Abstract
The opportunistic yeast Candida albicans and lactic acid bacteria Enterococcus faecalis are frequently co-isolated from various infection sites on the human body, suggesting a common interkingdom interaction. While some reports suggest an antagonism, the reason for their co-isolation therefore remains unclear. The purpose of this study was to undertake a detailed characterisation of this dual-species interaction. We used standard biofilm characterisation methodologies alongside an RNASeq analysis to assess the response of C. albicans to E. faecalis. We evaluated the relevance of pH to dual-species biofilm interactions and demonstrated that E. faecalis rapidly and significantly impacted C. albicans morphogenesis and biofilm formation, which was mirrored by levels of gene expression. These transcripts were enriched in amino acids biosynthesis and metabolism pathways in co-cultures, a finding that guided our investigation into pH related mechanism. We were able to demonstrate the direct role of E. faecalis induced low pH, which inhibited C. albicans hyphal morphogenesis and biofilm formation. The results suggest that the anti-candidal effect of E. faecalis is not based solely on a single mechanism, instead it may involve various mechanisms, which collectively reflects the complexity of interaction between C. albicans and E. faecalis and impacts treatment outcomes.
Keywords: Interkingdom, Biofilm, Candida albicans, Enterococcus faecalis, Supernatant, pH
Highlights
-
•
Candida albicans and Enterococcus faecalis are frequently co-isolated in human diseases.
-
•
C. albicans hyphal formation and biofilm formation is inhibited in dual-species with E. faecalis.
-
•
pH plays a key role in allowing C. albicans and E. faecalis to co-exist.
1. Introduction
Candida albicans biofilm infections are often polymicrobial, highlighting the importance of interkingdom interactions. These relationships may take the form of synergistic, antagonistic, or simply coexistence. It is well documented that C. albicans has the capacity to interact with various bacterial species, including Staphylococcus aureus, Streptococcus mutans and Pseudomonas aeruginosa [[1], [2], [3]]. Beyond the basis of these interactions, C. albicans influences the composition, virulence and dysbiosis of the bacterial microbiome [[4], [5], [6]]. Reciprocally, it has also been shown that C. albicans is in turn influenced by bacteria or bacterial products in terms of growth, virulence, resistance and phenotypic switch [[7], [8], [9]]. The pleiomorphic fungus C. albicans and Enterococcus faecalis are opportunistic pathogens that colonize various niches of the human body, such as the skin, oral cavity, vagina and gastrointestinal tract [[10], [11], [12], [13], [14]].
Endogenous in nature, C. albicans and E. faecalis can become pathogenetic due to impairment in host immunity or dysbiosis in microflora [15,16]. Infections caused by the opportunistic yeast C. albicans can range in severity from superficial oropharyngeal candidiasis to a serious life-threatening candidemia [17]. Likewise, translocation of E. faecalis from the gut to other body sites is associated with various infections, including endocarditis, meningitis, urinary tract infections and blood-stream fatal sepsis [16,18]. In particular, it has been observed that the oral microbiome of immunocompromised mice in oropharyngeal candidiasis model showed a shift towards E. faecalis dominance [19]. In the gut, C. albicans inoculated mice remained dominated by enterococci (mainly E. faecalis) compared with the non-infected candidal animals, in which the Enterococcus species were replaced by lactobacilli [20]. Therefore, it can be inferred that C. albicans and E. faecalis may favour the same ecological environment, or interact in a way that supports the survival of one another. Indeed, both microorganisms are frequently co-isolated from the root canals of the teeth and oral mucosa [21,22]. Notably, in endodontic literature these two pathogens are often considered the paradigm fungal and bacterial model to study, test and evaluate the effectiveness of endodontic antimicrobials or techniques [[23], [24], [25], [26]].
A variety of environmental conditions and microbial molecules have been shown to influence C. albicans hyphal morphogenesis and biofilm formation [27]. These include nutrient source, stressors, such as pH and temperature, and direct and indirect bacterial interactions [28]. RNASeq has been extensively used to analyse how C. albicans responds under these circumstances, which has dramatically enhanced our understanding of the transcriptional profiles of C. albicans response to weak organic acids [29], growth media, oxidative and nitrosative stresses [30], Lactobacillus [31], Streptococcus gordonii [7], Pseudomonas aeruginosa quorum sensing molecules [32], amongst others. The interaction between C. albicans and E. faecalis, and more specifically the effect of E. faecalis and its supernatant on C. albicans morphogenesis, biofilm formation and virulence has been the subject of several investigations [18,[33], [34], [35], [36], [37], [38], [39], [40]]. It was proposed that E. faecalis secretes an anti-candidal protein [40], and that it is the EntV bacteriocin that is specifically linked to the observed C. albicans inhibition [34]. Nevertheless, despite the well documented adverse effect of low pH and acid producing bacteria, such as lactobacilli, on C. albicans hyphal formation [31], the influence of the acidifying potential of the lactic acid bacterium E. faecalis remains largely unexplored. Therefore, the present in vitro study aimed to examine the phenotypic and transcriptomic biofilm interaction of C. albicans when co-cultured with E. faecalis. To the best of our knowledge this the first study that reveals E. faecalis induced changes in C. albicans biofilm transcriptome using RNA sequencing, and the impact that low pH has on this phenomenon.
2. Materials and methods
2.1. Microbial growth conditions and standardisation
All C. albicans and E. faecalis strains used in this study are listed in Table 1. C. albicans strains were incubated at 30 °C for 48 h on Sabouraud dextrose agar (SAB [Sigma–Aldrich, Dorset, UK]). E. faecalis strains were maintained on 5% v/v horse blood Columbia agar plate in 5% CO2 at 37 °C for 24 h. C. albicans overnight cultures were grown in yeast peptone dextrose ([YPD - Sigma-Aldrich, Dorset, UK]) with shaking at 120 rpm in an orbital shaker (IKA KS 4000 i control, Berlin, Germany) at 30 °C. For E. faecalis, Brain Heart Infusion ([BHI] Sigma–Aldrich, Dorset, UK) with incubation in 5% CO2 for 18 h at 37 °C was used. Cells were then harvested by centrifugation for 5 min at 3000 rpm and washed twice with PBS. Yeast cells were counted using a hemocytometer and standardized to (1 × 106 yeasts/mL). Bacterial cells were standardized using a spectrophotometer at an OD600nm of 0.3 (∼2 × 108 cells/mL).
Table 1.
List of used C. albicans and E. faecalis strains.
| C. albicans |
E. Faecalis |
||
|---|---|---|---|
| Name | Reference | Name | Reference |
| SC5314 | [41] | ATCC-29212 | [42] |
| BC023 (LBF) BC146 (HBF) |
[43] | NCTCC 5957 | National Collection of Type Cultures, Public Health Laboratory Service (www.phe-culturecollections.org.uk). |
| E1, E2, E3 | [44] | ||
| ER5/1 | [45] | ||
| ER35 | GDS culture collection | ||
| OS-16 | [44] | ||
| V583 | [46] | ||
| OGX-1 | [47] | ||
| J 42-7 | GDS culture collection | ||
| AA-OR 34 | [48] | ||
HBF = high biofilm former; LBF = low biofilm former; GDS = Glasgow Dental School.
2.2. Assessment of Candidaalbicans characteristics when co-cultured with Enterococcus faecalis
For phenotypic evaluation, C. albicans SC5314, high biofilm formers (HBF) and low biofilm formers (LBF) [49,50], and E. faecalis ER5/1, were standardized to 1 × 106 cells/mL for C. albicans and 1 × 107 cells/mL for E. faecalis in Todd-Hewitt broth (THB; Merck UK) supplemented with 10 mM menadione and 10 mg/mL hemin (Thermo Fisher). These were subsequently mixed 1:1 v/v with Roswell Park Memorial Institute (RPMI-1640 [Sigma–Aldrich, Dorset, UK]) (THB:RPMI), a media which has been shown to support the co-culture of C. albicans and bacterial species [51]. Mono-cultures of C. albicans and co-cultures of C. albicans with E. faecalis were created in a 24 well microtiter plates (Corning Incorporated, Corning, NY, USA) for 24 h in 5% CO2 at 37 °C. After incubation, biofilms were washed with PBS and stained with 5 μM calcofluor white (Invitrogen, Paisley, UK), a stain which specifically stains the chitin and beta-glucans of fungal cell wall [52]. Biofilms were incubated in the dark for 20 min and excess stain was washed with sterile water. 2% paraformaldehyde was used for 1 h to fix the stained biofilms which were then imaged using EVOS FL Cell Imaging system (Thermo Fisher Scientific, Waltham, MA, USA). Biofilm biomass of the same biofilms was also quantified using crystal violet stain as previously described [53].
2.3. Quantitative analysis using quantitative PCR
Mono- and dual-species biofilms of C. albicans SC5314 and E. faecalis ER5/1 and E2 were grown as described above in 6 well microtiter plates for 4, 6, 8 and 24 h in 5% CO2 at 37 °C. Biofilms were then removed by scraping into 1 mL of PBS before DNA extraction using QIAamp DNA mini kit, as per manufacturer's instructions (Qiagen, Crawley, UK). For qPCR, a mastermix containing Fast SYBR GreenER™ (Thermo Fisher Scientific, Paisley, UK), forward/reverse primers (Table 2), and UV treated RNase-free water was prepared, to which extracted DNA was added. Standard curves for each strain were also included. The used thermal cycles were 50 °C for 2 min, 95 °C for 2 min, 40 cycles of 95 °C for 3 s and 60 °C for 30 s using Step-One plus real time PCR machine and StepOne software V2.3 (Life Technologies, Paisley, UK). Colony forming equivalents (CFE) was calculated in relation to each species standard curve, as previously described [54]. Data obtained is from triplicates from three independent experiments.
