Abstract
Objective
Serum albumin to globulin ratio (AGR) is a marker of inflammatory disease, but its role in inflammatory bowel disease (IBD) remains unknown. The primary purpose of the present research was to explore the relationship between serum AGR and inflammatory bowel disease (IBD).
Methods
A total of 179 patients with ulcerative colitis (UC), 210 patients with Crohn’s disease (CD), and non-IBD controls (age- and gender-matched controls who have gastrointestinal (GI) symptoms) were enrolled in the research. Demographic data, endoscopic score, and serum biomarkers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, and Ca2+ were included. The Mayo score and the Harvey-Bradshaw Index (HBI) were applied to evaluate the disease activity of UC and CD, respectively.
Results
Serum AGR was significantly lower among IBD patients compared with non-IBD controls. There was a negative association between serum AGR and Mayo score in patients with UC (r = −0.413, p < 0.001), and serum AGR was also associated with HBI score in patients with CD (r = −0.471, p < 0.001). After adjusting other potential variables, low serum AGR (below-median) was independently associated with Mayo score (β = −0.196, p = 0.026) and HBI score (β = −0.162, p = 0.022), respectively. The area under the curve (AUC) for AGR to distinguish UC was 0.701, and the AUC of CD was 0.759. Based on the optimal cut-off value, multivariate logistic regression indicates that low AGR can differentiate UC from non-UC (OR = 2.564, 95% CI = 1.433–4.587, p = 0.002) and CD from non-CD (OR = 3.732, 95% CI = 1.640–8.492, p = 0.001).
Conclusion
AGR may become a promising candidate to help clinicians differentiate IBD and evaluate IBD disease activity. Inflammation and nutritional status might be the future directions to explore its mechanism.
Keywords: inflammatory bowel disease, biomarker, inflammation, malnutrition, activity evaluation
Introduction
Inflammatory bowel disease (IBD), which consists of ulcerative colitis (UC) and Crohn’s disease (CD), is a chronically relapsing inflammatory intestinal disorder with an incidence continuously ascending in recent years, especially in developing countries.1 The pathogenesis of IBD is undefined, complex, and not yet fully understood. It is thought to be due to the interaction of commensal bacteria (or their products), susceptibility genes, environmental factors (such as diet), and immune deficiencies.2,3 IBD has become a global public health problem that generates significantly higher morbidity and an enormous burden for health care systems.4–7 Moreover, IBD has a notable impact on patients’ physical and psychological health, making it critical to diagnose and treat as quickly as possible.6 In contrast, delays in referral and diagnosis seem prevalent in IBD.8 Endoscopy with pathological examination is the gold standard for diagnosing IBD and evaluating disease activity.9 The cross-sectional imaging offers complementary information of small bowel, which the endoscope can hardly reach.10 However, endoscopy tends to be least tolerable due to its considerable cost, inconvenience, and invasive risk, especially when routinely re-evaluated.11 Thus, developing a non-invasive method that is more convenient, tolerable, reliable, economical, and faster for accurately diagnosing IBD and monitoring IBD activity is of great importance. Currently, various biomarkers have been reported, including fecal calprotectin (FC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, and so on.12–14 FC and CRP have been widely studied and play a possible role in discriminating IBD from irritable bowel syndrome (IBS), evaluating disease activity, monitoring medication response, and so on.15 Whereas, the elevated levels of FC were not observed in CD patients even with large ulcers16 and were not a good predictor for IBD activity.9 Besides, CRP, as a non-specific reactant, was reported to discriminate IBD from IBS accurately. However, its prediction value in UC and postoperative recurrence of CD was limited.17,18
As mentioned previously, one of the critical characteristics of IBD is the deficit in the resolution of intestinal inflammation with extraintestinal involvement.19,20 In the last few years, several studies have demonstrated that serum AGR is a reliable indicator of poor prognosis in inflammatory disease and malignancy. AGR was negatively associated with chronic inflammation.21,22 As the main protein in human serum, albumin reflects nutritional status and acute-phase response to inflammation,23 and its levels decrease after the inflammatory stimulus.24 Globulin, another abundant serum protein, plays an essential part in immunity and inflammation.25 Current research has pointed out that globulin was positively associated with the severity of chronic inflammation26 and increased in the progressive IBD.14,24 The decreased albumin and increased globulin will substantially reduce AGR (calculated as albumin/globulin), suggesting its potential predictive value in inflammatory disease (such as IBD). However, the correlation between serum AGR and IBD has not been established.
Considering the vital role of inflammation in IBD and the strong association between inflammatory responses and AGR, we speculated that serum AGR is closely related to the development and severity of IBD, and serum AGR might hold potential for distinguishing IBD from other GI symptoms and evaluating disease activity.
Materials and Methods
Study Design
This research protocol was approved by the Medical Ethics Committee of Wenzhou Medical University’s First Affiliated Hospital, Wenzhou, China, and was conducted following the Declaration of Helsinki. Any information that could lead to patient identity was eliminated, and all data included in this research were anonymized; thus, the need for informed consent has been waived.27 In this cross-sectional study, 456 patients aged ≥18 years with a definitive diagnosis of IBD were consecutively screened from the First Hospital of Wenzhou Medical University from January 2012 to October 2020. Diagnosis of UC and CD was grounded on a combination of clinical manifestations, laboratory, imaging, endoscopic, and histopathologic results, consistent with the previous consensus.28,29 The exclusion criteria were as follows: (1) autoimmune disorders; (2) kidney dysfunction; (3) cancer; (4) acute or chronic infection; (5) hepatic diseases; (6) primary cholangitis; (7) non-classifiable IBD. Eventually, 389 patients with IBD (UC=179, CD=210) were eligible for study inclusion criteria. During the same period, sex- and gender-matched controls were found for UC, and CD patients, respectively (Non-UC=123, Non-CD=88), selected from patients admitted for similar IBD symptoms such as abdominal pain, diarrhea, and other GI symptoms. But they were diagnosed with other digestive diseases after admission (such as acute gastroenteritis, enterocolitis, irritable bowel syndrome, gastrointestinal dysfunction, ischemic enteritis, diverticulitis). The clinical disease activity of patients with CD and UC was evaluated applying the Harvey Bradshaw Index (HBI) score and the Mayo score, respectively, which were classified as active (HBI: total score ≥ 5; Mayo: total score> 2) and in remission (HBI: total score ≤ 4; Mayo: total score≤ 2).30–32 Endoscopic disease activity was assessed using Simplified Endoscopic Score for CD (SES-CD)33 and Mayo endoscopic score34 for UC. Endoscopic scores were obtained based on endoscopy reports written by experienced gastroenterologists who were blinded from this study. The endoscopic disease activity was defined as inactive (=0), mild (=1), moderate (=2), severe (=3) disease in both UC and CD.35
Data Collection
The demographic and clinical characteristics were extracted from the electronic medical record system, including age, gender, body mass index (BMI), current smoking, disease duration, clinical manifestations, medications, histopathological results, endoscopy reports and images. The venous blood was obtained and measured within 24h after admission (fasting). The albumin, CRP, ESR, WBC, Ca2+ were measured using Beckman AU5800 automatic biochemical analyzer. Serum globulin was obtained by calculating total serum protein minus serum albumin, and AGR was calculated by albumin/globulin.
