Highlights
-
•
Demyelination and increased axonal branching reduce the safety factor for impulse transmission.
-
•
Axonal hyperpolarization induced by voluntary contraction result in conduction block, and thereby fatigue in CIDP/SBMA patients.
-
•
Activity-dependent conduction block causes peripheral fatigue in neuromuscular diseases.
Keywords: Fatigue, Chronic inflammatory demyelinating polyneuropathy, Spinal and bulbar muscular atrophy, Activity-dependent hyperpolarization and conduction block
Abstract
Objective
Fatigue is a major disabling problem in patients with neuromuscular disorders. Both nerve demyelination and increased axonal branching associated with collateral sprouting reduce the safety factor for impulse transmission and could cause activity-dependent hyperpolarization and conduction block during voluntary contraction, and thus fatigue. This study aimed to investigate whether activity-dependent conduction block is associated with fatigue in demyelinating neuropathies and lower motor neuron disorders.
Methods
This study included 31 patients (17 with chronic inflammatory demyelinating polyneuropathy [CIDP] and 14 with spinal and bulbar muscular atrophy [SBMA]). Sixteen healthy subjects served as normal controls. Fatigue was assessed using the Fatigue Scale for Motor and Cognitive Functions (FSMC). Compound muscle action potential (CMAP) recording and nerve excitability testing after median nerve stimulation in the wrist were performed before and after maximal voluntary contraction of the abductor pollicis brevis for 1 min.
Results
Patients with CIDP/SBMA had prominent fatigue with higher FSMC motor scores (P < 0.0001) than normal controls. After voluntary contractions, CMAP amplitudes decreased significantly in four of the 17 patients with CIDP and one of the 14 patients with SBMA. The reduction in CMAP amplitude was associated with the fatigue score in the motor but not in the cognitive domain. After voluntary contraction, excitability testing showed axonal hyperpolarization in the normal and CIDP/SBMA groups.
Conclusions
In CIDP or SBMA, fatigue is caused by voluntary contraction-induced membrane hyperpolarization and conduction block, presumably due to the critically lowered safety factor due to demyelination or increased axonal branching.
Significance
Peripheral fatigue can be objectively assessed using CMAP amplitudes and nerve excitability testing.
1. Introduction
Fatigue is one of the most common and troublesome symptoms in patients with neurological disorders (Chaudhuri and Behan, 2004), and several mechanisms have been postulated. The major components of fatigue in neurological diseases are perception and performance (Kluger et al., 2013). Perceptions of fatigue have two aspects: homeostatic and psychological factors, while performance fatigability is composed of peripheral and central factors. Multiple mechanisms could be involved in single patients; therefore, it is difficult to evaluate fatigue. The Fatigue Scale for Motor and Cognitive Functions (FSMC) (Penner et al., 2009), which was invented to evaluate fatigue in multiple sclerosis, can separately assess cognitive and motor fatigue.
Patients with neuropathy or lower motor neuron disease also experience prominent fatigue. Previous studies have suggested the contribution of activity-dependent hyperpolarization and conduction block in demyelinating neuropathy and lower motor neuron disorders. Several studies applied nerve excitability testing during voluntary contractions in chronic inflammatory demyelinating polyneuropathy (CIDP) (Cappelen-Smith et al., 2000) and multifocal motor neuropathy (Kaji et al., 2000), and demonstrated that voluntary muscle contraction induces axonal membrane hyperpolarization, and when the safety factor for impulse transmission is critically reduced by demyelination, conduction block develops after muscle contractions.
Another study using stimulated-single fiber electromyography with high-frequency stimulation in spinal muscular atrophy and spinal bulbar muscular atrophy (SBMA) showed motor nerve conduction block with gradual prolongation of latencies by axonal hyperpolarization (Noto et al., 2013). Such a conduction block is induced by the reduced safety factor, presumably due to the increased axonal branching associated with collateral sprouting, and an action potential generated at one Ranvier node should depolarize multiple distal nodes to maintain saltatory conduction.
This study aimed to elucidate the relationship between the nature of fatigue and activity-dependent hyperpolarization/conduction block in patients with neuromuscular disease. We enrolled patients with CIDP and SBMA to validate the two mechanisms of reduced safety factors, demyelination, and increased axonal branching, respectively.