Table 2.
C. albicans and E. faecalis primers used for quantitative and real time qPCR.
| Primer | Gene name | Sequence (5′–3′) | Function |
|---|---|---|---|
| C. albicans | ITS | ITS3 - GCATCGATGAAGAACGCAGC | |
| ITS4 - TCCTCCGCTTATTGATATGC | |||
| ACT1 | F - AAGAATTGATTTGGCTGGTAGAGA | Housekeeping | |
| R - TGGCAGAAGATTGAGAAGAAGTTT | |||
| HWP1 | F- GCTCAACTTATTGCTATCGCTTATTACA | Hyphal wall protein | |
| R - GACCGTCTACCTGTGGGACAGT | |||
| ALS3 | F - CAACTTGGGTTATTGAAACAAAAACA | Adhesion | |
| R - AGAAACAGAAACCCAAGAACAACCT | |||
| ECE1 | F - GCTGGTATCATTGCTGATAT | Hyphae specific protein | |
| R - TTCGATGGATTGTTGAACAC | |||
| YWP | F - TCCGGTTCTGGTTCTGATTC | Yeast wall protein | |
| R - TACCGTGGACCGTAGTGACA | |||
| SAP2 | F- GAATTAAGAATTAGTTTGGGTTCAGTTGA | Secreted aspartyl proteases | |
| R - CCACAAGAACATCGACATTATCAGT | |||
| SAP5 | F - CCAGCATCTTCCCGCACTT | Secreted aspartyl proteases | |
| R - GCGTAAGAACCGTCACCATATTTAA | |||
| PLB1 | F - GGTGGAGAAGATGGCCAAAA | Phospholipase | |
| R - AGCACTTACGTTACGATGCAACA | |||
| HSP90 | F - GGTTGCTGATCACGTCCAAGTT | Heat shock protein | |
| R - AACTTACCACCAGCGTTAGATTCC | |||
| CDR1 | F - GTACTATCCATCAACCATCAGCACTT | Efflux pump | |
| R - GCCGTTCTTCCACCTTTTTGTA | |||
| MDR1 | F - TCAGTCCGATGTCAGAAAATGC | Efflux pump | |
| R - GCAGTGGGAATTTGTAGTATGACAA | |||
| ARG1 | F - CTTTGGTTTGTGCCACTGGG | Arginine synthesis | |
| R - TGCCGTTTCTCACGGTTGTA | |||
| ARG3 | F - TGCGTCTTCACAAACACCAC | Arginine and citrulline biosynthesis | |
| R - ATGATGCCGCTCCTTCAGTA | |||
| CPA1 | F - TGAAATGGTGCCTTGGTGGT | Arginine synthesis | |
| R - AAACGTTCTGGTGTTGCTGC | |||
| CPA2 | F - TGCTCAAGGTGTGGTGGTTT | Arginine synthesis | |
| R - TGGCATCACCGGAATGAACA | |||
| GPT1 | F - CAGTTCGGCTGGTACCACTT | Transmembrane transporter activity | |
| R - CGATACTGACATCACCCCCG | |||
| AAP1 | F - CTCGTCAACGGTCAACCAGA | Transmembrane transport | |
| R - TGCGCTATTGGGGCATTACA | |||
| E. faecalis | DDL | F - CAAACTG TTGGCATTCCACAA | housekeeping |
| R - TGGATTTCCTTTCCAGTC ACTTC | |||
| EntV | F - AGCTGCACAAAAGAAAGCCTG | Secreted bacteriocin | |
| R- GCTTAGCCCACATTGAACTGC |
2.4. Gene expression analysis of Candida albicans genes and Enterococcus faecalis EntV gene
For assessment of C. albicans related genes, biofilms of C. albicans SC5314 alone or in co-culture with E. faecalis ER5/1 were developed for 2 h, as described above. For E. faecalis EntV gene expression, biofilms of 12 E. faecalis strains were grown for 4 h in THB:RPMI 5% CO2 at 37 °C. After incubation, biofilms were removed by scraping, and RNA extracted using the MasterPure™ Yeast RNA Purification Kit (Cambio, Cambridge, UK) for yeast, as per the manufacturer's protocol, and with a TRIzol extraction for bacteria (Thermo Fisher Scientific, Paisley, UK). Extracted RNA was converted to cDNA with a high-capacity cDNA reverse transcription kit (Thermo Scientific, Loughborough, UK). Real-time PCR was carried as described above. The list of primers for genes assessed are located in Table 2. Gene expression was analysed for C. albicans using ΔΔCT method, after normalising CT values to ACT1 housekeeping gene [55]. EntV gene expression in E. faecalis strains was calculated as a percentage of expression in relation to ΔΔCT of the housekeeping gene. No-reverse transcription samples were included. Data obtained is from triplicates from three independent experiments.
2.5. Assessing the transcriptional response of Candida albicans to Enterococcus faecalis
Transcriptional analysis of C. albicans SC5314 in response to co-culture with E. faecalis ER5/1 was performed using RNA sequencing as previously described [31,56]. Briefly, C. albicans mono-cultures were grown in THB:RPMI media in T-75 cell culture flasks (Corning, USA) at a cellular density of 106 cells/mL for 4 h in 5% CO2 at 37 °C. Next, biofilms were washed with PBS and E. faecalis in cellular density of 107 cells/mL was added for additional 2, 4, 20 h. After each time point, biofilms washed and scraped in 1 mL of RNAlater (Thermo Scientific, Loughborough, UK). RNA was extracted using RiboPure™ RNA purification kit for yeast (Thermo Scientific, Loughborough, UK). The quantity and quality of extracted RNA were evaluated using Bioanalyzer system. RNA samples were submitted to Edinburgh Genomics facility where RNA sequencing was performed using Illumina NOVASeq6000 sequencing platform. The obtained raw fastq reads were trimmed to remove adaptors and poor-quality reads using Trimmomatic (V0.38). Reads were then aligned to a reference C. albicans genome (http://www.candidagenome.org) and the number of aligned reads per gene were counted. Differential expression analysis was then performed using DESeq2 in RStudio which also used to generate principal component analysis (PCA), volcano and heatmap plots.
To further evaluate transcriptional changes based on key findings, amino acid biosynthesis/metabolism related gene expression were analysed using RT-PCR. In this experiment, C. albicans SC5314 was grown in THB:RPMI in 6 well plates in 5% CO2 for 4 h. After incubation, supernatant was removed, and biofilms were washed with PBS. E. faecalis ER5/1 suspended in 3 different media conditions was then added to C. albicans biofilms. The media conditions used where THB:RPMI, THB:RPMI supplemented with amino acids (Sigma-Aldrich, Dorset, UK) and THB:RPMI supplemented with glucose (Sigma-Aldrich, Dorset, UK). Amino acids (l-glutamine, l-arginine and l-histidine) were added to THB:RPMI to reach a final concentration of 0.03, 0.4 and 0.6 g/L respectively. The final glucose concentration in THB:RPMI with glucose was 5 g/L. Supplemented media without E. faecalis was also added to C. albicans biofilms for mono-species biofilms. After incubation for an additional 2 h in 5% CO2 at 37 °C, biofilms washed, removed by scraping, RNA extracted and RT-PCR performed as described above using primers specific for ARG1, ARG3, CPA1, CPA2, GPT1 and AAP1 genes (Table 2).
2.6. Assessment of Candida albicans biofilm biomass with Enterococcus faecalis supernatant
Cell free supernatant from 12 E. faecalis strains was obtained by growing E. faecalis to a cellular density of 1 × 107 cells/mL in THB:RPMI in T-15 cell culture flasks (Corning, USA) for 24 h in 5% CO2 at 37 °C. Supernatant was centrifuged at 4000 rpm for 5 min before filtering with 0.2 μm syringe filter (Sartorius™ Minisart®, Fisher Scientific, Loughborough, UK). Next, a 9:1 v/v of supernatant to 10 times concentrated RPMI (10 × RPMI) media was then used to grow C. albicans SC5314, HBF and LBF in 24 well plate for 24 h in 5% CO2 at 37 °C (10 × RPMI was used to ensure any effects were not a result of nutrient depletion). Following incubation, biofilms were washed with PBS and dried at room temperature overnight. The biofilm biomass of the dry biofilms assessed with crystal violet stain.
Next, E. faecalis E2, ER5/1 and V583 were selected based on their differential level of EntV expression. E. faecalis supernatant from these strains was ultrafiltered using Amicon Ultra-0.5 Centrifugal Filter Unit with 3 kDa NMWCO pore size (Merck Life science, Gillingham, UK). As E. faecalis EntV was indicated to be larger than 3–10 kDa [34], the 3 kDa pore size was selected to ensure removal of any proteins larger than 3 kDa NMWCO from the supernatant. Following ultrafiltration, both normal and ultrafiltered supernatant were boiled for 10 min at 100 °C to heat inactivate any active proteins in the supernatant. Afterwards, the supernatant from each condition was mixed with 10 × RPMI at 9:1 ratio and used to grow C. albicans SC5314, HBF and LBF, as described above. The pH of each supernatant was also measured using pH meter (Fisher Scientific, Loughborough, UK). C. albicans biofilm biomass was assessed as described above.
2.7. pH modification of Enterococcus faecalis supernatant and growth media
The pH of E. faecalis E2, ER5/1 and V583 supernatant was quantified and shown to be ∼5 for all three strains. The pH of the supernatant was then adjusted to 6.0 and 7.0 using 1 M sodium hydroxide. The pH of THB:RPMI (normally has pH of 7.0) was also adjusted to 5.0 and 6.0 using 35% hydrochloric acid (Sigma–Aldrich, Dorset, UK) and 90% lactic acid (Sigma–Aldrich, Dorset, UK). pH modified supernatant was mixed with 10 × RPMI and used along with pH modified media to grow C. albicans SC5314, HBF and LBF. C. albicans biofilm biomass was assessed as described above.
