Abstract
Purpose
To describe a case of an immune-related adverse event associated with Atezolizumab therapy which was aggravated by ocular surgery.
Observations
A 59-year-old man treated with Atezolizumab for metastatic non-small-cell lung cancer developed a conjunctival hypertrophic lesion mistaken for metastatic tissue. Biopsy surgery induced fulminant and multifocal granulomatous conjunctival tissue growth and sterile corneal ulceration. The immune-related adverse event was refractory to topical therapy, with curative success only after introduction of systemic prednisone.
Conclusions
Atezolizumab use may be associated with severe and recalcitrant ocular surface inflammation with potential exacerbation after surgical interventions.
Keywords: Atezolizumab, Immune-related adverse event, Surgery, Ocular surface disease, Conjunctival granuloma
1. Introduction
Atezolizumab (Tecentriq, Genentech) is a monoclonal antibody of the immune checkpoint inhibitory class that specifically targets the programmed cell death ligand 1 (PD-L1) and is used for a broadening list of indications including the treatment in both early and late stages of lung cancer.1
However, based on their mechanism of action, immune-checkpoint inhibitors can “over-activate” the immune system leading to auto-immune toxicity in various organ systems summarized as “immune-related adverse events (irAEs).2 According to recent literature reviews, ocular irAEs occur in an estimated 1–4% of patients with dry eye disease (1–24%) and uveitis (1%) being most common.3, 4, 5, 6
Lung cancer is reported to be one of the tumors with the most frequent occurrence of irAEs of the eye.7
Here we present a case of severe granulomatous conjunctival inflammation and corneal ulceration with exacerbation by surgical intervention after atezolizumab use.
2. Case report
A 59-year old man presented to our clinic with suspicion of a conjunctival metastatic lesion. The patient had been diagnosed with lung cancer the year before, and had already undergone partial lobectomy and radiotherapy. As the most recent positron emission tomography–computed tomography (PET-CT) had shown signs of cerebral metastatic disease, he had been started on chemotherapy combined with atezolizumab treatment. He had completed 4 cycles of therapy (over a period of three months) at the time he first presented to our outpatient clinic. Further, his medical history was positive for type 2 diabetes mellitus, obstructive sleep apnea syndrome and arterial hypertension.
On exam, his right eye manifested a best-corrected visual acuity of 20/25 and a paralimbal round, hypertrophic, injected and inflamed lesion of the bulbar conjunctiva. A full serologic work-up excluded other (systemic) causes of chronic conjunctivitis including vasculitis. With suspicion of local metastatic transformation, a conjunctival biopsy was scheduled with suture-closure of the conjunctival defect and postoperative topical antibiotic therapy. The histological analysis provided no evidence of malignancy. It showed a non-cornified squamous epithelium with basal melanocytes, with a lamina propria showing basophil, fibrillary material with blood vessels. Two weeks later, the patient presented again, complaining of a rapidly growing painful lesion at the excision site of the right conjunctiva. On exam we identified three hypertrophic polyps in paralimbal arrangement with manifest feeder vessels as well as a pseudomembranous lesion in the sub-tarsal region of the lower lid (Fig. 1). The left eye was asymptomatic and showed a normal anterior segment exam and a best-corrected visual acuity of 20/20. Due to the history of a metastatic malignant disease of the patient, as well as distinct morphologic characteristics of the lesion itself suggesting malignancy (e.g. feeder vessel supply), it was decided to perform another surgery, which was a “no touch” conjunctival excision of the lesions in toto, with local cryotherapy and a same-eye conjunctival auto-graft transplantation at the paralimbal excision site, as well as an amnion-patch graft closure at the lower lid excision site. The surgery itself was uneventful.
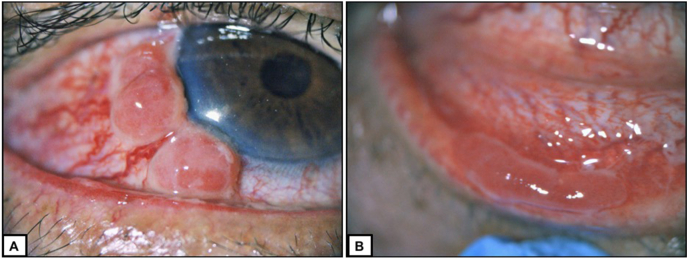
Fig. 1.
Pseudomembranous conjunctival granuloma.
