Highlights
-
•
Granulosa cell tumors (GCTs) are sex cord stromal tumors that normally arise from the ovaries.
-
•
Primary extraovarian GCTs are uncommon, but primary retroperitoneal GCTs are even rarer.
-
•
Management of extraovarian GCTs can include surgery, chemotherapy, and/or anti-hormone therapy.
-
•
Delays in care during the COVID-19 Pandemic may have led to advanced disease or progression of disease for cancer patients.
Keywords: Granulosa cell tumors, Extraovarian, Retroperitoneal, COVID-19 Pandemic, Delays in care
Abstract
Extraovarian granulosa cell tumors are rare with very few cases of isolated retroperitoneal granulosa cell tumors reported in the literature. Granulosa cell tumors are notorious for late recurrences and patients should have long term oncologic follow up. We describe a case of recurrent granulosa cell tumor of the retroperitoneum that originally presented as a renal mass, which has not been described before in the literature. Her management was delayed in part due to the COVID-19 Pandemic. Oncologists must be vigilant regarding the consequences of postponed care during this difficult time.
1. Introduction
Granulosa cell tumors (GCTs) are sex cord stromal tumors (SCST) that normally arise from the ovaries. They comprise 5% of all ovarian malignancies and 70% of malignant SCSTs (Chi et al., 2017). Patients may present with the following symptomatology: abnormal uterine or postmenopausal bleeding, abdominal distention and/or abdominal pain (Chi et al., 2017, Vasu et al., 2016, Al-Shraideh et al., 2012). These ovarian masses may also be incidental findings on imaging if patients are asymptomatic. GCTs usually produce estrogens and can be monitored with inhibin levels (Chi et al., 2017). Primary extraovarian GCTs are uncommon, but primary retroperitoneal GCTs are even rarer (Vasu et al., 2016, Al-Shraideh et al., 2012, Kim et al., 2001, Naniwadekar and Patil, 2010, Paul et al., 2009, Robinson et al., 1999, Soydinc et al., 2012, Rauniyar et al., 2021). Here we describe a case of primary retroperitoneal GCTs that originally presented as a renal mass. This patient had a recurrence during the COVID-19 pandemic that contributed to a delay in her care.
2. Case description
A 64 year old, Para 1, African, postmenopausal female presented to the emergency department in June 2021 with multiple abdominal masses. Her Eastern Cooperative Oncology Group (ECOG) status was 1 and body mass index: 32 kg/m2. Her past medical history was significant for hypertension and asthma. Her surgical history was significant for one cesarean delivery and a left nephrectomy along with resection of a retroperitoneal mass in May 2013. She did not have any known history of hysterectomy or salpingo-oophorectomy.
Upon interviewing the patient, she reported that she had a left nephrectomy in 2013 at an outside institution. That pathology report described a reddish brown hemorrhagic circumscribed mass measuring 14x10.5x6cm, which was excised along with the left kidney measuring 10x6.5x3cm. The pathology was significant for adult type granulosa cell tumor with round to oval tumor cells with vesicular nuclei and in many instances nuclear grooves. The tumor was diffusely positive for inhibin. Patient did not have follow up with a gynecologic oncologist afterward.
She subsequently presented to another outside institution in June 2020 with abdominal pain. She had a computed tomography (CT) scan of the abdomen/pelvis revealing a 12x6cm mass encompassing the spleen and multiple additional abdomino-pelvic masses. The uterus and ovaries were unremarkable. She had a CT guided core-needle biopsy of the peri-splenic mass. The pathology was significant for metastatic granulosa cell tumor. Immunostains were positive for inhibin, estrogen, and progesterone receptors. Patient then presented to our facility, almost one year later, in June 2021 and reported she was unaware of these results and could not follow up because she traveled to Nigeria in Africa in July 2020 and was not able to return until December 2020 because of international travel restrictions due to the COVID-19 Pandemic. The patient also reported that she did not receive any cancer care in Africa.