2.8. Assessment of Enterococcus faecalis inhibition of Candida albicans hyphal morphogenesis
Mono- and dual-cultures of C. albicans SC5314, HBF, LBF and E. faecalis E2, ER5/1 and V583 were prepared, as described above. Biofilms were washed with PBS and stained and imaged with calcofluor white at 2, 4, 6, 8 and 24 h, as described above. The pH of supernatant from each biofilm at the indicated time were also measured.
2.9. Statistical analysis
Figures and statistical analysis were performed using GraphPad Prism (version 7.0 d, GraphPad, La Jolla, CA, USA). To compare data sets and calculate significance, non-parametric Kruskal–Wallis test with Dunn's multiple comparison test was used. The ClueGO application in Cytoscape (Version: 3.8.0) software was used to generate gene ontology networks.
3. Results
3.1. Enterococcus faecalis impacts biomarkers of Candida albicans biofilms
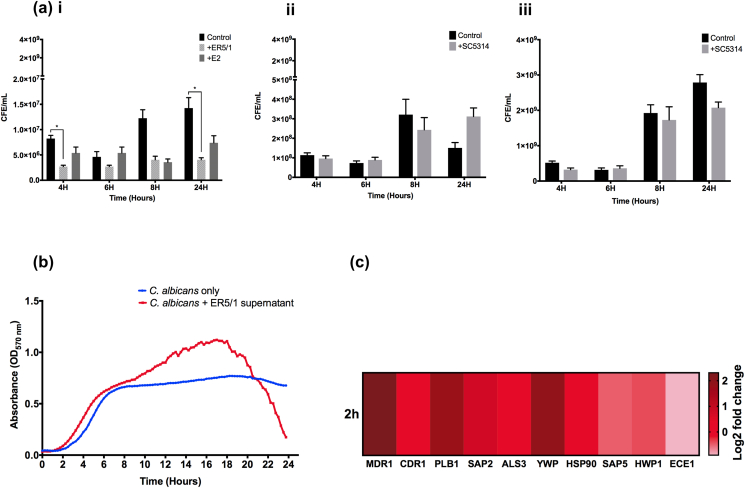
First, we investigated the general effect of E. faecalis on C. albicans hyphal morphogenesis, biofilm formation, growth, and expression of key genes related to biofilm formation, virulence and drug resistance. C. albicans SC5314, HBF and LBF were grown in mono-species biofilms or co-cultured with E. faecalis ER5/1 for 24 h. As reported by various studies [33,34,36,38,39], C. albicans hyphal morphogenesis was shown to be clearly inhibited in co-cultures, as shown in calcofluor stained images (Fig. 1a). C. albicans SC5314 (panel a [i]) and HBF (panel a [iii]) are known for their ability to form dense biofilm with tangled networks of hyphae [57,58]. This was obvious in mono--species biofilms, though in the presence of E. faecalis, less abundant and shorter hyphae are evident (panel a [ii], [iv]). The phenotype of C. albicans LBF (panel a [v], [vi]) remained unaltered. This phenotype of C. albicans usually grows in yeast form with occasional hyphae, therefore, it was not influenced by E. faecalis. To assess the effect of E. faecalis on C. albicans biofilm biomass, cell free E. faecalis supernatant supplemented with 10 × RPMI was used instead of live E. faecalis to ensure that the measured biofilm biomass is purely C. albicans biomass. E. faecalis supernatant was reported to have similar inhibitory effect on C. albicans as live E. faecalis [33]. The biofilm biomass, assessed using crystal violet assay, showed that E. faecalis supernatant significantly inhibited biofilm formation of C. albicans SC5314 and HBF compared with C. albicans only biofilms. Again, LBF remained unaffected (Fig. 1b).
Fig. 1.
E. faecalis inhibits C. albicans hyphal morphogenesis and biofilm formation in in vitro coculture. (a)C. albicans laboratory strain SC5314 (i, ii), HBF clinical isolates BC146 (ⅲ, ⅳ) and LBF clinical isolate BC023 (v, vi) were grown in THB:RPMI media in mono-culture (I, iii, v) or in co-culture with E. faecalis strain ER5/1 (ii, iv, vi) for 24 h. Biofilms were stained with calcofluor white. Scale bars are 100 μm. (b)C. albicans biofilm biomass measured using crystal violet stain after 24 h of incubation in mono-culture and with ER5/1 supernatant supplemented with 10x RPMI. Statistical significance was presented as ****p < 0.0001. Data obtained from triplicates of three independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, qPCR analysis was used to further quantify cell numbers during these interactions. E. faecalis was confirmed to inhibit the growth of C. albicans SC5314 compared with mono-species (Fig. 2a). This inhibition was notably more significant with E. faecalis ER5/1 than with that of E2. Whereas, the growth of E. faecalis remained unaffected by the presence of C. albicans, as there was no significant difference in E. faecalis CFE/mL between mono- and dual-species cultures at all time points for both strains, although there was a trend toward increased growth of ER5/1 in co-cultures at 24 h. Similar growth inhibition was observed in the kinetic growth curve of C. albicans grown in E. faecalis supernatant compared with C. albicans only at 24 h (Fig. 2b). The growth kinetics of C. albicans was characterised by steadily increasing growth up to 8 h, followed by a plateau where the growth remained stable over the remaining hours. C. albicans grown with E. faecalis supernatant showed a higher growth rate between the 8 and 20 h, after which the growth decreases sharply at 24 h compared with C. albicans only control. This matches the growth inhibition observed in qPCR analysis at 24 h in Fig. 2a(i).
Fig. 2.
E. faecalis inhibits C. albicans growth and induce change in C. albicans SC5314 genes associated with biofilm, virulence and resistance. (a) The growth of C. albicans SC5314 (i) E. faecalis ER5/1 (ii) and E. faecalis E2 (iii) in mono- and dual-species was assessed using qPCR at 4 h, 6 h 8 h and 24 h. Statistical significance was presented as * p < 0.05, ****p < 0.0001. (b) 24 h kinetic growth curve of C. albicans SC5314 only (blue) and C. albicans cultured in E. faecalis ER5/1 supernatant (red) at 570 nm absorbance (c) Heatmap of C. albicans SC5314 genes associated with biofilm, virulence and resistance was measured in mono-culture and in presence of E. faecalis at 2 h. Control = C. albicans only biofilms. Data shown is the mean log fold change relative to mono-species biofilms. Results represent data from three independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At the single transcript level, co-culturing C. albicans SC5314 with E. faecalis ER5/1 resulted in down-regulation of the hyphal related genes HWP1 and ECE1 in relation to C. albicans only at 2 h. Conversely, the adhesion gene ALS3 was upregulated, which may indicate that E. faecalis affects hyphal formation of C. albicans without affecting its adhesion to surfaces (Fig. 2c). SAP5 encoding SAP5 enzyme, which are expressed upon hyphal formation [59], is also downregulated in dual-species biofilms. In contrast, SAP2 and YWP, yeast typical genes, were upregulated. The gene expression patterns support the above-mentioned inhibition levels observed microscopically and by crystal violet assay. In addition, the effect of E. faecalis on C. albicans virulence was assessed using genes encoding proteolytic enzymes, PLB1 and SAP2 and those associated with drug resistance CDR1, MDR1 and HSP90. The upregulation of the genes PLB1 and SAP2 indicates enhanced virulence of C. albicans via the increase in proteolytic enzymes activities in the dual-species biofilms. Similarly, the efflux pump genes, CDR1, MDR1 and HSP90, which are directly linked to drug resistance were also upregulated. This upregulation in efflux pumps expression may refers to C. albicans attempt to remove intracellular toxic substances produced by E. faecalis, such as quorum sensing molecules, bacteriocins or metabolites. Overall, the above analysis indicates that E. faecalis inhibits C. albicans hyphal morphogenesis, biofilm formation and growth, while positively affecting enzymatic activity and drug resistance.
3.2. Transcriptomic analysis of Candida albicans response to Enterococcus faecalis demonstrated the importance of early interactions
We have shown E. faecalis to have a significant effect on various biological functions of C. albicans. In order to gain a more comprehensive overview of the molecular mechanisms of this interaction, a whole transcriptome sequencing (RNA-Seq) approach was employed. For this purpose, C. albicans SC5314 as mono-cultures and as co-cultures with E. faecalis ER5/1 were prepared at 2, 4 and 20 h, after the addition of E. faecalis to 4 h C. albicans biofilms, to enable a temporal analysis. This approach enabled us to capture gene expression levels at early, intermediate and late time points of microorganisms’ interaction.
The principal component analysis demonstrates the level of variance between C. albicans mono- and co-cultures at the different time points (Fig. 3a). The variance in clustering was the highest at 6 h (E. faecalis was added to 4 h pre-established C. albicans biofilms and samples were harvested after 2 h of further incubation). This indicates that C. albicans shows a quick transcriptional response to E. faecalis. The degree of variance then decreased at 8 and 24 h. Therefore, the subsequent analysis was focused on 6 h time point. In total, 186 genes were significantly and differentially expressed between mono- and dual-species biofilms at 6 h (with cut-off of 1.5 for fold change and 10e-3 for p-adjusted value). Of these, 112 genes were upregulated in dual-species and 74 were downregulated (Fig. 3b). The top 50 differentially expressed genes are shown in Fig. 3c, accompanied by a list of the top 10 upregulated genes in C. albicans mono-and co-cultures, and their function (if known) are shown in Table 3. However, most of these genes are of unknown function and were not involved in the enriched biological pathways within the subsequent enrichment analysis. The full list of all significantly differentially expressed genes in C. albicans mono- and dual-species biofilm are available in Table S1.