At week one after surgery, a low-inflamed, healing state and a decent position of the post-operatively placed therapeutic contact lens, the conjunctival autograft and the amnion patch were noted (Fig. 2). The histologic preparation results revealed the following: At the paralimbal excision site it showed a polypoid lesion formed by granulation tissue with dense inflammatory cells (lymphocytes, also plasma cells and neutrophile granulocytes) covered by normal epithelium, i.e. an acutely inflamed granulation-polyp. At the lower-lid location it similarly showed a granulation tissue with dense inflammatory cell infiltration with dominance of lymphocytes and neutrophile granulocytes, i.e. an acutely inflamed granulation-polyp. At all excision sites there was no evidence of malignant disease. Furthermore, the histologic preparation demonstrated no signs of linear immunoglobulin and/or complement deposition at the conjunctival epithelial basement membrane, and the inflammatory infiltrate was not mainly composed of macrophages and other monocytes, as would be expected in a case of ocular cicatricial pemphigoid.
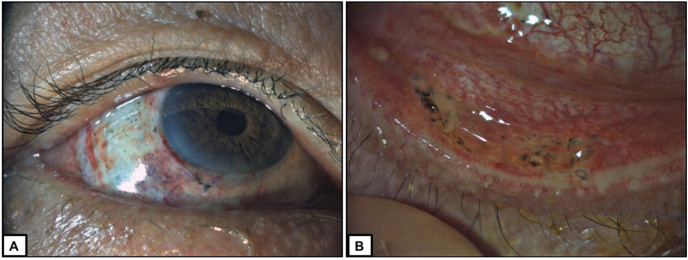
Fig. 2.
Post-operative appearance at week 1.
At this point, the patient further complained of a skin rash, especially at the scalp, which he had observed since one month. Therefore, the diagnosis of an immune-related adverse event due to atezolizumab treatment was suspected, and the patient was started on topical, unpreserved dexamethasone-dihydrogenphosphate (0.1%) six times daily, as well as antibiotic eye drops and topical cyclosporine (0.4%) three times daily for the right eye.
At week two, the therapeutic contact lens was no longer in place, the conjunctival autograft had been displaced showing bare, avascular scleral tissue, and there were first signs of a re-activation of inflammation with subtle sub-tarsal papillary conjunctivitis. The lower lid showed ongoing healing response with the amnion patch in place. We decided to increase the cyclosporine dose from 0.4% to 2% three times daily. Therapeutic contact lens placement was repeated.
At week three, fulminant recurrence of inflammatory pseudo-membranous tissue was noted at the borders of the paralimbal excision as well as at the site of the conjunctival autograft excision. The therapeutic contact lens had been lost again. In addition, inferior sterile corneal ulceration without infiltration had developed (Fig. 3). We placed a new therapeutic contact lens and decided to start systemic prednisone (1mg/kg) while instructing the patient to perform precise blood sugar monitoring. The decision to proceed with systemic instead of local prednisone (e.g. subconjunctival injection) was made due to the accompanying systemic adverse events including the skin rash which was reported by the patient. The introduction of systemic anti-inflammatory medication led to a slow recovery of the right eye with regression of the conjunctival pseudo-membranous tissue and gradual healing of the epithelial defect after further 3 weeks with tapering of prednisone therapy.
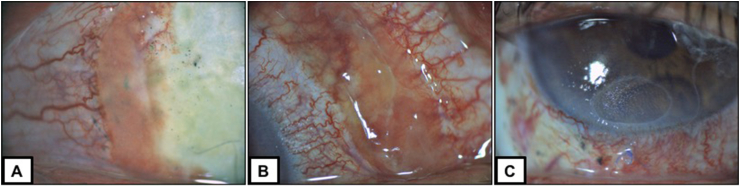
Fig. 3.
Post-operative findings at week 3.
3. Discussion and conclusions
The introduction of immune checkpoint inhibitor therapy has revolutionized cancer treatment for a growing spectrum of malignancies.
With their increasing use, we witness a rising incidence of associated inflammatory ocular side effects. There is extensive evidence from literature for irAEs with the use of nivolumab (anti-PD-1), pembrolizumab (anti-PD-1), and ipilimumab (anti-cytotoxic T-lymphocyte antigen-4),5 but studies and case reports on atezolizumab side effects are scarce. Bitton et al. described a case of 57-year of woman who developed bilateral cicatrizing conjunctivitis after ten infusions of atezolizumab treatment. The therapy was discontinued, and complications were under control with topical dexamethasone four times daily, systemic corticosteroid and scleral contact lenses.8 Other cases of dry eye disease were reported in a retrospective study by Fortes et al.9
Here we describe atezolizumab-associated severe pseudo-membranous, hypertrophic conjunctival inflammation with subsequent sterile corneal ulceration. As the corneal lesion showed no sign of infiltration or discharge, it was judged to be immune-related or of neurotrophic nature.
At this point it is important to highlight that sarcoidosis-like reactions induced by immune checkpoint inhibitors have been widely reported with occurrence in a similar time frame after medication as in our reported case. However, the histologic work-up excluded such suspicion here, as there was no evidence of non-caseous epithelioid cell granuloma typical of sarcoidosis in the histologic specimen.10
As the patient responded well to atezolizumab therapy, discontinuation of the treatment was not considered an option, even though additional systemic side effects in the form of a severe skin rash were noted throughout follow-up.
