Abstract
From genomic DNA of the clinical isolate Nocardia farcinica VIC, a 1.6-kb Sau3AI fragment was cloned and expressed in Escherichia coli JM109. The recombinant strain expressed a β-lactamase (pI, 4.6), FAR-1, which conferred high levels of resistance to amoxicillin, piperacillin, ticarcillin, and cephalothin. The hydrolysis constants (kcat, Km, Ki, and 50% inhibitory concentration) confirmed the MIC results and showed that FAR-1 activity is inhibited by clavulanic acid and at a low level by tazobactam and sulbactam. Moreover, FAR-1 β-lactamase hydrolyzes aztreonam (at a low level) without significant activity against ceftazidime, cefotaxime and imipenem. FAR-1 mature protein of molecular mass ca 32 kDa, has less than 60% amino acid identity with any other class A β-lactamases, being most closely related to PEN-A from Burkholderia cepacia (52%). A blaFAR-1-like gene was found in all studied N. farcinica strains, underlining the constitutive origin of this gene.
β-Lactamases are distributed almost ubiquitously in both gram-positive and gram-negative bacteria. Based on sequence analysis, these β-lactamases are divided into four molecular classes: A, B, C, and D (1, 11). Most of the gram-positive β-lactamases belong to Ambler class A, especially those derived from filamentous soil bacterial species, such as Streptomyces, Actinomadura, Bacillus, and Mycobacterium spp. (39).
Nocardia spp. are soil organisms that are opportunistic pathogens for humans and animals (4). The strains belonging to this genus, which includes at least 12 different species, are responsible for a wide spectrum of clinical diseases, especially in immunocompromised patients. The number of such isolates has increased twofold in France over the last 10 years (7). The isolates belonging to N. farcinica species represent about 25% of all Nocardia strains isolated in France (8). Moreover, the frequent presence of this species as a cause of disseminated human disease, its high virulence compared to the other Nocardia species, and its high degree of drug resistance warrant attempts to separate N. farcinica from the N. asteroides complex in clinical laboratories and to study their resistance profiles.
Sulfonamides have been the mainstay for nocardiosis treatment (38, 49, 60). Broad-spectrum cephalosporins, such as cefotaxime and imipenem combined with amikacin, are used to take potential advantage of these rapidly bactericidal agents (20, 38, 54). These antibiotics seemed to improve antibacterial treatment efficacy, although a β-lactamase activity is known to occur in several nocardiae, such as N. asteroides, N. brasiliensis, and N. farcinica (4, 20, 23, 54).
Partial biochemical characterizations of β-lactamases have been reported for N. farcinica strains (52), while no information is available concerning the molecular basis of Nocardia β-lactamases except for a nonpathogen N. lactamdurans isolate (16), a species which, in fact, now belongs to the Streptomyces genus.
In the present study, we have examined the β-lactamase activity of the N. farcinica VIC strain. We report the cloning and the sequence analysis of a novel class A β-lactamase, named FAR-1, and its distribution in several N. farcinica isolates.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this work are listed in Table 1. The N. farcinica strains were identified by conventional methods and by molecular techniques as described previously (34, 52) at the National Reference Center for Mycosis, Antifungal Therapy and Actinomycetes (Institut Pasteur, Paris, France).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| E. coli JM109 | endA1 hsdR17 gyrA96 Δ(lac proA) recAB1 relA supE44 thi F′ (lacIqlacZΔM15 proAB+ traΔ36) | Laboratory collection |
| pBK-CMV phagemid | Neomycinr and kanamycinr | Stratagene |
| N. farcinica VIC | The studied β-lactamase | This study |
| N. farcinica 94.0250 | Common susceptibility phenotypea | CIPb |
| N. farcinica 94.0664 | Common susceptibility phenotype and AMC intermediate | CIP |
| N. farcinica 95.0288 | Common susceptibility phenotype and AMC intermediate | CIP |
| N. farcinica 95.0684 | Common susceptibility phenotype and AMC intermediate | CIP |
| N. farcinica 96.0027 | Common susceptibility phenotype and AMC intermediate | CIP |
| N. farcinica 96.0087 | Common susceptibility phenotype and AMC intermediate | CIP |
| N. farcinica 96.0624 | Common susceptibility phenotype | CIP |
| N. farcinica 96.0691 | Common susceptibility phenotype | CIP |
| N. farcinica 96.0994 | Common susceptibility phenotype and IMP resistant | CIP |
| N. farcinica 96.1087 | Common susceptibility phenotype | CIP |
| N. farcinica 97.0244 | Common susceptibility phenotype | CIP |
| N. farcinica 3318 | Common susceptibility phenotype | ATCCc |
| R. equi ATCC 6939 | No β-lactamase producer | ATCC |
A “common susceptibility phenotype” is amoxicillin, ticarcillin, piperacillin, ceftriaxone, and cefotaxime resistant and amoxicillin-clavulanate (AMC) and imipenem (IMP) susceptible.