Fig. 3.
Overview of the transcriptional analysis of C. albicans SC5314 with E. faecalis ER5/1 at 4h, 6h and 24h. (a) The principal component analysis of data at all time points. (b) Volcano plot of the significantly differentially expressed genes of mono- and dual-species at 6 h. Genes located on the negative x-axis are the up regulated genes in mono-species biofilms. Genes on the positive x-axis are the up regulated genes in dual-species biofilms. (c) Heatmap of the top 50 significantly differentially expressed genes of mono- and dual-species biofilms at 6 h.
Table 3.
List of the top 10 upregulated genes in C. albicans mono-- and dual-species biofilms at 6 h.
| Gene ID | Gene Name | P value (adj) | Log2FC | |
|---|---|---|---|---|
| Up in C. albicans mono-species biofilms | CR_03580C_A | CR_03580C_A | 0.00140825 | −5.9714945 |
| CR_04510W_A | HXK2 | 4.07E-15 | −5.4994791 | |
| C2_01000W_A | HGT7 | 4.44E-05 | −5.436272 | |
| C3_01540W_A | C3_01540W_A | 8.47E-05 | −4.7324912 | |
| C4_00450C_A | PGA10 | 4.08E-07 | −4.3971164 | |
| C4_04720W_A | C4_04720W_A | 9.05E-09 | −4.1599839 | |
| C1_07160C_A | C1_07160C_A | 0.00283371 | −4.1157133 | |
| CR_07170W_A | CR_07170W_A | 2.39E-06 | −4.0613455 | |
| C6_00150W_A | ARD | 2.09E-09 | −4.0025723 | |
| CR_01910C_A | CR_01910C_A | 0.02841275 | −3.8845479 | |
| Up in C. albicans - faecalis dual-species biofilms | C4_05580C_A | C4_05580C_A | 1.65E-13 | 14.967598 |
| C1_02520W_A | SCW4 | 2.64E-22 | 14.6848791 | |
| C1_13080W_A | OP4 | 5.11E-18 | 14.2265626 | |
| C1_07580C_A | PRY1 | 4.27E-13 | 12.9814901 | |
| C1_05890W_A | C1_05890W_A | 2.05E-18 | 12.4438686 | |
| C2_09800C_A | C2_09800C_A | 2.21E-13 | 12.1176629 | |
| CR_10320W_A | CR_10320W_A | 1.21E-07 | 12.052965 | |
| C2_02230C_A | C2_02230C_A | 6.16E-19 | 11.4784176 | |
| C6_00070C_A | PGA25 | 8.75E-26 | 10.9038636 | |
| C1_09580C_A | LIP1 | 0.04085374 | 10.6107591 |
3.3. Amino acid biosynthesis and metabolism genes are upregulated by Enterococcus faecalis, regardless of glucose and amino acids concentration in the media
Gene ontology (GO) term analysis was then carried out to investigate the biological functions of the differentially expressed genes in mono- and dual-species biofilms. The upregulated genes in the dual-species were mainly responsible for amino acids biosynthesis/metabolism and transmembrane transport activity (Fig. 4a). For the downregulated genes, the main biological functions enriched were purine nucleotide inosine monophosphate (IMP)/nucleotides/purine related biosynthetic processes (Fig. 4b). A simple presentation of the GO term analysis is illustrated in Fig. S1. The increased amino acid uptake, biosynthesis and metabolism in response to E. faecalis can be an indication of amino acids or glucose starvation or a stress response. It was also evident that C. albicans down-regulated the purine nucleotide biosynthesis pathway, an essential precursor for the nucleic acids DNA and RNA [60]. This may indicate an inhibition in C. albicans growth when co-cultured with E. faecalis, as shown previously in (Fig. 2a and b).
Fig. 4.
Gene ontology (GO) enrichment analysis of significant biological, cellular, and molecular functions of mono- and dual-species biofilms at 6h. Amino acids biosynthesis/metabolism and purine related biosynthesis C. albicans genes expression in mono- and dual-species biofilms at 6 h. (a) Enriched biological processes within the upregulated genes in dual-species biofilms. (b) Enriched biological processes within the upregulated genes in mono-species biofilms with ≥10 associated genes. Only enriched pathways of a significant ≤0.05 are displayed. (c) Heatmap of key Amino acids biosynthesis/metabolism and transmembrane transport C. albicans genes in THB:RPMI media (media), THB:RPMI supplemented with glucose (+Glucose) or THB:RPMI supplemented with amino acids (+Amino acids) at 6 h. Data shown is the mean log fold change relative to mono-species biofilms. (d) Number of the unique and overlapped genes that are upregulated at 24 h in C. albicans co-cultured with E. faecalis or with L. crispatus.
Next, we aimed to investigate whether the up-regulated amino acid biosynthesis and metabolism was a result of E. faecalis induced amino acids or glucose starvation. To achieve this, C. albicans SC5314 was grown in THB:RPMI media for 4 h. Afterwards, E. faecalis was standardised to the desired cellular density in either THB:RPMI only, THB:RPMI supplemented with amino acids or with glucose and added to C. albicans biofilms. C. albicans mono-species biofilms with the three different media were also included. After additional incubation of 2 h, mono- and dual-species biofilms were analysed for the expression of amino acids biosynthesis and metabolism genes; ARG1, ARG3, CPA1, CPA2 and transmembrane transport related genes GPT1 and AAP1. Despite supplementation with amino acids and glucose, all the investigated genes were still upregulated in all the conditions in the dual-species biofilms (Fig. 4c). However, the level of expression of all genes was the highest in THB:RPMI media and lower in glucose supplemented THB:RPMI. Furthermore, the CaGcn4 gene, C. albicans functional homologue of S. cerevisiae Gcn4, which mediates C. albicans response to amino acids starvation [61], was not upregulated at any time point, as indicated in RNASeq analysis. These findings suggest that increased amino acid uptake, biosynthesis and metabolism is not caused by amino acids starvation, but instead, it is a direct stress response to E. faecalis, its released products, or the environment that it generates.
As recently published by our group [31], amino acid biosynthesis and breakdown related genes were also upregulated in dual-species biofilms of C. albicans and L. crispatus at 24 h. Indeed, there was 21 shared genes that were upregulated by both E. faecalis and L. crispatus at 24 h (Fig. 4d). Of note, the transcriptional response of C. albicans to L. crispatus was delayed (at 24 h) compared to its early response to E. faecalis (at 6 h).
Moreover, 10 out of 27 of the enriched GO biological processes in our dual-species biofilms, and 20 out of 22 of the enriched GO biological processes in our mono-species biofilms are also enriched in response to the weak organic acids [29]. In addition, many genes involved in arginine and lysin biosynthesis were induced in C. albicans under mild oxidative stress [7,30,62]. Considering that E. faecalis is a lactic acid bacteria [63], the similarities observed in C. albicans transcriptional response to E. faecalis, L. crispatus, and weak organic acids, and the fact that acidic environment favours yeast growth [[64], [65], [66]], then we hypothesized that C. albicans biofilm and hyphal morphogenesis inhibition observed in dual-species biofilms is pH dependant phenomenon.
3.4. Enterococcus faecalis induced Candida albicans biofilm formation inhibition is not dependent on bacteriocin EntV expression
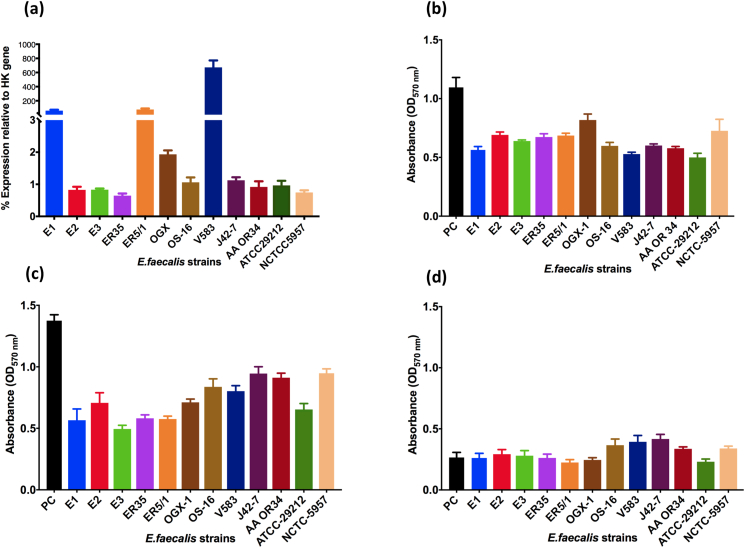
E. faecalis inhibition of C. albicans hyphal morphogenesis, biofilm formations and virulence are reported in various studies [33,34,36,38,39]. However, few investigated the mechanism of this interaction. Shekh and Roy (2012) first characterized and purified an anti-candida protein produced by E. faecalis, which was heat stable up to 90°C with molecular weight of approximately 43 KDa [40]. Graham and colleagues (2017) also identified the bacteriocin EntV produced by E. faecalis as a potent inhibitor of C. albicans biofilm formation and virulence [34]. Therefore, we investigated the expression of EntV gene in 12 different E. faecalis strains and correlated the level of EntV expression with the degree of C. albicans biofilm inhibition, as quantified by the crystal violet assay. There was a wide variation in the expression of EntV among the screened E. faecalis strains at 4 h (Fig. 5a). E. faecalis V583, showed the highest level of expression, followed by E. faecalis E1 and ER5/1. All other E. faecalis strains showed comparable expression levels. The inhibition of biofilm from C. albicans SC5314 (Fig. 5b), HBF (Fig. 5c) and LBF (Fig. 5d) was then assessed using the cell free supernatant from the 12 E. faecalis strains. Here the biomass inhibition was not correlated with the quantified EntV expression, and there was not a statistically significant difference in C. albicans biomass inhibition induced by the different E. faecalis strains. Moreover, the linear regression analysis showed that there is no correlation between the level of EntV expression of different E. faecalis strains and the biofilm biomass of C. albicans grown in the supernatant of the corresponding E. faecalis strains (Fig. S4).