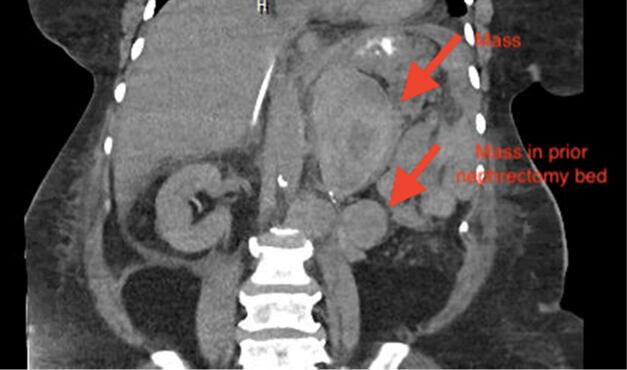
Since the patient returned to the United States, she had intermittent abdominal pain, but no vaginal bleeding. On patient’s current presentation in June 2021, CT scan of the abdomen/pelvis revealed multiple masses within the left abdomen, prior nephrectomy bed, retroperitoneum, and pelvis (Fig. 1a. and Fig. 1b.). Ultrasound of the pelvis demonstrated normal appearing uterus, normal appearing right ovary, left ovary not visible, however no adnexal masses identified on the left. Her pelvic examination was unremarkable with a normal sized uterus and no palpable adnexal masses. Inhibin B level was > 1300 pg/mL with no prior values to compare to. No further biopsies were performed and her abdomino-pelvic masses were attributed to metastatic granulosa cell tumors. Patient was eventually discharged to a rehabilitation facility. She returned for follow up with the gynecologic and medical oncologists and received seven cycles of carboplatin and paclitaxel. Her most recent inhibin B level in December 2021 was 388 pg/mL. A repeat CT scan at this time demonstrated retroperitoneal and left upper quadrant masses smaller in size, suggesting partial response. Patient is due to receive leuprolide and anastrazole due to the estrogen and progesterone receptor positivity of her tumor.
Fig. 1a.
Coronal view of CT scan of abdomen/pelvis demonstrating mass in patient’s left upper quadrant and prior nephrectomy bed.
Fig. 1b.
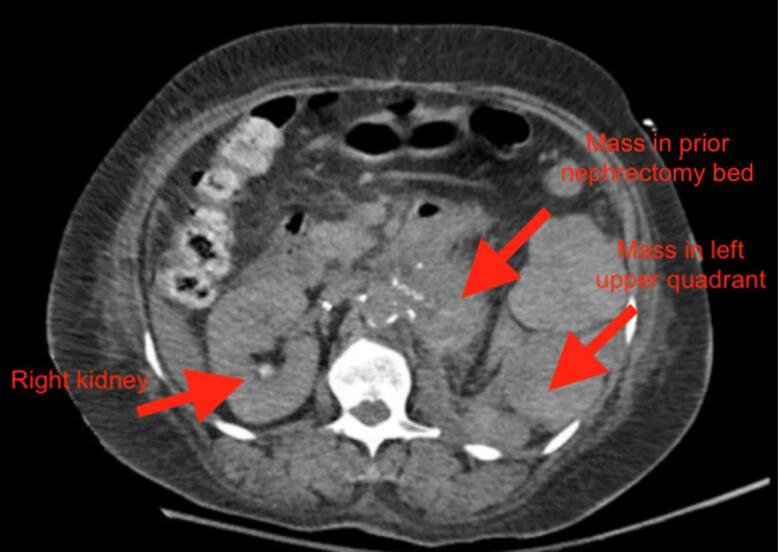
Axial view of CT scan of abdomen/pelvis demonstrating mass in patient’s left upper quadrant and prior nephrectomy bed.
3. Discussion
Extraovarian granulosa cell tumors are uncommon. (Naniwadekar and Patil, 2010) and (Paul et al., 2009) described cases of GCT presenting as mesenteric masses (Naniwadekar and Patil, 2010, Paul et al., 2009). (Robinson et al., 1999) described a case of GCT that arose from the pelvic side wall, but in close proximity of the ovary (Robinson et al., 1999). Another case of extraovarian GCT was described in which the mass was near the pelvic structures, including the bladder and pubic bone (Soydinc et al., 2012). Additionally, one unique case of extraovarian GCT was described to have originated from the cerebellum (Rauniyar et al., 2021). It is thought that GCTs that arise in locations other than the ovary are derived from the mesenchyme of the genital ridge, however the pathophysiology is not well understood (Al-Shraideh et al., 2012).
