Abstract
The BG line was originated from the cross between 2 Chinese indigenous breeds, Dongxiang blue eggshell, and Jiangshan black-bone, and has been bred to combine dark heavy black-bone body and high production of blue-shell eggs, into single dual-purpose line. Full-pedigree hens from 2 generations, G4 (n = 441) and G5 (n = 464), were reared in the same single-cage laying facility in 2019–2020 and 2020–2021, respectively. Starting from the first egg of each hen, its daily egg production was recorded until 300 days-of-age. Up to 7 "no-egg" days were considered normal laying breaks between clutches, whereas laying cessation of 8 or more days was considered Pause, and the laying pattern of each hen was assigned either with Pause or No-Pause. The other traits included PsDays: number of Pause days; AFE: age at first egg; EN300: eggs laid until 300 d; %L300: total laying rate (EN300/[300-AFE]); %Lnet: net laying rate (EN300/[300-AFE-PsDays]); ClLng: average clutch length; EW200 and EW300: average egg weight around 200 d and 300 d. Estimates of heritability (h2) of each trait, and phenotypic and genetic correlation between traits, were calculated in each generation using the animal model. Heritability estimates were calculated also by regressing the means of full-sib G5 hens on their G4 parents' means. Mean overall laying rate of all G4 hens was low (%L300 = 57%) because 53% of them had Pause in their laying pattern. In G5, incidence of Pause was higher (75%) due to a 3-wk cold stress, with mean %L300 = 54%. However, significant estimates of heritability and genetic correlations suggest that selection for low PsDays will reduce the incidence of Pause in BG hens and elevate the line's mean laying rate towards %L300 = 70%, as the No-Pause hens in G5. PsDays-free laying rate (%Lnet) was found to be highly correlated with the significantly heritable (h2≈0.4) clutch length (ClLng). Selection index combining the genetically independent low PsDays and high ClLng is expected to maximize egg production improvement in the BG line, and in similar populations derived from indigenous breeds.
Key words: laying pause, net laying rate, clutch length, egg weight, selection index
INTRODUCTION
Throughout evolution, egg production has been the basic fitness performance of chickens, and is economically very important since their domestication. Practically, egg production is the number of eggs laid up to a certain target age, which is the product of age at first egg (AFE) and the overall laying rate: the number of eggs laid from AFE to target age, divided by the number of days between these 2 ages. Thus, laying rate simply represent the ratio between the overall numbers of days with or without egg laid. Biologically, however, these numbers depend on the egg-laying pattern of clutches (consecutive days with eggs) and periods of no-laying days between them, and their length.
Wolc et al. (2019) estimated of heritability of clutch length in commercial Rohde Island Red (RIR) and White Leghorn (WL) lines; the estimates were medium (0.31–0.34) for average clutch length, and lower (0.20–0.29) for maximum clutch length. Similar estimates of heritability were reported for average and maximum clutch length (0.26–0.31 and 0.21–0.24, respectively) in 2 lines originated from Chinese indigenous chickens (Jin, 2010; Shen et al., 2019), whereas somewhat higher heritability (0.42) of average clutch length was found in 2 brown-egg dwarf lines selected for egg production over 16 generations (Chen and Tixier-Boichard, 2003). Higher heritability estimates were reported for number of clutches: 0.42 and 0.41 in commercial RIR and WL (Wolc et al., 2019), and 0.52 in Chinese Rugao Yellow chickens (Shen et al., 2019).
Clutch ends when the hen skip one or a few days before resuming egg laying and starting another clutch, and its length may vary from a single day to over a year (Warren, 1953; Preisinger, 2018). Up to 7 no-egg days between clutches are considered normal, whereas a temporary cessation of egg laying for 8 or more days have been defined as Winter Pause because of their winter occurrence (Jull, 1952; Warren, 1953). Due to the substantial reduction in overall egg production caused by such pauses, the genetic and non-genetic factors affecting their occurrence and length were thoroughly investigated in commercial WL and RIR hens during the 1930′s and 1940s (e.g., Lerner and Taylor, 1947; Hays, 1949, 1951). Lerner and Taylor (1947), by selecting for high egg production over 9 generations, reduced the incidence of Winter Pause from 54 to 26%, indicating that the tendency to such long pauses is hereditary. In a recent study (Wolc et al., 2019) on the genetic determination of egg-laying pattern in modern WL and RIR commercial egg-production lines, long pauses were not mentioned, suggesting that after many generations of intensive selection for high egg production, hens in modern commercial lines do not enter long pauses under normal rearing conditions. However, it is expected that chicken populations recently derived from poorly selected indigenous rural breeds may exhibit variable laying patterns, including long pauses similar to those of the commercial WL and RIR layers of the 1930s and 1940s.
In contrast to the low number of reports on egg-laying pattern, there were many studies on the genetics of the standard components of overall egg production: egg number, AFE, laying rate, and egg weight. These studies were conducted in intensely selected commercial and experimental egg-type chicken lines, and in populations of indigenous or native rural chickens. Medium estimates of heritability were reported for AFE in intensively selected RIR lines (0.45, Tongsiri et al., 2015; 0.51, Liu et al., 2019), White Plymouth Rock (WPR) line (0.44, Tongsiri et al., 2015), WL line and brown-egg dwarf line (0.32 and 0.55, Yi et al., 2014), whereas low heritability (0.16) was estimated for AFE in a population of Thai native chickens (Tongsiri et al., 2018). Low estimates of heritability were reported for egg number during various laying periods in intensively selected RIR lines (0.20, Tongsiri et al., 2015; 0.14–0.24, Liu et al., 2019), and WPR line (0.19, Tongsiri et al., 2015). Also in native and indigenous populations in Iran, Thailand, and India, the heritability estimates of egg number were low, 0.14 to 0.17 (Niknafs et al., 2012; Tongsiri et al., 2018; Ullengala et al., 2020). Low to medium estimates of heritability were reported for egg weight at various ages, ranging from 0.17 to 0.43 in native Iranian breed (Niknafs et al., 2012), 0.24 in native Indian male line (Ullengala et al., 2020), and 0.38, 0.43, 0.44 to 0.54 and 0.35 to 0.60 in RIR, WPR, WL and brown-egg dwarf lines, respectively (Yi et al., 2014; Tongsiri et al., 2015).
The present study is part of the ongoing development of BG, an experimental dual-purpose line originated from a cross between 2 Chinese indigenous breeds, and bred since 2015 for improved production of a unique combination of dark carcass and blue-shell eggs. The general interest in populations derived from indigenous breeds is increasing, following the efforts to improve their performances for meat production (Yang and Jiang, 2005) or for dual-purpose production (Ibrahim et al., 2019; Ibrahim, 2020), and to broaden the genetic basis of commercial chicken lines by capturing useful genetic variants that exist only in indigenous breeds (Athrey, 2020). The first paper in this series (Wang et al., 2021) presented and discussed the genetic parameters of body weight and skin darkness at various ages in the G4 generation of the BG line, and their potential use in selecting females and males for these 2 carcass-related traits. This paper covers the genetic parameters of laying pattern and egg production in 2 consecutive generations, G4 and G5, and the prospects to improve these traits by selective breeding.