Fig. 5.
Inhibition of C. albicans hyphal morphogenesis when cocultured with E. faecalis is not dependent on bacteriocin EntV expression. (a) Percentage of EntV expression in 12 E. faecalis strains relative to ddl housekeeping gene at 4 h with real time qPCR. The expression of ddl housekeeping gene was stable cross all the 12 E. faecalis strains with a variation within 2 cycle threshold (Ct) values. (b–d) Cell free E. faecalis supernatant obtained from 24 h incubation of E2, ER5/1 or V583 in THB:RPMI was supplemented with 10x RPMI. C. albicans biofilm biomass of the mono-culture (control) and C. albicans grown in 12 E. faecalis strains supernatant was assessed by crystal violet at 24 h (a) SC5314 (b) HBF (BC146) (c) LBF (BC023). Data obtained from triplicates of three independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In order to further investigate the effect of the EntV peptide on C. albicans, we employed ultrafiltration membranes with 3kD cut-off (EntV was identified as a 7.2-kDa peptide) [34] and heat inactivation of E. faecalis supernatant. We aimed to separate any proteins larger than 3kD and heat inactivate the supernatant. E. faecalis E2, ER5/1 and V583 were selected for the subsequent analysis based on their variation in EntV expression. The supernatant was then used in combination with 10x RPMI to grow C. albicans. Despite the ultrafiltration and the heat inactivation of E. faecalis supernatant, a significant inhibition in C. albicans biofilm biomass still evident in SC5314 (Fig. 6a) and HBF (Fig. 6b). Furthermore, ultrafiltration resulted in increased inhibition compared with normal supernatant in C. albicans SC5314 and HBF. Of note, the pH of the normal, ultrafiltered and heat inactivated supernatant was similar around 5.45 before incubation with C. albicans (Fig. S2). C. albicans is known for its ability to raise extracellular pH [67], and since the RPMI used in this work was unbuffered, the pH of all the conditions increased to around 6–6.3 after 24 h of incubation. Despite this rise in pH, the conditions remained in the acidic range and the biofilm biomass of C. albicans SC5314 and HBF was significantly lower in all supernatant containing conditions compared with the C. albicans only controls.
Fig. 6.
Inhibition of C. albicans biofilm formation by E. faecalis normal, ultrafiltered and boiled supernatant.C. albicans biofilm biomass of the mono-culture (control) and C. albicans grown in E. faecalis E2, ER5/1 and V583 supernatant supplemented with 10x RPMI as assessed by crystal violet at 24 h. Four types of each strain supernatant were used; normal, boiled, ultrafiltered and boiled ultrafiltered supernatant (a) SC5314 (b) HBF (c) LBF. Statistical significance was presented as * p < 0.05, ***p < 0.001, ****p < 0.0001. Results represent data from three independent experiments. PC = C. albicans only control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Candida albicans biofilm inhibition is pH dependent
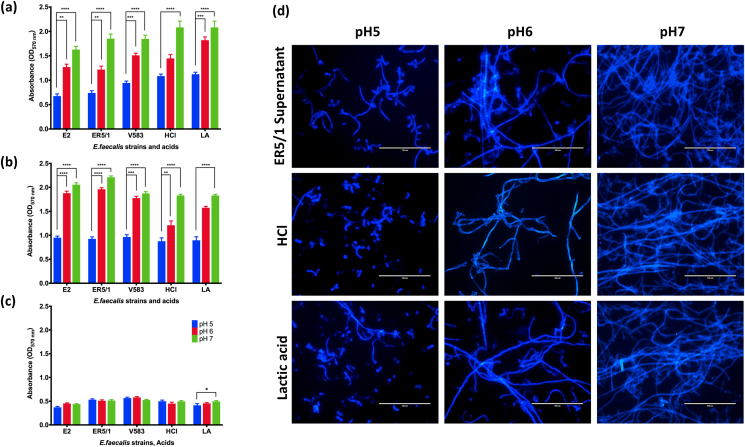
As suggested above, we aimed to investigate the effect of E. faecalis supernatant pH on C. albicans hyphal morphogenesis and biofilm formation. Firstly, we measured the pH of the E. faecalis cell free supernatant obtained from 24 h E. faecalis biofilms grown in THB:RPMI media. The pH of the supernatant was approximately 5.0. The pH of this supernatant and THB:RPMI media was then modified to different pH levels. Following the growth of C. albicans in various pH conditions, the biofilm biomass was assessed. Interestingly, there was a positive correlation between raising the pH of the E. faecalis supernatant and increased C. albicans biofilm biomass of SC5314 and HBF (Fig. 7a and b, respectively). C. albicans biofilm biomass was also increased with a pH rise of in pH modified media. In all conditions tested, the biomass was significantly higher at pH 7.0 compared to that of pH 5.0. More importantly, E. faecalis supernatant showed a similar effect trend on C. albicans biofilm formation to that of the pH modified media at the same pH value. This similarity is further phenotypically confirmed using calcofluor white images of the same conditions (Fig. 7d). More abundant and longer C. albicans hyphae can be observed with the rise in pH of both the ER5/1 supernatant and the media. As shown, with pH 5.0, yeast is the predominant phenotype of C. albicans. With pH increase to 6.0, more hyphae are evident, which becomes the dominant phenotype at pH 7.0. The biofilm biomass of LBF was not affected by pH modification of the supernatant and media except for lactic acid modified media, in which the biofilm biomass was significantly higher in pH 7.0 compared with pH 5.0 (Fig. 7c). These observations indicate the potential role of E. faecalis supernatant pH on C. albicans hyphal morphogenesis and biofilm formation.
Fig. 7.
The effect of pH modified E. faecalis supernatant and acid modified media on C. albicans biofilm biomass. Cell free E. faecalis supernatant of E2, ER5/1 or V583 supplemented with 10x RPMI used with its original pH 5 or adjusted to pH 6 and pH 7 using NaOH. THB:RPMI media also used at its normal pH 7 or adjusted to pH5 and pH6 using hydrochloric acid (HCl) or lactic acid (LA). pH modified supernatant and media was used to grow C. albicans SC5314 (a) HBF (b) and LBF (c) for 24 h and biofilm biomass assessed using crystal violet. (d)C. albicans SC5314 biofilms stained with Calcofluor white following incubation in the above-mentioned conditions for 24 h. Scale bars are 100 μm. Statistical significance was presented as * p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Results represent data from three independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Enterococcus faecalis induced pH drop correlates with inhibition of Candida albicans hyphal morphogenesis
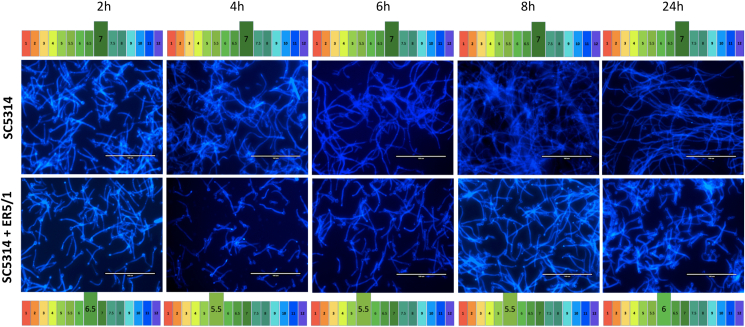
To gain insight into the chronology of C. albicans hyphal and biofilm formation in mono- and dual-species cultures with E. faecalis, the biofilm of both conditions is stained and imaged at 2, 4, 6, 8 and 24 h. The pH of the supernatant of each condition was also measured at the corresponding time points. It was shown that C. albicans starts to form germ tubes at the first hour of incubation in both conditions when the pH was 7.0 (Fig. 8). No differences were observed between the two conditions at 2 h, where true hyphae start to form and elongate whilst the pH of the mono-species remains neutral, whereas dropping to 6.5 in the co-culture. From 4 h onwards, the difference between the two conditions becomes visibly evident. Here the mono-species C. albicans biofilms showed denser and longer hyphae that continued to increase over time, whilst the pH remained neutral across all time points. Conversely, the abundance and length of the hyphae appeared to be unchanged from 4 to 24 h in the co-cultures. Interestingly, the 4 h time point also represents the point in which the media becomes more acidic and drops to 5.5 in the co-cultures. The pH of the co-culture remained acidic (pH 5.5) at 4, 6, and 8 h, and starts to rise slightly at 24 h to reach 6.0, which indicates some buffering attempts exerted by C. albicans. The pH values of C. albicans mono-species, E. faecalis mono-species and their co-culture at the different time points is available in Fig. S3. Collectively, these observations further confirm that E. faecalis induced inhibition of C. albicans hyphal morphogenesis and biofilm formation is based on the ability of E. faecalis to acidify the environment which in turns drives a predominantly yeast phenotype [68].
Fig. 8.
C. albicans hyphal formation and elongation in mono-culture and coculture with E. faecalis at 2, 4, 6, 8 and 24h.C. albicans SC5314 grown in THB:RPMI in mono- and dual-species biofilms with E. faecalis ER5/1. At every time point, supernatant removed, biofilms washed with PBS and stained with calcofluor white and supernatant's pH is measured. Images are for SC5314 and ER5/1 with Scale bars of 100 μm and pH measurements of the supernatant of each condition at the relevant time point.