2. Methods
2.1. Subjects
A total of 31 patients with CIDP (n = 17) or SBMA (n = 14) were included in this study. All patients with CIDP were diagnosed according to the European Federation of Neurological Societies/ Peripheral Nerve Society (EFNS/ PNS) criteria (Van den Bergh et al., 2010). All patients with CIDP were in the partial remitting phase after immune treatment. Patients with SBMA were diagnosed by genetic testing with an expanded CAG trinucleotide repeat in the first exon of the androgen receptor gene. Motor function was assessed using the overall neuropathy limitations scale (ONLS) (Graham and Hughes, 2006), grip strength, Medical Research Council-sum score (MRC-SS) (Kleyweg et al., 1991), 9 hole peg test (Oxford Grice et al., 2003), and 6-min walk test (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002). MRS-SS was bilaterally evaluated in the following six muscles: shoulder abduction, elbow flexion, wrist extensor, hip flexion, knee extension, and ankle dorsiflexion muscles, ranging from 0 to 60. Control data were obtained from 16 healthy subjects. None of the patients had a neurological disorder, systematic disease, or medication that affected peripheral nerve function.
Written informed consent was obtained from all participants. The procedures of the present study were approved by the Ethics Committee of the Chiba University School of Medicine.
2.2. Assessment of fatigue
Fatigability was assessed using the FSMC (Penner et al., 2009). The FSMS questionnaire is composed of cognitive and motor domains. The 20 statements were related to patients' subjective perception of fatigue, cognition, and motor by half; 10 questions related to motor function, such as the need for more frequent or longer rest during physical activity, and the remaining 10 questions focused on mental conditions such as the decrease in concentration under a stressful situation. Patients were asked to rate their level of agreement (toward 5) or disagreement (toward 0).
2.3. CMAP amplitude
Conventional nerve conduction studies (NCSs) were performed on the median nerve. The compound muscle action potential (CMAP) was recorded from the abductor pollicis brevis (APB) muscle after wrist stimulation. After routine testing, the CMAP amplitude was monitored after 1 min of maximal voluntary contraction (MVC). Maximal isometric voluntary abduction of the thumb against resistance was provided by the same investigator (AT) for 1 min. The cut-off value for the presence of an activity-dependent conduction block was determined using CMAP as the point corresponding to the mean – 2SD of the control value (expressed as a percentage of the baseline).
2.4. Nerve excitability testing
Motor nerve excitability measurements were performed in the median nerve of the wrist using a computerized program, the QTRAC software (copyright, Institute of Neurology, University College London, London, distributed by Digitimer Ltd) (Kiernan et al., 2000). The stimulus current was applied using an isolated linear bipolar constant current stimulator (DS5, Digitimer Ltd). Stimulation was performed with non-polarizable electrodes with a cathode at the wrist, which was 3 cm proximal to the wrist crease, and an anode electrode, which was placed 10 cm proximal to the stimulation electrode. The ground electrode was placed on the palm. CMAPs were recorded with active and reference electrodes placed in the belly and tendon of the APB muscle, respectively.
We used the “multitrack” protocol to continuously monitor and record the real-time excitability of motor axons in the median nerve at the wrist, as previously described (Bostock and Baker, 1988) (Bostock et al., 1998) (Grosskreutz et al., 1999). The nerve excitability indices comprised five measurements tracked by five different channels as follows: channels 1 and 5 were used to track the steepest portion of the stimulus–response curve of CMAPs, using long (1.0 ms) (channel 1) and short (0.2 ms) (channel 5) duration stimuli to calculate the strength-duration time constant (SDTC). Channels 2 and 3 monitored a stimulus following a depolarizing conditioning stimulus of 100 ms with a 40% threshold (channel 2) and a hyperpolarizing conditioning stimulus −40% of the threshold (channel 3). Channel 4 monitored superexcitability using a test stimulus delivered 6.3 ms after a supramaximal conditioning stimulus.
Subjects were monitored for 20 min, and real-time excitability was recorded. During the test, the subjects performed a 1 min isometric maximal voluntary abduction of the thumb in the same way as the NCS testing. Before applying MVC, a stable baseline recording was established for at least 4 min, and the stimuli were stopped during voluntary contraction.
2.5. Statistical analyses
All statistical analyses were performed using the JMP version 15 software. In analyses of clinical profiles, electrophysiological study parameters, and excitability properties, Student's t-test for unrelated samples, Fisher's exact test, or Dunnett's multiple comparison test was used to compare normal and disease cohorts. The correlation between FSMC scores and CMAP amplitude ratios of pre-/post-MVC or excitability indices in disease cohorts was tested using the Spearman’s rank correlation coefficient. Data are presented as mean (SEM). Differences were considered significant at p-values < 0.05.