MATERIALS AND METHODS
The BG Line
Breeding Process
The BG line has been bred at the Hangzhou Academy of Agricultural Sciences (Wang et al., 2021). Briefly, it originated from the segregating progeny of the cross between 2 Chinese indigenous breeds, Dongxiang blue eggshell chicken and Jiangshan black-bone chicken. The breeding program of the BG line has been aimed at combining black body (and feathers) and blue eggshell. Following molecular confirmation of homozygosity in the major genes for black body and blue eggshell in 2015, the BG line had been further expanded by 3 generations (G1 to G3) of random mating. In the year 2019, each of 35 random G3 sires was mated by artificial insemination with 3 to 6 randomly assigned non-sib G3 dams. The G4 full-pedigree progeny chicks were obtained in 3 consecutive hatches (May 1, 7, and 13, 2019), and they were marked by wing bands with pedigree-related identification numbers.
From the 441 G4 females that were alive at 300 d of age (300 d), 154 were selected to serve as dams of G5. They had the top values of an index with 2 carcass-related traits: body weight (BW) at 300 d (BW300) minus skin lightness (L*) at 250 d (Wang et al., 2021), and 2 egg production traits: % lay to 300 days (%L300) plus mean egg weight at 200 d (EW200). The index consisted of the standardized phenotypic values the 4 traits, with the following relative weights: BW = 30%, L* = 20%, %L = 35%, EW=15%. From the 318 G4 males that were alive at 300 d of age, 35 males were selected according to the same index, consisted of their own BW and L*, and the means of %L300 and EW200 of their full-sib sisters. Each of these 35 males, selected to serve as sires of G5, was mated by artificial insemination with 3 to 6 randomly assigned, but non-sib, selected G4 dams. As in G4, the G5 full-pedigree progeny chicks were obtained in three consecutive hatches (May 18 and 30, and June 5, 2020), and they were marked by wing bands with pedigree-related identification numbers.
Animal Management
In both generations, all the chicks were reared to 56 d of age in stack-style brooding batteries of group cages, and then moved to stack-style growing group cages for additional 50 d. The stocking density from 1 to 30 d, from 31 to 56 d, and from 57 to 105 d was 100, 50 and 25 birds per square meter, respectively. After the age of 105 d, females and males were housed in individual cages. When the hens started laying eggs, day length was increased artificially, in 30-min weekly increments, to 16 h of light per day. At each stage, from day-old chicks to laying hens, the same facilities and all management procedures (following the guidelines of Hy-Line International, https://www.hyline.com/) were used in G4 and G5. Major nutrients during brooding, growing and laying periods were 19.5% crude protein (CP) and 2900 Kcal/Kg metabolism energy (ME), 16.5% CP and 2,800 Kcal/Kg ME, 17.5% CP and 2850 Kcal/Kg ME, respectively. All rearing practices were approved by the animal ethics committee of Hangzhou Academy of Agricultural Sciences (Hangzhou, China).
Number of Females Per Generation and Family
Between the housing in individual cages at 105 d, and the termination of daily egg recording at 300 d, rate of mortality was approximately 3% in both generations. This rate is similar to the mortality in commercial layer farms (Fulton, 2017), indicating standard hygiene and overall rearing conditions. The data used in this study were taken only from the hens that lay at least one egg and were alive at 300 d of age: 441 in G4 and 464 in G5. The hens in both generations were progeny of 35 sires, each mated at random with 3 to 6 unrelated (non-sib) dams, 144 in G4 and 154 in G5. The average number of hens per sire and per dam was 12.5 and 3.0 in G4, 13.5 and 3.1 in G5. There were only a few dams with a single hen progeny, and one sire (in G5) with a single dam; their exclusion from the analyses hardly changed the results, and therefore data from all hens were included in this study.
Egg Production Measurements
Starting from the first egg of each hen, its daily egg production was recorded up to 300 days of age (300 d). In the rare cases where 2 eggs per hen were recorded on the same day, as happens when the exact time of egg recording slightly varies from day to day, the record of 1 egg was moved to the "no-egg" day before or after the "2-eggs" day. Clutch length was the number of consecutive days (also a single day) with an egg laid by the hen, ended by at least one no-egg day. Up to 7 no-eggs days were considered normal laying breaks between clutches, whereas temporary laying cessation of 8 or more days were considered Pause, following the definition of Winter Pause (Lerner and Taylor, 1947; Hays, 1949, 1951). For each hen, the following traits of egg production up to 300 days of age (300 d) were defined: Pause0/1 – hens without Pause in their laying pattern were assigned 0 and hens with Pause were assigned 1; PsDays – total number of Pause days; AFE – age at first egg; EN300 – number of eggs laid until 300 d; %L300 – laying rate from AFE to 300 d (EN300/[300-AFE]); %Lnet – laying rate during the in-lay days (EN300/[300-AFE-PsDays]); ClLng – average clutch length (in days); EW200 and EW300 – average weight of about 5 eggs collected from each hen around 200d and 300d, respectively (the few hens not laying around 200 d or 300 d were assigned the average EW200 or EW300 of their full-sib sisters).
Statistical Analysis
The inbreeding coefficient was determined in each generation of the BG line and found to be too low to affect performance neither to bias the estimates of genetic parameters (Wang et al., 2021). Estimates of heritability (h2) of each trait, and the genetic correlation (rG) and phenotype correlation (rP) between traits, were calculated in each generation using the individual animal model of ASReml 4.1 software (https://asreml.kb.vsni.co.uk/) with pedigree information (G3 parents of G4 hens, G3 grandparents and G4 parents of G5 hens) as random effects, and hatch as fixed effect. The significances of h2, rG and rP were determined by the likelihood ratio test of ASReml. Heritability estimate of each trait was calculated also from Parent-Offspring (P-O) association, by regressing the means of G5 hens in each full-sib family on the their mid-parent – the mean of their G4 dams and sires (each sire was assigned the means of his 3 to 5 full-sib sisters). These regressions, as well as chi-square tests and contrasts (t tests) between generations and between No-Pause vs. Pause hens, were conducted by the JMP software (https://www.jmp.com/).
RESULTS AND DISCUSSION
Incidence of Pause in the Laying Pattern
About 53% of the hens in G4 had Pause (temporary cessation of egg laying for more than 7 d) in their laying pattern up to 300 days of age (300 d), when egg recording was terminated in this study. This percentage (%Pause) is very similar to the incidence of Winter Pause in commercial WL and RIR lines during the 1930s and 1940s (Lerner and Taylor, 1947; Hays, 1949, 1951), whereas in generation G5, %Pause was significantly higher (75.6%) than in G4 (Table 1). It should be noted that only for hens that were laying on 300 d or the preceding week, the recorded Pauses ended by resumption of egg laying. However, for hens that were on Pause at 300 d, it is not known if the laying cessation was temporary that is, they were in a "real" Pause of unknown length, or they already terminated their yearly egg production. The percentage of these hens, that possibly stopped laying before 300 d, was 18.4% in G4 and decreased to 9.0% in G5 (Table 1).
Table 1.
The numbers and percentages of hens in each of two Pause categories, divided by the hen's status at 300 d (in laying or in Pause), in generations G4 and G5.
| Hen status at 300 d | Generation G4 (441 hens) |
Generation G5 (464 hens) |
||
|---|---|---|---|---|
| No-Pause | Pause | No-Pause | Pause | |
| In laying | 208 (47.2%) | 152 (34.4%) | 113 (24.4%) | 309 (66.6%) |
| In Pause | 0 | 81 (18.4%) | 0 | 42 (9.0%) |
| All hens | 208 (47.2%) | 233 (52.8%) | 113 (24.4%) | 351 (75.6%) |
The differences between generations in the frequencies of No-Pause vs. Pause hens were highly significant (P of Chi-square < 0.0001).
