Summary
Candida albicans, an oral fungal opportunistic pathogen, has shown the ability to colonize implant surfaces and has been frequently isolated from biofilms associated with dental implant-related infections, possibly due to its synergistic interactions with certain oral bacteria. Moreover, evidence suggests that this cross-kingdom interaction on implant can encourage bacterial growth, leading to increased fungal virulence and mucosal damage. However, the role of Candida in implant-related infections has been overlooked and not widely explored or even considered by most microbiological analyses and therapeutic approaches. Thus, we summarized the scientific evidence regarding the ability of C. albicans to colonize implant surfaces, interact in implant-related polymicrobial biofilms, and its possible role in peri-implant infections as far as biologic plausibility. Next, a systematic review of preclinical and clinical studies was conducted to identify the relevance and the gap in the existing literature regarding the role of C. albicans in the pathogenesis of peri-implant infections.
Subject areas: Mycology, microbiofilms
Graphical abstract
Mycology; Microbiofilms;
Introduction
Biofilms are highly structured microbial communities enmeshed in a three-dimensional extracellular matrix (ECM) (Costerton et al., 1995; Bowen et al., 2018) which provide several advantages to colonizing species such as reduced antimicrobial and host-response susceptibility (Costerton et al., 1995; Socransky and Haffajee, 2002; Flemming and Wingender, 2010). The microenvironment of polymicrobial biofilms creates a favorable condition for synergistic microbial coaggregation processes and the accumulation of pathogenic species responsible for triggering infectious diseases (Costerton et al., 1995; Bowen et al., 2018). Therefore, microorganisms growing in the biofilm have unique advantages facilitating specific cross-kingdom interactions, which have been associated with increased microbial virulence and host tissue damage (Peters et al., 2012; Diaz et al., 2014).
In the oral environment, the second largest and diverse microbiome in the human body (Dewhirst et al., 2010; Baker et al., 2017), indigenous microorganisms live in a symbiotic state with the host by adhering to biotic (Xu et al., 2017) or abiotic surfaces (Arciola et al., 2018). However, when left undisturbed, oral biofilms accumulate, mature, and lead to increased inflammation on surrounding tissues (Sultan et al., 2018; Rosier et al., 2018; Naginyte et al., 2019), consequently allow microbiological composition to shift to a more pathogenic state. Thus, it has been implicated as a critical factor in the pathogenesis of microbial infections (Bowen et al., 2018).
Although the evidence has focused mainly on the microbial infections related to oral surfaces, such as mucosal and dental surfaces (Bowen et al., 2018; Souza et al., 2020a), biofilms growing on implanted devices can also encourage persistent local infection, leading to implant failure (Arciola et al., 2018). Such biofilms are considered the main etiologic factor for inflammatory disease processes known as peri-implant mucositis and peri-implantitis on implant devices (Berglundh et al., 2018), the main reason for dental implant treatment failure (Salvi et al., 2017). These biofilm-related infections on implant surfaces have a high proportion of putative pathogens and reduced abundance of beneficial species, which are directly correlated with worse clinical measures (Lindhe et al., 1992; Lang et al., 1993; Shibli et al., 2008; Padial-Molina et al., 2016). Interestingly, although the interactions between yeast and bacteria have a key role in prevalent oral diseases, such as dental caries (Wan et al., 2021) and oral mucositis (Bertolini and Dongari-Bagtzoglou, 2019a; 2019b), it has not been well discussed by implant-related infections studies.
In vivo findings have shown a higher proportion of total bacteria in peri-implantitis biofilms, compared to healthy sites, but also high levels of Candida albicans (Canullo et al., 2017a, 2017b), the most frequent oral fungal opportunistic pathogen (Ghannoum et al., 2010) that forms biofilms on implanted materials and can cause disseminated infection (Kojic and Darouiche, 2004; Andes et al., 2004; Bertolini and Dongari-Bagtzoglou, 2019a, 2019b). Similarly, C. albicans has been shown to be associated with chronic periodontal disease sites (Vieira Colombo et al., 2016) and a dose-response seem to be present as disease severity increases from periodontal health, gingivitis and periodontitis, with an increase of fungal load for this last one. Although not frequently evaluated in human peri-implant disease studies, C. albicans can adhere and grow on implant surfaces and has been clinically isolated from biofilms associated with peri-implant disease (Leonhardt et al., 1999; Schwarz et al., 2015; Canullo et al., 2017a, 2017b).
Moreover, our group has shown that C. albicans promotes bacterial growth and increased virulence of biofilms growing on the implant surface, leading to significantly more mucosal damage (Souza et al., 2020b). Interestingly, Candida shows an enhanced effect on Streptococcus species when growing on the implant surface to increase matrix-related gene expression which can facilitate the creation of an anaerobic microenvironment (Souza et al., 2020c), thus allowing anaerobic bacteria to thrive. These interactions have been attributed to the pathogenic synergy between C. albicans and certain commensal bacteria, leading to more severe oral infection (Bertolini and Dongari-Bagtzoglou, 2019a; 2019b).
Therefore, although previous evidence suggests that Candida is present on teeth and implant-related biofilms, its possible role in the pathogenesis of peri-implant infections has not been sufficiently discussed. This review aims to discuss the current evidence about the presence and plausibility of C. albicans influence on implant-related infections and key factors to be considered for the therapeutic strategies.
Candida albicans: the prevalent oral opportunistic pathogen subgingival microbiota
C. albicans colonizes the oral mucosal surfaces of up to 75% of healthy individuals, being the most abundant fungus isolated and a critical component of healthy individuals' “core mycobiome” (Ghannoum et al., 2010). Albeit this opportunistic fungus is typically found in oral mucosal surfaces as a commensal organism, under certain predisposing conditions, it can lead to severe mucosal infections often associated with the mucosal resident bacterial species (Jenkinson and Lamont, 2005).
Although C. albicans colonization has been chiefly associated with mucosal tissues in the form of oropharyngeal and vaginal candidiasis (Bertolini and Dongari-Bagtzoglou, 2019a; 2019b), it has also been described within the subgingival sites of diabetic patients with periodontal disease (Sardi et al., 2012), as well as smokers and non-smokers with periodontal disease (Santhana Krishnan et al., 2020), a chronic inflammatory disease that affects teeth-supporting tissues in the oral cavity. In these patients, subgingival colonization of C. albicans was associated with the severity of periodontitis (Canabarro et al., 2013). In addition, a positive correlation was observed between Candida colonization and increasing pocket depth and attachment loss (Santhana Krishnan et al., 2020). More recently, it has been shown that during periodontal disease the teeth with higher inflammation and tissue destruction presented a mixed population of bacteria and fungi, including enterobacteria, C. albicans, Pseudomonas aeruginosa, Filifactor alocis, and others (Vieira Colombo et al., 2016).
With the advent of dental implants, it has been reported that the most common yeast species found around implant sites is C. albicans (Alrabiah et al., 2019), especially in patients with type 2 diabetes in which the fungus was found in around 74% of the subgingival samples collected, and with a significantly higher load than in patients without diabetes and peri-implant disease (Alsahhaf et al., 2019). When comparing the colonization in dental implant surfaces versus teeth, fungal species were more frequently identified at peri-implantitis and even health implant sites than at selected teeth in healthy patients, showing the ability of C. albicans to colonize titanium surfaces (Schwarz et al., 2015). Importantly, although C. albicans is the most prevalent and more often described Candida species in peri-implant diseased sites (Yeh et al., 2019; Pranno et al., 2021), with the advantage of new identification techniques, other non-albicans species have been recently identified on peri-implantitis sites such as Candida parapsilosis, Candida tropicalis, and Candida dubliniensis (Mendoza et al., 2021). Among these, C. albicans and C. dubliniensis are the only polymorphic species, which are able to form hyphae and/or pseudohyphae, creating robust biofilms, while C. parapsilosis and C. tropicalis cannot produce true hyphae, but can form pseudohyphae, depending on growth conditions. However, the literature is almost non-existing regarding the importance of morphology alone on Candida on virulence factors (Silva et al., 2012). Thus, for non-albicans Candida species, virulence factors are mostly associated with surface adherence, biofilm formation, antifungal resistance, and the secretion of some hydrolytic enzymes that serve to improve their persistence in the oral epithelium and lead to host cell damage. Secreted aspartyl proteinases, phospholipases, lipases, and hemolysins are the most frequent enzymes implicated in Candida species pathogenicity. Importantly, phospholipases contribute to host cell membrane damage and is able to expose additional receptors to facilitate fungal adherence (Dostal et al., 2005; Portela et al., 2010; Galan-Ladero et al., 2010).
Recently, it has been described that once C. albicans transition from its commensal (yeast) to pathogenic (hyphae) state, it is able to secrete a cytolytic peptide toxin, now known as candidalysin (Moyes et al., 2016a, 2016b). Before this, human fungal pathogens were not known to possess such toxins. Thus, once C. albicans transition from yeast to hyphae form within the biofilm, and secretes this pore-forming toxin (Naglik et al., 2019), not only epithelial cells become damaged, but this also exacerbates the host immune response, by increasing local neutrophil recruitment as an immunomodulatory pathway (Conti et al., 2016). This dual role of C. albicans candidalysin with epithelial destruction and immunomodulatory effect adds C. albicans in all six classifications of the host-microbe damage response framework previously published by Noverr group (Jabra-Rizk et al., 2016). Importantly, there seems to be a threshold level of hyphal burdens are required for full epithelial activation (Moyes et al., 2010a, 2010b), which correlate with earlier findings in periodontal disease associating fungal burdens with disease severity (Vieira Colombo et al., 2016) and with peri-implantitis studies showing that Candida spp. and other organisms were frequently found in higher loads at peri-implantitis sites when compared to healthy ones (Schwarz et al., 2015). Furthermore, C. albicans is able to secrete metallopeptidase, which degrades several constitutive proteins of mucosal barrier, such as collagen, fibronectin, and laminin (Rodier et al., 1999).
Thus, although it seems other non-albicans Candida species have been recently described to be present at peri-implantitis sites, there is mounting evidence pointing that C. albicans is the only one that fits in the host-microbe damage response framework and would be able to significantly contribute to tissue damage and immune modulation. Such factors locally contribute to changes associated with the local bacterial microbiome and reduction in the epithelial barrier integrity (Pappas et al., 2018). Perturbation of local and systemic host factors can then lead to C. albicans overgrowth, transition from yeast to hyphal state, and the development of mucosal invasive infections, initiated by epithelial damage and neutrophil recruitment (Vila et al., 2020).
Factors that modulate Candida spp. colonization on titanium-based and other dental materials surfaces
A wide range of implanted biomaterials used in clinical practice has been shown to support the colonization by Candida spp., including dental implant surfaces (Ramage et al., 2006; Souza et al., 2020d). In implant manufacturing, titanium (Ti) and its alloys represent the most commonly used materials owing to their favorable biomechanical and biocompatible properties (Cordeiro and Barão, 2017). However, Ti is a bioinert material that allows microbial surface interactions and biofilm accumulation. For Ti surfaces, the colonization of dental implants by different bacterial species has been widely reported in the literature (Cavalcanti et al., 2014; Chouirfa et al., 2019). By contrast, fungal adhesion has been barely discussed.
Some in vitro studies by our group and others have shown the ability of C. albicans to adhere to Ti surfaces and to readily form mono- and mixed-species biofilms (Cavalcanti et al., 2014, 2014, 2015, 2016a, 2016b, 2014; Martorano-Fernandes et al., 2020; Montelongo-Jauregui et al., 2018; Souza et al., 2020a, 2020b, 2020d). Similarly, Candida spp. are present in several sites in in vivo studies assessing microbiota from healthy (Canullo et al., 2015), peri-implantitis (Alsahhaf et al., 2019), and failed implants (Leonhardt et al., 1999). Therefore, Ti-based dental implants have been considered a potential substrate for C. albicans' adhesion and accumulation (Bürgers et al., 2010).