3. Results
Clinical profiles and motor function in normal controls and patients with CIDP or SBMA are shown in Table 1. The mean age was higher in patients with SBMA than in normal controls (p < 0.05), and all patients with SBMA were male. Motor function was impaired in the patient groups: grip strength in CIDP (p < 0.01) and SBMA (p < 0.0001), 9 hole peg time in CIDP (p < 0.05) and SBMA (p < 0.01), distance in the 6-min walk test in CIDP (p < 0.01) and SBMA (p < 0.0001) compared with normal controls.
Table 1.
Clinical profiles.
| Normal |
CIDP |
SBMA |
||
|---|---|---|---|---|
| (n = 16) | (n = 17) | (n = 14) | ||
| Age (years) | 48 (2.7) | 56 (4.7) | 61 (2.8) * | |
| Gender (male: female) | 11: 5 | 14: 3 | 14: 0 * | |
| Disease duration (months) | N.A. | 33 (5–105) | 148 (116–192) | |
| Motor function | ||||
| Grip strength (kg) (sum of both sides) | 78 (5.6) | 56 (6.3) ** | 33 (3.7) *** | |
| 9 hole peg (sec) (sum of both sides) | 45 (2.3) | 61 (5.5) * | 62 (2.8) ** | |
| 6 min walk (m) | 602 (20) | 409 (36) ** | 305 (37) *** | |
| MRC sum score | 60 (0) | 58 (0.97) | 52 (0.80) *** | |
| Fatigue scale for motor and cognitive functions | ||||
| sum score | 45 (3.7) | 54 (3.6) | 59 (3.7) * | |
| motor score | 23 (1.9) | 31 (2.2) * | 37 (2.1) *** | |
| cognitive score | 23 (2.0) | 23 (2.1) | 23 (2.0) | |
CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; SBMA, spinal and bulbar muscular atrophy; MRC sum score, medical research council sum score in the 12 muscles (0–60); NA, not applicable; Data are given as mean (SEM) or median (IQR). *p < 0.05, **p < 0.01, ***p < 0.0001; compared with normal.
3.1. Fatigue score
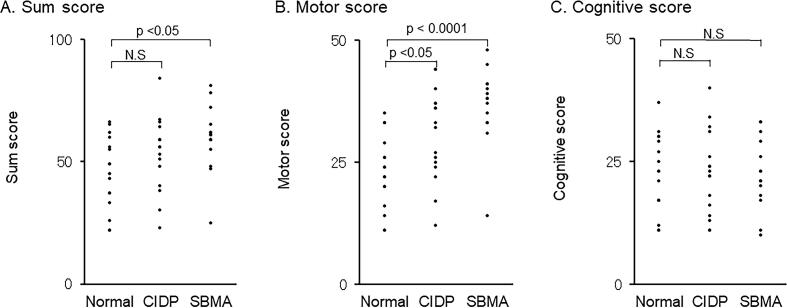
The fatigue scale (FSMC) results are shown in Fig. 1. The motor score of the FSMC was significantly higher in the CIDP (p < 0.01) and SBMA (p < 0.01) than in the normal controls. In contrast, there were no differences in the cognitive scores of FSMC between the normal, CIDP, and SBMA groups. These results indicated that patients with CIDP and SBMA had prominent performance fatigue, whereas the cognitive component did not significantly contribute to fatigue.
Fig. 1.
Fatigue scale for motor and cognitive functions scores. The scores of the Fatigue Scale for Motor and Cognitive Functions (FSMC) in 17 patients with chronic inflammatory demyelinating polyneuropathy (CIDP) and 14 patients with spinal and bulbar muscular atrophy (SBMA) were compared with those of 16 normal controls (NC). The sum score of the motor part is higher in the CIDP (p < 0.05) and SBMA (p < 0.0001) than in the NC group, whereas the score of the cognitive function is almost similar in the three groups. N.S.; not significant.