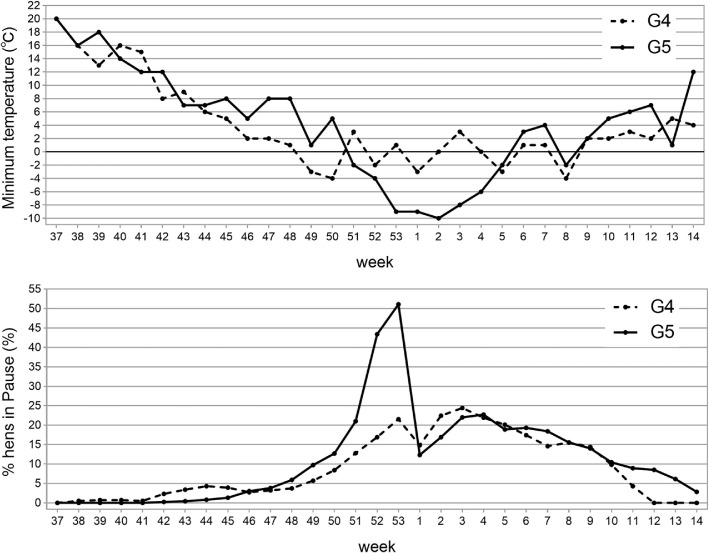
The term Winter Pause was used in Lerner and Taylor (1947) and Hays (1949, 1951), and in textbooks (Jull, 1952; Warren, 1953) because these pauses occurred mainly during the winter months (November, December, January, February). These months are characterized by less hours/day of natural light, but this seasonal variation is the same every year, whereas all the authors reported large year-to-year variation in %Pause. Among several biotic and a-biotic stressors suggested as potential causes of Pause, low temperatures were considered the main cause (Hays, 1949, 1951), and therefore, the minimal temperatures during the laying seasons of G4 and G5 were compared. The upper graph in Figure 1 shows the weekly averages of the daily minimum temperatures from mid-September to early April in the years 2019–2020 (generation G4) and 2020–2021 (generation G5), whereas the lower graph shows the weekly averages of %Pause among the hens in G4 and G5 during the same weeks. The lower graph shows that G4 and G5 had similar %Pause throughout most the weeks, except during the last 3 wk of 2020 (wk 51, 52, 53), when the %Pause of the G5 hens increased sharply up to 50%, before returning to around 20% (similar to G4) in the following weeks as many in-Pause G5 hens resumed egg production. The upper graph shows that during these weeks in the year 2020, there was a sharp drop, from 5°C down to −9°C, in the minimum temperatures recorded outside the hens' house. Temperatures were not recorded inside the house, but considering the house's simple materials, it is assumed that inside minimum temperatures were no more than 10°C above the outside temperatures, that is, the G5 hens were exposed to minimum ambient temperatures around the freezing point. The corresponding outside temperatures during these weeks in 2019–2020 fluctuated between 4°C and −4°C, suggesting that during the coldest weeks, the G4 hens (kept in the same house as the G5 hens) were exposed to ambient temperatures well above the freezing point. In a study with commercial WL and RIR lines of the late 1950s, egg production declined sharply when ambient temperatures dropped to around −7°C outside the chicken house and 2°C inside (Hays, 1958). This report, earlier ones by Hays (1949, 1951), and a recent one by Xie et al. (2017), support the suggestion that the elevation in %Pause among G5 hens was induced by low minimum temperatures. It should be noted that mortality during the wk 51 to 53 in 2020 was normal (0.6%), indicating that this cold stress was not lethal.
Figure 1.
The minimum weekly temperature (upper graph) and the percentage of hens in Pause (lower graph), from 37th week of 2019 to 14th week of 2020 (generation G4), and from 37th week of 2020 to 14th week of 2021 (generation G5).
The Effects of Pause on Egg Production Traits
Table 2 shows the means of the egg production traits calculated separately for the hens without Pause (No-Pause) and with Pause in generations G4 and G5, and the Pause effect (Pause minus No-Pause) on each trait. By definition, the No-Pause hens had no Pause days (PsDays = 0), whereas the Pause hens averaged 35.9 d in G4 and 42 d in G5. Compared to No-Pause hens, mean AFE of their counterparts with Pause was lower by 2.6 d (not significant) in G4 and by 5.2 d (significant) in G5 (Table 2). It appears that on the average, hens that started to lay earlier were more prone to pause, especially under cold stress (G5). The No-Pause hens in G4 and in G5 averaged substantially higher EN300 (99 and 98 eggs) than the Pause hens (71.6 and 72.5 eggs). Having Pause in the laying pattern reduced EN300 (by an average of about 26 eggs), reflecting the Pause-related PsDays, because as there are more PsDays in the laying pattern, there are less potential days for laying eggs. The overall rate of lay from AFE to 300 d (%L300) is the ratio between EN300 and 300-AFE, and due to the small difference in AFE between the No-Pause and Pause groups, their means of %L300 ranked similarly to their ranking by EN300. In G4 and G5, mean %L300 of the No-Pause hens (67.5 and 69.2%) were significantly higher than the corresponding means of the Pause hens (47.7 and 49.2%; Table 2).
Table 2.
Means of the egg production traits until 300 days-of-age (300 d), of the hens with No-Pause and the hens with Pause, and the Pause effects (Pause minis No-Pause), in G4 and G5 generations.
| Generation G4 |
Generation G5 |
|||||
|---|---|---|---|---|---|---|
| Trait | No-Pause | Pause | Pause effect | No-Pause | Pause | Pause effect |
| PsDays (d) | 0.0 | 35.9 | +35.9⁎⁎⁎ | 0.0 | 42.0 | +42.0⁎⁎⁎ |
| AFE (d) | 153.7 | 151.1 | −2.6 | 158.7 | 153.6 | −5.2⁎⁎ |
| EN300 (eggs) | 99.0 | 71.6 | −27.4⁎⁎⁎ | 98.0 | 72.5 | −25.5⁎⁎⁎ |
| %L300 (%) | 67.5 | 47.7 | −19.8⁎⁎⁎ | 69.2 | 49.2 | −20.0⁎⁎⁎ |
| %Lnet (%) | 67.5 | 62.7 | −4.8*** | 69.2 | 68.9 | 0.3 |
| ClLng (d) | 2.90 | 2.66 | −0.23* | 3.15 | 3.08 | −0.07 |
| EW200 (g) | 41.0 | 40.8 | −0.2 | 41.9 | 41.8 | −0.1 |
| EW300 (g) | 47.5 | 47.4 | −0.1 | 48.1 | 48.5 | +0.4 |
PsDays, number of Pause days until 300 d; AFE, age at first egg; EN300, number of eggs laid until 300d; %L300, overall laying rate from AFE to 300d; %LNet, laying rate during in-lay days; ClLng, clutch length, average number of days per clutch; EW200, average egg weight around 200d; EW300, average egg weight around 300 d.
*,**,***The Pause effect (Pause minus No-Pause) differ significantly from zero at P < 0.05, < 0.01 and < 0.001, respectively.
In view of known non-genetic factors that increase PsDays, and because %L300 is calculated from the ratio of EN300 divided by the total number of days from AFE to 300d including the PsDays, %Lnet was calculated for each hen from the ratio of EN300 divided by the number of non-Pause days (300-AFE-PsDays). In G4, mean %Lnet of the No-Pause and Pause hens were 67.7 and 62.7%, but in G5, mean %Lnet of the No-Pause and Pause hens were almost identical (69.2 and 68.9%), indicating that %Lnet successfully neutralized the cold-induced reduction in the overall laying rate in G5 (Table 2). Average clutch length (ClLng) of each hen reflects its laying pattern throughout the no-Pause periods, with larger clutches associated with higher potential for egg production (Wolc et al., 2019). Indeed the No-Pause and Pause means of ClLng ranked similarly to the ranking of %Lnet. In G4, Pause significantly reduced mean ClLng by 0.23 d whereas in G5 the Pause effect on ClLng was lower (−0.07d) and not significant (Table 2). In both generations, means of egg weight were very similar in the No-Pause and Pause groups at both ages (200 d and 300 d), with very low and nonsignificant Pause effect, ranging from −0.2 g to +0.4 g (Table 2).