CIP, Institut Pasteur Collection, Paris, France.
ATCC, American Type Culture Collection, Rockville, Md.
Antimicrobial agents and MIC determinations.
The antimicrobial agents used in this study were obtained from standard laboratory powders and were used immediately after their solubilization. The agents and their sources were as follows: amoxicillin, clavulanic acid, and ticarcillin (Smith Kline Beecham, Nanterre, France); aztreonam and cefepime (Bristol-Myers Squibb, Paris-La Défense, France); ceftazidime (GlaxoWellcome, Paris, France); cephalothin (Eli Lilly, Saint-Cloud, France); piperacillin and tazobactam (Lederle, Oullins, France); sulbactam (Pfizer, Orsay, France); benzylpenicillin and cefotaxime (Hoechst-Roussel, Paris, France); cefoxitin and imipenem (Merck Sharp & Dohme-Chibret, Paris, France); and meropenem (Zeneca, Paris, France).
MICs were determined by an agar dilution technique on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Paris, France) with a Steers multiple inoculator and an inoculum of 104 CFU (40). All plates were incubated at 37°C for 18 h. The MICs of β-lactams were determined alone or in combination with a fixed concentration of clavulanic acid (2 μg/ml), tazobactam (4 μg/ml), or sulbactam (8 μg/ml).
Cloning experiments and analysis of recombinant plasmids.
Genomic DNA of N. farcinica VIC was extracted as previously described (46). Restriction enzymes and other enzymes used in cloning experiments were from Amersham Pharmacia Biotech (Orsay, France). Fragments from Sau3AI partially digested genomic DNA were ligated into BamHI-restricted phagemid pBK-CMV (Stratagene, La Jolla, Calif.). Ligation was performed at a 1:2 vector/insert ratio at a final concentration of 200 ng of DNA in a ligation mixture containing 1 U of T4 DNA ligase at 4°C for 18 h. Recombinant plasmids were transformed by electroporation (Bio-Rad Gene Pulser II; Bio-Rad, Ivry-sur-Seine, France) into Escherichia coli JM109 electrocompetent cells. Antibiotic-resistant colonies were selected onto Trypticase soy (TS) agar plates containing amoxicillin (50 μg/ml) and kanamycin (30 μg/ml).
Recombinant plasmid DNA was obtained from 100 ml of TS broth overnight cultures grown in the presence of amoxicillin (100 μg/ml) at 37°C. The recombinant plasmid conferring resistance to amoxicillin was named pFAR-1. Plasmid DNAs were obtained by using Qiagen columns (Qiagen, Courtaboeuf, France). Plasmid mapping was performed after double restriction analysis. Fragment sizes were estimated according to the 1-kb and 100-bp molecular-weight DNA ladders (Amersham Pharmacia Biotech).
DNA sequencing and protein analysis.
The 1,543-bp cloned DNA fragment from pFAR-1 was sequenced on both strands by using an Applied Biosystems sequencer (ABI377). The nucleotide sequence and the deduced protein sequence were analyzed by using the software available over the internet at the National Center of Biotechnology Information website (41) and at Pedro’s Biomolecular Research Tools website (45). The Signalp program was used to screen for putative signal peptide within the deduced protein sequence of FAR-1 β-lactamase. Multiple protein sequence alignments were carried over the internet at the University of Cambridge by using the program CLUSTALW. The β-lactamases from the following strains were used for comparisons: Streptomyces clavuligerus (Scla) (46), Actinomadura sp. strain R39 (AR39) (27), Burkholderia cepacia (PEN-A) (56), Streptomyces lactamdurans (Slac) (17), Streptomyces badius (Sbad) (21), Mycobacterium tuberculosis (Mtub) (24), Mycobacterium fortuitum (blaF) (55), Bacillus licheniformis (Blip) (42), B. cereus (Bcer) (36), B. amyloliquefaciens (Bamy) (57), Klebsiella oxytoca (KOXY) (22), Pseudomonas aeruginosa (PER-1) (43), Staphylococcus aureus (PC-1) (15), and E. coli (TEM-1) (13).