3.2. CMAP amplitude and activity-dependent conduction block
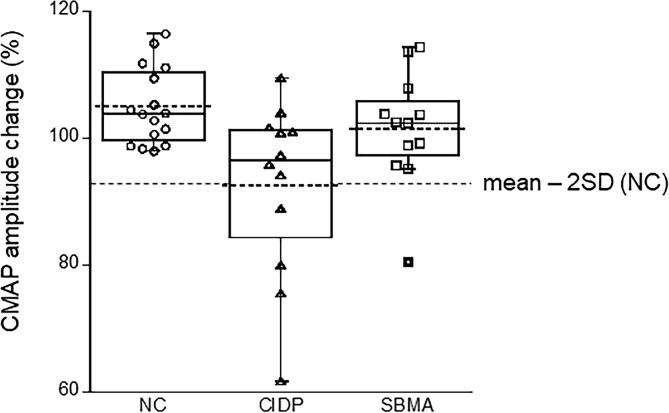
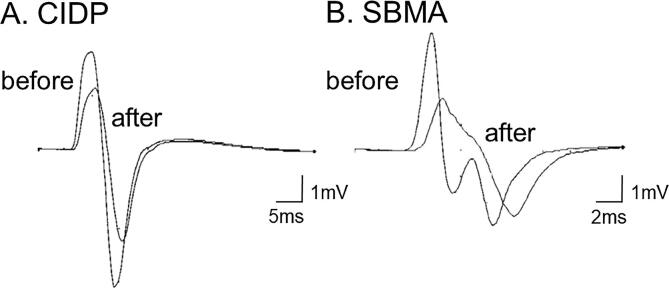
The results of the median motor NCSs are shown in Table 2. Baseline CMAP amplitudes were lower in patients with CIDP and SBMA than in normal controls. After 1 min of MVC, the mean decrease in CMAP amplitude was significantly greater in the CIDP group, while the mean values were similar in the normal and SBMA groups. Fig. 2 shows the changes in the CMAP amplitude in individual subjects. When the cut-off value for abnormal CMAP reduction was defined as the normal mean – 2SD (93%), activity-dependent conduction block was present in four (24%) of the 17 patients with CIDP and one (7%) of the 14 patients with SBMA. Representative CMAP waveforms in individual patients with CIDP and SBMA are shown in Fig. 3a & b, respectively.
Table 2.
Changes in CMAP amplitude and excitability indices following maximal voluntary contraction.
| Normal |
CIDP |
SBMA |
|||
|---|---|---|---|---|---|
| (n = 16) | (n = 17) | (n = 14) | |||
| Median nerve conduction study | |||||
| Distal latency (ms) | 3.5 (0.09) | 6.8 (0.57) *** | 4.4 (0.26) | ||
| CMAP amplitude (mV) | |||||
| Before MVC | 8.3 (0.54) | 5.0 (0.83) ** | 5.1 (0.45) ** | ||
| After MVC | 8.7 (0.54) | 4.6 (0.81) *** | 5.2 (0.50) ** | ||
| Ratio of post/pre MVC (%) | 105 (1.5) | 93 (4.0) ** | 101 (2.6) | ||
| Motor conduction velocity (m/s) | 58 (0.71) | 35 (13) *** | 53 (2.6) | ||
CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; SBMA, spinal and bulbar muscular atrophy; CMAP, compound muscle action potential; MVC, maximal voluntary contraction; data are presented as mean (SEM). *p < 0.05, **p < 0.01, ***p < 0.0001; compared with normal.
Fig. 2.
Changes in neurophysiological parameters following maximal voluntary contraction. Amplitude changes in compound muscle action potential (CMAP) of the abductor pollicis brevis muscle after 1 min of voluntary contraction in normal control (NC), chronic inflammatory demyelinating polyneuropathy (CIDP), and spinal and bulbar muscular atrophy (SBMA). The cut-off value of 93% (dotted line) is determined as the mean – 2 standard deviation based on the normal control data.
Fig. 3.
Representative superimposed compound muscle action potential recordings before and after maximal voluntary contraction. Representative compound muscle action potential (CMAP) recordings from the abductor pollicis brevis with stimulation at the wrist in chronic inflammatory demyelinating polyneuropathy (CIDP) (A) and spinal and bulbar muscular atrophy (SBMA) (B) subjects. The two superimposed waveforms are recorded before and after 1 min of maximal voluntary contraction to ensure the presence of an activity-dependent conduction block with decreased CMAP amplitude.