Summing up the aspect of laying pattern, the results in Table 2 show that overall egg number and laying rate were lower in hens with Pause compare to hens without Pause. These Pause effects are highly relevant to the main objective of this study – to evaluate the genetic merit of the G4 hens and males that were selected to serve as parents (dams and sires) of the G5 hens. This evaluation requires comparisons between the means of egg production traits in G4 and G5, but the comparisons in EN300 and %L300 are biased by the significantly higher %Pause in G5 than G4 (Table 1). The higher %Pause in G5 occurred due to incidental cold stress (Figure 1), and therefore studying the genetics and breeding aspects of egg production in G4 vs. G5 populations must account for the incidence of Pauses in the laying pattern of each individual hen, and their impacts on the hen's potential and actual egg production.
Means of G4 vs. G5, and the Selection Differentials of G4 Dams and Sires (Parents of G5)
The first column in Table 3 shows the means of the egg production traits calculated from all 441 G4 hens. The following columns show the means of the 154 G4 hens and 35 G4 males that were selected to be the dams and sires (mothers and fathers of the G5 hens). To calculate the means of the selected sires, each sire was assigned the means of his 3–5 full-sib sisters. For each G5 hen, the values of her dam and sire were averaged and presented in the Mid-parents column. The values in the next column are the selection differentials (SelDif), the mid-parents minus the corresponding means of all G4 hens. The means of all 464 G5 hens are presented in the next column, and the values in the column titled G5mG4 are the mean of G5 minus the mean of G4, an approximation of the response to the selection applied when the dams and sires were selected in G4. The last 4 columns show the standard deviations (SD) in G4 (all hens, dams, sires) and in G5.
Table 3.
Means and standard deviation (SD) of the egg production traits of generations G4 and G5 hens, and the selected G4 dams and sires of the G5 hens.
| Trait | G4 |
G5 All hens | G5mG44 | SD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All hens | Selected dams | Selected sires1 | Mid-parents2 | SelDif 3 | G4 all | G4 dams | G4 sires | G5 all | |||
| %Pause5 (%) | 52.8b | 47.4b | 40.2c | 43.8 | −9.0* | 75.6a | 22.8⁎⁎⁎ | — | — | — | — |
| PsDays (d) | 19.0b | 13.3c | 11.7c | 12.3 | −6.7⁎⁎ | 31.8a | 12.8⁎⁎⁎ | 24.3 | 17.8 | 9.3 | 25.6 |
| AFE (d) | 152.3b | 150.7b | 151.9b | 151.5 | −0.8 | 154.8a | 2.5* | 15.1 | 13.3 | 9.8 | 14.8 |
| EN300 (eggs) | 84.5b | 93.1a | 94.4a | 94.1 | 9.6⁎⁎⁎ | 78.7c | −5.8⁎⁎⁎ | 25.0 | 18.9 | 12.7 | 23.8 |
| %L300 (%) | 57.0b | 62.4a | 63.9a | 63.5 | 6.4⁎⁎⁎ | 54.1c | −2.9⁎⁎ | 15.5 | 11.4 | 8.5 | 15.1 |
| %Lnet (%) | 65.0b | 68.3a | 69.0a | 68.8 | 3.8⁎⁎⁎ | 69.0a | 4.0⁎⁎⁎ | 11.4 | 8.3 | 6.2 | 10.4 |
| ClLng (d) | 2.77b | 2.95ab | 3.21a | 3.10 | 0.33* | 3.10a | 0.33⁎⁎⁎ | 1.22 | 1.18 | 1.09 | 1.45 |
| EW200 (g) | 40.9b | 41.5a | 41.8a | 41.7 | 0.8* | 41.9a | 1.0⁎⁎⁎ | 3.0 | 2.6 | 1.6 | 3.0 |
| EW300 (g) | 47.4b | 48.0a | 48.5a | 48.2 | 0.8* | 48.4a | 1.0⁎⁎⁎ | 3.6 | 3.2 | 2.1 | 3.6 |
Differences between these means represent the selection differentials in G4, and the responses to the selection.
%Pause, percentage of hens with Pause; PsDays, number of Pause days until 300 d; AFE, age at first egg; EN300, number of eggs laid until 300 d; %L300, overall laying rate from AFE to 300 d; %LNet, laying rate during in-lay days; ClLng, average clutch length; EW200, average egg weight around 200 d; EW300, average egg weight around 300 d.
Each sire was assigned the means of his 3-to-5 full-sib sisters.
Mid-Parents is the average of the dam and sire of each G5 hen.
SelDif: the selection differential, i.e. the difference between the Mid-parents mean and the mean of all the hens in G4.
G5mG4: the mean of all G5 hens minus the mean of all G4 hens.
The differences in %Pause between groups were tested by Chi-square test of the numbers of hens with Pause or without Pause in each group.
For each trait, means with no common superscript, differ significantly at P < 0.05.
*,**,***The parameter (SelDif, G5mG4) estimate significantly different from zero at P < 0.05, P < 0.01, and P < 0.001, respectively.
There was no intentional selection against Pause, but the selection of G4 parents with higher laying rate favored No-Pause hens. Consequently mean %Pause of the dams (47.4%) and sires (40.2%) were lower than the 52.8% in the entire population of G4 hens, resulting in SelDif = −9% (Table 3). However, the high %Pause of G5 hens (75.6%) and the positive G5mG4 (22.8%) are not relevant to the selection applied in G4 because they reflect an incidental cold stress (Figure 1). Similarly, mean PsDays of the dams (13.3 d) and the sires (11.7 d) were significantly lower than mean PsDays of all G4 hens (19.0 d), resulting in significant SelDif of −6.7d, yet the G5mG4 was positive, +12.8 d (Table 3).
The AFE means of the dams and sires were similar to the mean of all G4 hens, and consequently SelDif of AFE was very small (−0.8 d) and not significant (Table 3). This could be expected because AFE was not a selection criterion in G4, yet the G5 hens started to lay, on the average, a bit later (G5mG4 = 2.5d). The difference between 154.8d (G5) and 152.3d (G4) was statistically significant, but it cannot be attributed to genetic changes, because G4 and G5 hens started to lay in different years (2019 vs. 2020) under similar but not identical environments. The same reservation regarding the genetic interpretation of G5mG4 vs. SelDif holds for all traits, although some are known to be less affected by environmental conditions.