Statistical Analysis
SPSS (version 26.0, Chicago, IL) and GraphPad Prism (version 8.0, GraphPad Software, USA) were applied for the statistical analyses. We used the Kolmogorov–Smirnov test to check the normality of continuous variables. Mean ± standard deviation expressed the normally distributed variables, while non-normally distributed data were expressed as medians (interquartile ranges). The numbers and percentages are utilized to represent noncontinuous variables. The chi-square test was used to compare categorical variables. The Student’s t-test was performed for normally distributed variables, and non-normally distributed variables were analyzed using the Mann–Whitney U-test. We employed Spearman’s correlation analysis to verify the relationship between the AGR, BMI, CRP, ESR, WBC and disease activity. The difference of AGR among the high endoscopic scores group (2 or 3) and the low endoscopic scores group (0 or 1) was also compared using t-test to explore its correlation with disease severity in endoscopy.5
What’s more, we applied multiple linear regression to estimate the independent predictive ability of serum AGR as continuous variables and dichotomous variables (truncation value was the median), respectively, on Mayo score and HBI score. The area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the accuracy of serum AGR in distinguishing IBD, and the optimal cut-offs for serum AGR were evaluated based on ROC curves using Youden's index. According to the optimal cut-offs, serum AGR was divided into low AGR and high AGR. Low AGR’s ability to distinguish IBD from non-IBD was evaluated by multivariate logistic regression analysis to adjust confounding variables such as age, sex, ESR, CRP, WBC, Ca2+. Results were shown as adjusted odds ratio (OR) (95% confidence interval (CI)). The p-value of less than 0.05 (two-tailed) was considered statistically significant.
Results
Basic Characteristics of Baseline Data
This study included 179 patients with UC, 123 patients with non-UC, 210 patients with CD, and 88 patients with non-CD. Gender and age between patients with IBD and non-IBD were not statistically different. Compared to patients with non-UC, BMI, albumin, AGR, Ca2+ were significantly lower (p < 0.05), while ESR, CRP, WBC were considerably higher in patients with UC (p < 0.05). Similarly, BMI, albumin, AGR, Ca2+ were significantly lower in patients with non-CD (p < 0.05), while ESR, CRP, WBC were significantly higher in patients with CD (p < 0.05). Noteworthy, the proportion of current smokers among IBD (statistically significant in CD) was lower. And other demographic and clinical characteristics between IBD and the controls are displayed in Table 1.
Table 1.
Characteristics of Patients with IBD and Non-IBD Cohorts
| UC (n = 179) | Non-UC (n = 123) | CD (n = 210) | Non-CD (n = 88) | |
|---|---|---|---|---|
| Sex (Male/Female) | 95/84 | 63/60 | 149/61 | 56/32 |
| Age (yr) | 49.20 ± 15.20 | 46.20 ± 13.80 | 31.40 ± 10.46 | 33.28 ± 9.51 |
| BMI (kg/m2) | 20.80 (19.20,22.80)a | 22.20 (20.00,24.20) | 19.10 (17.30,21.40)b | 21.60 (19.50,24.50) |
| Current smoking (n,%) | 25 (14.0%) | 26 (21.1%) | 19 (9.0%)b | 17 (19.3%) |
| Duration years (yr) | 1.00 (0.30,4.00) | 1.00 (0.50;4.00) | ||
| ESR (mm/h) | 18.00 (6.00,26.00)a | 7.00 (3.00,15.00) | 19.00 (9.00,34.00)b | 5.50 (2.00,12.00) |
| CRP (mg/L) | 5.45 (2.89,18.34)a | 3.13 (2.11,8.91) | 9.79 (3.16,28.63)b | 3.13 (2.12,9.60) |
| WBC (109/L) | 6.94 (5.53,8.61)a | 6.23 (5.10,7.83) | 6.50 (5.11,8.37)b | 6.26 (5.30,7.98) |
| Albumin (g/L) | 36.99 ± 6.14a | 40.25 ± 4.67 | 38.26 ± 5.53b | 41.31 ± 4.40 |
| Globulin (g/L) | 30.42 ± 4.51a | 28.27 ± 4.06 | 31.54 ± 5.26b | 27.93 ± 4.07 |
| AGR | 1.24 ± 0.27a | 1.45 ± 0.27 | 1.25 ± 0.30b | 1.51 ± 0.27 |
| Ca2+ (mmol/L) | 2.17 (2.10,2.26)a | 2.25 (2.16,2.32) | 2.21 ± 0.13b | 2.25 ± 0.13 |
| Clinical score (n, %) | ||||
| Remission | 9 (5.0%) | 78 (37.1%) | ||
| Active disease | 170 (95.0%) | 132 (62.9%) | ||
| Endoscopic score (%) | 0/1/2/3 5.0/19.0/48.1/27.9 |
0/1/2/3 17.1/22.9/42.4/17.6 |
||
| Age at diagnosis (%) | A1/A2/A3 2.9/84.2/12.9 |
|||
| Disease Location (%) | E1/E2/E3 24.0/33.0/43.0 |
L1/L2/L3/L4 25.7/33.3/39.5/1.5 |
||
| Disease behavior (%) | B1/B2/B3/P 51.4/20.5/4.8/23.3 | |||
| Medications (n, %) | ||||
| 5-ASA | 162 (90.5%) | 64 (30.48%) | ||
| Antibiotics | 46 (25.7%) | 1 (0.48%) | ||
| Steroids | 53 (29.6%) | 26 (12.38%) | ||
| Immunosuppression | 18 (10.1%) | 49 (23.33%) | ||
| Biological therapy | 8 (4.5%) | 106 (50.48%) | ||
| Disease categories (n, %) | ||||
| Acute gastroenteritis | 25 (20.3%) | 21 (23.9%) | ||
| Enterocolitis | 32 (26.0%) | 28 (31.8%) | ||
| Gastrointestinal dysfunction | 48 (39%) | 34 (38.6%) | ||
| Ischemic enteritis | 11 (8.9%) | 1 (1.1%) | ||
| Diverticulitis | 7 (5.7%) | 4 (4.5%) |
Note: ap < 0.05 compared with non-UC; bp < 0.05 compared with non-CD. Endoscopic score: Mayo endoscopic score (UC): 0- inactive, 1- mild, 2- moderate, 3- severe disease; SES-CD score (CD): 0- inactive- ≤2, 1- mild- 3–6, 2- moderate- 7–15, 3- severe- ≥16. Age at diagnosis, disease location, and behavior were categorized using the Montreal classification: A (age at diagnosis): A1- ≤16, A2- 17–40, A3- >40; L (location): L1- ileal disease, L2- colonic disease, L3- ileocolonic disease, L4- isolated upper disease; B (behavior): B1- nonstructuring/ penetrating phenotype, B2- structuring phenotype, B3- penetrating phenotype, P- perianal phenotype. E (extension): E1- ulcerative proctitis, E2- left-side colitis, E3- extensive colitis. The chi-square test was used to compare categorical variables. Student’s t-test and the Mann–Whitney U-test were used accordingly. Data were shown as Mean ± standard deviation or medians (interquartile ranges).