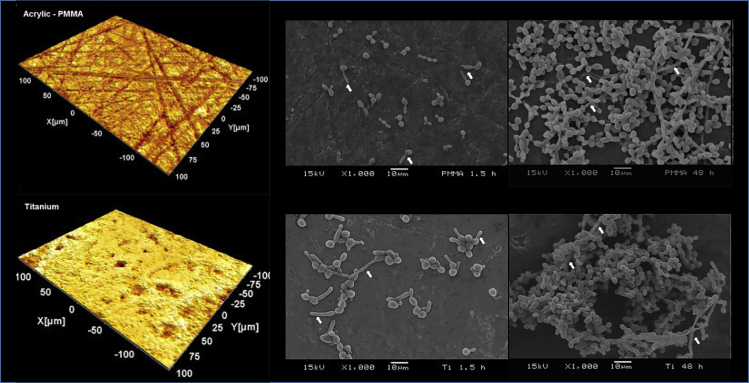
Because C. albicans is a common colonizer of the oral tissues (Ghannoum et al., 2010), any oral surface or abiotic material placed in the oral environment is expected to act as a substrate for Candida adhesion. Importantly, not all Ti surfaces are created equal and surface properties, such as surface roughness (Li et al., 2013), surface free energy, and chemical composition (Bürgers et al., 2010), can modulate Candida adhesion and accumulation. Interestingly, a previous study (Cavalcanti et al., 2016a; 2016b) has shown that different dental materials (acrylic and titanium) with similar surface roughness parameters allowed similar Candida accumulation once exposed to oral environment fluids (saliva and blood plasma) (Figure 1). Recently, Mouhat et al. (2020) found that lower surface free energy is also a key parameter inducing less Candida accumulation (24 h) on the Ti surface. Later on, our group corroborated their results by developing a superhydrophobic Ti surface which significantly reduced Candida adhesion (Souza et al., 2020c).
Figure 1.
Relationship between material/substrate type and Candida biofilm development
Representative microscopy of acrylic (Poly(methyl methacrylate) - PMMA) and titanium surface topography evaluated by white light 3D profilometry (left side)
Scanning electron microscopy (SEM) images (right side) at 1.5 h (adhesion) and 48 h (maturation) of C. albicans biofilm formed on PMMA (top) and titanium (bottom). White arrows indicate the presence of hyphae, which were prevalent in both materials at 48 h. Overall, the similar surface roughness of both materials and the presence of environment fluids (saliva and blood plasma) culminated in no difference for Candida adhesion and growth for the two tested substrates. Reprinted (adapted) from ref (Cavalcanti et al., 2016a; 2016b); Copyright (2016), with permission from Elsevier (License number: 5117881126618).
However, attention must be brought to the fact that although physical-chemical properties directly affect microbial adhesion on biomaterials, any surface placed in the mouth is immediately coated by a protein layer from saliva or blood plasma. Such protein adsorption on the surfaces has been considered the first biological response in the human body, which is responsible for mediating subsequent cellular events, such as microbial and host cell adhesion (Kalasin and Santore, 2009; Rabe et al., 2011; Mukai et al., 2020), and even osseointegration process of dental implants (Romero-Gavilan et al., 2018)
Older in vitro studies did not use a pre-coated Ti surfaces with saliva or blood plasma, prior bacterial/fungal adhesion. As a result, biofilm development and composition was significantly affected by the type of substratum, including metallic, non-metallic, and mucosal surfaces (Frade and Arthington-skaggs, 2011; Fernández-Rivero et al., 2017; Xu et al., 2017). Perhaps, the results from a previous study (Cavalcanti et al., 2016a; 2016b) showing similar fungal adhesion to different dental materials (acrylic and titanium) were not only related to similar surface roughness but also due to the pre-coating with saliva and blood plasma that could have equalized the surface of such materials.
However, in vivo findings still show significant differences for materials presenting distinct surface characteristics, such as machined Ti and polished zirconia. The latter presented significantly lower Candida adhesion (do Nascimento et al., 2013), which may be explained by the composition of initial saliva protein adhesion, as shown by others (Yoshida and Hayakawa, 2016). The salivary protein lactoferrin (protein with antimicrobial and antifungal activity against a range of pathogens) (Curvelo et al., 2019) has a better binding to zirconia and PMMA surfaces than to titanium and stainless steel surfaces, possibly explaining why fungal biofilm grows better on titanium and stainless steel surfaces.
Previous evidence has shown that the protein layer adhered to Ti material can modulate Candida adhesion and accumulation (Bürgers et al., 2010; Cavalcanti et al., 2016a, 2016b; Mouhat et al., 2020). Importantly, Ti material coated with human saliva, or specific proteins, such as mucin, showed higher C. albicans adhered to the surface than non-coated (Bürgers et al., 2010). Saliva coating actually led to a significantly higher expression of C. albicans virulence genes such as ALS1, ALS3, and HWP1, which are associated with hyphal formation (Cavalcanti et al., 2016a; 2016b). Corroborating these results and further dissecting the specific role of salivary or plasma proteins on biofilm formation, we recently showed that Ti coated with proteins from blood plasma showed an increased level of adhered bacteria when compared to saliva, which is explained by the specificity of the proteomic profile of blood plasma (Souza et al., 2020e).
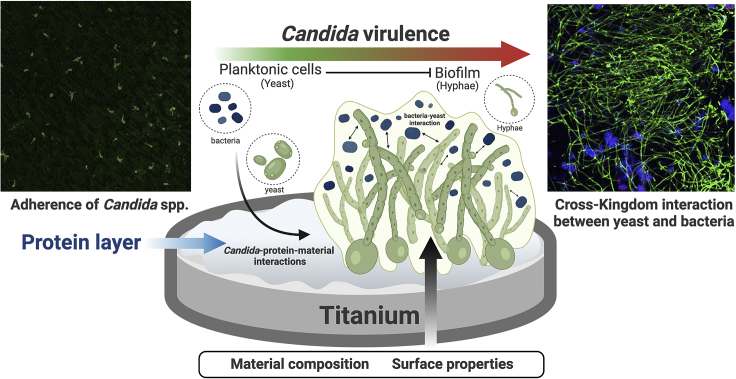
Such results must now be experimentally validated for C. albicans and mixed biofilms because the initial bacterial colonizers may also interfere with C. albicans biofilm formation. The current evidence suggests that Candida adhesion, morphology, and expression of virulence factors are influenced by the surface characteristics and the presence of a protein layer on the surface, which can favor (mucin) or hinder (lactoferrin) biofilm formation. Some factors affecting Candida attachment and accumulation on Ti material are summarized in Figure 2.
Figure 2.
Schematic representation of Candida spp. attachment and accumulation on Ti surface
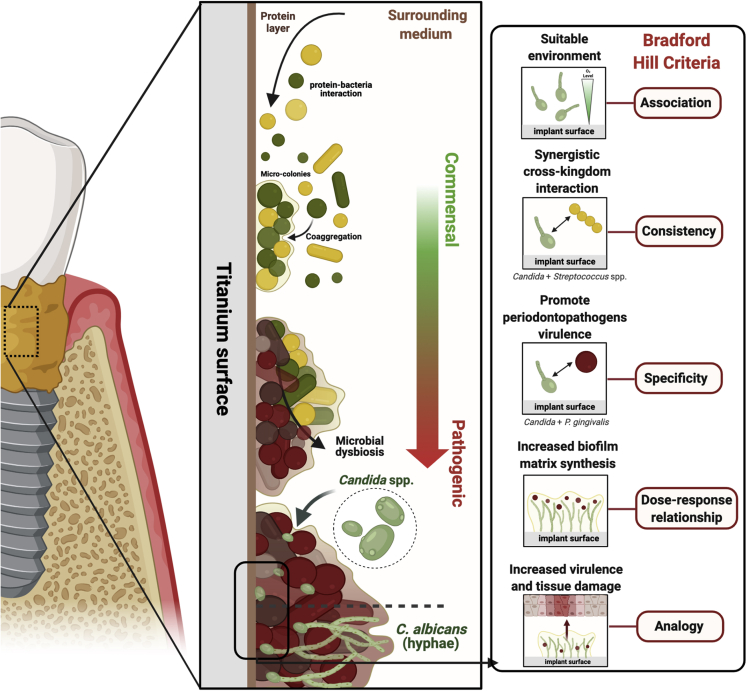
After implantation, the Ti-based implant surface is immediately coated by the protein layer. Consequently, microbial-material interactions promote Candida adhesion (confocal images, left side; scale: 50 μm/time: 2 h - stained by immuno-FISH green). Moreover, fungal biofilm growth is modulated by surface and microorganism properties and environmental conditions, leading to the transition of Candida morphology from yeast to hyphae in single or multi-species biofilms. The cross-kingdom interaction between Candida and Streptococcus on implant surface may promote biofilm growth. It is also possible to observe in the confocal image (right side) the mixed biofilm stained by immuno-FISH of Candida albicans (green) and Streptococcus oralis stained by Streptococcus-specific probe conjugated to Alexa 405 (blue) growing on Ti surface (scale: 50 μm/time: 72h). Reprinted (adapted) from refs (Souza et al., 2020b; 2020d). Created with BioRender.com (license number: FH22TBHYN9).
Finally, besides the surface parameters of Ti and other dental materials in modulating initial salivary or plasma protein adhesion, the fungal cell wall proteins also play a role in colonizing biotic and abiotic surfaces. In biotic surfaces, epithelial cells recognize cell wall differences between yeast (mostly commensal) and hyphal (more pathogenic on mucosal surfaces) C. albicans cells. Specifically, β-glucans on the surface of the fungal pathogen C. albicans activate mitogen-activated protein kinase (MAPK) (Swidergall et al., 2018) and initiate a biphasic MAPK response, modulating gene transcription factors that lead to NF-κB activation. Thereafter, cytokines and chemokines secreted by epithelial cells in response to C. albicans hyphal recognition lead to recruitment and activation of immune cells. For example, IL-8 is one of the key chemokines involved in initial neutrophil chemotaxis to the infection site (Casale and Carolan, 1999), with later cell activation by GM-CSF, G-CSF, and IL-1, allowing the activation of respiratory burst and local release of reactive oxygen species (ROS) (Romani, 2011). In addition, mucosal dendritic cells are also recruited to process fungal antigens and activate T-cell immunity (Naglik et al., 2014). Once recruited, Th17 cells will differentiate and produce IL-17 to further increase local neutrophil activity (Huppler et al., 2014). Together, these innate and adaptive immune response pathways are responsible for controlling C. albicans infection and tissue invasion and clearing the mucosal surfaces; moreover, ROS and proteases such as elastase produced by neutrophils may also contribute to epithelial damage (Sampson, 2000; Fournier and Parkos, 2012). Thus, Candida overgrowth is able to create an excessive recruitment and accumulation of activated neutrophils leading to mucosal injury and disease.
On abiotic surfaces, hydrophobic cell wall proteins from Candida, such as Csh1p, mediates Candida attachment to immobilized fibronectin and endothelial cells (Singleton et al., 2001; Glee et al., 2001), but its effect on implant surface adhesion has not been evaluated yet. In addition, extracellular polymers synthesized by bacterial exoenzymes can also increase C. albicans adhesion on hydroxyapatite surface (Gregoirie et al., 2011), although its effect on Ti remains to be elucidated. In this context, our group has previously shown a significant increase in fungal attachment when C. albicans was inoculated on a preformed biofilm of Streptococcus oralis growing with sucrose, the substrate for extracellular polymers production (Souza et al., 2020b). Thus, there is evidence that the microorganism overgrowth on biotic surfaces can modulate an immune response and create mucosal damage, while on abiotic surfaces initial protein adhesion (from saliva or plasma) can modulate biofilm characteristics. Future studies must consider all to elucidate the factors that lead to Candida accumulation on implanted Ti devices and to verify the interplay between biofilm accumulation and mucosal tissue immune response.
Cross-kingdom interaction between Candida and bacteria on oral polymicrobial biofilms
The human oral cavity contains several niches for microbial colonization, such as the oral mucosa, teeth, tongue, implant surface, and restorative materials (Diaz et al., 2012a; Xu et al., 2014a; Bertolini et al., 2015; Cavalcanti et al., 2016a, 2016b; Souza et al., 2020a; Barao et al., 2022). Each niche favors a certain microenvironment that modulates the tridimensional structure and composition of the formed biofilm, varying significantly in terms of complexity and diversity depending on the location, surface characteristics, and microenvironment nutritional sources (Seidel et al., 2020). Such polymicrobial biofilm communities include bacteria, fungi, and archaea species in close contact, providing several opportunities for physical and metabolic cross-kingdom interactions among distinct species (Egland et al., 2004; Kim et al., 2008; Ghannoum et al., 2010; Belda-Ferre et al., 2012). These cell-cell polymicrobial interactions are likely to affect community assembly and may modulate the microbial profile in terms of resistance and resilience ability to promote a steady state of commensalism or trigger biofilm pathogenicity and infection in disease development (Diaz et al., 2014).