Although total number of eggs (EN300) is the trait of economic importance, the G4 hens and males (based on their full-sisters) that served as dams and sires of G5 were selected according to their overall laying rate, %L300, and the effect of this selection is apparent in both traits. In EN300, the means of the dams and sires were around 94 eggs (Mid-parents = 94.1), significantly higher than 84.5 eggs, the mean of all G4 hens. Similarly, mean %L300 of the dams and sires (Mid-parents = 63.5%) were significantly higher than 57.0%, the mean of all G4 hens (Table 3). In spite of the substantial and highly significant SelDif in EN300 and %L300, 9.6 eggs and 6.4%, the means of G5 were significantly lower than G4, with G5mG4 equal −5.8 eggs and −2.9% lay. However, mean egg production in G5 was reduced due to higher %Pause and more PsDays (Table 2), in agreement with many reports (e.g., Lerner and Taylor, 1947; Hays, 1949, 1951). Since this elevation in PsDays of G5 hens was due to the incidental cold stress in G5, the negative values of G5mG4 in EN300 and %L300 do not reflect a genetic response to the selection in G4. Therefore, %Lnet was used to compare the laying rate of the hens in G5 and their predecessors in G4. Mean %Lnet of the G4 dams and sires (Mid-parents = 68.8%) were significantly higher than the mean of all G4 hens (65.0%), indicating that the selection of dams and sires by %L300 resulted also in a significant SelDif (3.8%) in %Lnet (Table 3). With the effects of Pause and PsDays neutralized, mean %Lnet in G5 (69.0%) was significantly higher than in G4, indicating a true genetic response to the selection for higher laying rate, as applied in G4.
Reported to be associated with total egg number (Chen and Tixier-Boichard, 2003; Wolc et al., 2010, 2019), mean clutch length (ClLng) has been used to describe laying pattern of individual hens. As for %Lnet, the means of ClLng were similar in the No-Pause and Pause hens in G5 (Table 2), allowing unbiased comparison between G4 and G5 in the present study. Mean ClLng of the dams and sires (Mid-parents = 3.1 d) were significantly higher than the mean of all G4 hens, indicating that the selection of dams and sires by %L300 resulted also in a moderate (0.33 d) but significant SelDif in ClLng (Table 3). As in the case of %Lnet, mean ClLng in G5 (3.10 d) was significantly higher than in G4 (2.77 d), indicating a true genetic improvement in clutch length, in response to the selection for higher laying rate in G4, thus proving the positive genetic association between these 2 traits.
Selection of the G4 parents (of G5) was done by an index that included %L300 and EW200 as egg production traits (along with BW and skin darkness, Wang et al., 2021). Accordingly, mean EW200 of the dams and the sires were significantly higher than the mean of all G4 hens (40.9 g) and with Mid-parents = 41.7 g, the significant SelDif was 0.8 g (Table 3). With the known high correlation between EW at different ages, EW300 means of all G4 hens, dams, sires and Mid-parent were ranked similarly to those of EW200, and SelDif was the same in the two ages. The EW200 and EW300 means of the G5 hens, 41.9 g and 48.4 g, were significantly higher than the corresponding means of the G4 hens, indicating significant genetic response to the selection on egg weight. These results could be expected because egg weight is known to be highly heritable (Yi et al., 2014; Tongsiri et al., 2015), and in view of the independence of EW and Pause (Table 2).
Two aspects should be mentioned concerning the phenotypic standard deviations (SD) of the four groups for each trait (Table 3). First, as could be expected, the SD of the selected dams and sires were lower than those of all G4 hens, mainly in the %L300 and EW200, the selected traits, and to a lesser extent in the other traits associated with egg production and egg weight. Second, the SD values of all G4 hens and all G5 hens were very similar in all traits, indicating that in spite of genetic and environmental differences between the two generations, both had quite similar phenotypic variances (SD2) in these egg-production traits.
Heritability Estimates
Heritability (h2) estimates of the egg production traits, and their standard errors (SE), are presented in Table 4. The availability of data from 2 consecutive generations (G4 and G5), allowed to calculate h2 by the traditional approach of Parent-Offspring regression (regressing G5 hens on their G4 mid-parents values), and separately in each generation by the more-recent pedigree-based Animal Model (Falconer and Mackay, 1996). In generation G4, one-generation pedigree (G3 parents) was available, whereas in generation G5, the h2 calculation used 2-generation pedigree (G3 grandparents and G4 parents). In G5, h2 estimates (and their SE) were calculated also with one-generation pedigree (only G4 parents), and the values (data not shown) were very similar to those from 2-generation pedigree. This similarity suggests that h2 estimates calculated by one-generation pedigree in G4 were as reliable as the h2 estimated calculated by 2-generation pedigree in G5.
Table 4.
The heritability (h2) and standard error (SE) of the egg production traits, calculated separately from the data of generations G4 (441 hens) and G5 (464 hens) using the animal model, and from the regression of G5 hens on their G4 mid-parents (G5/G4)1.
| Trait | h2(G4) | h2(G5) | h2(G5/G4) | SE(G4) | SE(G5) | SE(G5/G4) |
|---|---|---|---|---|---|---|
| Pause0/12 | 0.120 | 0.243* | 0.223⁎⁎ | 0.076 | 0.098 | 0.076 |
| PsDays | 0.042 | 0.280⁎⁎ | 0.383⁎⁎⁎ | 0.062 | 0.102 | 0.145 |
| AFE | 0.258⁎⁎ | 0.499⁎⁎⁎ | 0.305⁎⁎⁎ | 0.095 | 0.115 | 0.089 |
| EN300 | 0.152 | 0.307⁎⁎ | 0.247* | 0.080 | 0.101 | 0.118 |
| %L300 | 0.133 | 0.331⁎⁎ | 0.425⁎⁎⁎ | 0.079 | 0.106 | 0.121 |
| %Lnet | 0.284⁎⁎ | 0.277⁎⁎ | 0.336⁎⁎ | 0.103 | 0.102 | 0.115 |
| ClLng | 0.358⁎⁎⁎ | 0.439⁎⁎⁎ | 0.392⁎⁎⁎ | 0.103 | 0.117 | 0.105 |
| EW200 | 0.416⁎⁎⁎ | 0.373⁎⁎ | 0.589⁎⁎⁎ | 0.110 | 0.114 | 0.098 |
| EW300 | 0.495⁎⁎⁎ | 0.355⁎⁎ | 0.710⁎⁎⁎ | 0.113 | 0.108 | 0.086 |
Pause0/1, 0, No-Pause, 1, Pause; PsDays, number of Pause days until 300 d; AFE, age at first egg; EN300, number of eggs laid until 300d; %L300, overall laying rate from AFE to 300d; %LNet, laying rate during in-lay days; ClLng, average clutch length; EW200, average egg weight around 200 d; EW300, average egg weight around 300 d.
Calculated from 154 pairs of full-sib G5 hens' means regressed on the mean of their dams and sires.
Pause0/1: hens without Pause were assigned 0 and hens with Pause were assigned 1.
*,**,***The heritability estimate is significant at P < 0.05, P < 0.01, and P < 0.001, respectively.
For the estimation of heritability, the tendency to pause (of each individual hen) was considered a binary threshold trait (Falconer and Mackay, 1996). Hens without Pause were assigned 0 and hens with Pause were assigned 1, hence the trait was termed Pause0/1. The h2 of Pause0/1 was low and not significant in G4 (h2(G4) = 0.120), but 2-times larger and significant in G5 (h2(G5) = 0.243). Similar and significant h2 of Pause0/1 was obtained from the regression of G5 hens of their G4 mid-parents (h2(G5/G4) = 0.223; Table 4). Lerner and Taylor (1947) and Hays (1949, 1951) reported that the multiyear tendency for winter pause had low h2, similar to h2(G4) in the present study. It is suggested that the specific tendency to pause under cold stress in G5 had further genetic variation which resulted in significantly higher h2(G5). The significant h2 estimates of the binary Pause0/1 support the assumption that it is a threshold trait with underlying continuous variation in the tendency for Pause (Falconer and Mackay, 1996). In such trait, the actual expression of this tendency (here, stay No-Pause or enter Pause) depends on environmental parameters, like the cold stress in G5. This genetic nature of Pause0/1 is supported by Lerner and Taylor (1947) results: they reported substantial year-to-year fluctuations in absolute levels of %Pause in 3 lines genetically differing in their tendency to Pause, but the differences in %Pause between the lines were similar in all years. The similarity between h2(G5/G4) and h2(G5), in spite of the lower h2(G4), also supports the suggestion that Pause0/1 is a threshold trait. Data from more generations (i.e., years) of the BG line will be used to confirm the genetic nature of the tendency to pause, and how its expression is affected by environmental factors.