4. Discussion
There is a growing fascination of how C. albicans interacts with bacterial species within complex interkingdom communities [31,56,69,70]. These studies have been instrumental in furthering our understanding of how this opportunistic yeast co-operates, or otherwise, with key bacterial species. Our previous work has shown how interactions with different species of Lactobacillus leads to differential health outcomes in patients with recurrent vulvovaginal candidiasis, and that denture wearing patients have resilient populations of C. albicans that support bacterial biofilm populations [31,71]. In this new study we describe changes in C. albicans growth, phenotype, virulence and transcriptome when co-cultured with E. faecalis in vitro. In agreement with previous in vitro and in vivo studies, our preliminary investigation showed an inhibition of C. albicans growth, hyphal morphogenesis and biofilm formation in dual-species biofilms [18,[33], [34], [35], [36], [37], [38], [39], [40]]. Subsequent RNASeq showed that E. faecalis rapidly and significantly alters C. albicans gene expression. Our enrichment analysis findings and the degree of overlapping with responses observed to lactobacilli and weak organic acids directed our interest toward the role of pH and stress response in this inter-kingdom interaction.
At the outset of this work we reviewed the published mechanisms of interaction between C. albicans and E. faecalis, where the secreted bacteriocin EntV was reported as a potent inhibitor of C. albicans hyphal morphogenesis, biofilm formation, and virulence, albeit with limited strain selection [34,40]. Despite this carefully characterised phenomenon we were unable to demonstrate a correlation between the expression of EntV and the level of inhibition of C. albicans biofilm biomass, suggesting that an alternative additional interaction was important. It has been reported that for optimal functionality EntV requires post-translational gelatinase processing [36], though our own assessment of gelatinase activity showed there was no difference observed between the strains with positive activity, and those lacking this property in supressing C. albicans biofilm (data not shown). Moreover, despite the heat inactivation and the removal of any E. faecalis supernatant proteins of molecular weight of more than 3 kDa, C. albicans hyphal and biofilm formation was not recovered, again suggestive of another mechanism. EntV is a heat stable peptide, therefore, recovery of C. albicans biofilm activities was not anticipated following heat treatment. However, the increased inhibition associated with ultrafiltered supernatants was surprising. There is no clear explanation of why ultrafiltration enhanced biofilm inhibition, however, it is possible that the proteins and molecules from the supernatant had a subtle buffering capacity, so removal of these exposed C. albicans solely to a pH effect. We surmised that differences in growth media and the E. faecalis strains used in our work could account for the difference in findings. The ability of E. faecalis to metabolise nutrients and thereby produce organic acids may differ according the media used. Metabolising THB:RPMI by E. faecalis resulted in a significant drop in pH after 24 h. However, similar drop may be not be achieved with other growth media such as artificial saliva used in EntV based studies. Indeed, post-translational modification following gene expression may explain the absence of correlation between the high EntV gene expression in three of the E. faecalis strains and the levels of C. albicans biofilm inhibition. It is also possible that the low expression of EntV gene in most E. faecalis strains was sufficient, or reached an expression threshold, beyond which any extra expression fails to cause more inhibition of C. albicans biofilm formation. We cannot rule out the possibility that EntV requires undefined optimal conditions for full functionality, including a specific pH range. Therefore, it is possible that raising the supernatant pH may have impaired the functionality of EntV and renders it less effective in inhibiting C. albicans biofilm formation. However, the evidence gathered does not support previous reports showing the singular importance of EntV in preventing C. albicans hyphal growth and biofilm formation [34].
To investigate for alternative mechanisms, we conducted a large-scale transcriptional analysis, and in doing so revealed the possibility of pH dependent mechanisms. Amino acid starvation was excluded for the reasons explained above. Some studies reported enrichment of C. albicans amino acids biosynthesis/metabolism in response to acids [29], lactic acid producing bacteria [31] and to oxidative stress [30,62]. Moreover, the vast majority of enriched pathways in C. albicans mono-species biofilms were also enriched in the response to the weak organic acids [29]. Upregulation in arginine biosynthesis and oxidative stress related genes were also shown in C. albicans when internalized by macrophages [62]. However, none of the reported oxidative stress genes in their study was upregulated in our dual-species biofilm. This further focussed the subsequent investigation to pH dependant mechanisms.
C. albicans tolerates and adapts to a wide pH range, and this dynamic feature plays an important role in changing gene expression and morphology [64,72]. In an acidic environment, C. albicans grows as yeast cells, while neutral and alkaline environment induces filamentation [68]. In low pH, C. albicans uses amino acids as a carbon source in glucose limiting environment and produce ammonia which in turn rises the extracellular pH and induce hyphal morphogenesis. This ability of C. albicans to raise the extracellular pH was also observed in our results. As shown in Figs. 8 and S3, despite the early drop, the pH of dual-species biofilms started to rise at 24 h. Therefore, E. faecalis induced inhibition of C. albicans can be time and media dependant where the ability of E. faecalis to acidify the media and the time required for C. albicans to raise the extracellular pH and restore hyphal morphogenesis play a major role. C. albicans environment alkalization was also associated with upregulation in arginine biosynthesis [67]. This finding further supports the close association between amino acid biosynthesis upregulation and pH response in C. albicans. Moreover, C. albicans utilization of amino acids for environment alkalization is repressed by glucose. This may explain the reduced expression of amino acid biosynthesis and transport genes in glucose supplemented media noted in our data shown in Fig. 4c.
As E. faecalis favours the growth in the yeast form of C. albicans, it suppresses the latter's virulence. We showed previously that C. albicans is more susceptible to endodontic irrigants when co-cultured with E. faecalis compared with its mono-species biofilm [73]. Ishijima and colleagues (2014) also showed that oral administration of heat killed E. faecalis, in the form of the commercially available probiotic, protected immunocompromised mice from oral candidiasis [37]. They reasoned this protection to the direct binding of heat killed E. faecalis to C. albicans yeast, pseudohypha and hyphae, and therefore reduces C. albicans adherence to surfaces. This is another proposed mechanism of C. albicans and E. faecalis interaction. Likewise, it has been shown that the survival of Caenorhabditis elegans was significantly higher when co-infected with C. albicans and E. faecalis compared with the infection with single species. Similar results were also reported by Cruz and colleagues [33], which indicates that C. albicans and E. faecalis are inhibiting one another's virulence, and instead enhance commensalism. When considering endodontic infections of the root canals of teeth where these two microorganisms are frequently co-isolated from recurrent infections, this interkingdom interaction may influence their persistence in the root canals. The standard endodontic treatment regimen of root canals involves dressing the canals with calcium hydroxide as antimicrobial medicament between treatment visits. The antimicrobial efficiency of calcium hydroxide is attributed to its high alkalinity (pH 12.5) [74]. Although, most endodontic pathogens are sensitive to calcium hydroxide, C. albicans and E. faecalis are shown to be tolerant [75]. The ability of E. faecalis to produce lactic acid may account for its ability, and C. albicans through coexistence, to neutralize and survive the high alkalinity of calcium hydroxide.
Finally, we show the importance of E. faecalis induced pH change as inducer of C. albicans inhibition of hyphal and biofilm formation. However, the interkingdom interaction between C. albicans and E. faecalis is dynamic and complicated process that is further influenced by the environment in which this interaction takes place. Therefore, it is expected that this interaction between the two microorganisms can vary in different environments such as in the root canals of teeth and the gut. It is plausible to consider that the biofilm microenvironment within root canals has a positive influence in their ability to coexist and take advantage of the dentinal tubules [76]. The low pH can drive dissolution and enlargement of the tubules, which can then enhance invasive capacity. When C. albicans elevates the pH within this microenvironment, then its ability to induce hyphae and invade into the tubules is enhanced [77]. Furthermore, it cannot be claimed that E. faecalis effect is based solely on pH and subsequently, the other reported mechanisms namely, EntV bacteriocin [34], anticandidal protein [40] and direct binding [37] cannot be excluded. Notably, we observed that despite a downregulation of hyphal regulated genes HWP1 and ECE1, we did not observe an expected reciprocal downregulation of the key adhesin ALS3. This may indicate that the presence of E. faecalis stimulates C. albicans to maintain its extracellular adhesins to support potential interactions, or at least the organisms within the same microenvironments.
Overall, it is more reasonable to consider that the effect of E. faecalis on C. albicans is a collective effect of a combination of different mechanisms that ultimately manifested by C. albicans inhibition. How the interaction between C. albicans and E. faecalis fits for potential antimicrobial strategies remains unclear and should be further explored. In addition, to gain a full picture of this interkingdom interaction, the impact of C. albicans on E. faecalis's biology should be also evaluated.
5. Conclusions
This study highlights the importance that the microenvironment can make in driving dysbiosis or rebiosis within complex interkingdom interactions. We cannot assume antagonism from a simple phenotypic change, but instead we need to carefully assess whether this change benefits the competing species. We have shown that modulation of the pH microenvironment influences these perceived enemies to become friends (frenemies) and drive co-existence to support invasion of dentinal tubules.
CRediT authorship contribution statement
Om Alkhir Alshanta: participated in study design, experimental procedures, data analysis, and wrote the manuscript. Khawlah Albashaireh: participated in study design, experimental procedures and data analysis. Emily McKloud: participated in study design, experimental procedures and data analysis. Christopher Delaney: participated in study design, experimental procedures and bioinformatic data analysis. Ryan Kean: conceived the study, participated in study design, and was responsible for producing the final manuscript. William McLean: conceived the study, participated in study design, and was responsible for producing the final manuscript. Gordon Ramage: conceived the study, participated in study design, and was responsible for producing the final manuscript. All authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would also like to acknowledge the funding support of the BBSRC Industrial CASE PhD studentship for Christopher Delaney (BB/P504567/1).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioflm.2022.100072.