Cross-kingdom interactions between the opportunistic fungus C. albicans and the oral streptococci, predominant microorganisms in the oral cavity, have become of growing interest because they have been known to play important roles in the pathogenesis of mucosal infections and teeth carious lesions (Ghannoum et al., 2010; Kraneveld et al., 2012; Diaz et al., 2012a; Xu et al., 2014a; Bertolini and Dongari-Bagtzoglou, 2019a, 2019b; Kim et al., 2021). Importantly, mitis group streptococci (Streptococcus gordonii, S. oralis, Streptococcus sanguinis, and Streptococcus mitis) have been termed “accessory pathogens” due to their ability to form multi-species biofilms and to enhance the virulence of the biofilms where they reside (Whitmore and Lamont, 2011). These species have been described as early colonizers, as they drive the subsequent colonizers leading to pathogenic polymicrobial biofilm phenotypes when associated with Candida (Rickard et al., 2003; Diaz et al., 2012a, 2012b; Xu et al., 2014a, 2014b).
This cross-kingdom interaction can occur in several ways, such as adhesive and coaggregation processes, inter-kingdom signaling, and metabolic interactions (Xu et al., 2014b; Bertolini and Dongari-Bagtzoglou, 2019a, 2019b). Interestingly, Diaz et al. (2012a) has shown that although S. oralis displays poor ability to form mucosal biofilms on its own in a single-species biofilm, under a flow condition, the presence of C. albicans enhanced the bacteria attachment and growth, creating a much more robust biofilm, with significantly epithelial damage and C. albicans invasion through the mucosa. Results suggested that S. oralis and C. albicans are commensal organisms growing separately but synergistically affect pathogenic potential. Furthermore, despite the belief that commensal streptococcal species protect the host against candida-related infections (Liljemark and Gibbons, 1973), it has been widely reported that C. albicans can synergize with certain Streptococcus spp. leading to exacerbated local pro-inflammatory host response increasing the severity of oral mucosal infection and promoting epithelial damage and barrier breach.
It is known that during polymicrobial biofilm formation, Streptococcus and Candida communicate with each other by using chemical signaling and metabolites that modulate cell behavior (Miller and Bassler, 2001; Bamford et al., 2009; Ramsey et al., 2011). Even in a well-regulated biofilm community, these signaling communication metabolites, such as quorum-sensing system, can manipulate the hierarchical biofilm architecture depending on the microbial species playing the “leader” or “accessory” role in the biofilm growth (Xu et al., 2014b; Whitmore and Lamont, 2011)—oral streptococci and C. albicans may regulate gene expression and recognition pattern to diverse other microorganisms occupying the same ecological niches. Nevertheless, because bacteria can produce numerous chemical signs molecules, it is of utmost importance to highlight that the result of Candida–bacterial interaction toward the promotion or suppression of yeast virulence and pathogenicity is not solely driven by the cross-kingdom communication but also rely on the influence of the environmental condition, microbial communities load and composition, and host immune response. Most of the studies on the pathogenic interactions between C. albicans and oral bacteria focused only on individual bacterial species. Very few investigated the interactions between C. albicans and polymicrobial biofilms in health and disease essential to closely represent the complexity of the biofilms found in the human body, especially around peri-implant infections.
In this context, in vivo murine models have shown that Candida infection of immunosuppressed hosts contributed to changes in the oral microbiome, leading to mucosal bacterial overgrowth of certain species, such as Enterococcus faecalis, leading to significantly dysbiosis and reduced diversity (Bertolini and Dongari-Bagtzoglou, 2019a; 2019b; Bertolini et al., 2019). Although enterococci are traditionally considered transient commensals in the oral cavity and carriage low rates, the oral carriage rate of Enterococcus species (predominantly E. faecalis) with underlying systemic disease rises significantly (more than 80%) (Gonçalves et al., 2009). Polymicrobial infections with C. albicans and bacteria have shown higher morbidity and mortality in immunosuppressed patients (Boktour et al., 2004; Puig-Asensio et al., 2015). Therefore, these medically compromised patients are some of the most high-risk populations for Candida–bacteria interaction, and such biofilms should be further studied in polymicrobial infections. It is well established that commensal anaerobic bacteria are critical in limiting Candida intestinal colonization in mice, and colonization levels are generally proportional to the level of antibiotic depletion of anaerobic bacteria (Koh, 2013). However, an antagonistic relationship between oral anaerobic bacteria and C. albicans has not been established in the oral mucosa.
Implant-related infection: an emergent and prevalent disease
Once a microbial biofilm forms on dental implant surface, immune-mediated biological factors and environmental conditions can induce a deleterious shift in the balance of the normally stable resident microbiome. It allows a significant increase in bacterial loads and can lead to progressive inflammatory destruction of the peri-implant-surrounding tissues (Belibasakis and Manoil, 2021). These conditions are the main reason for dental implant treatment failure showing a high prevalence, with more than 40% of implants affected by mucositis and more than 22% affected by peri-implantitis (Salvi et al., 2017).
In the oral cavity, peri-implant mucositis is characterized by inflammation in the mucosa around dental implants. Still, its progression and subsequent progressive loss of supporting bone are known as peri-implantitis (Berglundh et al., 2018). Both conditions are considered a “biofilm-associated pathological condition” and, therefore, there is strong evidence that biofilm is the main etiological factor for dental implant-related infections (Berglundh et al., 2018). Furthermore, clinical signs of disease progression have been linked to increased microbial loads, microbiological changes, and transition to a more pathogenic biofilm on dental implant surfaces (Shibli et al., 2008; Padial-Molina et al., 2016).
Compared to orthopedic implants, dental implants are unique because they have a trans-mucosal component (i.e., abutment devices) that penetrates the soft tissue between the anchoring bone and the functional dental prosthesis (Wang et al., 2016). Consequently, the long-term success of dental implants depends on both osseointegration and soft tissue stability (Spriano et al., 2018). Hence, it is crucial to prevent biofilm formation on the Ti surface to avoid polymicrobial infections and tissue damage.
Nonetheless, factors influencing the transition from a health-associated oral to a disease-associated microbiome are still not fully understood. Up to now, it is believed that multifactorial components are to be associated with the inflammatory process (Marsh et al., 2011), such as poor oral hygiene (Serino and Ström, 2009), lack of regular maintenance (Frisch et al., 2014), carbohydrate consumption (Souza et al., 2019), Ti particles release (Souza et al., 2020f), and even extracellular polymers of biofilm matrix (Costa et al., 2020). All these factors are pointed out as potential players in the pathogenicity of oral biofilms inducing the overgrowth of putative species.
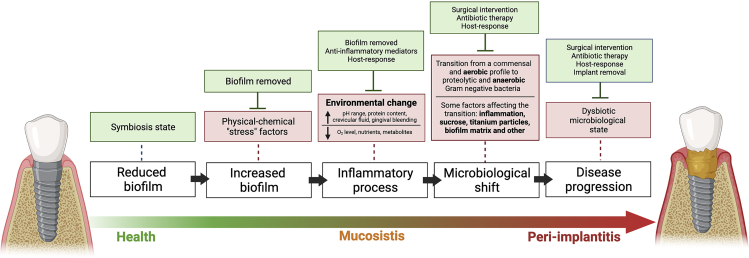
An adaptation from the “ecological plaque hypothesis”, previously published by our group (Souza et al., 2020g) on tooth surfaces, has been applied to describe a transition from a healthy state to a dysbiotic state in implant-related infections (Figure 3). According to this model, increased biofilm accumulation acts as a “stress” factor triggering an inflammatory process that leads to changes in the local microenvironment, favoring proteolytic and anaerobic Gram-negative bacterial overgrowth (Marsh et al., 2011). Such a concept has been recently discussed by Scannapieco and Dongari-Bagtzoglou (2021), establishing that periodontal diseases are a result of an increase in the biomass of a highly diverse microbiota that has evolved. Interestingly, different factors mentioned above have been directly linked to the disease process on implant-related biofilms. They can lead to an increased bacterial biomass, such as carbohydrate consumption and extracellular polymers. Thus, biofilm formation, maturation, and increased bacterial load are complex processes determined not only by the surface properties, host-response, and environmental conditions (Cheng et al., 2019) but also the cell-to-cell interactions that can create synergistic or antagonistic effects in polymicrobial biofilms. It may explain the lack of consensus regarding the best protocol treatment for peri-implantitis disease.
Figure 3.
Schematic representation of the “ecological plaque hypothesis” concerning peri-implant disease, adapted from Marsh et al. (2011), Rosier et al. (2018), and Souza et al., 2020a, 2020b, 2020c, 2020d, 2020e, 2020f
Increased biofilm accumulation on implant surface triggers an inflammatory process that changes the environment leading to microbiological shift and disease progression, as shown by red boxes.
Other factors can also favor the microbiological shift on biofilms growing on titanium surfaces, such as carbohydrate (sucrose exposure). However, some factors can control biofilm accumulation and inflammatory response, shown in green boxes, such as surgical and antimicrobial intervention and host response.
This mature and pathogenic biofilm associated with implant infections has a polymicrobial composition directly linked to clinical signs of disease (Lindhe et al., 1992; Lang et al., 1993; Shibli et al., 2008). Peri-implant disease microbial communities show a change in microbiological diversity, compared to healthy sites, in which lower levels of Prevotella and Leptotrichia and higher levels of Actinomyces, Peptococcus, Campylobacter, nonmutans Streptococcus, Butyrivibrio, and Streptococcus mutans have been described (Kumar et al., 2012). In addition, increased levels of Treponema forsythia, Treponema denticola, F. nucleatum, P. intermedia, P. micros, Candida rectus, E. corrodens, C. albicans, P. nigrescens, Candida gracilis, Candida ochracea, Candida concisus, S. spp., A. odontolyticus, V. parvula, and E. faecalis in peri-implantitis have been shown by in vivo study (Canullo et al., 2017a, 2017b).
The consensus report of the 7th European Workshop on Periodontology recognized several risk factors associated with peri-implant diseases (Berglundh et al., 2018; Schwarz et al., 2018). The well-known risk factors are the history of chronic periodontitis, poor oral hygiene status, and no regular maintenance care after implant therapy (Gurgel et al., 2017; Heitz-Mayfield, 2008; Schwarz et al., 2018). Different from periodontal diseases, data identifying “smoking” and “diabetes” as potential risk factors/indicators for peri-implantitis are inconclusive (Berglundh et al., 2018; Schwarz et al., 2018). However, a direct cause-and-effect relationship is not entirely understood, and further well-designed prospective longitudinal studies are recommended to provide reliable evidence. Additionally, there is limited evidence linking peri-implantitis to other factors such as keratinized mucosa, genetic trails, systemic conditions (other than diabetes), iatrogenic factors, occlusal overload, Ti particles, and others (Berglundh et al., 2018; Schwarz et al., 2018). Therefore, the importance of such factors should also be further evaluated.
Regarding Candida spp. infections, some specific aspects, including chronic hyperglycemia, habitual tobacco smoking, and poor oral hygiene skills, have been associated with an increased oral Candida colonization on implanted devices (Alrabiah et al., 2019; Ramage et al., 2006). These risk factors have also been shown to increase the risk of peri-implant diseases (Alrabiah et al., 2019). It is notable that Candida spp. infections share many risk factors/indicators with peri-implant diseases (Alqahtani, 2020). A combination of Candida biofilms with pre-existent risk conditions can enhance the likelihood of peri-implant disease development and progression. Moreover, several other risk indicators have also been associated with marginal bone loss (French et al., 2019). To date, knowledge of the participation of Candida infection in disorders of bone remodeling is limited. Still, it has been described for arthritis and osteomyelitis of the jaw (Daya Attie et al., 2018), mainly in patients with immunodeficiency (Gamaletsou et al., 2015). In the absence of sufficient data, it appears reasonable to suggest that Candida would act as a modifying agent in chronic inflammation, corroborating to the increased accumulation of bacterial pathogenic species around dental implants, which can enhance tissue damage and modulate the bone resorption response (Lafuente-ibáñez de Mendoza et al., 2021).
The possible role of Candida on implant-related infections
Although not considered a target pathogen commonly investigated by peri-implantitis studies, C. albicans has been identified in biofilms on implant surfaces in in vitro, in situ, and in vivo studies (do Nascimento et al., 2013; Souza et al., 2020a, 2020b, 2020d). Because Candida is an opportunistic pathogen mainly growing in medically or immunocompromised patients, conditions that may not directly affect the peri-implantitis risk (Vissink et al., 2018), this microorganism has not been straight linked to implant-related infections as a causative microorganism. However, as shown by a previous systematic review of observational studies, Candida has been found in high frequency (ranging from 3% to 76.7%) in the sulcular fluid of implants with peri-implantitis (Mendoza et al., 2021).