Abbreviations: UC, ulcerative colitis; CD, Crohn’s disease; BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; WBC, white blood cell; AGR, albumin to globulin ratio; SES-CD, Simplified endoscopic activity score for Crohn’s disease.
Serum AGR in IBD and Non-IBD Groups
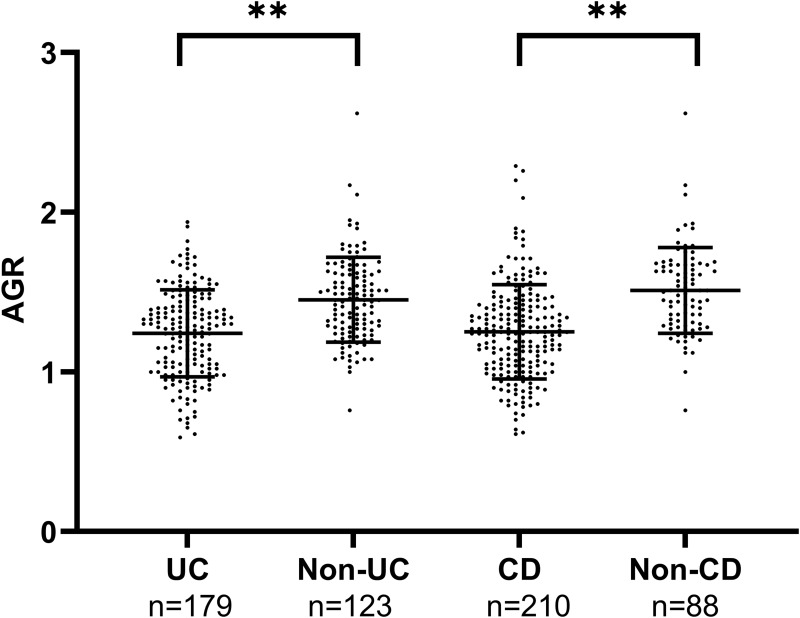
Serum AGR significantly decreased in UC patients compared with non-UC patients (1.24 ± 0.27 vs 1.45 ± 0.27, p < 0.01). A similar result was observed in CD and non-CD (1.25 ± 0.30 vs 1.51 ± 0.27, p < 0.01) (Table 1 and Figure 1).
Figure 1.
The difference in serum AGR between UC patients and non-UC patients, CD patients, and non-CD patients.
Note: **p < 0.01, Student’s t-test.
Correlation Between CRP, ESR, WBC, BMI, and Serum AGR
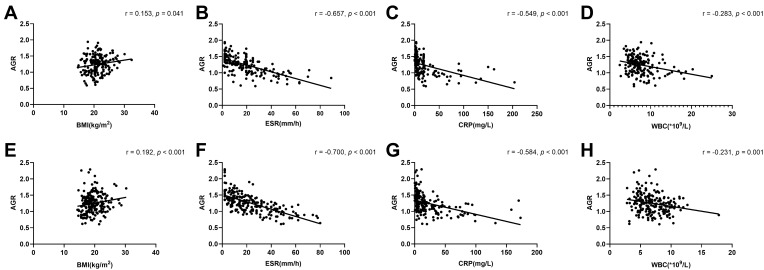
Serum AGR was found to be inversely associated with CRP, ESR, WBC levels (r = −0.549, p < 0.001; r = −0.657, p < 0.001; r = −0.283, p < 0.001), and positively associated with BMI (r = 0.153, p = 0.041) in patients with UC (Table 2, Figure 2). Likewise, serum AGR was negatively correlated with CRP, ESR, WBC levels (r = −0.584, p < 0.001; r = −0.700, p < 0.001; r = −0.231, p = 0.001), and positively associated with BMI (r = 0.192, p < 0.001) in patients with CD. Whereas BMI was not associated with other inflammatory indicators in both UC and CD. (Tables 2 and 3, Figure 2).
Table 2.
Correlation Between BMI, Serum Biomarkers, and Disease Activity in UC Patients
| Parameters | AGR | CRP | ESR | WBC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |||
| AGR | – | – | – | – | – | – | – | – | ||
| CRP | −0.549 | < 0.001 | – | – | – | – | – | – | ||
| ESR | −0.657 | < 0.001 | 0.621 | < 0.001 | – | – | – | – | ||
| WBC | −0.283 | < 0.001 | 0.336 | < 0.001 | 0.286 | < 0.001 | – | – | ||
| Mayo score | −0.413 | < 0.001 | 0.342 | < 0.001 | 0.352 | < 0.001 | 0.243 | 0.001 | ||
| BMI | 0.153 | 0.041 | 0.028 | 0.710 | −0.076 | 0.314 | −0.045 | 0.549 | ||
Note: Spearman correlation analysis was used.
Abbreviations: UC, ulcerative colitis; AGR, albumin to globulin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; BMI, body mass index.
Figure 2.
The correlation between serum AGR and BMI, other inflammation markers. (A) BMI, (B) ESR level, (C) CRP level, (D) WBC level of UC patients were shown by scatter plot; the correlation between serum AGR and (E) BMI, (F) ESR level, (G) CRP level, (H) WBC level of CD patients were shown by scatter plot.
Note: Spearman correlation analysis was used.
Table 3.
Correlation Between Serum Biomarkers and Disease Activity in CD Patients
| Parameters | AGR | CRP | ESR | WBC | ||||
|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |
| AGR | – | – | – | – | – | – | – | – |
| CRP | −0.584 | < 0.001 | – | – | – | – | – | – |
| ESR | −0.700 | < 0.001 | 0.677 | < 0.001 | – | – | – | – |
| WBC | −0.231 | 0.001 | 0.381 | < 0.001 | 0.329 | < 0.001 | – | – |
| HBI score | −0.471 | < 0.001 | 0.696 | < 0.001 | 0.479 | < 0.001 | 0.307 | < 0.001 |
| BMI | 0.192 | < 0.001 | −0.069 | 0.323 | −0.111 | 0.109 | −0.061 | 0.383 |
Note: Spearman correlation analysis was used.
Abbreviations: CD, Crohn’s disease; AGR, albumin to globulin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell; BMI, body mass index. Spearman correlation analysis was used.
