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 2005;162(7):1256–1265. [DOI] [PubMed] [Google Scholar]
- [24].Galvez-Sanchez CM, Montoro CI, Duschek S, Del Paso GAR. Pain catastrophizing mediates the negative influence of pain and trait-anxiety on health-related quality of life in fibromyalgia. Qual Life Res 2020;29(7):1871–1881. [DOI] [PubMed] [Google Scholar]
- [25].Gandhi W, Rosenek NR, Harrison R, Salomons TV. Functional connectivity of the amygdala is linked to individual differences in emotional pain facilitation. Pain 2020;161(2):300–307. [DOI] [PubMed] [Google Scholar]
- [26].Gellatly R, Beck AT. Catastrophic thinking: A transdiagnostic process across psychiatric disorders. Cognitive Therapy and Research 2016;40(4):441–452. [Google Scholar]
- [27].Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS Jr., Kennedy DN, Faraone SV, Tsuang MT. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biological psychiatry 2007;61(8):935–945. [DOI] [PubMed] [Google Scholar]
- [28].Goodwin H, Yiend J, Hirsch CR. Generalized Anxiety Disorder, worry and attention to threat: A systematic review. Clinical psychology review 2017;54:107–122. [DOI] [PubMed] [Google Scholar]
- [29].Grillon C Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological psychiatry 2002;52(10):958–975. [DOI] [PubMed] [Google Scholar]
- [30].Harvie DS, Moseley GL, Hillier SL, Meulders A. Classical conditioning differences associated with chronic pain: a systematic review. The Journal of Pain 2017;18(8):889–898. [DOI] [PubMed] [Google Scholar]
- [31].Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013;136:2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press, 2017. [Google Scholar]
- [33].Heathcote LC, Simons LE. Stuck on pain? Assessing children’s vigilance and awareness of pain sensations. European journal of pain (London, England) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heathcote LC, Timmers I, Kronman CA, Mahmud F, Hernandez JM, Bentley J, Youssef AM, Pine DS, Borsook D, Simons LE. Brain signatures of threat-safety discrimination in adolescent chronic pain. Pain 2020;161(3):630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognit Ther Res 2012;36(5):427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain 2017;158(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain 2016;157(9):1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev 2006;30(2):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Krabbe S, Grundemann J, Luthi A. Amygdala Inhibitory Circuits Regulate Associative Fear Conditioning. Biological psychiatry 2018;83(10):800–809. [DOI] [PubMed] [Google Scholar]
- [40].Kucyi A, Davis KD. The dynamic pain connectome. Trends in neurosciences 2015;38(2):86–95. [DOI] [PubMed] [Google Scholar]
- [41].Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar function reflects common individual differences in emotion and pain regulation success. J Cogn Neurosci 2012;24(1):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America 2011;108(11):4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lau JYF, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry 2008;47(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Le Borgne M, Boudoukha AH, Petit A, Roquelaure Y. Chronic low back pain and the transdiagnostic process: How do cognitive and emotional dysregulations contribute to the intensity of risk factors and pain? Scand J Pain 2017;17:309–315. [DOI] [PubMed] [Google Scholar]
- [45].Linton SJ. A Transdiagnostic Approach to Pain and Emotion. J Appl Biobehav Res 2013;18(2):82–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depression and anxiety 2012;29(4):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res 2006;83(2–3):155–171. [DOI] [PubMed] [Google Scholar]
- [48].Martucci KT, Mackey SC. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology 2018;128(6):1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McCracken LM. “Attention” to pain in persons with chronic pain: A behavioral approach. Behavior Therapy 1997;28(2):271–284. [Google Scholar]
- [50].McEvoy PM, Watson H, Watkins ER, Nathan P. The relationship between worry, rumination, and comorbidity: evidence for repetitive negative thinking as a transdiagnostic construct. J Affect Disord 2013;151(1):313–320. [DOI] [PubMed] [Google Scholar]
- [51].Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences 2011;15(10):483–506. [DOI] [PubMed] [Google Scholar]