Moreover, clinical evidence has shown higher levels of C. albicans in peri-implantitis sites than healthy ones (Leonhardt et al., 1999; Schwarz et al., 2015), and presence of Candida spp. in more than 50% of peri-implant lesions. Interestingly, even for healthy implant sites, Candida has shown a higher frequency than healthy teeth with a history of periodontitis (Schwarz et al., 2015), suggesting that dental implant surface and local environment may favor Candida colonization.
Although some evidence suggests the role of C. albicans in the etiopathogenesis of peri-implantitis (Alqahtani, 2020), Candida may act as a risk factor for microbial infection, playing a key role in the biofilm virulence, which would then lead to disease progression and tissue damage. In addition, diabetic patients, who have a higher risk for Candida infections (Zomorodian et al., 2016), show a higher frequency of C. albicans and worse clinical signs of peri-implantitis than in comparison to non-diabetic patients with peri-implantitis (Alsahhaf et al., 2019). In fact, diabetic patients with poor glycemic control and chronic periodontitis tend to present higher loads of Candida spp. in the subgingival area than healthy individuals with chronic periodontitis or diabetic patients with good glycemic control and chronic periodontitis (Matic Petrovic et al., 2019). Therefore, presence of Candida in the implant-related biofilms can worsen disease progression and prognostic for medically compromised patients, which needs further investigation.
Although Candida–bacterial interactions have been associated with oral infections such as oral mucositis and dental caries, limited information is available regarding periodontitis and peri-implantitis. Interestingly, high densities of yeasts for periodontitis were found only in patients with moderate and severe chronic periodontitis (Canabarro et al., 2013). Such finding brings up the Bradford Hill criteria for causation (Hill, 1965), as we try to establish epidemiologic evidence of a causal relationship between high loads of C. albicans and the development or progression of peri-implantitis.
Similar bacterial groups have been described for periodontitis and peri-implantitis, with thirty-one “core species” present in >90% sites, Streptococcus infantis/mitis/oralis and Fusobacterium sp., the most prevalent. Among periodontitis and peri-implantitis sites, many putative “periodontopathogens” such as Prevotella, Porphyromonas, Tannerella, Bacteroidetes, and Treponema spp. have been described (Yu et al., 2019). Although authors suggested some difference in their proportions among periodontitis and peri-implantitis sites, inter-subject variations outweighed such differences. These results were recently corroborated by a review (Retamal-Valdes et al., 2019) which concluded that there are more similarities than differences among bacterial species that colonize peri-implant and periodontitis sites in disease. Thus, it is important to establish if there is a synergistic effect between such pathogens and the fungus C. albicans in diseased peri-implant sites to determine C. albicans' role in this chronic inflammatory disease.
Among the periodontopathogens, in vitro results show that Porphyromonas gingivalis seems to have an increased ability to invade human gingival epithelial cells and gingival fibroblasts in the presence of C. albicans (Tamai et al., 2011). Moreover, recent evidence (Sztukowska et al., 2018) points to a coadhesion mediated by specific proteins between C. albicans and P. gingivalis, resulting in major changes in gene expression by P. gingivalis, which could govern increased biofilm virulence. Current in vivo evidence suggests that at implant sites, fungal organisms were significantly correlated with P. micra and T. forsythia (Schwarz et al., 2015). T. forsythia is directly related to the etiopathogenesis of implant-related infections and present in high abundance in sub-mucosal biofilm samples of peri-implantitis subjects (Shibli et al., 2008). Although Candida has been found in implant-related infections, the pathogenic role played by Candida–bacterial interactions in implant-related biofilms remains unclear, requiring additional mechanistic and in vivo studies. Available evidence suggests a synergistic interaction between C. albicans and oral bacteria that potentially increases the virulence of polymicrobial biofilms (O’Donnell et al., 2015; Montelongo-Jauregui and Lopez-Ribot 2018).
In regards to the “core species” present on periodontitis and peri-implantitis, the cross-kingdom interaction between Candida and Streptococcus species has been widely investigated, and some effects may be applied to implant-related infections (Souza et al., 2020a, 2020b, 2020c, 2020d, 2020e, 2020f). Our group has shown high levels of certain bacterial members of the yellow microbial periodontal complex on biofilms growing in situ on Ti surface (Souza et al., 2019), represented mainly by Streptococcus species of the mitis group (S. oralis, S. mitis, S. gordonii, and S. sanguinis). Interestingly, these bacteria are present in biofilms in both early and late stages of peri-implantitis (Kumar et al., 2012) and represent about 60%–90% of initial colonizers in biofilms formed on a dental surface (Rickard et al., 2003; Diaz et al., 2006) and also dominate the oral mucosa of healthy individuals (Diaz et al., 2012a). Thus, mitis Streptococcus species have been shown to form robust, hyper-virulent biofilms with C. albicans (Diaz et al., 2012b; Ricker et al., 2014), resulting in increased biofilm growth and pathogenic synergy able to cause significant mucosal damage (Xu et al., 2014a, 2017; Bertolini et al., 2015; Xu et al., 2016). A central mechanism in this pathogenic synergy is activating the Efg1 filamentation pathway in C. albicans by streptococci, which upregulates tissue invasion by hyphae, mucosal inflammation, and expression of microbial coaggregation-promoting adhesins (Bertolini et al., 2015; Xu et al., 2017). Because both organisms, Candida and Streptococcus from mitis groups, have been found in abundance in implant-related biofilms, the same pathway is expected, which needs to be experimentally evaluated for implant surface.
Using a titanium-mucosal interface model, we evaluated the interaction between Candida and Streptococcus from mitis group (Souza et al., 2020d). Interestingly, C. albicans promoted bacterial biofilms of all mitis Streptococcus species on the Ti surface, and bacterial species upregulated the efg1 hypha-associated gene in C. albicans, which led to increased mucosal damage (Souza et al., 2020d). Moreover, the interaction of multi-species biofilms with organotypic mucosal surfaces led to the release of growth-suppressing mediators of Candida, which may represent a homeostatic defense mechanism of the oral mucosa against fungal overgrowth. These findings provide novel insights into biofilms on biomaterials that may play an important role in the pathogenesis of mucosal infections around dental implants and should be considered by in vivo studies.
Another important parameter that suggests that Candida plays a role in implant-related infections is the effect on the biofilm matrix. Biofilm matrix provides a unique environment for microbial growth, promoting coaggregation, antimicrobial resistance, and nutrient source (Flemming and Wingender, 2010; Bowen et al., 2018). Streptococcal extracellular exoenzymes, known as glucosyltransferases (Gtfs), synthesize glucan polymers which contribute to the extracellular matrix forming the scaffold for the three-dimensional architecture of biofilms with several advantages for microbial accumulation and have been implicated as virulence factors (Kopec et al., 1997; Bowen and Koo, 2011).
Interestingly, previous evidence has shown that C. albicans increases extracellular biofilm matrix formation by upregulating gtf expression when inoculated with S. mutans (Falsetta et al., 2014). We recently showed that C. albicans increased bacterial biomass of mixed biofilms with S. oralis strain encoding gtf gene for biofilms growing on biotic and abiotic surfaces (Souza et al., 2020b). Moreover, C. albicans positively affected matrix-related polymers synthesis, which enhanced Candida colonization on abiotic surfaces. Surprisingly, this cross-kingdom interaction was modulated by the surface where the biofilm was growing, because only for Ti surface Candida upregulated gtf expression by S. oralis (Souza et al., 2020b), suggesting that Ti surface is a suitable surface for this interaction.
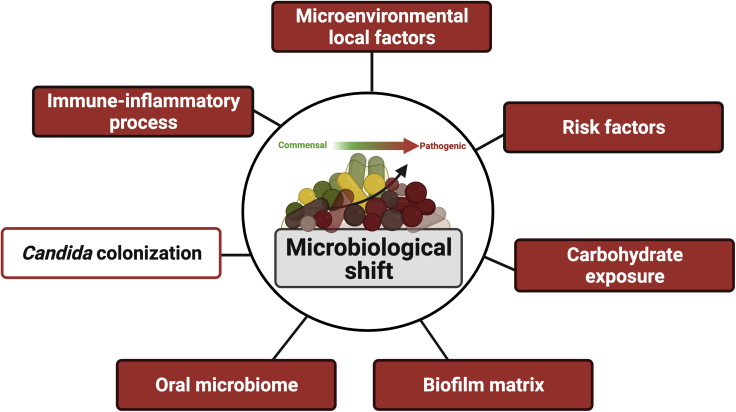
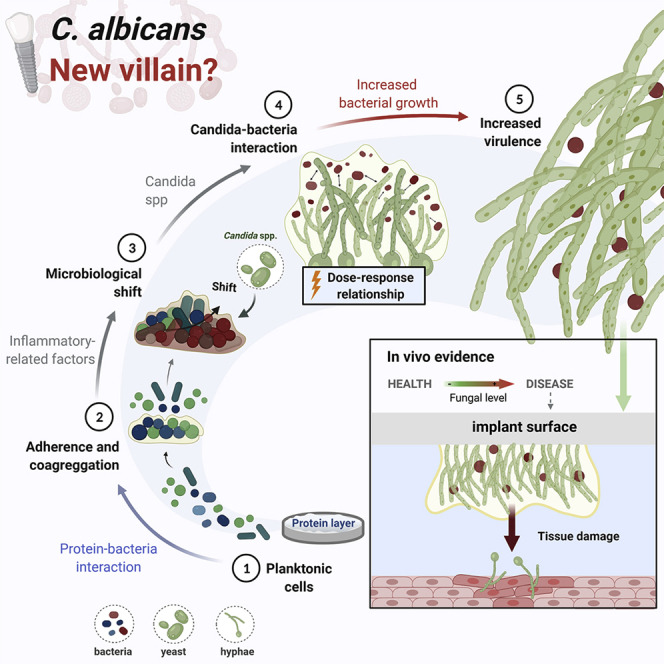
In this context, our group has shown that matrix-enriched biofilms promoted bacterial accumulation, led to a dysbiosis on biofilms growing on Ti surface, and increased even strict anaerobic species related to peri-implant infections (Costa et al., 2020). Moreover, extracellular polymers increased biofilm virulence promoting higher host cell damage and reduced antimicrobial susceptibility (Costa et al., 2020). Therefore, because Candida upregulates gtf genes by Streptococcal species and increases extracellular polymers synthesis, which has been recognized as an essential virulence factor on implant-related infections, an effect of Candida in the pathogenesis of peri-implantitis is expected. Thus, considering the direct impact of Candida on bacterial growth and extracellular polymers synthesis, parameters that lead to microbiological shift, this fungal may be viewed as an important factor enhancing microbiological dysbiosis on implant-related biofilms, mainly considering its effect on the mucosal microbiome modulation (Bertolini and Dongari-Bagtzoglou, 2019a; 2019b; Bertolini et al., 2019) (Figure 4).
Figure 4.
Different factors directly affect microbiological shift on implant-related biofilms from a commensal to a pathogenic profile, such as extracellular biofilm matrix, inflammatory process, and carbohydrate exposure
Because Candida colonization promotes biofilm accumulation and virulence factors, this opportunistic pathogen should be considered an additional factor leading to microbiological shift on implant-related biofilms, which must be tested experimentally. Created with BioRender.com (license number: ZV22TBI7ME).
Therefore, although Candida may not trigger the initiation of the peri-implantitis process, it certainly can play a role in disease progression and increased biofilm virulence in subgingival sites. However, it remains overlooked by implant-related infection studies that currently focus on the bacterial microbiome and usually leave out the mycobiome component.
The current knowledge regarding the cross-kingdom synergistic interaction between Candida and bacteria suggests Candida’s key role in promoting biofilm virulence and disease progression with expected greater tissue damage on peri-implantitis. Thus, the biological plausibility of Candida’s role on peri-implantitis is based on the following hypothesis and findings: (1) Although temporality has not been evaluated as far as fungal (or bacterial) colonization preceding disease, there is evidence of identification of C. albicans on affected teeth and implant sites; (2) Existing knowledge about Candida interactions with certain bacteria highly associated with peri-implantitis, such as P. gingivalis, promoting bacterial growth, virulence, and the ability to invade host cells; (3) Consistency across studies for periodontitis and peri-implantitis showing cross-kingdom and synergistic interaction with bacterial species, forming a hyper-virulent biofilms with increased bacterial biomass, up-regulating hyphae-related genes, exacerbated inflammatory response, and tissue damage; (4) Biological gradient showing high Candida loads are found in more severely affected diseased sites associated with increased extracellular polymers synthesis by bacterial species, enhancing biofilm matrix which has several advantages for microbial accumulation, leading to increased microbial loads, dysbiosis, and virulence; (5) Analogy from other situations in which under oxygen deprivation, such as subgingival implant sites, C. albicans shows an increased virulence promoting microbial infection (Lopes et al., 2018) (Figures 5A-5E).