Data availability
Sequenced transcriptome data has been deposited to the NCBI sequence read archive (SRA) database and can be found under accession number PRJNA731052.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Barplot of GO over representation of significant biological, cellular, and molecular functions of mono and dual species biofilms at 6h. (a) Enriched pathways in dual species biofilms. (b) Enriched pathways in C. albicans mono species biofilms.
pH measurements of normal, boiled, ultrafiltered and boiled ultrafiltered supernatant of E. faecalis after incubation with C. albicans for 24h (a) SC5314 (b) HBF (c) LBF.
pH change of the C. albicans and E. faecalis monocultures and dual species cultures at 2h, 4h, 6h, 8h and 24h. (a) pH measurements of C. albicans SC5314, E. faecalis ER5/1 and their coculture. (b–d) pH values of 3 C. albicans and 3 E. faecalis mono and dual species cultures at the indicated time points. (b)C. albicans SC5314 with E. faecalis E2, ER5/1 and V583. (c)C. albicans HBF. (d)C. albicans LBF.
Linear regression analysis for the correlation of EntV expression in 12 E. faecalis strains and biofilm biomass of C. albicans grown in the corresponding E. faecalis supernatant. (a) SC5314 (b) HBF (BC146) (c) LBF (BC023). The p-value for SC5314, HBF and LBF was 0.2352, 0.7894 and 0.2273 respectively.
References
- 1.Kean R., Rajendran R., Haggarty J., Townsend E.M., Short B., Burgess K.E., et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front Microbiol. 2017;8:258. doi: 10.3389/fmicb.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J., Kim D., Zhou X., Ahn S.-J., Burne R.A., Richards V.P., et al. RNA-seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front Microbiol. 2017;8:1036. doi: 10.3389/fmicb.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Méar J.-B., Kipnis E., Faure E., Dessein R., Schurtz G., Faure K., et al. Candida albicans and Pseudomonas aeruginosa interactions: more than an opportunistic criminal association? Med Maladies Infect. 2013;43(4):146–151. doi: 10.1016/j.medmal.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Janus M., Crielaard W., Volgenant C., Van Der Veen M., Brandt B., Krom B. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9(1):1270613. doi: 10.1080/20002297.2016.1270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolini M., Dongari-Bagtzoglou A. Oral mucosal immunity and microbiome. Springer; 2019. The relationship of Candida albicans with the oral bacterial microbiome in health and disease; pp. 69–78. [DOI] [PubMed] [Google Scholar]
- 6.Zhai B., Ola M., Rolling T., Tosini N.L., Joshowitz S., Littmann E.R., et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med. 2020;26(1):59–64. doi: 10.1038/s41591-019-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutton L.C., Paszkiewicz K.H., Silverman R.J., Splatt P.R., Shaw S., Nobbs A.H., et al. Transcriptional landscape of trans‐kingdom communication between C andida albicans and S treptococcus gordonii. Mol Oral Microbiol. 2016;31(2):136–161. doi: 10.1111/omi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Liu Y., Benhamou R.I., Sanchez H., Simón-Soro Á. Li Y., et al. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018;12(6):1427–1442. doi: 10.1038/s41396-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Sobue T., Thompson A., Xie Z., Poon K., Ricker A., et al. Streptococcal co‐infection augments C andida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014;16(2):214–231. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashem S.W., Kaplan D.H. Skin immunity to Candida albicans. Trends Immunol. 2016;37(7):440–450. doi: 10.1016/j.it.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rindum J., Stenderup A., Holmstrup P. Identification of Candida albicans types related to healthy and pathological oral mucosa. J Oral Pathol Med. 1994;23(9):406–412. doi: 10.1111/j.1600-0714.1994.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 12.Achkar J.M., Fries B.C. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–273. doi: 10.1128/cmr.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason K.L., Downward J.R.E., Falkowski N.R., Young V.B., Kao J.Y., Huffnagle G.B., et al. Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis. Infect Immun. 2012;80(1):150–158. doi: 10.1128/IAI.05162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuita N.I.A., Huycke M.M. 2014. (Enterococcal Disease, Epidemiology, and Implications for Ireatment. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection). [Internet] [Google Scholar]
- 15.Hube B. From commensal to pathogen: stage-and tissue-specific gene expression of Candida albicans. Curr Opin Microbiol. 2004;7(4):336–341. doi: 10.1016/j.mib.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Mason K.L., Stepien T.A., Blum J.E., Holt J.F., Labbe N.H., Rush J.S., et al. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio. 2011;2(3) doi: 10.1128/mBio.00065-11. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yapar N. Epidemiology and risk factors for invasive candidiasis. Therapeut Clin Risk Manag. 2014;10:95. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamoorthy A.L., Lemus A.A., Solomon A.P., Valm A.M., Neelakantan P. Interactions between Candida albicans and Enterococcus faecalis in an organotypic oral epithelial model. Microorganisms. 2020;8(11):1771. doi: 10.3390/microorganisms8111771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertolini M., Munoz R.V., Archambault L., Shah S., Souza J.G.S., Costa R.C., et al. Mucosal bacteria modulate Candida albicans virulence in oropharyngeal candidiasis. mBio. 2021;12(4) doi: 10.1128/mBio.01937-21. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason K.L., Erb Downward J.R., Falkowski N.R., Young V.B., Kao J.Y., Huffnagle G.B. Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis. Infect Immun. 2012;80(1):150–158. doi: 10.1128/IAI.05162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovac J., Kovac D., Slobodnikova L., Kotulova D. Enterococcus faecalis and Candida albicans in the dental root canal and periapical infections. Bratisl Lek Listy. 2013;114(12):716–720. [PubMed] [Google Scholar]
- 22.Dahlén G., Blomqvist S., Almståhl A., Carlén A. Virulence factors and antibiotic susceptibility in enterococci isolated from oral mucosal and deep infections. J Oral Microbiol. 2012;4(1):10855. doi: 10.3402/jom.v4i0.10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Freitas Lima S.M., da Costa Sousa M.G., de Souza Freire M., de Almeida J.A., de Castro Cantuária A.P., e Silva T.A.M., et al. Immune response profile against persistent endodontic pathogens Candida albicans and Enterococcus faecalis in vitro. J Endod. 2015;41(7):1061–1065. doi: 10.1016/j.joen.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Vaghela D.J., Kandaswamy D., Venkateshbabu N., Jamini N., Ganesh A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: as intracanal medicaments against Enterococcus faecalis and Candida albicans: an in vitro study. J Conserv Dent: J Comput Dynam. 2011;14(2):182. doi: 10.4103/0972-0707.82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghivari S.B., Bhattacharya H., Bhat K.G., Pujar M.A. Antimicrobial activity of root canal irrigants against biofilm forming pathogens-An in vitro study. J Conserv Dent: J Comput Dynam. 2017;20(3):147. doi: 10.4103/JCD.JCD_38_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noites R., Pina-Vaz C., Rocha R., Carvalho M.F., Gonçalves A., Pina-Vaz I. Synergistic antimicrobial action of chlorhexidine and ozone in endodontic treatment. BioMed Res Int. 2014;2014 doi: 10.1155/2014/592423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kean R., Delaney C., Rajendran R., Sherry L., Metcalfe R., Thomas R., et al. Gaining insights from Candida biofilm heterogeneity: one size does not fit all. J Fungi (Basel) 2018;4(1) doi: 10.3390/jof4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell L.E., Millhouse E., Sherry L., Kean R., Malcolm J., Nile C.J., et al. Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS Yeast Res. 2015;15(7) doi: 10.1093/femsyr/fov077. [DOI] [PubMed] [Google Scholar]
- 29.Cottier F., Tan A.S.M., Chen J., Lum J., Zolezzi F., Poidinger M., et al. The transcriptional stress response of Candida albicans to weak organic acids. G3: Gene Genet. Genomes. 2015;5(4):497–505. doi: 10.1534/g3.114.015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno V.M., Wang Z., Marjani S.L., Euskirchen G.M., Martin J., Sherlock G., et al. Comprehensive annotation of the transcriptome of the human fungal pathogen Candida albicans using RNA-seq. Genome Res. 2010;20(10):1451–1458. doi: 10.1101/gr.109553.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKloud E., Delaney C., Sherry L., Kean R., Williams S., Metcalfe R., et al. Recurrent vulvovaginal candidiasis: a dynamic interkingdom biofilm disease of Candida and Lactobacillus. mSystems. 2021;6(4) doi: 10.1128/mSystems.00622-21. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandara H., Wood D., Vanwonterghem I., Hugenholtz P., Cheung B., Samaranayake L.P. Fluconazole resistance in Candida albicans is induced by Pseudomonas aeruginosa quorum sensing. Sci Rep. 2020;10(1):1–17. doi: 10.1038/s41598-020-64761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz M.R., Graham C.E., Gagliano B.C., Lorenz M.C., Garsin D.A. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun. 2013;81(1):189–200. doi: 10.1128/IAI.00914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham C.E., Cruz M.R., Garsin D.A., Lorenz M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc Natl Acad Sci Unit States Am. 2017;114(17):4507–4512. doi: 10.1073/pnas.1620432114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garsin D.A., Lorenz M.C. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microb. 2013;4(5):409–415. doi: 10.4161/gmic.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown A.O., Graham C.E., Cruz M.R., Singh K.V., Murray B.E., Lorenz M.C., et al. Antifungal activity of the Enterococcus faecalis peptide EntV requires protease cleavage and disulfide bond formation. mBio. 2019;10(4) doi: 10.1128/mBio.01334-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishijima S.A., Hayama K., Ninomiya K., Iwasa M., Yamazaki M., Abe S. Protection of mice from oral candidiasis by heat-killed enterococcus faecalis, possibly through its direct binding to Candida albicans. Med Mycol J. 2014;55(1):E9–E19. doi: 10.3314/mmj.55.e9. [DOI] [PubMed] [Google Scholar]