Association Between Serum AGR and Disease Activity
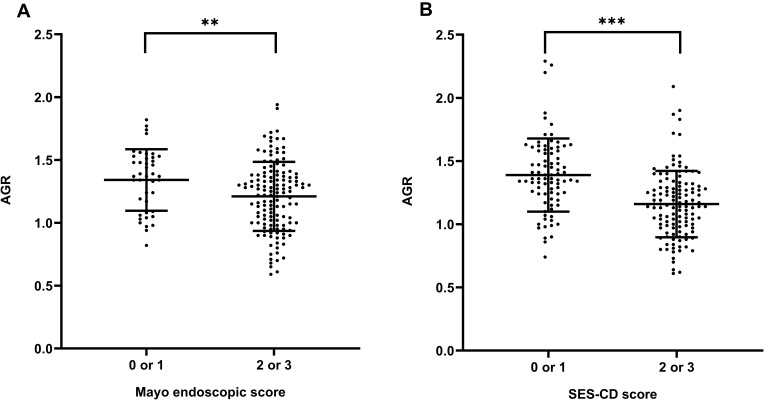
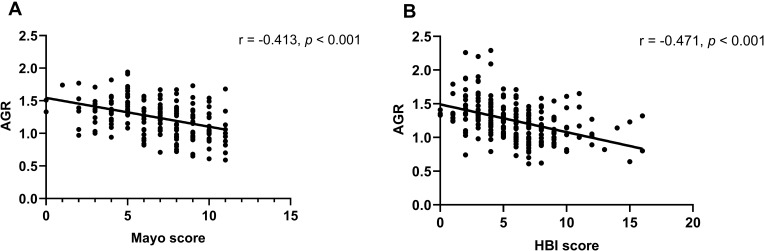
Employing the composite IBD endoscopy score,5 serum AGR significantly decreased in the UC patients with high endoscopic score (2 or 3) compared with low endoscopic score (0 or 1) (p < 0.01, Figure 3), as well as in the CD patients (p < 0.001, Figure 3). Besides, it has been proved that the Mayo score is widely used in UC due to good test-retest reliability, responsiveness, and correlating well with clinical features, endoscopic appearances, and biomarkers of UC activity. Likewise, the Harvey-Bradshaw Index (HBI) score is widely used in CD, which has great construct validity employing clinical and biochemical evaluation.36 Therefore, we conducted the correlation analysis and established the regression model using the HBI and Mayo score to evaluate disease activity. There was a highly negative correlation between serum AGR and Mayo score in patients with UC (r = −0.413, p < 0.001, Table 2 and Figure 4), similarly between serum AGR and HBI score in patients with CD (r = −0.471, p < 0.001, Table 3 and Figure 4). In addition, we found that CRP, ESR and WBC levels were positively associated with disease activity in patients with UC (r = 0.342, p < 0.001; r = 0.352, p < 0.001; r = 0.243, p = 0.001, Table 2), as well as in patients with CD (r = 0.696, p < 0.001; r = 0.479, p < 0.001; r = 0.307, p < 0.001, Table 3). We conducted multiple linear regression to explore further whether serum AGR was an independent predictor of IBD disease activity. The linear regression unadjusted model indicated that serum AGR below-median (≤ 1.28) and Ca2+ were negatively related to Mayo score. In contrast, CRP, ESR, WBC were positively related to Mayo score in patients with UC (model 1: unadjusted p < 0.05, Table 4). After adjusting age, gender, BMI, current smoking, disease duration, disease extension, CRP, ESR, WBC, Ca2+, only serum AGR below-median (≤ 1.28) remained independently related to Mayo score (model 2: β = −0.196, p = 0.026, Table 4). Similar results were found in patients with CD. There was an inverse association between serum AGR below-median (≤ 1.24), Ca2+, and HBI score. In contrast, a positive association between CRP, ESR, WBC, and HBI score without adjustment (model 1: unadjusted p<0.05, Table 5). After adjusting the confounding variables in CD, serum AGR below-median (≤ 1.24) also independently associated with HBI score (model 2: β = −0.162, p = 0.022, Table 5). Furthermore, as a continuous variable, serum AGR remained significant after adjusting confounding variables in the assessment of disease activity in both UC and CD patients (model 2: β = −0.244, p = 0.017, Table 4; model 2: β = −0.204, p = 0.007, Table 5, respectively). In addition, CRP was an independent predictor for disease activity in patients with CD (model 2: β = 0.356, p < 0.001, Table 5).
Figure 3.
Distributions between serum AGR and endoscopic score in IBD patients. (A) Mayo endoscopic score in UC patients (0 or 1, n = 43; 2 or 3, n = 136), (B) SES-CD score in CD patients (0 or 1, n = 84; 2 or 3, n = 126). AGR was shown by binary ordered endoscopic disease activity, using a composite IBD endoscopy score (0 or 1 indicating inactive or mild disease and 2 or 3 indicating moderate or severe disease, respectively).
Note: **p < 0.01, ***p < 0.001. Student’s t-test.
Figure 4.
Correlation between serum AGR and Mayo score in UC patients (A), HBI score in CD patients (B) by using a scatter plot.
Note: Spearman correlation analysis was used.
Table 4.
Linear Regression Analysis of Serum AGR with Disease Activity in UC Patients
| Model 1a | p-value | Model 2b | p-value | |
|---|---|---|---|---|
| Standardized Coefficients β (95% CI) | Standardized Coefficients β (95% CI) | |||
| AGR | −0.411 (−5.083, −2.568) | < 0.001 | − 0.244 (−4.119, −0.414) | 0.017 |
| AGR≤1.28 | −0.352 (−2.485, −1.078) | < 0.001 | − 0.196 (−1.865, −0.119) | 0.026 |
| CRP | 0.267 (0.010, 0.033) | < 0.001 | 0.030 (−0.011, 0.016) | 0.727 |
| ESR | 0.335 (0.029, 0.072) | < 0.001 | 0.132 (−0.007, 0.047) | 0.139 |
| WBC | 0.220 (0.056, 0.273) | 0.003 | 0.073 (−0.061, 0.170) | 0.351 |
| Ca2+ | −0.284 (−7.868, −2.611) | < 0.001 | − 0.144 (−5.537, 0.214) | 0.069 |
Note: Model 1a: unadjusted. Model 2b: adjusted for age, gender, BMI, current smoking, disease duration, disease extension, CRP, ESR, WBC, Ca2+.
Abbreviations: UC, ulcerative colitis; CI, confidence interval; BMI, body mass index; AGR, albumin to globulin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Table 5.
Linear Regression Analysis of Serum AGR with Disease Activity in CD Patients
| Model 1a | p-value | Model 2b | p-value | |
|---|---|---|---|---|
| Standardized Coefficients β (95% CI) | Standardized Coefficients β (95% CI) | |||
| AGR | −0.430 (−5.772, −3.196) | < 0.001 | −0.204 (−3.674, −0.581) | 0.007 |
| AGR≤1.24 | −0.396 (−3.210, −1.666) | < 0.001 | −0.162 (−1.844, −0.145) | 0.022 |
| CRP | 0.523 (0.042, 0.066) | < 0.001 | 0.356 (0.022, 0.051) | < 0.001 |
| ESR | 0.431 (0.054, 0.097) | < 0.001 | 0.083 (−0.013, 0.042) | 0.296 |
| WBC | 0.282 (0.210, 0.573) | < 0.001 | 0.040 (−0.114, 0.225) | 0.517 |
| Ca2+ | −0.186 (−7.852, −1.271) | 0.007 | −0.010 (−3.233, 2.756) | 0.875 |
Note: Model 1a: unadjusted. Model 2b: adjusted for age, gender, BMI, current smoking, disease duration, disease localization, disease behavior, CRP, ESR, WBC, Ca2+.