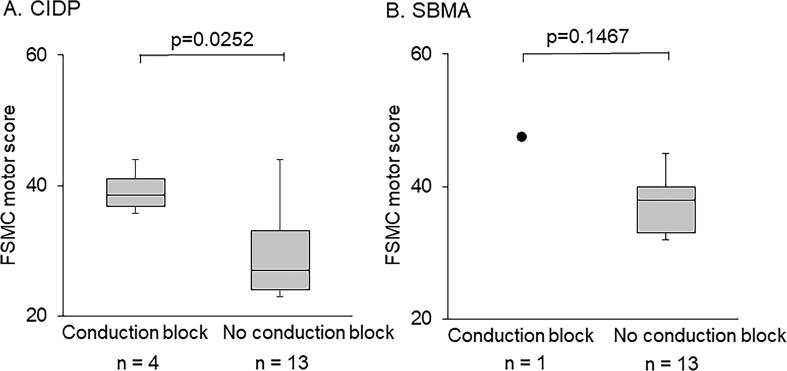
Patients with activity-dependent conduction block had significantly higher FSMC motor scores than those without it (Fig. 4). In the SBMA group, a single patient with activity-dependent conduction block showed a high FSMC motor score.
Fig. 4.
Comparison of the FSMC motor score between the activity-dependent conduction block positive and negative groups. The motor scores of the Fatigue Scale for Motor and Cognitive Functions (FSMC) of activity-dependent conduction block (ADCB) positive and negative groups for patients with chronic inflammatory demyelinating polyneuropathy (CIDP) and spinal and bulbar muscular atrophy (SBMA). The box plot shows the median and IQR of the third and first quartiles. The notches in the box plots indicate the maximum and minimum values. In both the CIDP and SBMA groups, the ADCB-positive subgroup experienced more significant fatigue than the ADCB-opposing subgroups (CIDP; p = 0.0252).
Correlations between FSMC scores and CMAP amplitude ratios of pre-/post-MVC showed moderate R values with mild tendency (R = −0.34976, p = 0.0939).
3.3. Nerve excitability testing
The baseline data for nerve excitability testing are presented in Supplementary Table 1. Briefly, the excitability properties of CIDP were characterized by greater threshold changes in threshold electrotonus, presumably due to decreased myelin resistance by demyelination (Cappelen-Smith et al., 2001). Patients with SBMA showed longer SDTC, suggesting increased persistent nodal sodium currents, possibly due to collateral sprouting (Shibuya et al., 2020), consistent with previous reports.
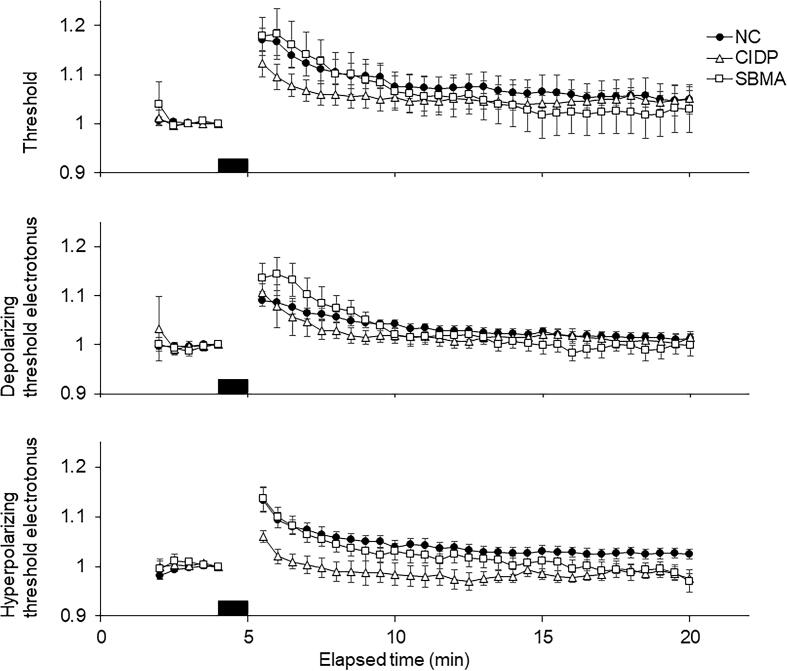
Changes in excitability indices after voluntary contraction are shown in Table 3 and Fig. 5. In all the normal, CIDP, and SBMA groups, the major changes were similar: increased threshold current, shortened SDTC, fanning out in threshold electrotonus, and increased superexcitability, all of which indicated that the axons were hyperpolarized after voluntary contraction (Kuwabara et al., 2001). At 1, 2, 3, 4, and 5 min after voluntary contraction, the ratios of depolarizing threshold electrotonus post-/pre-MVC in SBMA and hyperpolarizing threshold electrotonus in CIDP were greater and milder than in normal controls (all, p < 0.05), respectively. The changes in the hyperpolarizing threshold electrotonus and hyperpolarizing threshold electrotonus in CIDP were milder than those in the other two groups, suggesting mild hyperpolarization caused by reduced impulses passing through the wrist due to proximal conduction block.