Our case highlights two important aspects, which require consideration during treatment of patients with immune-checkpoint inhibitors. First of all, surgical intervention may trigger or aggravate irAEs,11 as was observed in this particular case after the biopsy intervention. However, ocular irAE presentation can be deceptive in many ways, and surgical intervention cannot be circumvented where metastatic tissue transformation must be ruled out. However, in retrospect, in this particular case, after removal of the primary lesion, the use of topical steroids or steroid-antibiotic combination drugs might would have helped to mitigate the inflammatory cascade presented in this case.
Furthermore, these irAEs may be severe and recalcitrant. Topical cortisone and T-cell specific therapy may not be curative in all cases, and systemic anti-inflammatory treatment should therefore not be withheld for too long.
Ophthalmologists are expected to encounter many patients with potential irAE in the upcoming years. Therefore, an interdisciplinary approach with oncology is of paramount importance, because if appropriate therapy is started promptly, ocular toxicity may be reversed and severe complications avoided without the need for discontinuation of potentially life-saving immune checkpoint inhibitor therapy.
Patient consent
Consent to publish the case report was obtained from the patient.
Declaration of competing interest
The authors have no relevant financial, non-financial or proprietary interests to declare.
A: Three hypertrophic, inflamed lesions in paralimbal conjunctival location with feeder vessel supply can be discriminated. The adjacent cornea is clear and inconspicuous.
B: A homologous pseudomembranous lesion of the subtarsal inferior conjunctiva.
A: Proper position of therapeutic contact lens, conjunctival auto-graft and conjunctival sutures.
B: Decent position of the amnion patch graft and conjunctival sutures with low-grade inflammation.
A: Pseudomembranous conjunctival regrowth at the paralimbal excision site with lost of auto-graft and avascular scleral tissue.
B: Conjunctival pseudomembranous tissue at the auto-graft sampling location.
C: Inferior sterile corneal ulceration without infiltration and lost of therapeutic contact lens.
Acknowledgments and
No funding or grant support.
References
- 1.Lai L.T., Zhan Z.Y., Feng M., Li F., Lai L.F., Zhong L.X. Immune checkpoint inhibitors for the management of advanced non-small-cell lung carcinoma: a meta-analysis. Anti Cancer Drugs. 07 2020;31(6):637–645. doi: 10.1097/CAD.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M., Brahmer J.R., Callahan M.K., et al. Immune-related adverse events of checkpoint inhibitors. Expert. Rev. Anticancer. Ther. Apr. 05 07 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalvin L.A., Shields C.L., Orloff M., Sato T., Shields J.A. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 06 2018;38(6):1063–1078. doi: 10.1097/IAE.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Rahman O., Oweira H., Petrausch U., et al. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. Apr. 2017;17(4):387–394. doi: 10.1080/14737140.2017.1296765. [DOI] [PubMed] [Google Scholar]
- 5.Park R.B., Jain S., Han H., Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocul. Surf. 2021;20:115–129. doi: 10.1016/j.jtos.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Wei X. Ocular immune-related adverse events associated with immune checkpoint inhibitors in lung cancer. Front Immunol. 2021;12:701951. doi: 10.3389/fimmu.2021.701951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M.M., Seleme N., Chen J.J., et al. Neuro-ophthalmic complications in patients treated with CTLA-4 and PD-1/PD-L1 checkpoint blockade. J Neuro Ophthalmol. Dec 01 2021;41(4):519–530. doi: 10.1097/WNO.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 8.Bitton K., Michot J.M., Barreau E., et al. Prevalence and clinical patterns of ocular complications associated with anti-PD-1/PD-L1 anticancer immunotherapy. Am J Ophthalmol. 2019;6(202):109–117. doi: 10.1016/j.ajo.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Fortes B.H., Liou H., Dalvin L.A. Ophthalmic adverse effects of immune checkpoint inhibitors: the Mayo Clinic experience. Br. J. Ophthalmol. 2021;105(9):1263–1271. doi: 10.1136/bjophthalmol-2020-316970. [DOI] [PubMed] [Google Scholar]
- 10.Gkiozos I., Kopitopoulou A., Kalkanis A., Vamvakaris I.N., Judson M.A., Syrigos K.N. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 08 2018;13(8):1076–1082. doi: 10.1016/j.jtho.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.J., Lee J.S., Lee J., et al. Factors associated with ocular adverse event after immune checkpoint inhibitor treatment. Cancer Immunol Immunother. Dec 2020;69(12):2441–2452. doi: 10.1007/s00262-020-02635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]