Abbreviations: CD, Crohn’s disease; CI, confidence interval; BMI, body mass index; AGR, albumin to globulin ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Value of Serum AGR to Discriminate IBD from Non-IBD Group
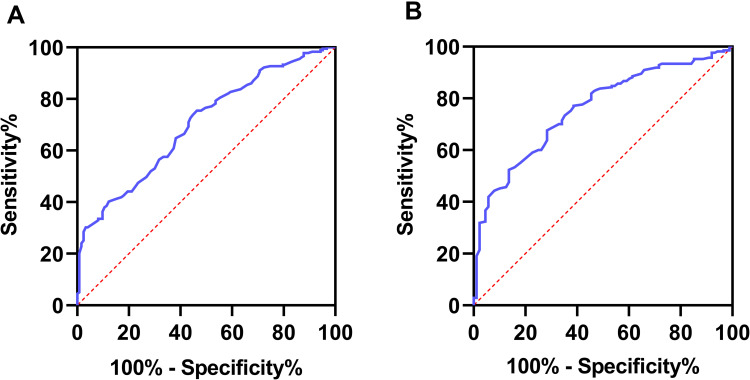
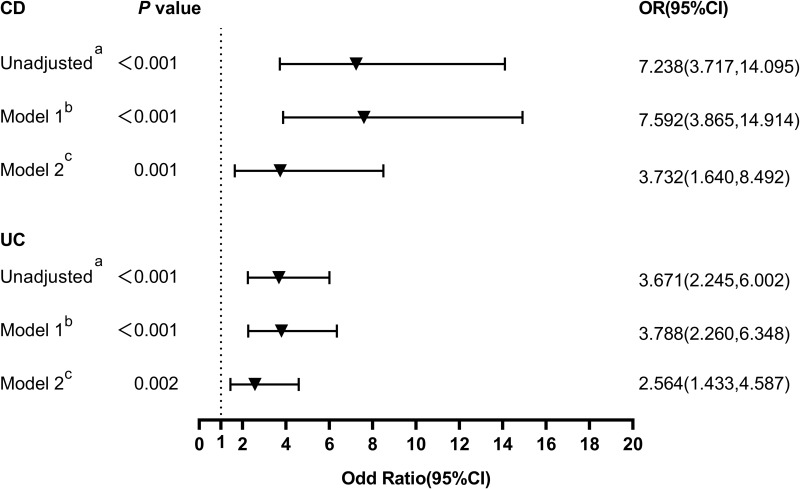
ROC analyses were used to assess the ability of serum AGR to distinguish IBD from non-IBD patients. According to the ROC curve, the optimum cut-off value for serum AGR as a diagnostic marker of UC and CD was 1.413 and 1.262, respectively (sensitivity 0.545, specificity 0.754, AUC = 0.701, 95% CI 0.643–0.759, p < 0.001; sensitivity 0.864, specificity 0.533, AUC = 0.759, 95% CI 0.702–0.815, p < 0.001; respectively, Figure 5). The multiple logistic regression analysis showed that low serum AGR below the optimal cut-off was the risk factor with UC and CD patients (≤ 1.413, ≤ 1.262, respectively) without adjustment (unadjusted: OR 3.671, 95% CI 2.245–6.002, p < 0.001; unadjusted: OR 7.238, 95% CI 3.717–14.095, p < 0.001; respectively, Figure 6). After adjusting for age and gender, low serum AGR remained the risk factor in both UC and CD (Model 1: OR 3.788, CI 2.260–6.348, p < 0.001; Model 1: OR 7.592, CI 3.865–14.914, p < 0.001; respectively, Figure 6). After further adjusting for BMI, current smoking, ESR, CRP, WBC, Ca2+, low AGR still was an independent risk factor with UC and CD (Model 2: OR 2.564, CI 1.433–4.587, p = 0.002; Model 2: OR 3.732, CI 1.640–8.492, p = 0.001; respectively, Figure 6).
Figure 5.
Receiver operator characteristic analysis of albumin to globulin ratio for distinguishing UC from non-UC (A) and CD from non-CD (B).
Figure 6.
Multivariate adjusted odds ratios for distinguishing inflammatory bowel disease (UC and CD) from non-inflammatory bowel disease. Unadjusteda: unadjusted model. Model 1b: adjusted for age, sex. Model 2c: adjusted for covariates from Model 1 and adjusted for BMI, current smoking, ESR, CRP, WBC, Ca2+.
Discussion
The initial idea of the present study was to discover a novel IBD-specific biomarker that can help distinguish IBD from other patients with GI symptoms. To our knowledge, it is the first time to explore the correlation between AGR and IBD. Our research demonstrated that low serum AGR was independently related to the presence and the disease activity of IBD.
Serum AGR is the calculated ratio of serum albumin to serum globulin. Serum albumin, a negative acute-phase reactant, is widely applied to evaluate the inflammatory and nutritional condition of the human body.37,38 Hypoalbuminemia is correlated with malnutrition, hepatopathy, kidney disease, and all kinds of inflammation.22,39,40 Low serum albumin was associated with severe colitis and can help the early decision-making process for colectomy or intravenous cyclosporine Treatment.13,41 Globulin level is closely related to the individuals’ immune and inflammatory state.22,37 Globulin consists of various immunoglobulins and acute-phase proteins such as CRP, amyloid A protein, α1 antitrypsin.42 In chronic inflammation, these globulin components are upregulated with the stimulation of pro-inflammatory cytokines, like IL-6 and TNF-α.23,42 Previous studies reported that increased immunoglobulin and acute-phase proteins, including CRP, amyloid A protein, α1 antitrypsin, were significantly elevated in active IBD.14,43 In addition, increased serum β2-microglobulin was associated with the activity of IBD.7 Shiraishi et al44 found a positive association of endoscopic activity and a negative association of mucosal healing with serum globulin in patients with UC. Serum AGR, as a predictor, was widely applied in the evaluation of malignancies and inflammation. In the last few years, a considerable volume of published research has described that low serum AGR was a negative predictor of overall survival rate in multiple malignant tumors, including breast cancer,45 gastric cancer,46 cholangiocarcinomas,47 and so on. Chronic inflammation is correlated with AGR, and current studies manifested that AGR was an essential predictive factor of mortality about chronic kidney disease,22,40 rheumatoid arthritis, and bronchiectasis,48 acute exacerbation of chronic obstructive pulmonary disease (COPD),49 coronavirus disease-19 (COVID-19),50 microscopic polyangiitis51 and so on. Overall, the reverse relationship between globulin and albumin in the inflammatory and Infectious responses drastically reduced the AGR, implying that the AGR is a strong indicator of the inflammatory state and infection. In the present study, we have also identified this association in IBD. In addition, the advantage of the AGR over albumin and globulin alone is that it terrifically eliminates the effects of dehydration and fluid retention, which is prevalent in IBD patients.52 AGR is a ratio instead of an absolute value. It can provide a true reflection of the nutritional and inflammatory status of the body.22,37,47 Moreover, AGR was a promising predictor because it’s readily accessible and cheap in clinical applications.