Table 3.
Changes in excitability indices following maximal voluntary contraction (expressed as a ratio of post-/pre-MVC).
| Normal |
CIDP |
SBMA |
||
|---|---|---|---|---|
| (n = 16) | (n = 17) | (n = 14) | ||
| Excitability measurements | ||||
| Threshold | 1.17 (0.024) | 1.12 (0.027) | 1.18 (0.038) | |
| Strength-duration time constant | 0.95 (0.014) | 0.90 (0.023) | 0.95 (0.026) | |
| Depolarizing threshold electrotonus (100 ms) | 1.09 (0.013) | 1.11 (0.017) | 1.14 (0.028) | |
| Hyperpolarizing threshold electrotonus (100 ms) | 1.13 (0.024) | 1.06 (0.012) * | 1.14 (0.024) | |
| Superexcitability | 1.17 (0.076) | 1.27 (0.16) | 1.47 (0.20) | |
Data are presented as mean (SEM). *p < 0.05, compared to normal.
Fig. 5.
Changes in excitability parameters following a maximal voluntary contraction in each group. Comparison of changes in excitability parameters recorded from the median nerve before, during, and after maximal voluntary contraction of the abductor pollicis brevis for 1 min. The black horizontal bar indicates the period of maximal voluntary contraction. Each trace represents the mean data for 12 normal controls (filled circles), 17 patients with CIDP (open triangles), and 14 patients with SBMA (open squares). Data were averaged over consecutive 30 s intervals. All data are normalized to precontraction. Threshold current using test stimuli of 1.0 ms duration (A). Threshold electrotonus (TE) showed greater threshold changes in both depolarizing (B) and hyperpolarizing (C). The error bars represent the standard error of the mean (SEM). These findings suggest hyperpolarizing changes after maximal voluntary contraction. The changes were more significant in NC and SBMA than in CIDP.
Correlation analyses between motor scores of FSMC and excitability indices in disease cohorts did not show significant relationships.
4. Discussion
Our results confirmed that patients with CIDP and SBMA experienced prominent fatigue of performance and showed that some patients with both diseases actually developed activity-dependent conduction block, which was defined in the present study by voluntary contraction-induced axonal hyperpolarization, and that patients with the presence of conduction block had more severe fatigability. Moreover, our results suggest that axons are hyperpolarized following voluntary contraction in healthy subjects and in both diseases.
We hypothesized that there are two mechanisms for the reduced safety factor for impulse transmission: demyelination and increased axonal branching; therefore, patients with representative demyelinating neuropathy, CIDP, and those with chronic denervation and re-innervation by collateral sprouting, SBMA, were investigated in this study. Regardless of the mechanisms of the decrease in safety factors, slight axonal hyperpolarization could induce conduction block and, thus, muscle fatigue. It has been reported that during MVC, high-frequency impulse transmission (usually up to 30 Hz) causes a build-up of Na + ions inside the axon. The electrogenic Na+/K+ pump, which exchanges three Na+ ions for two K+ ions, yields a net positive electrogenic charge and is activated to restore the Na+/K+ balance across the membrane. As a result, the axonal membrane hyperpolarizes due to the stoichiometry of the pump. This physiological process in daily activity can cause an activity-dependent conduction block if the safety factor is reduced by pathology. Conduction blocks with membrane hyperpolarization probably result in fatigue. This study did not show a significant relationship between motor scores of FSMC and excitability indices in disease cohorts. We think that the development of conduction blocks largely depends on the baseline safety factor. As shown in Fig. 5, voluntary contraction causes a 10–20% increase in the threshold (hyperpolarization), which means that the conduction block may occur only when the safety factor decreases to nearly 1.2. Theoretically, no linear relationship is expected between membrane potential and fatigue.