Figure 5.
Schematic representation of the role of Candida to promote biofilm accumulation and virulence with expected higher tissue damage on peri-implantitis
The biological plausibility hypothesis of Candida’|'s role on peri-implantitis was considered based on current evidence and Bradford Hill criteria.
(A–E) Implant surface and surrounding microenvironment (i.e., low oxygen level) seem a suitable site for Candida colonization, mainly after protein pellicle adsorption; (B) Candida interactions with bacteria highly associated with peri-implantitis, such as P. gingivalis, promoting bacterial growth, virulence, and the ability to invade host cells; (C) Cross-kingdom and synergistic interaction with Streptococcus species, group highly found in healthy and disease implant sites, forming a hyper-virulent biofilm with increased bacterial biomass, upregulating hyphae-related genes, exacerbated inflammatory response, and tissue damage; (D) Candida increases the extracellular polymers synthesis by bacterial species, enhancing biofilm matrix which has several advantages for microbial accumulation, leading to microbial dysbiosis and increased virulence; therefore, high Candida count is expected to lead to increased biofilm matrix synthesis; (E) The effect on bacteria growth and biofilm virulence lead to increased tissue damage. Created with BioRender.com (license number: CA22TEEYEL).
Current in vivo evidence of C. albicans colonization in implant-related biofilms
To further dissect this research topic regarding the ability of C. albicans to colonize implant surfaces and play a role on peri-implantitis, systematic reviews are an essential tool to synthesize the scientific information available, enhancing the validity of the findings of individual studies while at the same time detecting areas of uncertainty that require further research. Keeping this in mind, we conducted a systematic review of in vivo studies that evaluated the presence or level of Candida on Ti surface (Supplemental material). The initial search identified a total of 1,287 references collected from all databases. Then, 181 duplicates were removed, and in the title and abstract screening, 1,057 records were excluded according to the eligibility criteria. Out of a total of 98 articles thoroughly assessed in full text, 74 were considered not eligible according to the inclusion/extrusion criteria checklist. Consequently, 24 in vivo studies were included in this systematic review (Supplemental material).
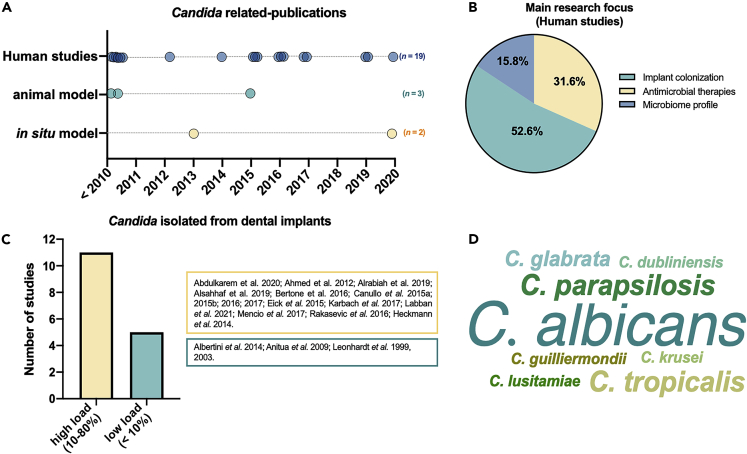
Most of the studies included (more than 60%) were published between 2010 and 2020, showing an increasing interest for microbiological evaluations of biofilms formed on Ti surface considering the presence of Candida. Among included studies, three were animal models, two in situ studies with humans, and 19 were clinical studies (Figure 6A). Most in vivo studies aimed to evaluate the microbiome profile (52.6%) of biofilm accumulated on the implant surface (Figure 6B). Although some studies evaluated specific therapeutic approaches to reduce microbial colonization, the effect on Candida was considered. Interestingly, most of the studies showed that Candida spp. were found in high load (10%–80%) on implant surface in terms of implants contaminated with these organisms (Figure 6C), and C. albicans was the species most commonly evaluated and identified (Figure 6D), followed by C. parapsilosis and C. tropicalis.
Figure 6.
Results of the systematic review to identify in vivo studies evaluating the presence or level of Candida on the implant surface
(A) Twenty-four in vivo studies were included among animal, in situ, and human models.
(B) The main research focus of included studies.
(C) The number of studies describing high (10%–80%) or low (<10%) Candida load on the implant surface.
(D) Word cloud graph of Candida species found on implant surface according to the number of studies.
The animal studies considered models evaluating microbiological infection in dogs (Shibli et al., 2003), monkeys (Eke et al., 1998), and mice (Kucharíková et al., 2016). Two studies considered the implant insertion in the oral cavity to mimic oral conditions (Eke et al., 1998; Shibli et al., 2003) and, therefore, implants were exposed to the oral environment with saliva, and oral microorganisms (Eke et al., 1998) and microbial accumulation were promoted by ligatures (Shibli et al., 2003).
All studies evaluated the presence of Candida and other microbial species, mainly periodontopathogens. Two studies considered the normal oral flora for microbial colonization (Eke et al., 1998; Shibli et al., 2003). One study (Kucharíková et al., 2016) used a C. albicans' infection model to evaluate the ability of newly developed implant surface to reduce Candida colonization, showing a reduction of 80% for caspofungin (CAS)-coated titanium discs. There is a high heterogeneity of studies in terms of experimental models applied, such as infection source, time of evaluation, and even sites or type of implant insertion, which makes hard some comparisons (Supplemental material). Unfortunately, the studies considered microbiological techniques only to evaluate the presence or level of Candida. Further animal studies should consider infection models to assess the mechanism of fungal colonization and the cross-kingdom interaction between Candida and important implant-related pathogens on the Ti surface. Moreover, information related to the effect of Candida on biofilms growing on implant surface in terms of microbial load, virulence factors, and consequent mucosal damage needs to be experimentally tested to better determine biological gradient through dose-response mechanisms (Supplemental material).
For studies with humans, two in situ studies (do Nascimento et al., 2013; Koch et al., 2020) were included considering the biofilm accumulation on implant surface in the oral environment using Ti substrate on oral devices for 24 h. Interestingly, both studies showed that Ti surface was colonized by different Candida species, including C. albicans, even for only 24 h of biofilm formation, indicating that this material may be a suitable substrate for Candida adhesion (Supplemental material). However, none of the studies was able to re-create a subgingival environment closely resembling the microenvironment of a peri-implant sulcus.
As for animal studies, in vivo studies with humans also showed a high diversity of experimental designs, making it difficult to draw conclusions. All in vivo studies with humans included adult patients, mainly healthy individuals, but also considered diabetic patients (Labban et al., 2021; Alsahhaf et al., 2019), submitted to radiation therapy (Karbach et al., 2007), with the previous history of oral cancer (Ahmed et al., 2012), and liver transplant recipients (Heckmann et al., 2004). In terms of oral condition, totally and partially edentulous patients (Eick et al., 2016), with and without peri-implantitis (Leonhardt et al., 1999; Rakašević et al., 2016) and with periodontitis, were investigated. The use of paper points was the common technique for microbial collection. Quantitative analysis was done through CFU, DNA probe technology, and real-time PCR to identify and quantify the microorganisms.
Overall, in vivo studies with humans showed the ability of Candida to colonize the implant surface because the fungal was identified in biofilms formed on implant surface or samples from surrounding sites (i.e., sulcular fluid). However, the findings were inconsistent, because some studies found Candida species were equally frequent in disease and healthy sites (Ahmed et al., 2012), but others with higher frequency for peri-implantitis sites (Bertone et al., 2016; Alrabiah et al., 2019).
For studies showing a higher frequency of Candida on disease sites, C. albicans was the most abundant fungal species (Bertone et al., 2016; Canullo et al., 2016; Alrabiah et al., 2019). Only two studies (Leonhardt et al., 2003; Rakašević et al., 2016) did not find the presence of Candida, but one of them (Rakašević et al., 2016) tested antimicrobial strategies for biofilm removal, which explain the findings. Interestingly, only three studies (Labban et al., 2021; Alrabiah et al., 2019; Alsahhaf et al., 2019) did not inform the evaluation of other microbial species, such as bacteria, which suggest that Candida is an emergent pathogen that has been considered by microbiological analysis of biofilms formed on implant surface (Supplemental material). As mentioned above, although some implant-related studies have assessed the presence of Candida in the microbial findings, it has focused only on the presence or level of this organism.
The unraveling role of Candida on peri-implantitis opens a broad opportunity for further studies focusing on: (1) The presence and level of Candida in different periods/stages of disease progression; (2) Candida–bacteria interactions on peri-implantitis sites; (3) Virulence factors upregulated and correlated with Candida level; (4) Omics analysis to identify the effect of Candida presence and level on microbial composition and metabolism; (5) Effect on tissue invasion and damage; and (6) Microscopic analysis to identify its direct effect on biofilm structure and architecture.
Concluding remarks and future perspectives
Candida has been overlooked by implant-related infection studies. It is an opportunistic pathogen affecting mainly medically compromised patients. Interestingly, Candida spp. infections share a significant number of risk factors/indicators with the peri-implant diseases, indicating possible association. The current evidence and our review suggest that this organism has to be considered a new villain in the pathogenesis of dental implant-related infections, because it may play an important role in the progression of peri-implantitis by modulating the host immune response and the virulence of periodontal pathogens often found in diseased sites and candidalysin role should be further investigated in this scenario. In vivo findings show that implant surface is a suitable substrate for Candida colonization and growth. Furthermore, this fungal has been found in higher levels in biofilms from peri-implantitis sites, compared to healthy ones, showing biological plausibility. Therefore, although the evidence is limited, the current literature shows clearly that Candida has been found on implant infection sites and the in vitro and animal studies have shown the synergistic relationship of Candida and bacteria highly related to these infections.
The microbiome related to peri-implantitis has been widely investigated considering bacterial species, which are highlighted to be the main microorganisms triggering inflammatory processes and leading to increased tissue damage. Still, few studies correlated fungal loads and created a temporal sequence of fungal colonization and disease progression. Considering the current evidence and conditions that promote Candida growth in the oral environment, the fungal presence may not directly affect the etiopathogenesis of peri-implantitis. Still, it can exacerbate inflammatory response from the host epithelial receptors and increase tissue damage via synergism with bacterial species.
Thus, evidence suggests that C. albicans can indirectly affect the disease progression with a significant effect on microbiological and clinical findings because this organism can interact synergistically with bacteria present in peri-implant sites promoting increased microbial load and biofilm virulence. Previous studies have shown that Candida–bacteria interaction leads to exacerbated inflammatory response and mucosal damage (Xu et al., 2014a, 2014b; 2017; Diaz et al., 2012a; Souza et al., 2020a, 2020b, 2020d). Such evidence is also available by analogy on periodontitis severity, which correlated with higher fungal loads. However, additional well-defined studies are necessary to elucidate the role of Candida further before true causality can be defined.
Unfortunately, most of the knowledge evaluating the interaction of Candida with common oral bacteria has not considered biofilms growing on the Ti surface, which may modulate this relationship (Souza et al., 2020a, 2020b). Moreover, although the cross-kingdom interaction between Candida and Streptococcus species has been widely explored, such interaction with putative periodontal pathogens related to implant-related infections needs increased attention by further studies. Additionally, although there is no consensus regarding the best therapeutic protocol for peri-implantitis (Heitz-Mayfield and Mombelli, 2014), the current modalities have not considered the effect of antifungal, even for the development of new surfaces with antimicrobial abilities (Souza et al., 2020c). Thus, further studies should consider the evaluation of Candida’s role triggering or promoting microbiological shift on dental implants, inflammatory process, and immune response, as well the synergistic relationship with pathogens highly related to peri-implantitis. Moreover, in vivo evidence may consider the presence of Candida on therapeutic approach to achieve an optimal treatment protocol for these infections.
Therefore, the current evidence suggests that C. albicans, a frequent fungal found on the implant surface and peri-implantitis sites with the ability to increase the bacterial load and biofilm accumulation, is an emergent villain on implant-related infections, affecting indirectly the disease progression and tissue damage due to its effect on bacteria growth and biofilm virulence.
Limitations of the study
The current evidence regarding the role of Candida in dental implant-related infections is limited and mainly originated from in vitro and animal studies. Moreover, dental implants can be made with different biomaterials, which affect directly microbial adhesion and accumulation and were not considered in our systematic review. Finally, the studies included in our systematic review have some limitations, such as: (1) a high heterogeneity of studies in terms of experimental models applied; (2) the studies did not consider Candida virulence factors and tissue damage; (3) some studies did not specify the Candida species; (4) different microbiological methods were used to quantify Candida and bacteria species. In addition, we did not conduct a qualitative analysis of the included studies.