The number of PsDays was strongly affected by Pause, suggesting that this trait's very low h2(G4) (0.042) was related to the low h2(G4) of Pause0/1 (Table 4). This logic is supported by the similar h2(G5) of Pause0/1 and PsDays (0.243 and 0.280, respectively). The h2(G5/G4) of PsDays was even higher (0.383), further indication of the potential to select for lower Pause0/1 and less PsDays. Overall egg production, both EN300 and %L300, was also strongly affected by Pause (Table 2). Accordingly, the three heritability estimates (h2(G4), h2(G5), h2(G5/G4)) of these traits were quite similar to the corresponding h2 of Pause0/1: low (0.133 and 0.152) in G4 and moderate (0.307 and 0.331) in G5, with h2(G5/G4) being intermediate (0.247) for EN300 and higher (0.425) for %L300 (Table 4). The h2 estimates of EN300 and %L300 in G4 were similar to the corresponding estimates reported by Wolc et al. (2010), Niknafs et al. (2012), Tongsiri et al. (2015), Liu et al. (2019), and Shen et al. (2019).
With %Lnet expressing the potential laying rate independent of Pause, the h2(G4) and h2(G5) estimates for this trait were very similar (0.284 and 0.277) and h2(G5/G4) was somewhat higher (0.336); all three estimates were significant (Table 4). The 3 h2 estimates for clutch length (ClLng) were somewhat higher, ranging from 0.358 to 0.439 (Table 4). Similar estimates of h2 (0.31 and 0.34) were found recently for clutch length in commercial lines of RIR and WL (Wolc et al., 2019) and slightly lower estimate (0.23) was obtained in an experimental line (Wolc et al., 2010). The significant moderate-high heritability of %Lnet and ClLng, both neutralized from Pause effects, were reflected in the results presented in Table 3: significant SelDif for %Lnet (3.8%) and slightly higher response (G5mG4 = 4%), and also for ClLng, SelDif and G5mG4 were similar, 0.33d.
For AFE, the heritability estimates were high in G5 (h2(G5) = 0.499), whereas moderate estimates were obtained for h2(G4) and h2(G5/G4) (0.258 and 0.305). Similar h2 of AFE, ranging from 0.32 to 0.55, were reported by Niknafs et al. (2012), Yi et al. (2014), Tongsiri et al. (2015), Liu et al. (2019), Wolc et al. (2010, 2019) and Shen et al. (2019).
The heritability estimates of egg weight, both EW200 and EW300, were moderate in G5 (0.355–0.373) to high in G4 (0.416-0.495), and h2(G5/G4) (the regression of G5 on G4) was further higher, 0.589 for EW200 up to 0.710 for EW300. These high heritability estimates are in agreement with many studies: 0.42 (Wolc et al., 2010), 0.43 (Niknafs et al., 2012), 0.38 to 0.43 (Tongsiri et al., 2015); and 0.35 to 0.60 (Yi et al., 2014). Moreover, the moderate/high h2 of EW are in agreement with the actual response to the selection of the G4 parents of G5; they were selected by an index that included EW200, and indeed mean EW200 and mean EW300 of the G5 hens were significantly higher than the corresponding means in G4 (Table 3). In the case of the BG line, there is no need to select further on egg weight, because the means of EW200 and EW300 in G5 meet the market requirements.
Phenotypic and Genetic Correlations Between Traits
Table 5 presents all the phenotypic correlations (rP, above diagonal) and genetic correlations (rG, below diagonal) between the eight continuous traits, in generations G4 and G5. The correlations were calculated by the ASReml software using the same animal model and pedigrees that were used to calculate the heritability estimates (previous section). The SE of the correlations, also calculated by ASReml, are presented in Table 6. In most trait-combinations, the phenotypic correlations were similar in G4 and G5, whereas the genetic correlations in G4 and G5 were similar in some trait-combinations, and quite different in others. Because Pause is a binary categorical trait, its association with the other traits was determined by comparing the means of the No-Pause and Pause hens (Table 2).
Table 5.
The phenotype correlation (rP, above the diagonal) and genetic correlation (rG, below the diagonal) between the egg production traits in G4 and G5 generations.
| Trait | Gen. | PsDays | AFE | EN300 | %L300 | %Lnet | ClLng | EW200 | EW300 |
|---|---|---|---|---|---|---|---|---|---|
| PsDays | G4 | −0.007 | −0.735⁎⁎⁎ | −0.814⁎⁎⁎ | −0.232⁎⁎⁎ | −0.082 | −0.091* | −0.057 | |
| G5 | −0.070 | −0.753⁎⁎⁎ | −0.853⁎⁎⁎ | −0.147⁎⁎ | −0.112* | −0.017* | 0.049 | ||
| AFE | G4 | −0.336 | −0.429⁎⁎⁎ | −0.094 | −0.108* | −0.129⁎⁎ | 0.110* | 0.130* | |
| G5 | −0.446* | −0.416⁎⁎⁎ | −0.082* | −0.077 | −0.109 | 0.177⁎⁎⁎ | 0.190⁎⁎⁎ | ||
| EN300 | G4 | −0.182 | −0.592* | 0.935⁎⁎⁎ | 0.695⁎⁎⁎ | 0.503⁎⁎⁎ | 0.032 | −0.028 | |
| G5 | −0.565* | −0.377* | 0.935⁎⁎⁎ | 0.586⁎⁎⁎ | 0.467⁎⁎⁎ | −0.092* | −0.150⁎⁎ | ||
| %L300 | G4 | −0.474 | −0.180 | 0.900* | 0.730⁎⁎⁎ | 0.508⁎⁎⁎ | 0.072 | 0.016 | |
| G5 | −0.815⁎⁎⁎ | 0.084 | 0.891⁎⁎⁎ | 0.606⁎⁎⁎ | 0.490⁎⁎⁎ | −0.040* | −0.097* | ||
| %Lnet | G4 | −0.292 | −0.373 | 0.940⁎⁎⁎ | 0.967⁎⁎⁎ | 0.760⁎⁎⁎ | 0.068 | 0.013 | |
| G5 | −0.245 | −0.298 | 0.813⁎⁎⁎ | 0.745⁎⁎⁎ | 0.765⁎⁎⁎ | −0.040 | −0.050 | ||
| ClLng | G4 | −0.417* | −0.473* | 0.974⁎⁎⁎ | 0.991⁎⁎⁎ | 0.958⁎⁎⁎ | −0.065 | −0.101 | |
| G5 | −0.546* | −0.329 | 0.978⁎⁎⁎ | 0.932⁎⁎⁎ | 0.868⁎⁎⁎ | −0.113* | −0.083 | ||
| EW200 | G4 | 0.265 | 0.162 | −0.148 | −0.129 | −0.008 | −0.074 | 0.809⁎⁎⁎ | |
| G5 | 0.414 | 0.107 | −0.510* | −0.540* | −0.498* | −0.233 | 0.801⁎⁎⁎ | ||
| EW300 | G4 | 0.218 | 0.291 | −0.200 | −0.116 | 0.029 | −0.136 | 0.967⁎⁎⁎ | |
| G5 | 0.431* | 0.059 | −0.536* | −0.594⁎⁎ | −0.513* | −0.315 | 0.950⁎⁎⁎ |
PsDays, number of Pause days until 300 d; AFE, age at first egg; EN300, number of eggs laid until 300d; %L300, overall laying rate from AFE to 300 d; %LNet, laying rate during in-lay days; ClLng, average clutch length; EW200, average egg weight around 200 d; EW300, average egg weight around 300 d.