- 38.Hassan M.N., Tartor Y.H., Ashour A.F., Elmowalid E.A. Enterococcus faecalis cell-free supernatant inhibits hyphal morphogenesis and biofilm formation in Candida albicans. Zagazig Vet. J. 2018;46(2):128–135. [Google Scholar]
- 39.Bachtiar E.W., Dewiyani S., Akbar S.M.S., Bachtiar B.M. Inhibition of Candida albicans biofilm development by unencapsulated Enterococcus faecalis cps2. J. Dent. Sci. 2016;11(3):323–330. doi: 10.1016/j.jds.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shekh R.M., Roy U. Biochemical characterization of an anti-Candida factor produced by Enterococcus faecalis. BMC Microbiol. 2012;12(1):132. doi: 10.1186/1471-2180-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonzi W.A., Irwin M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134(3):717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim E.B., Kopit L.M., Harris L.J., Marco M.L. Am soc microbiol. 2012. Draft genome sequence of the quality control strain Enterococcus faecalis ATCC 29212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coco B., Bagg J., Cross L., Jose A., Cross J., Ramage G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol Immunol. 2008;23(5):377–383. doi: 10.1111/j.1399-302X.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 44.Sedgley C., Lennan S., Clewell D. Prevalence, phenotype and genotype of oral enterococci. Oral Microbiol Immunol. 2004;19(2):95–101. doi: 10.1111/j.0902-0055.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 45.Johnson E.M., Flannagan S.E., Sedgley C.M. Coaggregation interactions between oral and endodontic Enterococcus faecalis and bacterial species isolated from persistent apical periodontitis. J Endod. 2006;32(10):946–950. doi: 10.1016/j.joen.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Sahm D.F., Kissinger J., Gilmore M.S., Murray P.R., Mulder R., Solliday J., et al. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shelburne C.E., An F.Y., Dholpe V., Ramamoorthy A., Lopatin D.E., Lantz M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother. 2007;59(2):297–300. doi: 10.1093/jac/dkl495. [DOI] [PubMed] [Google Scholar]
- 48.Sedgley C., Buck G., Appelbe O. Prevalence of Enterococcus faecalis at multiple oral sites in endodontic patients using culture and PCR. J Endod. 2006;32(2):104–109. doi: 10.1016/j.joen.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell L.E., Alalwan H.K.A., Kean R., Calvert G., Nile C.J., Lappin D.F., et al. Candida albicans biofilm heterogeneity does not influence denture stomatitis but strongly influences denture cleansing capacity. J Med Microbiol. 2017;66(1):54–60. doi: 10.1099/jmm.0.000419. [DOI] [PubMed] [Google Scholar]
- 50.Sherry L., Rajendran R., Lappin D.F., Borghi E., Perdoni F., Falleni M., et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014;14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montelongo-Jauregui D., Srinivasan A., Ramasubramanian A.K., Lopez-Ribot J.L. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva. Front Microbiol. 2016;7:686. doi: 10.3389/fmicb.2016.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gil-Bona A., Reales-Calderon J.A., Parra-Giraldo C.M., Martinez-Lopez R., Monteoliva L., Gil C. The cell wall protein Ecm33 of Candida albicans is involved in chronological life span, morphogenesis, cell wall regeneration, stress tolerance, and host–cell interaction. Front Microbiol. 2016;7(64) doi: 10.3389/fmicb.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jose A., Coco B.J., Milligan S., Young B., Lappin D.F., Bagg J., et al. Reducing the incidence of denture stomatitis: are denture cleansers sufficient? J Prosthodont: Implant Esthetic Reconstr Dent. 2010;19(4):252–257. doi: 10.1111/j.1532-849X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell L.E., Smith K., Williams C., Nile C.J., Lappin D.F., Bradshaw D., et al. Dentures are a reservoir for respiratory pathogens. J Prosthodont. 2016;25(2):99–104. doi: 10.1111/jopr.12342. [DOI] [PubMed] [Google Scholar]
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Short B., Delaney C., McKloud E., Brown J.L., Kean R., Litherland G.J., et al. Investigating the transcriptome of Candida albicans in a dual-species Staphylococcus aureus biofilm model. Front Cell Infect Microbiol. 2021;11:791523. doi: 10.3389/fcimb.2021.791523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramage G., VandeWalle K., López-Ribot J.L., Wickes B.L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214(1):95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 58.Alshanta O.A., Shaban S., Nile C.J., McLean W., Ramage G. Candida albicans biofilm heterogeneity and tolerance of clinical isolates: implications for secondary endodontic infections. Antibiotics. 2019;8(4):204. doi: 10.3390/antibiotics8040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White T.C., Agabian N. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J Bacteriol. 1995;177(18):5215–5221. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daignan-Fornier B., Pinson B. Yeast to study human purine metabolism diseases. Cells. 2019;8(1):67. doi: 10.3390/cells8010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripathi G., Wiltshire C., Macaskill S., Tournu H., Budge S., Brown A.J.P. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 2002;21(20):5448–5456. doi: 10.1093/emboj/cdf507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenz M.C., Bender J.A., Fink G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3(5):1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehmeti I., Solheim M., Nes I.F., Holo H. Enterococcus faecalis grows on ascorbic acid. Appl Environ Microbiol. 2013;79(15):4756–4758. doi: 10.1128/AEM.00228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet. 2003;44(1):1–7. doi: 10.1007/s00294-003-0415-2. [DOI] [PubMed] [Google Scholar]
- 65.Vazquez-Munoz R., Dongari-Bagtzoglou A. Anticandidal activities by Lactobacillus species: an update on mechanisms of action. Frontiers in Oral Health. 2021:47. doi: 10.3389/froh.2021.689382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dos Santos A.L.S. HIV aspartyl protease inhibitors as promising compounds against Candida albicans André Luis Souza dos Santos. World J Biol Chem. 2010;1(2):21. doi: 10.4331/wjbc.v1.i2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vylkova S., Carman A.J., Danhof H.A., Collette J.R., Zhou H., Lorenz M.C. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2(3) doi: 10.1128/mBio.00055-11. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis D., Wilson R.B., Mitchell A.P. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20(3):971–978. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez J.C. The interplay between gut bacteria and the yeast Candida albicans. Gut Microb. 2021;13(1):1979877. doi: 10.1080/19490976.2021.1979877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santus W., Devlin J.R., Behnsen J. Crossing kingdoms: how the mycobiota and fungal-bacterial interactions impact host health and disease. Infect Immun. 2021;89(4) doi: 10.1128/iai.00648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delaney C., O'Donnell L.E., Kean R., Sherry L., Brown J.L., Calvert G., et al. Interkingdom interactions on the denture surface: implications for oral hygiene. Biofilm. 2019;1:100002. doi: 10.1016/j.bioflm.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hollomon J.M., Grahl N., Willger S.D., Koeppen K., Hogan D.A., Mitchell A.P. Global role of cyclic AMP signaling in pH-dependent responses in Candida albicans. mSphere. 2016;1(6) doi: 10.1128/mSphere.00283-16. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alshanta O.A., Alqahtani S., Shaban S., Albashaireh K., McLean W., Ramage G. Comparison of three endodontic irrigant regimens against dual-species interkingdom biofilms: considerations for maintaining the status quo. Antibiotics. 2020;9(9) doi: 10.3390/antibiotics9090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siqueira J., Jr., Lopes H. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32(5):361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 75.Waltimo T., Siren E., Orstavik D., Haapasalo M. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int Endod J. 1999;32(2):94–98. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 76.Ran S., Wang J., Jiang W., Zhu C., Liang J. Assessment of dentinal tubule invasion capacity of Enterococcus faecalis under stress conditions ex vivo. Int Endod J. 2015;48(4):362–372. doi: 10.1111/iej.12322. [DOI] [PubMed] [Google Scholar]
- 77.Al-Nazhan S., Al-Sulaiman A., Al-Rasheed F., Alnajjar F., Al-Abdulwahab B., Al-Badah A. Microorganism penetration in dentinal tubules of instrumented and retreated root canal walls. In vitro SEM study. Restor Dent Endod. 2014;39(4):258–264. doi: 10.5395/rde.2014.39.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Barplot of GO over representation of significant biological, cellular, and molecular functions of mono and dual species biofilms at 6h. (a) Enriched pathways in dual species biofilms. (b) Enriched pathways in C. albicans mono species biofilms.
pH measurements of normal, boiled, ultrafiltered and boiled ultrafiltered supernatant of E. faecalis after incubation with C. albicans for 24h (a) SC5314 (b) HBF (c) LBF.
pH change of the C. albicans and E. faecalis monocultures and dual species cultures at 2h, 4h, 6h, 8h and 24h. (a) pH measurements of C. albicans SC5314, E. faecalis ER5/1 and their coculture. (b–d) pH values of 3 C. albicans and 3 E. faecalis mono and dual species cultures at the indicated time points. (b)C. albicans SC5314 with E. faecalis E2, ER5/1 and V583. (c)C. albicans HBF. (d)C. albicans LBF.
Linear regression analysis for the correlation of EntV expression in 12 E. faecalis strains and biofilm biomass of C. albicans grown in the corresponding E. faecalis supernatant. (a) SC5314 (b) HBF (BC146) (c) LBF (BC023). The p-value for SC5314, HBF and LBF was 0.2352, 0.7894 and 0.2273 respectively.
Data Availability Statement
Sequenced transcriptome data has been deposited to the NCBI sequence read archive (SRA) database and can be found under accession number PRJNA731052.