The possible mechanism to explain this correlation has not been elucidated yet. We speculated that AGR could reflect the inflammation response in the digestive tract and the nutritional status of IBD patients. Our study demonstrated that serum AGR was correlated with CRP, ESR, and WBC (both served as markers of inflammation), and low serum AGR was associated with severe endoscopic inflammation, supporting the potential connection of serum AGR with inflammation. What’s more, numerous studies have shown that immune cells and cytokines play a significant role in the pathogenesis of IBD. Abnormal intestinal microbiota or antigen activate immune cells, such as macrophages, T cells, and innate lymphoid cells (ILCs), producing large amounts of cytokines, both pro-and anti-inflammatory, which regulate inflammatory processes.53–55 The pro-inflammatory cytokines, such as IL-1, IL-6, IL-8, TNF-α activate immune cells and nonimmune cells to produce more pro-inflammatory cytokines, triggering immune and inflammatory cascade reactions. Cytokines not only boosted intestinal inflammation but elicited systemic effects in IBD patients, such as hypoproteinemia by decreasing albumin mRNA expression and reducing albumin synthesis in hepatocytes.56 These cytokines also affect the capillary and increase vascular permeability, promoting the loss of albumin and accelerating albumin degradation in extravascular space like the intestinal tract.57–59 Furthermore, the increased permeability to antigens could stimulate antibody formation and elicit elevated immunoglobulins,43,60 which could partially account for the high serum globulin levels. In short, one underlying mechanism could be the inflammation in IBD contributing to hypoalbuminemia and elevated globulin levels.
Additionally, malnutrition occurred frequently in patients with IBD (especially with CD) due to oral intake reduction, malabsorption, nutrient losses from the gastrointestinal tract, and medication side effects.61 Diet is one of the critical factors in forming a normal intestinal microenvironment, and current studies have reported its possible association with IBD.62,63 Dietary patterns can exert either anti-inflammatory or pro-inflammatory effects.63 A dietary pattern characterized by red meat, finely processed foods, and saturated fatty acids,64 might trigger a pro-inflammatory environment by altering gut microbiota and impairing gut barrier function. High fat, high sugar, low fiber, gluten may also influence the dynamic balance of host immunity by increasing intestinal permeability, increasing pro-inflammatory markers, and decreasing Treg cell levels.62–64 However, short-chain fatty acids (SCFA) contribute to the diversity of the intestinal microbiota64 and play an immunomodulatory role in intestinal inflammation, including reducing chemokines production by neutrophils and macrophages and inhibiting the expression of inflammatory mediators such as cytokines and adhesion molecules.62 Recently, diet therapy has been widely studied in the treatment of IBD (especially in children).64,65 Besides, it has been reported that most patients changed their diet after being diagnosed with IBD,66 and restrictive diet behaviors and reduced appetite increase the risk of malnutrition.65
BMI and serum albumin have been widely used readouts to evaluate the nutritional status and have the greatest predictive value among different indicators of malnutrition in patients with active IBD. In the present study, BMI and serum levels of albumin were lower among IBD patients, and BMI was positively associated with serum AGR rather than CRP, ESR, WBC in the IBD population, indicating that AGR could be more exclusively affected by malnutrition in IBD, which may partially support our hypothesis of malnutrition. So we speculate that AGR has the potential advantage over other inflammatory markers because it may reflect both inflammation and nutritional status. What’s more, the complex interactions between inflammatory cytokines release and nutrients have been expounded. Firstly, the inflammation response of IBD patients could affect nutrition status. Appetite suppression caused by inflammation may be mediated by increased release of IL-1 from the inflamed gut and central 5-hydroxytryptamine release.67,68 Secondly, nutrients may modulate inflammatory responses through the intestinal ecosystem and act as cell membrane components to mediate the expression of proteins involved in inflammatory processes, such as cytokines, adhesion molecules, inducible nitric oxide synthase (iNOS).69 Micronutrient deficiencies are more common in malnourished patients (such as IBD).66 Micronutrients may be essential for tissue repair, oxidative stress resistance, and lipid peroxidation inhibition.69 For example, it has been demonstrated that vitamin D plays a critical role in immunity and its levels are negatively correlated with intestinal inflammation. Vitamin D receptors are found in macrophages, T cells, and other immune cells, which strengthen anti-inflammatory effects and reduce the production of pro-inflammatory factors. Zinc is a cofactor for several enzymes and exerts anti-inflammatory effects by protecting against free radical-mediated cell damage.67,70 Future studies about comprehensive nutritional assessments are warranted to verify the relationship between nutrition and AGR, including clinical, anthropometric, and body composition.
In this study, it was interesting to discover that serum CRP and ESR were more related to disease activity than AGR in CD patients (correlation analysis). Still, only CRP and AGR remained significant after adjustment. In contrast, AGR appeared to be more relevant to disease activity than CRP and ESR in UC patients, and only AGR was an independent predictor. To summarize, CRP levels seemed to be more associated with disease activity of CD patients than UC patients, which is consistent with the previous studies.12,14 Nevertheless, AGR correlates well with disease activity in both CD patients and UC patients.
To date, abundant biomarkers were examined in IBD diagnosis and disease activity in IBD, but none was ideal. Fecal calprotectin is a significant fecal marker for IBD, while it has not been promoted and applied in many hospitals because of its high cost and time-consuming. CRP is more suitable for diagnosing CD than UC, and the interindividual differences of genes lead to the variation of CRP production.14,71 ESR is not fast enough to peak, and the response to inflammatory changes was slower.14 Compared to them, AGR may become a promising candidate to help clinicians to differentiate IBD and evaluate disease activity in IBD because it is a fast, tolerable, cheap, and conveniently accessible marker.
Intriguingly, the proportion of current smoking was lower in both UC and CD patients than other GI symptom controls, replicating several studies in China.72,73 Though it is generally believed that current smoking could be risky for CD but protective in UC,74 it might be heterogeneity across race, diet, and geography that might account for this inconsistency.73,75 More importantly, the present study included other GI symptoms patients instead of healthy individuals as control, which could be another reason for this difference.
There are some limitations to our research. First, our sample size is a bit small. It might exist some confounders not included affecting the result. Second, this was a cross-sectional study from a single center, which would have some selective bias. In the future, a prospective study with a larger scale is required to verify our results and explore the relationship between AGR and the prognosis/reoccurrence of IBD. Third, our research may not be extrapolated to all age/region/racial groups due to the study design. Finally, we did not record the diet information in patients with IBD, and cytokines were not measured to assess disease activity. In addition, because our results and speculation were descriptive and preliminary, comprehensive nutritional assessment and cytokines measurement in IBD patients should be conducted to provide additional information.
Conclusion
In conclusion, our study demonstrates that low serum AGR is associated with active disease in IBD patients, and serum AGR can help distinguish IBD patients from patients with other GI symptoms. AGR may become a promising candidate to help clinicians differentiate IBD and evaluate disease activity in IBD. Although the mechanisms involved still need much clarification, the inflammation and nutritional status might be the future directions to explore.