Hybridization experiments.
Genomic DNAs from Actinomycetes strains were extracted as previously described (33). Extracted DNAs were heat denatured for 5 min in boiling water and then chilled on ice. Two microliters (corresponding to about 2 μg of DNA) of denatured DNAs was applied to a nylon membrane (Hybond N+; Amersham, Courtaboeuf, France) and UV cross-linked for 2 min. The membrane was incubated for 1 h at 42°C in a prehybridization solution containing 100 μg of salmon sperm DNA per ml, 5× Denhardt solution, 0.5% sodium dodecyl sulfate (SDS), 3 × SSC and 30% formamide. The DNA probe consisting of the 700-bp HincII-SmaI fragment from recombinant plasmid pFAR-1 (Fig. 1) was radiolabelled with [32P]dATP and [32P]dCTP with a random-primer DNA labeling kit (Boehringer Mannheim, Meylan, France). Hybridizations were performed at 42°C overnight in a Hybaid oven (Hybaid, Teddington, United Kingdom). Then, two washes were performed successively in the following two solutions: 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% SDS for 15 min at 50°C and 1× SSC–0.5% SDS for 15 min at 50°C. Autoradiography was achieved by exposing the membranes to Kodak films at −80°C for 18 h with intensifying screens.
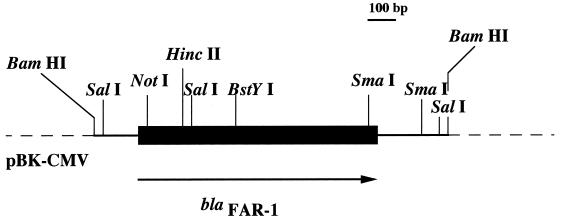
FIG. 1.
Restriction endonuclease schematic map of the recombinant plasmid pFAR-1, which codes the β-lactamase FAR-1 from N. farcinica VIC. The thin line represents the cloned insert from N. farcinica VIC; the dotted lines indicate vector pBK-CMV, and the thick line represents the studied β-lactamase gene, with the arrow indicating its translational orientation.
Isoelectric focusing.
Cultures of E. coli (pFAR-1) were grown overnight at 37°C in 100 ml of TS broth containing amoxicillin (100 μg/ml) and kanamycin (30 μg/ml). N. farcinica cultures were grown in TS broth at 35°C for 72 h in an aerobic atmosphere. Bacterial suspensions were disrupted by sonification (two times for 20 s at 20 Hz; Vibra Cell 300 Phospholyser; Bioblock, Illkirch, France) and centrifuged (30 min, 10,000 × g, 4°C). The supernatants containing the enzyme extract were subjected to isoelectric focusing (IEF) on a pH 3.5 to 9.5 ampholin polyacrylamide gel (Amersham Pharmacia Biotech) for 36 h at 10 W of constant power on a flatbed apparatus (FBE-3000; Amersham Pharmacia Biotech). The focused β-lactamases were detected by overlaying the gel with 1 mM nitrocefin (Oxoid, Paris, France) in 100 μM phosphate buffer (pH 7.0). The pI values were determined and compared to those of known β-lactamases.
β-Lactamase purification.
A 1-liter culture of E. coli JM109(pFAR-1) was grown overnight. The bacteria were harvested for 10 min at 6,000 × g, and the bacterial pellet was resuspended in 15 ml of 20 mM Bis-Tris {[bis(2-hydroxyethyl imino]tris(hydroxymethyl) methane}, pH 5.5, at 4°C. The bacterial cells were disrupted by ultrasonic treatment as described above. Residual cells and debris were removed by centrifugation (48,000 × g for 30 min at 4°C). Nucleic acids were precipitated by the addition of 0.2 M (7% [vol/vol]) spermin and centrifugation at 100,000 × g for 60 min at 4°C. The supernatant was dialyzed overnight at 4°C against 2 liters of 50 mM Bis-Tris buffer (pH 6.0) and was loaded onto a column (1.6 cm by 5 cm) of Q-Sepharose Fast Flow (Amersham Pharmacia Biotech) equilibrated in the Bis-Tris buffer. The β-lactamase was eluted with a linear NaCl gradient (0 to 500 mM). Fractions containing activity, which was detected with nitrocefin, were obtained after 40 min at 0.3 ml/min at an NaCl concentration of 250 mM. The most active fractions were pooled, dialyzed against 100 mM phosphate buffer (pH 7.0), concentrated (Centrisart-C30 microcentrifuge filters; Sartorius, Goettingen, Germany), and stored at 4°C until enzymatic testing. Purity was assessed by electrophoresis on an SDS–12% polyacrylamide gel stained with Coomassie blue R-250 (Sigma Chemical Co., St. Louis, Mo.). The total protein concentration was estimated with the Bio-Rad protein assay.