*,**,***The correlation coefficient is significant (differ from zero) at P < 0.05, P < 0.01, and P < 0.001, respectively.
Table 6.
Standard errors (SE) of the phenotype correlation (rP, above the diagonal) and genetic correlation (rG, below the diagonal) shown in Table 5.
| Trait | Gen. | PsDays | AFE | EN300 | %L300 | %Lnet | ClLng | EW200 | EW300 |
|---|---|---|---|---|---|---|---|---|---|
| PsDays | G4 | 0.048 | 0.023 | 0.017 | 0.046 | 0.048 | 0.048 | 0.048 | |
| G5 | 0.052 | 0.023 | 0.014 | 0.049 | 0.051 | 0.051 | 0.051 | ||
| AFE | G4 | 0.527 | 0.040 | 0.048 | 0.050 | 0.050 | 0.050 | 0.051 | |
| G5 | 0.209 | 0.043 | 0.052 | 0.051 | 0.053 | 0.052 | 0.051 | ||
| EN300 | G4 | 0.549 | 0.244 | 0.006 | 0.025 | 0.036 | 0.050 | 0.050 | |
| G5 | 0.166 | 0.193 | 0.007 | 0.032 | 0.037 | 0.051 | 0.050 | ||
| %L300 | G4 | 0.429 | 0.347 | 0.070 | 0.023 | 0.036 | 0.049 | 0.050 | |
| G5 | 0.085 | 0.222 | 0.047 | 0.031 | 0.039 | 0.052 | 0.051 | ||
| %Lnet | G4 | 0.538 | 0.259 | 0.090 | 0.084 | 0.021 | 0.051 | 0.052 | |
| G5 | 0.270 | 0.230 | 0.119 | 0.136 | 0.021 | 0.051 | 0.050 | ||
| ClLng | G4 | 0.556 | 0.230 | 0.150 | 0.148 | 0.059 | 0.052 | 0.052 | |
| G5 | 0.216 | 0.201 | 0.105 | 0.089 | 0.072 | 0.052 | 0.052 | ||
| EW200 | G4 | 0.519 | 0.238 | 0.290 | 0.312 | 0.244 | 0.220 | 0.018 | |
| G5 | 0.246 | 0.216 | 0.216 | 0.219 | 0.239 | 0.224 | 0.018 | ||
| EW300 | G4 | 0.472 | 0.217 | 0.269 | 0.291 | 0.232 | 0.207 | 0.033 | |
| G5 | 0.235 | 0.218 | 0.208 | 0.206 | 0.250 | 0.220 | 0.043 |
PsDays, number of Pause days until 300 d; AFE, age at first egg; EN300, number of eggs laid until 300d; %L300, overall laying rate from AFE to 300 d; %LNet, laying rate during in-lay days; ClLng, average clutch length; EW200, average egg weight around 200 d; EW300, average egg weight around 300 d.
The correlations between PsDays and overall egg production to 300d (EN300 and %L300) were highly negative (ranging from −0.735 to −0.853), because hens with more PsDays had less potential days to lay eggs. The genetic correlations (rG) between these traits were highly negative (−0.565 and −0.815) in generation G5 but weaker in G4, supporting the conclusion regarding a genetic component in the Pause response to cold stress in G5. In both generations, the rP and rG between PsDays and %Lnet were also negative but much weaker, ranging from −0.147 to −0.292. The rP of PsDays with AFE and with ClLng were very low, between -0.112 and 0.049 (Table 5). However, most rG between PsDays and these traits were higher and significant in G5 generation: −0.446 with AFE and −0.546 with ClLng, suggesting that early-maturing hens, and hens with shorter clutches, were more prone to pause under cold stress.
As could be expected, rP (−0.592 in G4, -0.416 in G5) and rG (−0.429 in G4, -0.377 in G5) between AFE and EN300 were significant and negative (Table 5), because hens that start to lay earlier (lower AFE) have longer laying period to 300 d. The rP between AFE and ClLng were low (−0.129 in G4, −0.109 in G5), but the corresponding rG were higher (−0.473 in G4, −0.329 in G5) and significant. These negative correlations indicate a favorable breeding-wise association; with early-maturing hens (lower AFE) tending to have longer clutches (higher ClLng). The rP between the 2 rate-of-lay traits, %L300 and %Lnet, were very high (0.730 in G4, 0.606 in G5), and the rG between these traits were further higher (0.967 in G4, 0.745 in G5). With EN300 in the numerator of both %L300 and %Lnet, the estimates of rG of EN300 with these 2 %L traits were very high in both generations, ranging from 0.813 to 0.940 (Table 5), supporting the conclusion that %Lnet was an accurate expression of the genetic potential for egg production. The expected very high rP and rG between egg number and laying rate were reported also by Niknafs et al. (2012) and Shen et al. (2019). However, both laying-rate measurements, %L300 and %Lnet, are directly affected by the incidence and length of laying pause, known to depend on environmental factors and vary considerably between years. In contrast, clutch length (ClLng) is independent of Pause, and was reported to be associated with total egg number (Chen and Tixier-Boichard, 2003; Wolc et al., 2010, 2019). Also in the present study, the rG between ClLng and EN300 was very high (0.974 and 0.978), and similarly highly correlated with %L300 and %Lnet (Table 5). With these very high rG values, along with a significant negative rG with PsDays, clutch length appears to be the best selection criterion for higher egg production in the BG line and similar populations, as well as in commercial lines (Wolc et al, 2019).
The rP between the egg production traits (EN300, %L300, %Lnet) and the two EW traits (EW200, EW300) were low in both generations, ranging from -0.150 to 0.068, similar to the low rP between EW and PsDays. The low and nonsignificant rG between egg production and EW in G4 are in agreement with previous reports (Wolc et al., 2010; Niknafs et al., 2012), whereas in G5, the rG between EW and the egg production traits ranged from −0.498 to −0.594, and the rG between EW300 and PsDays was 0.431 (all significant, Table 5). Having these significant genetic correlations only in G5 and not in G4, suggests that they are not consistent characteristics of the BG line, but rather related to the non-genetic difference between the 2 generations. The cold stress in G5 (Figure 1) is assumed to cause higher incidence of pause and elevation in PsDays, possibly due to cold-related shortage of body energy resulting from higher rate of body heat dissipation, as suggested by Xie et al. (2017). It appears that under cold stress, hens with genetic potential to produce higher egg mass (more eggs and/or heavier eggs) were more prone to pause, possibly because they could not meet the higher body energy requirement needed to maintain their inherent level of reproduction. Further observations are required, under controlled ambient temperatures and feeding treatments, to clarify this interpretation.
CONCLUSIONS
The overall objective of this study was to evaluate the prospects to improve egg production (along with BW and skin darkness) of the BG line, an experimental population derived from a cross between 2 indigenous Chinese lines, and bred to serve as a dual-purpose line. With its indigenous genetic background, the genetic potential for egg production in the BG population, with overall laying rate (%L300) around 57%, is inferior (as expected) to modern commercial layer lines. Individual recording of daily egg laying revealed that the relatively low mean egg production resulted from a Pause (more than a week without laying) in the laying pattern of 53% of the G4 hens, and higher incidence (75%) in G5, probably due to a cold stress.