Funding Statement
There is no funding to report.
Abbreviations
UC, ulcerative colitis; CD, Crohn’s disease; AGR, albumin to globulin ratio; BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; WBC, white blood cell; SES-CD, Simplified Endoscopic Activity Score for Crohn’s Disease; OR, odds ratio; CI, confidence interval.
Ethics Approval and Consent to Participate
This research protocol was approved by the Medical Ethics Committee of Wenzhou Medical University’s First Affiliated Hospital, Wenzhou, China. It was conducted according to the guidelines of the Declaration of Helsinki. All data included in this research were anonymized; thus, the need for informed consent has been waived.27
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Yanyan Wang and Chengyong Li are co-first authors for this study. The authors have no conflicts of interest to declare in this work.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528 [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]
- 4.Acarturk G, Acay A, Demir K, Ulu MS, Ahsen A, Yuksel S. Neutrophil-to-lymphocyte ratio in inflammatory bowel disease - as a new predictor of disease severity. Bratisl Lek Listy. 2015;116(4):213–217. doi: 10.4149/bll_2015_041 [DOI] [PubMed] [Google Scholar]
- 5.Bourgonje AR, von Martels JZH, Gabriels RY, et al. A combined set of four serum inflammatory biomarkers reliably predicts endoscopic disease activity in inflammatory bowel disease. Front Med. 2019;6:251. doi: 10.3389/fmed.2019.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byron C, Cornally N, Burton A, Savage E. Challenges of living with and managing inflammatory bowel disease: a meta-synthesis of patients’ experiences. J Clin Nurs. 2020;29(3–4):305–319. doi: 10.1111/jocn.15080 [DOI] [PubMed] [Google Scholar]
- 7.Yılmaz B, Köklü S, Yüksel O, Arslan S. Serum beta 2-microglobulin as a biomarker in inflammatory bowel disease. World J Gastroenterol. 2014;20(31):10916–10920. doi: 10.3748/wjg.v20.i31.10916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahon S, Lahmek P, Lesgourgues B, et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J Crohns Colitis. 2014;8(9):964–969. doi: 10.1016/j.crohns.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 9.Dulai PS, Peyrin-Biroulet L, Danese S, et al. Approaches to integrating biomarkers into clinical trials and care pathways as targets for the treatment of inflammatory bowel diseases. Gastroenterology. 2019;157(4):1032–1043.e1031. doi: 10.1053/j.gastro.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 10.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164. doi: 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 11.Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(8):1425–1433. doi: 10.1097/MIB.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 12.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57(11):1518–1523. doi: 10.1136/gut.2007.146357 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95(2):359–367. doi: 10.1111/j.1572-0241.2000.t01-1-01790.x [DOI] [PubMed] [Google Scholar]
- 14.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55(3):426–431. doi: 10.1136/gut.2005.069476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149(5):1275–1285 e1272. doi: 10.1053/j.gastro.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 16.Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015;50(7):841–847. doi: 10.3109/00365521.2015.1008035 [DOI] [PubMed] [Google Scholar]
- 17.Sonoyama H, Kawashima K, Ishihara S, et al. Capabilities of fecal calprotectin and blood biomarkers as surrogate endoscopic markers according to ulcerative colitis disease type. J Clin Biochem Nutr. 2019;64(3):265–270. doi: 10.3164/jcbn.18-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boschetti G, Laidet M, Moussata D, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol. 2015;110(6):865–872. doi: 10.1038/ajg.2015.30 [DOI] [PubMed] [Google Scholar]
- 19.Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16(9):531–543. doi: 10.1038/s41575-019-0172-4 [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Wang HE, Bai YM, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70(1):85–91. doi: 10.1136/gutjnl-2020-320789 [DOI] [PubMed] [Google Scholar]
- 21.Niedziela JT, Hudzik B, Szygula-Jurkiewicz B, et al. Albumin-to-globulin ratio as an independent predictor of mortality in chronic heart failure. Biomark Med. 2018;12(7):749–757. doi: 10.2217/bmm-2017-0378 [DOI] [PubMed] [Google Scholar]
- 22.Zeng M, Liu Y, Liu F, Peng Y, Sun L, Xiao L. Association between albumin-to-globulin ratio and long-term mortality in patients with chronic kidney disease: a cohort study. Int Urol Nephrol. 2020;52(6):1103–1115. doi: 10.1007/s11255-020-02453-7 [DOI] [PubMed] [Google Scholar]
- 23.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 24.Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill LA, Bodnar TS, Weinberg J, Hammond GL. Corticosteroid-binding globulin is a biomarker of inflammation onset and severity in female rats. J Endocrinol. 2016;230(2):215–225. doi: 10.1530/JOE-16-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu PP, Hsieh YP, Kor CT, Chiu PF. Association between albumin-globulin ratio and mortality in patients with chronic kidney disease. J Clin Med. 2019;8(11):11. doi: 10.3390/jcm8111991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong M, Braun KL, Chang RM. Native Hawaiian preferences for informed consent and disclosure of results from genetic research. J Cancer Educ. 2006;21(1 Suppl):S47–52. doi: 10.1207/s15430154jce2101s_10 [DOI] [PubMed] [Google Scholar]
- 28.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133(5):1670–1689. doi: 10.1053/j.gastro.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 29.Ooi CJ, Fock KM, Makharia GK, et al. The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010;25(3):453–468. doi: 10.1111/j.1440-1746.2010.06241.x [DOI] [PubMed] [Google Scholar]
- 30.D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of Medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132(2):763–786. doi: 10.1053/j.gastro.2006.12.038 [DOI] [PubMed] [Google Scholar]
- 31.Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25. doi: 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- 32.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/S0140-6736(80)92767-1 [DOI] [PubMed] [Google Scholar]
- 33.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512. doi: 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 34.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 35.Abedin N, Seemann T, Kleinfeld S, et al. Fecal eosinophil cationic protein is a diagnostic and predictive biomarker in young adults with inflammatory bowel disease. J Clin Med. 2019;8(12):12. doi: 10.3390/jcm8122025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alrubaiy L, Rikaby I, Sageer M, Hutchings HA, Williams JG. Systematic review of the clinical disease severity indices for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(10):2460–2466. doi: 10.1097/MIB.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 37.Guo HW, Yuan TZ, Chen JX, Zheng Y. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: a meta-analysis. PLoS One. 2018;13(1):e0189839. doi: 10.1371/journal.pone.0189839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y, Chen W, Gu M, et al. Serum globulin and albumin to globulin ratio as potential diagnostic biomarkers for periprosthetic joint infection: a retrospective review. J Orthop Surg Res. 2020;15(1):459. doi: 10.1186/s13018-020-01959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feketea GM, Vlacha V. The diagnostic significance of usual biochemical parameters in Coronavirus Disease 19 (COVID-19): albumin to globulin ratio and CRP to albumin ratio. Front Med. 2020;7:566591. doi: 10.3389/fmed.2020.566591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J, Kim HJ, Kim J, Choi YB, Shin YS, Lee MJ. Predictive value of serum albumin-to-globulin ratio for incident chronic kidney disease: a 12-year community-based prospective study. PLoS One. 2020;15(9):e0238421. doi: 10.1371/journal.pone.0238421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelbmann CM. Prediction of treatment refractoriness in ulcerative colitis and Crohn’s disease–do we have reliable markers? Inflamm Bowel Dis. 2000;6(2):123–131. doi: 10.1097/00054725-200005000-00009 [DOI] [PubMed] [Google Scholar]