Kinetic measurements.
All kinetic measurements were performed at 30°C in 100 mM sodium phosphate (pH 7.0). The initial rates of hydrolysis were determined spectrophotometrically with a Pharmacia UV2000 spectrophotometer. The following wavelengths and absorption coefficients were used: for benzylpenicillin and amoxicillin, 232 nm (Λe = 1,100 M−1 cm−1); for ticarcillin, 235 nm (Λe = 1,050 M−1 cm−1); for piperacillin, 235 nm (Λe = 1,070 M−1 cm−1); for cephalothin, 262 nm (Λe = 7,960 M−1 cm−1); for cephaloridin, 255 nm (Λe = 9,360 M−1 cm−1); for ceftazidime, 260 nm (Λe = 8,660 M−1 cm−1); for cefuroxime, 262 nm (Λe = 7,800 M−1 cm−1); for cefotaxime, 365 nm (Λe = 6,260 M−1 cm−1); and for aztreonam, 318 nm (Λe = 640 M−1 cm−1). Kinetic parameters were determined by recording the initial rates at different substrate concentrations and by analyzing the results with the regression analysis program LEONARA written by Cornish-Bowden (17). The kcat and Km values were estimated by using a nonlinear least-squares regression method with dynamic weights (17). The Ki and the 50% inhibitory concentration (IC50) were determined, the latter value referring to the clavulanate, tazobactam, and sulbactam concentrations that reduced the hydrolysis rate of 100 μM benzylpenicillin by 50% under conditions in which the enzyme was preincubated with various concentrations of inhibitor for 5 min at 30°C before addition of the substrate.
Determination of relative molecular mass.
The relative molecular mass of pFAR-1 plasmid β-lactamase was estimated by SDS-PAGE analysis. Crude extracts and marker proteins were boiled for 10 min in a 1% SDS–3% mercaptoethanol solution and then subjected to electrophoresis on a 12% gel (200 V, 4 h, room temperature) (32). Renaturation of β-lactamase activity after the denaturing electrophoresis was performed as described previously (37).
Nucleotide sequence accession number.
The nucleotide sequence data reported here will appear in the GenBank nucleotide database under accession number AF024601.
RESULTS
Strain isolation.
N. farcinica VIC isolate was recovered from a brain abscess of a 53-year-old patient hospitalized in 1997 at the Hospital Bicêtre, Le Kremlin-Bicêtre, France. Prior to strain isolation, the patient had received empiric treatment with amoxicillin and gentamicin followed by treatment with imipenem, amikacin, and trimethoprim-sulfamethoxazole. Trimethoprim-sulfamethoxazole was continued for 6 months. Based on the preliminary results of disk diffusion agar assays, N. farcinica VIC was found to be resistant to penicillin, amoxicillin, ticarcillin, and piperacillin; to piperacillin plus tazobactam; to all cephalosporins (including ceftazidime and cefotaxime); and to aztreonam. It was susceptible to clavulanic acid plus either amoxicillin or ticarcillin and to imipenem. This clinical strain was also resistant to all aminoglycosides except amikacin and to macrolides but remained susceptible to sulfonamides, trimethoprim-sulfamethoxazole, and ciprofloxacin.
Cloning of the β-lactamase gene.
Partially Sau3AI-digested DNA from N. farcinica VIC was cloned into the BamHI site of pBK-CMV. Recombinant E. coli strains were selected onto amoxicillin- and kanamycin-containing TS agar plates. Only two recombinant E. coli strains were obtained from which plasmids were extracted and analyzed. The inserts were estimated to be of similar size (ca. 1.6 kb). A restriction map was generated for one of them, pFAR-1 (Fig. 1).
β-Lactam resistance phenotype of E. coli harboring pFAR-1.
Using a double-disk diffusion assay, E. coli JM109(pFAR-1) revealed a moderate synergy between aztreonam and clavulanic acid disks that was not observed with aztreonam combinations containing tazobactam or sulbactam.