Acknowledgments
AcknowledgmentValentim A. R. Barão was supported by the São Paulo Research Foundation (FAPESP) (#2020/05231-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (#304853/2018-6 and 307471/2021-7). Bruna E. Nagay was supported by FAPESP (#2019/17238-6). Raphael Cavalcante Costa was supported by FAPESP (#2020/10436-4). Victoria Abdo was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível (Finance code 001). Graphical abstract created and adapted from “Wnt Signaling During Cardiomyocyte Differentiation” template, by BioRender.com (2022 – license number: CZ23IPRJV5).
Author contributions
Conceptualization, J.G.S.S., M.B., and V.A.R.B.; Methodology, J.G.S.S., R.C.C., B.E.N., A.A.S., V.L.A., B.R.V., and N.C.; Investigation, J.G.S.S., M.B., R.C.C., and B.E.N.; Writing—Original Draft, J.G.S.S., B.E.N., and M.B.; Writing—Review & Editing, M.B., R.C.C., B.E.N., J.G.S.S., and V.A.R.B.; Supervision, J.G.S.S., M.B., M.F., J.A.S., and V.A.R.B.; Funding Acquisition, J.G.S. and V.A.R.B.
Declaration of interest
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.103994
Contributor Information
João G.S. Souza, Email: joao.gabriel@prof.ung.br.
Valentim A.R. Barão, Email: vbarao@unicamp.br.
Supplemental information
References
- Ahmed A., Chambers M.S., Goldschmidt M.C., Habib A., Lei X., Jacob R.F. Association between microbial flora and tissue abnormality around dental implants penetrating the skin in reconstructed oral cancer patients. Int. J. Oral Maxill. Implants. 2012;27:684–694. [PubMed] [Google Scholar]
- Alqahtani F. Role of oral yeasts in the etiopathogenesis of peri-implantitis: an evidence-based literature review of clinical studies. Arch. Oral Biol. 2020;111:104650. doi: 10.1016/j.archoralbio.2020.104650. [DOI] [PubMed] [Google Scholar]
- Alrabiah M., Alshagroud R.S., Alsahhaf A., Almojaly S.A., Abduljabbar T., Javed F. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant Dent Relat. Res. 2019;21:781–785. doi: 10.1111/cid.12760. [DOI] [PubMed] [Google Scholar]
- Alsahhaf A., Al-Aali K.A., Alshagroud R.S., Alshiddi I.F., Alrahlah A., Abduljabbar T., Javed F., Vohra F. Comparison of yeast species in the subgingival oral biofilm of individuals with type 2 diabetes and peri-implantitis and individuals with peri-implantitis without diabetes. J. Periodontol. 2019;90:1383–1389. doi: 10.1002/JPER.19-0091. [DOI] [PubMed] [Google Scholar]
- Andes D., Nett J., Oschel P., Albrecht R., Marchillo K., Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004;72:6023–6031. doi: 10.1128/IAI.72.10.6023-6031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Bor B., Agnello M., Shi W., He X. Ecology of the oral microbiome: beyond bacteria. Trends Microbiology. 2017;25:362–374. doi: 10.1016/j.tim.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford C.V., d'Mello A., Nobbs A.H., Dutton L.C., Vickerman M.M., Jenkinson H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009;77:3696–3704. doi: 10.1128/IAI.00438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barao V.A.R., Costa R.C., Shibli J., Bertolini M., Souza J.G.S. Emerging titanium surface modifications: The war against polymicrobial infections on dental implants. Braz. Dent. J. 2022;33:1–12. doi: 10.1590/0103-6440202204860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda-Ferre P., Alcaraz L.D., Cabrera-Rubio R., Romero H., Simón-Soro A., Pignatelli M., Mira A. The oral metagenome in health and disease. ISME J. 2012;6:46–56. doi: 10.1038/ismej.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belibasakis G.N., Manoil D. Microbial community-driven etiopathogenesis of peri-implantitis. J. dental Res. 2021;100:21–28. doi: 10.1177/0022034520949851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E., et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45:S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- Bertolini M.M., Xu H., Sobue T., Nobile C.J., Del Bel Cury A.A., Dongari-Bagtzoglou A. Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes. Mol. Oral Microbiol. 2015;30:307–322. doi: 10.1111/omi.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M., Ranjan A., Thompson A., Diaz P.I., Sobue T., Maas K., Dongari-Bagtzoglou A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019;22:e1007717. doi: 10.1371/journal.ppat.1007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M., Dongari-Bagtzoglou A. The dysbiosis and inter-kingdom synergy model in oropharyngeal candidiasis, a new perspective in pathogenesis. J. Fungi (Basel, Switzerland) 2019;5:87. doi: 10.3390/jof5040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M., Dongari-Bagtzoglou A. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. Adv. Exp. Med. Biol. 2019;1197:69–78. doi: 10.1007/978-3-030-28524-1_6. [DOI] [PubMed] [Google Scholar]
- Bertone A.M., Rosa A.C., Nastri N., Santillán H.D., Ariza Y., Iovannitti C.A., Jewtuchowicz V.M. Genetic-relatedness of peri-implants and buccal Candida albicans isolates determined by RAPD-PCR. Relación genética de aislamientos de Candida albicans por RAPD-PCR en surcos peri-implantarios de cavidad bucal. Acta odontologica latinoamericana: AOL. 2016;29:197–205. [PubMed] [Google Scholar]
- Boktour M.R., Kontoyiannis D.P., Hanna H.A., Hachem R.Y., Girgawy E., Bodey G.P., Raad I.I. Multiple-species candidemia in patients with cancer. Cancer. 2004;101:1860–1865. doi: 10.1002/cncr.20573. [DOI] [PubMed] [Google Scholar]
- Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–89. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen W.H., Burne R.A., Wu H., Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:69–86. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgers R., Hahnel S., Reichert T.E., Rosentritt M., Behr M., Gerlach T., Handel G., Gosau M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010;6:2307–2313. doi: 10.1016/j.actbio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Canabarro A., Valle C., Farias M.R., Santos F.B., Lazera M., Wanke B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J. Periodontal Res. 2013;48:428–432. doi: 10.1111/jre.12022. [DOI] [PubMed] [Google Scholar]
- Canullo L., Peñarrocha-Oltra D., Covani U., Botticelli D., Serino G., Penarrocha M. Clinical and microbiological findings in patients with peri-implantitis: a cross-sectional study. Clin. Oral Implants Res. 2016;27:376–382. doi: 10.1111/clr.12557. [DOI] [PubMed] [Google Scholar]
- Canullo L., Peñarrocha-Oltra D., Covani U., Rossetti P.H. Microbiologic and clinical findings of implants in healthy condition and with peri-implantitis. Int. J. Oral Maxill. Implants. 2015;30:834–842. doi: 10.11607/jomi.3947. [DOI] [PubMed] [Google Scholar]
- Canullo L., Peñarrocha M., Monje A., Catena A., Wang H.L., Peñarrocha D. Association between clinical and microbiologic cluster profiles and peri-implantitis. Int. J. Oral Maxill. Implants. 2017;32:1054–1064. doi: 10.11607/jomi.6043. [DOI] [PubMed] [Google Scholar]
- Canullo L., Radovanović S., Delibasic B., Blaya J.A., Penarrocha D., Rakic M. The predictive value of microbiological findings on teeth, internal and external implant portions in clinical decision making. Clin. Oral Implants Res. 2017;28:512–519. doi: 10.1111/clr.12828. [DOI] [PubMed] [Google Scholar]
- Casale T.B., Carolan E.J. Combination of IL-8 plus TNF alpha induces additive neutrophil migration. Allergy Asthma Proc. 1999;20:361–363. doi: 10.2500/108854199778251852. [DOI] [PubMed] [Google Scholar]
- Cavalcanti I.M., Ricomini Filho A.P., Lucena-Ferreira S.C., da Silva W.J., Paes Leme A.F., Senna P.M., Del Bel Cury A.A. Salivary pellicle composition and multispecies biofilm developed on titanium nitrided by cold plasma. Arch. Oral Biol. 2014;59:673–695. doi: 10.1016/j.archoralbio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Cavalcanti Y.W., Morse D.J., da Silva W.J., Del-Bel-Cury A.A., Wei X., Wilson M., Milward P., Lewis M., Bradshaw D., Williams D.W. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling. 2015;31:27–38. doi: 10.1080/08927014.2014.996143. [DOI] [PubMed] [Google Scholar]
- Cavalcanti Y.W., Wilson M., Lewis M., Williams D., Senna P.M., Del-Bel-Cury A.A., da Silva W.J. Salivary pellicles equalise surfaces' charges and modulate the virulence of Candida albicans biofilm. Arch. Oral Biol. 2016;66:129–140. doi: 10.1016/j.archoralbio.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Cavalcanti Y.W., Wilson M., Lewis M., Del-Bel-Cury A.A., da Silva W.J., Williams D.W. Modulation of Candida albicans virulence by bacterial biofilms on titanium surfaces. Biofouling. 2016;32:123–134. doi: 10.1080/08927014.2015.1125472. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Feng G., Moraru C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: a review. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouirfa H., Bouloussa H., Migonney V., Falentin-Daudré C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019;83:37–54. doi: 10.1016/j.actbio.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Conti H.R., Bruno V.M., Childs E.E., Daugherty S., Hunter J.P., Mengesha B.G., et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host & Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro J.M., Barão V. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C, Mater. Biol. Appl. 2017;71:1201–1215. doi: 10.1016/j.msec.2016.10.025. [DOI] [PubMed] [Google Scholar]
- Costa R.C., Souza J., Bertolini M., Retamal-Valdes B., Feres M., Barão V. Extracellular biofilm matrix leads to microbial dysbiosis and reduces biofilm susceptibility to antimicrobials on titanium biomaterial: an in vitro and in situ study. Clin. Oral Implants Res. 2020;31:1173–1183. doi: 10.1111/clr.13663. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Lewandowski Z., Caldwell D.E., Korber D.R., Lappin-Scott H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Curvelo J.A.R., Moraes D.C., Anjos C.A.D., Portela M.B., Soares R.M.A. Histatin 5 and human lactoferrin inhibit biofilm formation of a fluconazole resistant Candida albicans clinical isolate. Acad. Bras Cienc. 2019;91 doi: 10.1590/0001-3765201920180045. [DOI] [PubMed] [Google Scholar]
- Daya Attie M., Anderson I.A., Portnof J. Mandibular osteomyelitis associated with Candida albicans in marijuana and heroin abusers. Ann. Maxill. Surg. 2018;8:355–357. doi: 10.4103/ams.ams_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P.I., Chalmers N.I., Rickard A.H., Kong C., Milburn C.L., Palmer R.J., Jr., Kolenbrander P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P.I., Dupuy A.K., Abusleme L., Reese B., Obergfell C., Choquette L., Dongari-Bagtzoglou A., Peterson D.E., Terzi E., Strausbaugh L.D. Using high throughput sequencing to explore the biodiversity in oral bacterial communities. Mol. Oral Microbiol. 2012;27:182–201. doi: 10.1111/j.2041-1014.2012.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P.I., Strausbaugh L.D., Dongari-Bagtzoglou A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P.I., Xie Z., Sobue T., Thompson A., Biyikoglu B., Ricker A., Ikonomou L., Dongari-Bagtzoglou A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012;80:620–632. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento C., Pita M.S., Pedrazzi V., de Albuquerque Junior R.F., Ribeiro R.F. In vivo evaluation of Candida spp. adhesion on titanium or zirconia abutment surfaces. Arch. Oral Biol. 2013;58:853–861. doi: 10.1016/j.archoralbio.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Dostal J., Dlouhá H., Malon P., Pichova I., Hrušková-Heidingsfeldová O. The precursor of secreted aspartic proteinase Sapp1p from Candida parapsilosis can be activated both autocatalytically and by a membrane-bound processing proteinase. Biol. Chem. 2005;386:791–799. doi: 10.1515/BC.2005.093. [DOI] [PubMed] [Google Scholar]
- Egland P.G., Palmer R.J., Jr., Kolenbrander P.E. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc. Natl. Acad. Sci. U S A. 2004;101:16917–16922. doi: 10.1073/pnas.0407457101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick S., Ramseier C.A., Rothenberger K., Brägger U., Buser D., Salvi G.E. Microbiota at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implants Res. 2016;27:218–225. doi: 10.1111/clr.12588. [DOI] [PubMed] [Google Scholar]
- Eke P.I., Braswell L.D., Fritz M.E. Microbiota associated with experimental peri-implantitis and periodontitis in adult Macaca mulatta monkeys. J. Periodontol. 1998;69:190–194. doi: 10.1902/jop.1998.69.2.190. [DOI] [PubMed] [Google Scholar]
- Falsetta M.L., Klein M.I., Colonne P.M., Scott-Anne K., Gregoire S., Pai C.H., Gonzalez-Begne M., Watson G., Krysan D.J., Bowen W.H., Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Rivero M.E., Del Pozo J.L., Ramírez P., Valentín E., Ruiz-Gaitán A., Pemán J., Cantón E. Time-kill assays of amphotericin B plus anidulafungin against Candida tropicalis biofilms formed on two different biomaterials. Int. J. Artif. Organs Adv. Online Publ. 2017;41:23–27. doi: 10.5301/ijao.5000652. [DOI] [PubMed] [Google Scholar]
- Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Fournier B.M., Parkos C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- Frade J.P., Arthington-Skaggs B.A. Effect of serum and surface characteristics on Candida albicans biofilm formation. Mycoses. 2011;54:e154–e162. doi: 10.1111/j.1439-0507.2010.01862.x. [DOI] [PubMed] [Google Scholar]
- French D., Grandin H.M., Ofec R. Retrospective cohort study of 4,591 dental implants: analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019;90:691–700. doi: 10.1002/JPER.18-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch E., Ziebolz D., Vach K., Ratka-Krüger P. Supportive post-implant therapy: patient compliance rates and impacting factors: 3-year follow-up. J. Clin. Periodontol. 2014;41:1007–1014. doi: 10.1111/jcpe.12298. [DOI] [PubMed] [Google Scholar]
- Galan-Ladero M.A., Blanco M.T., Sacristan B., Fernandez-Calderon M.C., Perez-Giraldo C., Gomez-Garcia A.C. Enzymatic activities of Candida tropicalis isolated from hospitalized patients. Med. Mycol. 2010;48:207–210. doi: 10.3109/13693780902801242. [DOI] [PubMed] [Google Scholar]
- Gamaletsou M.N., Rammaert B., Bueno M.A., Sipsas N.V., Moriyama B., Kontoyiannis D.P., Roilides E., Zeller V., Taj-Aldeen S.J., Miller A.O., et al. Candida arthritis: analysis of 112 pediatric and adult cases. Open Forum Infect. Dis. 2015;3 doi: 10.1093/ofid/ofv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M.A., Jurevic R.J., Mukherjee P.K., Cui F., Sikaroodi M., Naqvi A., Gillevet P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glee P.M., Cutler J.E., Benson E.E., Bargatze R.F., Hazen K.C. Inhibition of hydrophobic protein-mediated Candida albicans attachment to endothelial cells during physiologic shear flow. Infect. Immun. 2001;69:2815–2820. doi: 10.1128/IAI.69.5.2815-2820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves L.S., Souto R., Colombo A.P. Detection of Helicobacter pylori, Enterococcus faecalis, and Pseudomonas aeruginosa in the subgingival biofilm of HIV-infected subjects undergoing HAART with chronic periodontitis. Eur. J. Clin. Microbiol. Infect. Dis. official Publ. Eur. Soc. Clin. Microbiol. 2009;28:1335–1342. doi: 10.1007/s10096-009-0786-5. [DOI] [PubMed] [Google Scholar]
- Gregoire S., Xiao J., Silva B.B., Gonzalez I., Agidi P.S., Klein M.I., Ambatipudi K.S., Rosalen P.L., Bauserman R., Waugh R.E., Koo H. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011;77:6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel B., Montenegro S., Dantas P., Pascoal A., Lima K.C., Calderon P. Frequency of peri-implant diseases and associated factors. Clin. Oral Implants Res. 2017;28:1211–1217. doi: 10.1111/clr.12944. [DOI] [PubMed] [Google Scholar]
- Heckmann S.M., Heckmann J.G., Linke J.J., Hohenberger W., Mombelli A. Implant therapy following liver transplantation: clinical and microbiological results after 10 years. J. Periodontol. 2004;75:909–913. doi: 10.1902/jop.2004.75.6.909. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L.J. Peri-implant diseases: diagnosis and risk indicators. J. Clin. Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L.J., Mombelli A. The therapy of peri-implantitis: a systematic review. Int. J. Oral Maxill. Implants. 2014;29:325–345. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- Hill A.B. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler A.R., Conti H.R., Hernández-Santos N., Darville T., Biswas P.S., Gaffen S.L. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J. Immunol. (Baltimore, Md.) 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabra-Rizk M.A., Kong E.F., Tsui C., Nguyen M.H., Clancy C.J., Fidel P.L., Jr., Noverr M. Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infect. Immun. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson H.F., Lamont R.J. Oral microbial communities in sickness and in health. Trends Microbiology. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Kalasin S., Santore M.M. Non-specific adhesion on biomaterial surfaces driven by small amounts of protein adsorption. Colloids Surfaces. B, Biointerfaces. 2009;73:229–236. doi: 10.1016/j.colsurfb.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Karbach J., Callaway A., Willershausen B., Wagner W., Geibel M.A., Al-Nawas B. Antibiotic resistance testing of the total implant-associated micro-flora and its pure isolates. Eur. J. Med. Res. 2007;12:120–128. PMID: 17507308. [PubMed] [Google Scholar]
- Kim H.E., Dhall A., Liu Y., Bawazir M., Koo H., Hwang G. Intervening in symbiotic cross-kingdom biofilm interactions: a binding mechanism-based nonmicrobicidal approach. mBio. 2021;12 doi: 10.1128/mBio.00651-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Boedicker J.Q., Choi J.W., Ismagilov R.F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc. Natl. Acad. Sci. United States America. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Burkovski A., Zulla M., Rosiwal S., Geißdörfer W., Dittmar R., Grobecker-Karl T. Pilot study on the use of a laser-structured double diamond electrode (DDE) for biofilm removal from dental implant surfaces. J. Clin. Med. 2020;9:3036. doi: 10.3390/jcm9093036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A.Y. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot. Cel. 2013;12:1416–1422. doi: 10.1128/EC.00196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic E.M., Darouiche R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec L., Vacca-Smith A., Bowen W. Structural aspects of glucans formed in solution and on the surface of hydroxyapatite. Glycobiology. 1997;7:929–934. doi: 10.1093/GLYCOB/7.7.929. [DOI] [PubMed] [Google Scholar]
- Kraneveld E.A., Buijs M., Bonder M., Visser M., Keijser B., Crielaard W., Zaura E. The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PLoS ONE. 2012;7:e42770. doi: 10.1371/journal.pone.0042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharíková S., Gerits E., De Brucker K., Braem A., Ceh K., Majdič G., Španič T., Pogorevc E., Verstraeten N., Tournu H., et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016;71:936–945. doi: 10.1093/jac/dkv437. [DOI] [PubMed] [Google Scholar]
- Kumar P.S., Mason M.R., Brooker M.R., O'Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012;39:425–433. doi: 10.1111/j.1600-051X.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labban N., Alfouzan A.F., Al Taweel S.M., ALRabiah M.A., Assery M.K. Role of antimicrobial photodynamic therapy in reducing whole salivary oral yeasts colonization in type-2 diabetic and non-diabetic patients with and without dental implants. Photodiagnosis Photodyn Ther. 2021;33:102183. doi: 10.1016/j.pdpdt.2021.102183. [DOI] [PubMed] [Google Scholar]
- Lafuente-Ibáñez de Mendoza I., Cayero-Garay A., Quindós-Andrés G., Aguirre-Urizar J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant dentistry. 2021;7 doi: 10.1186/s40729-021-00338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N.P., Brägger U., Walther D., Beamer B., Kornman K.S. Ligature-induced peri-implant infection in cynomolgus monkeys. I. Clinical and radiographic findings. Clin. Oral Implants Res. 1993;4:2–11. doi: 10.1034/j.1600-0501.1993.040101.x. [DOI] [PubMed] [Google Scholar]
- Leonhardt A., Dahlén G., Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J. Periodontol. 2003;74:1415–1422. doi: 10.1902/jop.2003.74.10.1415. [DOI] [PubMed] [Google Scholar]
- Leonhardt A., Renvert S., Dahlén G. Microbial findings at failing implants. Clin. Oral Implants Res. 1999;10:339–345. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo T., Zhao J., Wang J. Effects of polishing methods on Candida albicans adhesion on cast pure titanium surfaces. Implant dentistry. 2013;22:546–551. doi: 10.1097/ID.0b013e3182a03ce9. [DOI] [PubMed] [Google Scholar]
- Liljemark W.F., Gibbons R.J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect. Immun. 1973;8:846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J., Berglundh T., Ericsson I., Liljenberg B., Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin. Oral Implants Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- Lopes J.P., Stylianou M., Backman E., Holmberg S., Jass J., Claesson R., Urban C.F. Evasion of immune surveillance in low oxygen environments enhances Candida albicans virulence. mBio. 2018;9 doi: 10.1128/mBio.02120-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P.D., Moter A., Devine D.A. Dental plaque biofilms: communities, conflict and control. Periodontology. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- Martorano-Fernandes L., Rodrigues N.C., Borges M.H., Cavalcanti Y., Almeida L.F. Interkingdom interaction between C. albicans and S. salivarius on titanium surfaces. BMC Oral Health. 2020;20 doi: 10.1186/s12903-020-01334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic Petrovic S., Radunovic M., Barac M., Kuzmanovic Pficer J., Pavlica D., Arsic Arsenijevic V., Pucar A. Subgingival areas as potential reservoirs of different Candida spp in type 2 diabetes patients and healthy subjects. PLoS ONE. 2019;14:e0210527. doi: 10.1371/journal.pone.0210527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza I.L.I., Cayero-Garay A., Quindos-Andres G., Aguirre-Urizar J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant Dentistry. 2021;7:73. doi: 10.1186/s40729-021-00338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Montelongo-Jauregui D., Lopez-Ribot J.L. Candida interactions with the oral bacterial microbiota. J. Fungi (Basel, Switzerland) 2018;4:122. doi: 10.3390/jof4040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelongo-Jauregui D., Srinivasan A., Ramasubramanian A.K., Lopez-Ribot J.L. An in vitro model for Candida albicans⁻Streptococcus gordonii biofilms on titanium surfaces. J. fungi (Basel, Switzerland) 2018;4:66. doi: 10.3390/jof4020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouhat M., Moorehead R., Murdoch C. In vitro Candida albicans biofilm formation on different titanium surface topographies. Biomaterial Invest. Dentistry. 2020;7:146–157. doi: 10.1080/26415275.2020.1829489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes D.L., Runglall M., Murciano C., Shen C., Nayar D., Selvam T., et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes D.L., Runglall M., Murciano C., Shen C., Nayar D., Thavaraj S., Kohli A., Islam A., Mora-Montes H., Challacombe S.J., Naglik J.R. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes D.L., Wilson D., Richardson J.P., Mogavero S., Tang S.X., Wernecke J., Höfs S., Gratacap R.L., Robbins J., Runglall M., et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;7:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes D.L., Wilson D., Richardson J.P., Mogavero S., Tang S.X., Wernecke J., et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y., Torii M., Urushibara Y., Kawai T., Takahashi Y., Maeda N., Ohkubo C., Ohshima T. Analysis of plaque microbiota and salivary proteins adhering to dental materials. J. Oral biosciences. 2020;62:182–188. doi: 10.1016/j.job.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Naginyte M., Do T., Meade J., Devine D.A., Marsh P.D. Enrichment of periodontal pathogens from the biofilms of healthy adults. Scientific Rep. 2019;9:5491. doi: 10.1038/s41598-019-41882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik J.R., Gaffen S.L., Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019;52:100–109. doi: 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik J.R., Richardson J.P., Moyes D.L. Candida albicans pathogenicity and epithelial immunity. PLoS Pathog. 2014;10:e1004257. doi: 10.1371/journal.ppat.1004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell L.E., Millhouse E., Sherry L., Kean R., Malcolm J., Nile C.J., Ramage G. Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS yeast Res. 2015;15 doi: 10.1093/femsyr/fov077. [DOI] [PubMed] [Google Scholar]