However, significant estimates of heritability were obtained from the G5 data, and from the G5/G4 regression, for the tendency to pause (Pause0/1) and for the number of pause days (PsDays). Because selection for a threshold trait (Pause0/1) is problematic, PsDays should be used as a continuous expression of the tendency to pause. By including low PsDays in a selection index aimed at improving egg production, the No-Pause hens (with PsDays = 0) will have a priority, followed by hens with short Pauses (low PsDays). By eradicating Pause from the laying pattern of the BG hens, their current mean laying rate is already about 70%, as the No-Pause hens in G5.
In addition to selection against Pause, potential laying rate of the BG hens can be improved by selection for high %Lnet and/or for longer clutches (ClLng), also a component of the laying pattern. The genetic correlations between these traits were very high (0.958 in G4 and 0.868 in G5), but the h2 estimates of ClLng were higher and more significant. Thus, a selection index combining low PsDays and high ClLng is recommended for improving the genetic potential for egg production of the BG population. Due to very short (if at all) history of intensive selection for high egg production, populations derived from rural indigenous breeds are characterized by higher tendency to pause and by short clutches. Therefore, selection based on laying pattern, against pause and for longer clutches, will be especially useful in such populations, which are becoming more important in view of the efforts to improve their performances, and to utilize useful genetic variants from indigenous breeds.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the Agricultural and Social Development Research Project of Hangzhou [grant numbers 20162012A04, 2016].
DISCLOSURES
The authors declare that they have no conflicts of interest to this manuscript entitled “Genetics and breeding of a black-bone and blue eggshell chicken line. 2. Laying patterns and egg production in two consecutive generations”.
REFERENCES
- Athrey G. In: Pages 317–330 in Animal Agriculture. Sustainability, Challenges and Innovations. Bazer F.W., Lamb G.C., Wu G., editors. Academic Press; London, UK: 2020. Poultry genetics and breeding. [Google Scholar]
- Chen C.F., Tixier-Boichard M. Estimation of genetic variability and selection response for clutch length in dwarf brown-egg layers carrying or not the naked neck gene. Genet. Sel. Evol. 2003;35:219–238. doi: 10.1186/1297-9686-35-2-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D.S., Mackay T. 4th ed. Addison Wesley Longman; Harlow, UK: 1996. Introduction Quantitative Genetics. [Google Scholar]
- Fulton R.M. Causes of normal mortality in commercial egg-laying chickens. Avian Dis. 2017;61:289–295. doi: 10.1637/11556-120816-RegR. [DOI] [PubMed] [Google Scholar]
- Hays F.A. Temperature and viability as related to winter pause incidence and duration. Poult. Sci. 1949;28:894–897. [Google Scholar]
- Hays F.A. Further Studies on environmental and hereditary factors affecting winter pause incidence and duration. Poult. Sci. 1951;30:100–105. [Google Scholar]
- Hays F.A. Laying house temperature and egg production. Poult. Sci. 1958;37:592–595. [Google Scholar]
- Ibrahim D., Goshu G., Esatu W., Cahaner A. Dual-purpose production of genetically different chicken crossbreeds in Ethiopia. 2. Egg and meat production of the final-crossbreed females and males. Poult. Sci. 2019;98:3405–3417. doi: 10.3382/ps/pez137. [DOI] [PubMed] [Google Scholar]
- Ibrahim D. Dual-purpose production of genetically different chicken crossbreeds in Ethiopia. Int. Hatchery Pract. 2020;35:15–17. [Google Scholar]
- Jin P. Huazhong Agricultural University; China: 2010. Quantitative Genetic Analysis on Traits of Clutch and Egg Performance in Xinhua E Strain. (in Chinese with English abstract) [Google Scholar]
- Jull M.A. John Wiley & Sons Inc.; New York: 1952. Poultry Breeding. [Google Scholar]
- Lerner I.M., Taylor L.W. Further observations on winter pause in single comb White Leghorn pullets. Poult. Sci. 1947;26:198–205. [Google Scholar]
- Liu Z., Yang N., Yan Y.Y., Li G.Q., Liu A.Q., Wu G.Q., Sun C.J. Genome-wide association analysis of egg production performance in chickens across the whole laying period. BMC Genet. 2019;20:67. doi: 10.1186/s12863-019-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknafs S., Nejati-Javaremi A., Mehrabani-Yeganeh H., Fatemi S.A. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop. Anim. Health Prod. 2012;44:1437–1443. doi: 10.1007/s11250-012-0084-6. [DOI] [PubMed] [Google Scholar]
- Preisinger, R. 2018. Innovative layer genetics to improve egg production. Lohmann Information. 52:4-11. [DOI] [PubMed]
- Shen M.M., Qu L., Wang X.G., Dou T.C., Ma M., Wang K.H. Analysis on genetic parameters of clutch trait in Rugao yellow chicken. China Poult. 2019;41:5–9. (in Chinese with English abstract) [Google Scholar]
- Tongsiri S., Jeyaruban M.G., van der Werf J.H.J. Genetic parameters for egg production traitsin purebred and hybrid chicken in a tropical environment. Br. Poult. Sci. 2015;56:613–620. doi: 10.1080/00071668.2015.1099614. [DOI] [PubMed] [Google Scholar]
- Tongsiri S., Jeyaruban M.G., Hermesch S., van der Werf J.H.J., Li L., Chormai T. Genetic parameters and inbreeding effects for production traits of Thai native chickens. Asian-Australas J. Anim. Sci. 2018;32:930–938. doi: 10.5713/ajas.18.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullengala R., Prince L.L.L., Paswan C., Haunshi S., Chatterjee R. Variance component analysis of growth and production traits in Vanaraja male line chickens using animal model. Anim. Biosci. 2020;00:1–11. doi: 10.5713/ajas.19.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Cahaner A., Lou L.F., Zhang L., Ge Y., Li Q.H., Zhang X.D. Genetics and breeding of a black-bone and blue eggshell chicken line. 1. Body weight, skin color, and their combined selection. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D.C. The Macmillan Company; New York: 1953. Practical Poultry Breeding. [Google Scholar]
- Wolc A., Bednarczyk M., Lisowski M., Szwaczkowski T. Genetic relationships among time of egg formation, clutch traits and traditional selection traits in laying hens. J. Anim. Feed Sci. 2010;19:452–459. [Google Scholar]
- Wolc A., Jankowski T., Arango J., Settar P., Fulton J.E., O'Sullivan N.P., Dekkers J.C.M. Investigating the genetic determination of clutch traits in laying hens. Poult. Sci. 2019;98:39–45. doi: 10.3382/ps/pey354. [DOI] [PubMed] [Google Scholar]
- Xie S.S., Yang X.K., Gao Y.H., Jiao W.J., Li X.H., Li Y.J., Ning Z.H. Performance differences of Rhode Island Red, Bashang Long-tail Chicken, and their reciprocal crossbreds under natural cold stress. Asian-Australas J. Anim. Sci. 2017;30:1507–1514. doi: 10.5713/ajas.16.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Jiang R.S. Recent advances in breeding for quality chickens. Worlds Poult. Sci. J. 2005;61:373–381. [Google Scholar]
- Yi G.Q., Liu W.B., Li J.Y., Zheng J.X., Qu L.J., Xu G.Y., Yang N. Genetic analysis for dynamic changes of egg weight in two chicken lines. Poult. Sci. 2014;93:2963–2969. doi: 10.3382/ps.2014-04178. [DOI] [PubMed] [Google Scholar]