The present study first applied FSMC to patients with CIDP and SBMA and discovered that fatiguability results from physical (peripheral motor) rather than cognitive factors. The term “fatigue” is used in many ways. FSMC has been developed to separately evaluate the physical and cognitive aspects of fatigue in multiple sclerosis (Penner et al., 2009) and has recently been applied to other neurological diseases (Hubacher et al., 2012). Items in the FSMC for the motor subscale are composed of assessments mainly for skillfulness, stamina, muscle strength, speed, and physical energy. It is not surprising that patients with CIDP and SBMA experience noncognitive fatigue in such items.
There are several limitations to the present study. First, the included patients with CIDP were already treated and in the remitting phase. This led to a failure to evaluate the maximal fatigue in the active phase. Second, conduction blocks after MVC have not been defined in prior studies, and clear cut-off values have not been adequately established. In the present study, correlation analyses did not find significant relationships between fatigue scores and conduction block, probably due to the small sample size. As such, the cut-off value was defined as mean – 2SD, which was calculated from healthy subject data, but it is difficult to validate this cut-off value. In future studies, this issue should be addressed. Third, previous studies targeting several nerves and comparing distal and proximal CMAP areas and duration in demyelinating neuropathies suggested MVC-induced temporal dispersion and rare ADCB (Straver et al., 2011a, Straver et al., 2011b). In contrast, our previous study, which used a single fiber electromyogram, revealed apparent ADCB in SBMA (Noto et al., 2013). In the present study, one patient with SBMA who met the criteria for ADCB showed a decrease in CMAP amplitude and prolonged CMAP duration (Fig. 3). The CMAP decrement might come partly from conduction blocks and partly from dispersion. Fourth, in the present study, CMAP was only recorded from APB, which might underestimate fatigue and ultimately do not reflect conduction blocks in patients with SBMA, who usually have proximal dominant muscle weakness. The mean CMAP amplitude decrease was significantly greater in the CIDP group than in the normal group, whereas the mean values were similar in the normal and SBMA groups. However, fatigue scores were the most prominent in SBMA. This discrepancy may be due to proximal weakness in the SBMA. Future studies may better target distal muscles as well as proximal muscles. Finally, while ADCB was found in a limited number of patients, fatigue was prominent in patients with CIDP and SBMA. ADCB might be only one part of fatigue, and several other factors may contribute to it. Additionally, several changes in excitability indices after MVC were milder in CIDP and greater in SBMA, compared with those of healthy subjects, but these changes were not accompanied by other indices. We believe that it is difficult to interpret the meaning of these changes. This might be due to the small sample size. As such, these should be addressed in a large cohort of patients.
The findings of the present study suggest that in neuromuscular disorders, fatigue is caused by different pathologies, including nerve demyelination and increased axonal branching. However, irrespective of the mechanisms, the fact that slight axonal hyperpolarization by physiological activity leads to fatigue could provide new insights into symptomatic treatment. Digitalis, an inhibitor of electrogenic Na+/K+ pumps, may reduce the extent of membrane hyperpolarization. A previous study applied digoxin to patients with multiple sclerosis and reduced symptoms with improved evoked potentials (Kaji et al., 1990). In the near future, this may have to be applied to neuromuscular disorders in clinical trials. However, appropriate, adequate, and step-by-step studies, including correlations between measures that are believed to reflect membrane potential with conduction block and fatigue and proximal muscle assessment, should be performed before these trials. Separately, serum potassium levels largely affect the resting membrane potential, and therefore some diets could alter membrane potential. The modulation of the axonal membrane potential could be a treatment option for severe muscle fatigue. We would like to conduct such trials.
5. Ethics approval
Approval was obtained from the Ethics Committee of the Chiba University School of Medicine. The procedures used in this study adhered to the tenets of the Declaration of Helsinki.
Author contributions
AT and KS designed the study. KS, SM, YS, TS, YK, KN, HK, and MP collected clinical, neurophysiological, and genetic data. AT analyzed the data. AT, KS, and SK wrote the manuscript. SK supervised the study.