- [52].Meulders A. Fear in the context of pain: Lessons learned from 100 years of fear conditioning research. Behav Res Ther 2020;131:103635;epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [53].Miller MM, Meints SM, Hirsh AT. Catastrophizing, pain, and functional outcomes for children with chronic pain: a meta-analytic review. Pain 2018;159(12):2442–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Murray CB, Groenewald CB, de la Vega R, Palermo TM. Long-term impact of adolescent chronic pain on young adult educational, vocational, and social outcomes. Pain 2020;161(2):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nahman-Averbuch H, Schneider VJ 2nd, Chamberlin LA, Kroon Van Diest AM, Peugh JL, Lee GR, Radhakrishnan R, Hershey AD, King CD, Coghill RC, Powers SW. Alterations in Brain Function After Cognitive Behavioral Therapy for Migraine in Children and Adolescents. Headache 2020;60(6):1165–1182. [DOI] [PubMed] [Google Scholar]
- [56].Newby JM, McKinnon A, Kuyken W, Gilbody S, Dalgleish T. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clinical psychology review 2015;40:91–110. [DOI] [PubMed] [Google Scholar]
- [57].Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One 2012;7(2):e31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peng X, Lau WKW, Wang C, Ning L, Zhang R. Impaired left amygdala resting state functional connectivity in subthreshold depression individuals. Sci Rep 2020;10(1):17207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004;43(6):897–905. [DOI] [PubMed] [Google Scholar]
- [60].Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005;48(2):175–187. [DOI] [PubMed] [Google Scholar]
- [61].Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain 2014;155(11):2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9(5):745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp 2010;31(2):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 2009;10(6):423–433. [DOI] [PubMed] [Google Scholar]
- [65].Russell JD, Marsee MA, Weems CF. Developmental Variation in Amygdala Volumes: Modeling Differences Across Time, Age, and Puberty. Biol Psychiatry Cogn Neurosci Neuroimaging 2021;6(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Simons LE, Moulton EA, Linnman C, Carpino EA, Becerra L, Borsook D. The human amygdala and pain: Evidence from neuroimaging. Human brain mapping 2012;35(2):527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel a, Serrano P, Sethna N, Berde C, Becerra L, Borsook D. The responsive amygdala: Treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 2014. Sep;155(9):1727–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Simons LE, Vlaeyen JWS, Declercq L, A MS, Beebe J, Hogan M, Li E, Kronman CA, Mahmud F, Corey JR, Sieberg CB, Ploski C. Avoid or engage? Outcomes of graded exposure in youth with chronic pain using a sequential replicated single-case randomized design. Pain 2020;161(3):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain 2006;7(4):261–271. [DOI] [PubMed] [Google Scholar]
- [70].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- [71].Spielberger CD, Edwards CD, Lushene RE, Monturi J, Platzek D. The state-trait anxiety inventory for children (preliminary manual). Palo Alto: Consulting Psychologists Press, 1973. [Google Scholar]
- [72].Stegmann Y, Reicherts P, Andreatta M, Pauli P, Wieser MJ. The effect of trait anxiety on attentional mechanisms in combined context and cue conditioning and extinction learning. Sci Rep 2019;9(1):8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17(1):52–64. [DOI] [PubMed] [Google Scholar]
- [74].Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain 2016;157(8):1588–1589. [DOI] [PubMed] [Google Scholar]
- [75].Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008;59(6):1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009;47(3):821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16(1):39–58. [DOI] [PubMed] [Google Scholar]
- [78].Wang J, Zhang J, Rong M, Wei X, Zheng D, Fox PT, Eickhoff SB, Jiang T. Functional topography of the right inferior parietal lobule structured by anatomical connectivity profiles. Hum Brain Mapp 2016;37(12):4316–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- [80].Yizhar O, Klavir O. Reciprocal amygdala-prefrontal interactions in learning. Curr Opin Neurobiol 2018;52:149–155. [DOI] [PubMed] [Google Scholar]
- [81].Zhu R, Tian S, Wang H, Jiang H, Wang X, Shao J, Wang Q, Yan R, Tao S, Liu H, Yao Z, Lu Q. Discriminating Suicide Attempters and Predicting Suicide Risk Using Altered Frontolimbic Resting-State Functional Connectivity in Patients With Bipolar II Disorder. Front Psychiatry 2020;11:597770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zidda F, Andoh J, Pohlack S, Winkelmann T, Dinu-Biringer R, Cavalli J, Ruttorf M, Nees F, Flor H. Default mode network connectivity of fear- and anxiety-related cue and context conditioning. Neuroimage 2018;165:190–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