MICs of the β-lactams for E. coli JM109(pFAR-1) were compared to those for N. farcinica VIC and for E. coli JM109 (Table 2). MIC values were consistent with the results obtained from the disk diffusion assay. Aztreonam, as opposed to the extended-spectrum cephalosporins, had decreased MICs for E. coli JM109(pFAR-1). All β-lactams which had increased MICs for E. coli JM109(pFAR-1) compared to E. coli JM109 had reduced MICs in the presence of clavulanic acid, whereas tazobactam and sulbactam failed to inhibit totally the β-lactamase activity.
TABLE 2.
MICs of β-lactams for N. farcinica VIC, E. coli JM109 harboring recombinant plasmid pFAR-1, and reference strain E. coli JM109
| β-Lactam(s)a | β-Lactam MIC (μg/ml) for:
|
||
|---|---|---|---|
| N. farcinica VIC | E. coli JM109(pFAR-1) | E. coli JM109 | |
| Benzylpenicillin | 512 | NDb | ND |
| Benzylpenicillin + CLA | 8 | ND | ND |
| Benzylpenicillin + TZB | 128 | ND | ND |
| Benzylpenicillin + SUL | 256 | ND | ND |
| Amoxicillin | 64 | 256 | 2 |
| Amoxicillin + CLA | 1 | 8 | 2 |
| Amoxicillin + TZB | 64 | 256 | 2 |
| Amoxicillin + SUL | 64 | 128 | 2 |
| Ticarcillin | 256 | 512 | 2 |
| Ticarcillin + CLA | 8 | 16 | 1 |
| Ticarcillin + TZB | 128 | 512 | 1 |
| Ticarcillin + SUL | 256 | 256 | 2 |
| Piperacillin | 512 | 32 | 1 |
| Piperacillin + CLA | 32 | 2 | 0.5 |
| Piperacillin + TZB | 256 | 32 | 0.5 |
| Piperacillin + SUL | 256 | 16 | 0.5 |
| Cephalothin | 128 | 8 | 4 |
| Cephalothin + CLA | 64 | 8 | 2 |
| Cephalothin + TZB | 128 | 4 | 2 |
| Cephalothin + SUL | 128 | 4 | 2 |
| Cefoxitin | 128 | 4 | 4 |
| Cefoxitin + CLA | 64 | 4 | 4 |
| Cefoxitin + TZB | 64 | 4 | 4 |
| Cefoxitin + SUL | 64 | 4 | 4 |
| Ceftazidime | >512 | 0.25 | 0.25 |
| Ceftazidime + CLA | >512 | 0.25 | 0.25 |
| Ceftazidime + TZB | >512 | 0.25 | 0.25 |
| Ceftazidime + SUL | >512 | 0.25 | 0.25 |
| Cefotaxime | 256 | 0.06 | 0.06 |
| Cefotaxime + CLA | 128 | 0.06 | 0.06 |
| Cefotaxime + TZB | 128 | 0.06 | 0.06 |
| Cefotaxime + SUL | 256 | 0.06 | 0.06 |
| Aztreonam | >512 | 1 | 0.12 |
| Aztreonam + CLA | 512 | 0.12 | 0.06 |
| Aztreonam + TZB | 512 | 1 | 0.06 |
| Aztreonam + SUL | 512 | 0.5 | 0.06 |
| Cefepime | 128 | 0.12 | 0.06 |
| Cefepime + CLA | 64 | <0.06 | 0.06 |
| Cefepime + TZB | 128 | <0.06 | 0.06 |
| Cefepime + SUL | 128 | <0.06 | 0.06 |
| Imipenem | 1 | 0.06 | 0.06 |
| Imipenem + CLA | 1 | 0.06 | 0.06 |
| Imipenem + TZB | 1 | 0.06 | 0.06 |
| Imipenem + SUL | 1 | 0.06 | 0.06 |
| Meropenem | 0.25 | <0.06 | <0.06 |
| Meropenem + CLA | 0.25 | <0.06 | <0.06 |
| Meropenem + TZB | 0.25 | <0.06 | <0.06 |
| Meropenem + SUL | 0.25 | <0.06 | <0.06 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml; SUL, sulbactam at a fixed concentration of 8 μg/ml.
ND, not determined.
Sequence analysis of the N. farcinica β-lactamase gene.