- 42.Li K, Fu W, Bo Y, Zhu Y. Effect of albumin-globulin score and albumin to globulin ratio on survival in patients with heart failure: a retrospective cohort study in China. BMJ Open. 2018;8(7):e022960. doi: 10.1136/bmjopen-2018-022960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodgson HJ, Jewell DP. The humoral immune system in inflammatory bowel disease. II. Immunoglobulin levels. Am J Dig Dis. 1978;23(2):123–128. doi: 10.1007/BF01073186 [DOI] [PubMed] [Google Scholar]
- 44.Shiraishi K, Furukawa S, Yagi S, et al. Serum globulin is associated with endoscopic findings and mucosal healing in Japanese patients with ulcerative colitis. Dig Dis Sci. 2021;67(1):233–240. doi: 10.1007/s10620-021-06834-5 [DOI] [PubMed] [Google Scholar]
- 45.Xuan Q, Yang Y, Ji H, et al. Combination of the preoperative albumin to globulin ratio and neutrophil to lymphocyte ratio as a novel prognostic factor in patients with triple negative breast cancer. Cancer Manag Res. 2019;11:5125–5131. doi: 10.2147/CMAR.S195324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozkaya Y, Erdem GU, Demirci NS, et al. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35(2):275–282. doi: 10.1080/03007995.2018.1479683 [DOI] [PubMed] [Google Scholar]
- 47.Lin Q, Lin ZH, Chen J, et al. Prognostic significance of preoperative albumin-to-globulin ratio in patients with cholangiocarcinoma. Curr Res Transl Med. 2017;65(2):83–87. doi: 10.1016/j.retram.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 48.Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. 2014;25(11):2260–2266. doi: 10.1093/annonc/mdu274 [DOI] [PubMed] [Google Scholar]
- 49.Qin J, Qin Y, Wu Y, et al. Application of albumin/globulin ratio in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2018;10(8):4923–4930. doi: 10.21037/jtd.2018.07.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Y, Li T, Zheng C, Jin S. The role of albumin/globulin ratio in discharged COVID-19 patients with re-positive nucleic acid detection. J Inflamm Res. 2020;13:713–717. doi: 10.2147/JIR.S270305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn SS, Yoo J, Jung SM, Song JJ, Park Y-B, Lee S-W. Clinical role of albumin to globulin ratio in microscopic polyangiitis: a retrospective monocentric study. Clin Rheumatol. 2018;38(2):487–494. doi: 10.1007/s10067-018-4292-y [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Li S, Hu X, et al. The prognostic value of serum albumin-globulin ratio in early-stage non-small cell lung cancer: a retrospective study. Cancer Manag Res. 2019;11:3545–3554. doi: 10.2147/CMAR.S191288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi: 10.1038/nri3661 [DOI] [PubMed] [Google Scholar]
- 54.Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16(1):26–42. doi: 10.5217/ir.2018.16.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craven MD, Washabau RJ. Comparative pathophysiology and management of protein-losing enteropathy. J Vet Intern Med. 2019;33(2):383–402. doi: 10.1111/jvim.15406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao MJ, Wei XL, Sheng H, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int. 2017;2017:3083267. doi: 10.1155/2017/3083267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1(8432):781–784. doi: 10.1016/S0140-6736(85)91447-3 [DOI] [PubMed] [Google Scholar]
- 58.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53(8):789–803. doi: 10.1046/j.1365-2044.1998.00438.x [DOI] [PubMed] [Google Scholar]
- 60.Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med. 2014;1:24. doi: 10.3389/fmed.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–347. doi: 10.1016/j.clnu.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 62.Lo CH, Lochhead P, Khalili H, et al. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2020;159(3):873–883 e871. doi: 10.1053/j.gastro.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naqvi SA, Taylor LM, Panaccione R, et al. Dietary patterns, food groups and nutrients in Crohn’s disease: associations with gut and systemic inflammation. Sci Rep. 2021;11(1):1674. doi: 10.1038/s41598-020-80924-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh MS, Hsu WH, Wang JW, et al. Nutritional and dietary strategy in the clinical care of inflammatory bowel disease. J Formos Med Assoc. 2020;119(12):1742–1749. doi: 10.1016/j.jfma.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 65.Eder P, Niezgódka A, Krela-Kaźmierczak I, Stawczyk-Eder K, Banasik E, Dobrowolska A. Dietary support in elderly patients with inflammatory bowel disease. Nutrients. 2019;11(6):6. doi: 10.3390/nu11061421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casanova MJ, Chaparro M, Molina B, et al. Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J Crohns Colitis. 2017;11(12):1430–1439. doi: 10.1093/ecco-jcc/jjx102 [DOI] [PubMed] [Google Scholar]
- 67.Ling SC, Griffiths AM. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2000;3(5):339–344. doi: 10.1097/00075197-200009000-00003 [DOI] [PubMed] [Google Scholar]
- 68.Gassull MA. Nutrition and inflammatory bowel disease: its relation to pathophysiology, outcome and therapy. Dig Dis. 2003;21(3):220–227. doi: 10.1159/000073339 [DOI] [PubMed] [Google Scholar]
- 69.Gassull MA. Review article: the role of nutrition in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):79–83. doi: 10.1111/j.1365-2036.2004.02050.x [DOI] [PubMed] [Google Scholar]
- 70.Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21(4):315–319. doi: 10.1016/j.ejim.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 71.Chen YH, Wang L, Feng SY, Cai WM, Chen XF, Huang ZM. The relationship between C-reactive protein/albumin ratio and disease activity in patients with inflammatory bowel disease. Gastroenterol Res Pract. 2020;2020:3467419. doi: 10.1155/2020/3467419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang L, Xia B, Li J, et al. Risk factors for ulcerative colitis in a Chinese population: an age-matched and sex-matched case-control study. J Clin Gastroenterol. 2007;41(3):280–284. doi: 10.1097/01.mcg.0000225644.75651.f1 [DOI] [PubMed] [Google Scholar]
- 73.Wang P, Hu J, Ghadermarzi S, et al. Smoking and inflammatory bowel disease: a comparison of China, India, and the USA. Dig Dis Sci. 2018;63(10):2703–2713. doi: 10.1007/s10620-018-5142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta GA; 2014. [Google Scholar]
- 75.Thomas T, Chandan JS, Li VSW, et al. Global smoking trends in inflammatory bowel disease: a systematic review of inception cohorts. PLoS One. 2019;14(9):e0221961. doi: 10.1371/journal.pone.0221961 [DOI] [PMC free article] [PubMed] [Google Scholar]