- Padial-Molina M., López-Martínez J., O'Valle F., Galindo-Moreno P. Microbial profiles and detection techniques in peri-implant diseases: a systematic review. J. Oral Maxill. Res. 2016;7:e10. doi: 10.5037/jomr.2016.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat. Rev. Dis. primers. 2018;4 doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Peters B.M., Jabra-Rizk M.A., O'May G.A., Costerton J.W., Shirtliff M.E. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012;25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela M.B., Souza I.P.R., Abreu C.M., Bertolini M., Holandino C., Alviano C.S., Santos A.L.S., Soares R.M.A. Effect of serine-type protease of Candida spp. isolated from linear gingival erythema of HIV-positive children: critical factors in the colonization. J. Oral Pathol. Med. 2010;39:753–760. doi: 10.1111/j.1600-0714.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- Pranno N., Cristalli M.P., Mengoni F., Sauzullo I., Annibali S., Polimeni A., Monaca G.L. Comparison of the effects of air-powder abrasion, chemical decontamination, or their combination in open-flap surface decontamination of implants failed for peri-implantitis: an ex vivo study. Clin. Oral Investig. 2021;25:2667–2676. doi: 10.1007/s00784-020-03578-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Asensio M., Ruiz-Camps I., Fernández-Ruiz M., Aguado J.M., Muñoz P., Valerio M., Delgado-Iribarren A., Merino P., Bereciartua E., Fortún J., Cuenca-Estrella M., Almirante B., CANDIPOP Project, Geih-Gemicomed Seimc, REIPI Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin. Microbiol. Infect. official Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015;21:491. doi: 10.1016/j.cmi.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Rabe M., Verdes D., Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interf. Sci. 2011;162:87–106. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Rakašević D., Lazić Z., Rakonjac B., Soldatović I., Janković S., Magić M., Aleksić Z. Efficiency of photodynamic therapy in the treatment of peri-implantitis – a three-month randomized controlled clinical trial. Srpski arhiv za celokupno lekarstvo. 2016;144:9–10. [PubMed] [Google Scholar]
- Ramage G., Martínez J.P., López-Ribot J.L. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS yeast Res. 2006;6:478–484. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- Ramsey M.M., Rumbaugh K.P., Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011;7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal-Valdes B., Formiga M.C., Almeida M.L., Fritoli A., Figueiredo K.A., Westphal M., Gomes P., Feres M. Does subgingival bacterial colonization differ between implants and teeth? A systematic review. Braz. Oral Res. 2019;33:064. doi: 10.1590/1807-3107bor-2019.vol33.0064. [DOI] [PubMed] [Google Scholar]
- Rickard A.H., Gilbert P., High N.J., Kolenbrander P.E., Handley P.S. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11 doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Ricker A., Vickerman M., Dongari-Bagtzoglou A. Streptococcus gordonii glucosyltransferase promotes biofilm interactions with Candida albicans. J. Oral Microbiol. 2014;6:94–100. doi: 10.3402/jom.v6.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier M.H., Moudni B.E., Kauffman-Lacroix C., Daniault G., Jacquemin J.L. A Candida albicans metallopeptidase degrades constitutive proteins of extracellular matrix. FEMS Microbiol. Lett. 1999;177:205–210. doi: 10.1111/j.1574-6968.1999.tb13733.x. [DOI] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- Romero-Gavilan F., Araújo-Gomes N., Sánchez-Pérez A.M., García-Arnáez I., Elortza F., Azkargorta M., de Llano J.J.M., Carda C., Gurruchaga M., Suay J., Goñi I. Bioactive potential of silica coatings and its effect on the adhesion of proteins to titanium implants. Colloids Surf B Biointerfaces. 2018;162:316–325. doi: 10.1016/j.colsurfb.2017.11.072. [DOI] [PubMed] [Google Scholar]
- Rosier B.T., Marsh P.D., Mira A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dental Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- Salvi G.E., Cosgarea R., Sculean A. Prevalence and mechanisms of peri-implant diseases. J. Dental Res. 2017;96:31–37. doi: 10.1177/0022034516667484. [DOI] [PubMed] [Google Scholar]
- Sampson A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000;30:22–27. doi: 10.1046/j.1365-2222.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- Santhana Krishnan G., Naik D., Uppoor A., Nayak S., Baliga S., Maddi A. Candidal carriage in saliva and subgingival plaque among smokers and non-smokers with chronic periodontitis-a cross-sectional study. PeerJ. 2020;8:e8441. doi: 10.7717/peerj.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi J.C., Duque C., Höfling J.F., Gonçalves R.B. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Med. Mycol. 2012;50:467–475. doi: 10.3109/13693786.2011.633233. [DOI] [PubMed] [Google Scholar]
- Scannapieco F.A., Dongari-Bagtzoglou A. Dysbiosis revisited: understanding the role of the oral microbiome in the pathogenesis of gingivitis and periodontitis: a critical assessment. J. Periodontol. 2021;92:1071–1078. doi: 10.1002/JPER.21-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F., Becker K., Rahn S., Hegewald A., Pfeffer K., Henrich B. Real-time PCR analysis of fungal organisms and bacterial species at peri-implantitis sites. Int. J. Implant dentistry. 2015;1:9. doi: 10.1186/s40729-015-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F., Derks J., Monje A., Wang H. Peri-implantitis. J. Clin. Periodontol. 2018;45:S246–S266. doi: 10.1002/JPER.16-0350. [DOI] [PubMed] [Google Scholar]
- Seidel C.L., Gerlach R.G., Wiedemann P., Weider M., Rodrian G., Hader M., Frey B., Gaipl U.S., Bozec A., Cieplik F., et al. Defining metaniches in the oral cavity according to their microbial composition and cytokine profile. Int. J. Mol. Sci. 2020;21:8218. doi: 10.3390/ijms21218218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino G., Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin. Oral Implants Res. 2009;20:169–174. doi: 10.1111/j.1600-0501.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- Shibli J.A., Martins M.C., Lotufo R.F., Marcantonio E., Jr. Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int. J. Oral Maxill. Implants. 2003;18:383–390. [PubMed] [Google Scholar]
- Shibli J.A., Melo L., Ferrari D.S., Figueiredo L.C., Faveri M., Feres M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral Implants Res. 2008;19:975–982. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- Silva S., Negri M., Henriques M., Oliveira R., Williams D.W., Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- Singleton D.R., Masuoka J., Hazen K.C. Cloning and analysis of a Candida albicans gene that affects cell surface hydrophobicity. J. Bacteriol. 2001;183:3582–3588. doi: 10.1128/JB.183.12.3582-3588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D. Dental biofilms: difficult therapeutic targets. Periodontology. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- Souza J., Bertolini M.M., Costa R.C., Nagay B.E., Dongari-Bagtzoglou A., Barão V. Targeting implant-associated infections: titanium surface loaded with antimicrobial. iScience. 2020;24:102008. doi: 10.1016/j.isci.2020.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J., Bertolini M., Costa R.C., Cordeiro J.M., Nagay B.E., de Almeida A.B., Retamal-Valdes B., Nociti F.H., Feres M., Rangel E.C., Barão V. Targeting pathogenic biofilms: newly developed superhydrophobic coating favors a host-compatible microbial profile on the titanium surface. ACS Appl. Mater. Inter. 2020;12:10118–10129. doi: 10.1021/acsami.9b22741. [DOI] [PubMed] [Google Scholar]
- Souza J., Bertolini M., Costa R.C., Lima C.V., Barão V. Proteomic profile of the saliva and plasma protein layer adsorbed on Ti-Zr alloy: the effect of sandblasted and acid-etched surface treatment. Biofouling. 2020;36:428–441. doi: 10.1080/08927014.2020.1769613. [DOI] [PubMed] [Google Scholar]
- Souza J., Bertolini M., Thompson A., Barão V., Dongari-Bagtzoglou A. Biofilm interactions of Candida albicans and mitis group streptococci in a titanium-mucosal interface model. Appl. Environ. Microbiol. 2020;89 doi: 10.1128/AEM.02950-19. e02950–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J., Bertolini M., Thompson A., Mansfield J.M., Grassmann A.A., Maas K., Caimano M.J., Barao V., Vickerman M.M., Dongari-Bagtzoglou A. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020;14:1207–1222. doi: 10.1038/s41396-020-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza J., Costa Oliveira B.E., Bertolini M., Lima C.V., Retamal-Valdes B., de Faveri M., Feres M., Barão V. Titanium particles and ions favor dysbiosis in oral biofilms. J. Periodontal Res. 2020;55:258–266. doi: 10.1111/jre.12711. [DOI] [PubMed] [Google Scholar]
- Souza J., Cury J.A., Ricomini Filho A.P., Feres M., Faveri M., Barão V. Effect of sucrose on biofilm formed in situ on titanium material. J. Periodontol. 2019;90:141–148. doi: 10.1002/JPER.18-0219. [DOI] [PubMed] [Google Scholar]
- Spriano S., Yamaguchi S., Baino F., Ferraris S. A critical review of multifunctional titanium surfaces: new frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta biomaterialia. 2018;79:1–22. doi: 10.1016/j.actbio.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Sultan A.S., Kong E.F., Rizk A.M., Jabra-Rizk M.A. The oral microbiome: a Lesson in coexistence. PLoS Pathog. 2018;14:e1006719. doi: 10.1371/journal.ppat.1006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidergall M., Solis N.V., Lionakis M.S., Filler S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat. Microbiol. 2018;3:53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztukowska M.N., Dutton L.C., Delaney C., Ramsdale M., Ramage G., Jenkinson H.F., Nobbs A.H., Lamont R.J. Community development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. mBio. 2018;9:e00202–e00218. doi: 10.1128/mBio.00202-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai R., Sugamata M., Kiyoura Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. pathogenesis. 2011;51:250–254. doi: 10.1016/j.micpath.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Vieira Colombo A.P., Magalhães C.B., Hartenbach F.A., Martins do Souto R., Maciel da Silva-Boghossian C. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb. pathogenesis. 2016;94:27–34. doi: 10.1016/j.micpath.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Vila T., Sultan A.S., Montelongo-Jauregui D., Jabra-Rizk M.A. Oral candidiasis: a disease of opportunity. J. fungi (Basel, Switzerland) 2020;6:15. doi: 10.3390/jof6010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissink A., Spijkervet F., Raghoebar G.M. The medically compromised patient: are dental implants a feasible option? Oral Dis. 2018;24:253–260. doi: 10.1111/odi.12762. [DOI] [PubMed] [Google Scholar]
- Wan S.X., Tian J., Liu Y., Dhall A., Koo H., Hwang G. Cross-kingdom cell-to-cell interactions in cariogenic biofilm initiation. J. dental Res. 2021;100:74–81. doi: 10.1177/0022034520950286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Miron R.J. Health, maintenance, and recovery of soft tissues around implants. Clin. Implant dentistry Relat. Res. 2016;18:618–634. doi: 10.1111/cid.12343. [DOI] [PubMed] [Google Scholar]
- Whitmore S.E., Lamont R.J. The pathogenic persona of community-associated oral streptococci. Mol. Microbiol. 2011;81:305–314. doi: 10.1111/j.1365-2958.2011.07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Jenkinson H.F., Dongari-Bagtzoglou A. Innocent until proven guilty: mechanisms and roles of Streptococcus-Candida interactions in oral health and disease. Mol. Oral Microbiol. 2014;29:99–116. doi: 10.1111/omi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Sobue T., Bertolini M., Thompson A., Dongari-Bagtzoglou A. Streptococcus oralis and Candida albicans Synergistically Activate μ-Calpain to Degrade E-cadherin From Oral Epithelial Junctions. J. Infect. Dis. 2016;15:925–934. doi: 10.1093/infdis/jiw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Sobue T., Bertolini M., Thompson A., Vickerman M., Nobile C.J., Dongari-Bagtzoglou A. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence. 2017;8:1602–1617. doi: 10.1080/21505594.2017.1326438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Sobue T., Thompson A., Xie Z., Poon K., Ricker A., Cervantes J., Diaz P.I., Dongari-Bagtzoglou A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014;16:214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H.C., Lu J.J., Chang S.,C., Ge M.C. Identification of microbiota in peri-implantitis pockets by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Scientific Rep. 2019;9:774. doi: 10.1038/s41598-018-37450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida E., Hayakawa T. Adsorption analysis of lactoferrin to titanium, stainless steel, zirconia, and polymethyl methacrylate using the quartz crystal microbalance method. Biomed. Res. Int. 2016;2016:1–7. doi: 10.1155/2016/3961286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.L., Chan Y., Zhuang L., Lai H.C., Lang N.P., Keung Leung W., Watt R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral Implants Res. 2019;30:760–766. doi: 10.1111/clr.13459. [DOI] [PubMed] [Google Scholar]
- Zomorodian K., Kavoosi F., Pishdad G.R., Mehriar P., Ebrahimi H., Bandegani A., Pakshir K. Prevalence of oral Candida colonization in patients with diabetes mellitus. J. de mycologie medicale. 2016;26:100–103. doi: 10.1016/j.mycmed.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.