Only a handful of cases of extraovarian GCT have been published in the literature to have originated from the retroperitoneum (Paul et al., 2009, Vasu et al., 2016, Al-Shraideh et al., 2012, Kim et al., 2001). Our case is unique in that the GCT presented as a renal mass, which has not previously been described to our knowledge. Although our patient did not have a prior hysterectomy with salpingo-oophorectomy to confirm that the mass did not originate from her ovaries, we were reassured that her gynecologic structures were normal based on her examination and imaging eight years after her initial diagnosis.
The staging for ovarian GCTs is surgical and the same as that used for epithelial ovarian cancer; stage is also the most important prognostic factor for GCTs (Chi et al., 2017). Ovarian GCTs are usually localized and indolent with recurrence at extended time intervals from primary therapy. For patients who recur, the median time to recurrence is 6 years and median survival after recurrence is 5.6 years (Chi et al., 2017). For extraovarian GCTs, the staging is not as clear and recurrence rates are unknown. For these reasons, management may be challenging. It would be reasonable to perform an excisional procedure for a localized mass similar to our case at her primary presentation. A clinician may consider performing a hysterectomy with bilateral salpingo-oophorectomy for staging purposes and to establish an origin of disease. After the time of initial presentation for our patient, she was lost to follow up and a gynecologic surgery was not performed. For more advanced or recurrent cases that are not surgically resectable, adjuvant chemotherapy may be considered. Adjuvant chemotherapy should include platinum based therapy, including either carboplatin and paclitaxel, etoposide and cisplatin (EP), or bleomycin, etoposide, and cisplatin (BEP) (Network and Cancer, 2021). Our patient received carboplatin and paclitaxel and she tolerated it well with partial tumor response. Other treatment options may involve anti-hormone agents such as gonadotropin-releasing hormone (GnRH) agonists and aromatase inhibitors, depending on the presence of hormone receptors in a tumor. Our patient’s tumor was found to be estrogen and progesterone receptor positive, thus she will be receiving leuprolide acetate (GnRH agonist) and anastrazole (aromatase inhibitor). Most studies have supported the use of these medications as single agent therapy and not in combination (Fishman et al., 1996, Freeman and Modesitt, 2006). However, there is some evidence of synergy with dual anti-endocrine therapy in hormone positive breast cancer and even with recurrent granulosa cell tumors (Mehta et al., 2019, Keskin et al., 2012). Further, leuprolide and anastrazole will be used due to the large burden of disease.
Our patient had various delays in care, which worsened her disease recurrence. First, our patient did not have the appropriate follow up with a gynecologic or medical oncologist after her primary diagnosis was established approximately 9 years ago. It is unclear why she was initially lost to follow up, given English was her preferred language and she did have health insurance throughout this time. However, other barriers to care that may have contributed to her disease burden could have been a lack of understandable communication, low level of education and health literacy, limited family support at the time of initial presentation, and low socio-economic status as our patient was unemployed for several years. Addressing these health disparities could have allowed for more streamlined healthcare and appropriate compliance. Healthcare providers should help patients with access to care and understanding of their disease processes. Ideally, surveillance should have included close long term follow up with physical exams and trending of tumor markers (Network and Cancer, 2021). Moreover, her disease relapsed about 1–2 years ago and she was not only unaware of her results, but also unable to travel back from Africa to the United States due to the COVID-19 Pandemic. It has been established that gynecologic oncology treatments have been modified or delayed due to COVID-19 (Piedimonte et al., 2021). With travel restrictions during the pandemic and other barriers, imperative medical care has been hindered for many and many patients have also been lost to follow up. Since travel restrictions have been lifted and patients feel safer presenting to hospitals, clinicians are now seeing the consequences of postponed care, similar to our case.
In summary, there is a paucity of information on GCTs of extraovarian origin. We present an interesting case of retroperitoneal GCT that initially presented as a renal mass. Our patient had recurrent disease and her management was delayed in part due to the COVID-19 virus and travel restrictions. Gynecologic and medical oncologists should be vigilant about the consequences of delayed care during this pandemic. Her management has included carboplatin and paclitaxel, and will now include leuprolide acetate and anastrazole.
4. Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chi D.S., Berchuck A., Dizon D.S., Yashar C., Gershenson D.M., Dowdy S.C., et al. Wolters Kluwer.; Principles and Practice of Gynecologic Oncology: 2017. Ovarian Sex Cord-Stromal Tumors; pp. 724–728. [Google Scholar]
- Vasu P.P., Leelamma J.P., Mohammed B.A., Yesodharan J. Primary granulosa cell tumor of retroperitoneal origin: A rare presentation with emphasis on cytomorphology. J. Cytol. 2016;33:52–54. doi: 10.4103/0970-9371.175527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shraideh Y., Mahfooz A.B., Moazin M., Aslam M., Alhazmi A., Alshakweer W. Primary Retroperitoneal Granulosa Cell Tumor. UroToday. International J. 2012;05(06) [Google Scholar]
- Kim S.H., Park H.J., Linton J.A., Shin D.H., Yang W.I., Chung W.Y., Kim Y.T. Extraovarian granulosa cell tumor. Yonsei. Med. J. 2001;42(3):360. doi: 10.3349/ymj.2001.42.3.360. [DOI] [PubMed] [Google Scholar]
- Naniwadekar M.R., Patil N.J. Extraovarian granulosa cell tumor of mesentery: a case report. Patholog. Res. Int. 2010;2010 doi: 10.4061/2010/292606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P.C., Chakraborty J., Chakrabarti S., Chattopadhyay B. Extraovarian granulosa cell tumor. Indian J. Pathol. Microbiol. 2009;52:231–233. doi: 10.4103/0377-4929.48928. [DOI] [PubMed] [Google Scholar]
- Robinson J.B., Im D.D., Logan L., McGuire W.P., Rosenshein N.B. Extraovarian granulosa cell tumor. Gynecol. Oncol. 1999;74(1):123–127. doi: 10.1006/gyno.1999.5375. [DOI] [PubMed] [Google Scholar]
- Soydinc H.E., Sak M.E., Evsen M.S., Bozkurt Y., Keles A. Unusual case of extraovarian granulosa cell tumor. Eur. Rev. Med. Pharmacol. Sci. 2012;16(Suppl 4):30–31. [PubMed] [Google Scholar]
- Rauniyar S., Shen Z., Wang L., Gu J., Pengjin M., Yu R. Primary granulosa cell tumor of cerebellum: A rare case report. Interdisciplinary Neurosurgery. 2021;23:100992. [Google Scholar]
- National Comprehensive Cancer Network. Ovarian Cancer, including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 1.2021). 2021.
- Fishman A., Kudelka A.P., Tresukosol D., Edwards C.L., Freedman R.S., Kaplan A.L., et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J. Reprod. Med. 1996;41:393–396. [PubMed] [Google Scholar]
- Freeman S.A., Modesitt S.C. Anastrozole therapy in recurrent ovarian adult granulosa cell tumors: a report of 2 cases. Gynecol. Oncol. 2006;103(2):755–758. doi: 10.1016/j.ygyno.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Mehta R.S., Barlow W.E., Albain K.S., Vandenberg T.A., Dakhil S.R., Tirumali N.R., Lew D.L., Hayes D.F., Gralow J.R., Linden H.M., Livingston R.B., Hortobagyi G.N. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N. Engl. J. Med. 2019;380(13):1226–1234. doi: 10.1056/NEJMoa1811714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin S., Bengisu E., Tuzlali S., Aydiner A. Complete response in a patient with granulosa cell tumor treated with a combination of leuprolide and tamoxifen. Onkologie. 2012;35(7-8):451–453. doi: 10.1159/000341078. [DOI] [PubMed] [Google Scholar]
- Piedimonte S., Li S., Laframboise S., Ferguson S.E., Bernardini M.Q., Bouchard-Fortier G., et al. Gynecologic oncology treatment modifications or delays in response to the COVID-19 pandemic in a publicly funded versus privately funded North American tertiary cancer center. Gynecol. Oncol. 2021 doi: 10.1016/j.ygyno.2021.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]