Funding
This study did not receive a specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
Drs. Shibuya, Misawa, Suzuki, Suich, and Kuwabara received research support from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Dr. Kuwabara received research support from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, Ministry of Health, Labor and Welfare of Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2022.02.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-7. [DOI] [PubMed]
- Bostock H., Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988;462(2):354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H., Cikurel K., Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21(2):137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Cappelen-Smith C., Kuwabara S., Lin C.-Y., Mogyoros I., Burke D. Activity-dependent hyperpolarization and conduction block in chronic inflammatory demyelinating polyneuropathy. Ann. Neurol. 2000;48(6):826–832. [PubMed] [Google Scholar]
- Cappelen-Smith C., Kuwabara S., Lin C.S., Mogyoros I., Burke D. Membrane properties in chronic inflammatory demyelinating polyneuropathy. Brain. 2001;124:2439–2447. doi: 10.1093/brain/124.12.2439. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Graham R.C., Hughes R.A. A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J. Neurol. Neurosurg. Psychiatry. 2006;77(8):973–976. doi: 10.1136/jnnp.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J., Lin C., Mogyoros I., Burke D. Changes in excitability indices of cutaneous afferents produced by ischaemia in human subjects. J. Physiol. 1999;518(1):301–314. doi: 10.1111/j.1469-7793.1999.0301r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubacher M., Calabrese P., Bassetti C., Carota A., Stöcklin M., Penner I.-K. Assessment of post-stroke fatigue: the fatigue scale for motor and cognitive functions. Eur. Neurol. 2012;67(6):377–384. doi: 10.1159/000336736. [DOI] [PubMed] [Google Scholar]
- Kaji R., Happel L., Sumner A.J. Effect of digitalis on clinical symptoms and conduction variables in patients with multiple sclerosis. Ann. Neurol. 1990;28:582–584. doi: 10.1002/ana.410280419. [DOI] [PubMed] [Google Scholar]
- Kaji R., Bostock H., Kohara N., Murase N., Kimura J., Shibasaki H. Activity-dependent conduction block in multifocal motor neuropathy. Brain. 2000;123:1602–1611. doi: 10.1093/brain/123.8.1602. [DOI] [PubMed] [Google Scholar]
- Kiernan M.C., Burke D., Andersen K.V., Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23(3):399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kleyweg R.P., Van Der Meché F.G.A., Schmitz P.I.M. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- Kluger B.M., Krupp L.B., Enoka R.M. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Lin C.-Y., Mogyoros I., Cappelen‐Smith C., Burke D. Voluntary contraction impairs the refractory period of transmission in healthy human axons. J. Physiol. 2001;531(1):265–275. doi: 10.1111/j.1469-7793.2001.0265j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto Y.-I., Misawa S., Mori M., Kawaguchi N., Kanai K., Shibuya K., Isose S., Nasu S., Sekiguchi Y., Beppu M., Ohmori S., Nakagawa M., Kuwabara S. Prominent fatigue in spinal muscular atrophy and spinal and bulbar muscular atrophy: evidence of activity-dependent conduction block. Clin. Neurophysiol. 2013;124(9):1893–1898. doi: 10.1016/j.clinph.2012.12.053. [DOI] [PubMed] [Google Scholar]
- Oxford Grice K., Vogel K.A., Le V., Mitchell A., Muniz S., Vollmer M.A. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am. J. Occup. Ther. 2003;57(5):570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Penner I.K., Raselli C., Stöcklin M., Opwis K., Kappos L., Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. 2009;15(12):1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Misawa S., Uzawa A., Sawai S., Tsuneyama A., Suzuki Y.-I., Suichi T., Kojima Y., Nakamura K., Kano H., Prado M., Kuwabara S. Split hand and motor axonal hyperexcitability in spinal and bulbar muscular atrophy. J. Neurol. Neurosurg. Psychiatry. 2020;91(11):1189–1194. doi: 10.1136/jnnp-2020-324026. [DOI] [PubMed] [Google Scholar]
- Straver D.C.G., van den Berg L.H., Franssen H. Activity-dependent conduction block in chronic inflammatory demyelinating polyneuropathy. J. Neurol. Sci. 2011;300(1-2):33–38. doi: 10.1016/j.jns.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Straver D.C.G., van den Berg L.H., van den Berg-Vos R.M., Franssen H. Activity-dependent conduction block in multifocal motor neuropathy. Muscle Nerve. 2011;43(1):31–36. doi: 10.1002/mus.21843. [DOI] [PubMed] [Google Scholar]
- Van den Bergh P.Y.K., Hadden R.D.M., Bouche P., Cornblath D.R., Hahn A., Illa I., Koski C.L., Léger J.-M., Nobile-Orazio E., Pollard J., Sommer C., Van Doorn P.A., Van Schaik I.N. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur. J. Neurol. 2010;17(3):356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.