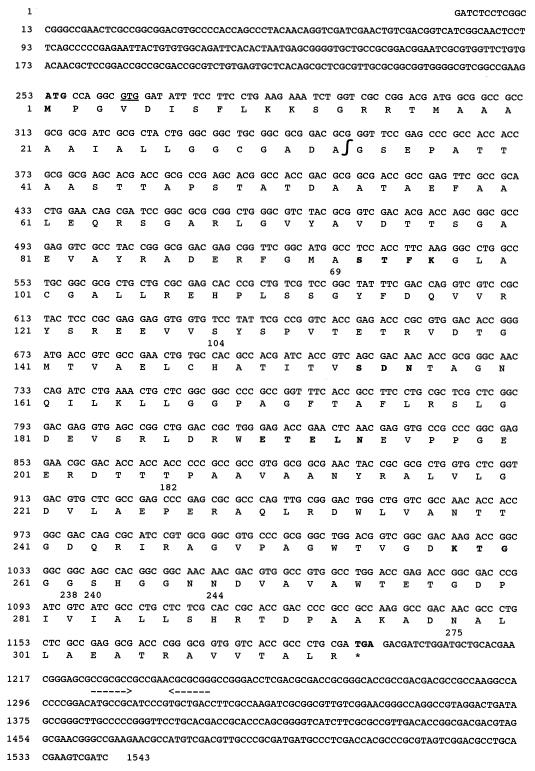
The nucleotide sequence of the β-lactamase gene and its deduced amino acid sequence are shown (Fig. 2). Analysis of the 1,543-bp insert for coding regions revealed a sufficiently large open reading frame (ORF) of 939 bp from nucleotide 252 to nucleotide 254. A putative ATG initiation codon at positions 252 to 254 could have been retained but no putative ribosome-binding site (RBS) was found immediately upstream. Another putative GTG initiation codon at positions 261 to 263 was preceded 9 bp upstream by a putative RBS (GAAGA, positions 249 to 253; Fig. 2). Inverted repeats (positions 1225 to 1230 [CCGCGC] and positions 1238 to 1244 [GCGCGG]) were found downstream from the ORF and may act as a Rho-dependent transcriptional terminator. Conversely, no putative sequence for any bacterial promoter was found upstream from the ORF.
FIG. 2.
Nucleotide sequence of the 1,543-bp fragment of pFAR-1 containing the β-lactamase coding region. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. The start and stop codons and the five structural elements characteristic of class A β-lactamases are in boldface. Additionally, a putative second start site (GTG) is underlined. Inverted repeat sequences are underlined by convergent arrows. The “∫” symbol indicates the putative cleavage site for the leader peptide. The 1,543 bp are numbered successively, and the amino acid numbering is according to the method of Ambler (1). Important amino acid positions of the deduced protein FAR-1 compared to those of TEM-1 are numbered; Ambler positions 69, 104, 182, 238, 240, 244, and 275.
The overall GC content of the ORF was 72.2%, which is close to the expected range of G+C ratio of the Nocardia genus (64 to 72%) (35). It results (as in genes from Streptomyces sp. ([21], Actinomadura sp. [27], or Mycobacterium tuberculosis [24]) in a highly biased codon usage with a remarkable codon preference for guanosine or cytosine at the third position (>95%), with NNC codons being used in 59.6% of the cases, NNG codons being used in 35% of the cases, NNT codons being used in 2% of the cases, and NNA codons being used in 3.4% of the cases.
The deduced protein sequence of the ORF was 313 amino acids long. A signal peptide with a putative cleavage site located between the 33rd and the 34th amino acids was found (Fig. 2). The resulting protein of 280 amino acids had a theoretical molecular mass of 30 kDa and a computed pI value of 4.7 (5, 6), which fits the experimentally determined pI and molecular mass values of 4.6 and 32 kDa, respectively. Moreover, the amino acid sequence at positions 25 to 29 (LLGGC) resembled the consensus sequence of an attachment site of a prokaryotic membrane lipoprotein (26).
Within the mature protein sequence, FAR-1, a serine-threonine-phenylalanine-lysine tetrad (S-T-F-K) was found at amino acid positions 70 to 73, according to Ambler numbering (1); this tetrad included the conserved serine and lysine amino acid residues characteristic of β-lactamases possessing a serine-active site or penicillin-binding proteins (Fig. 2) (29). Three structural elements characteristic of class A β-lactamases were found: serine-aspartic acid-asparagine (S-D-N) at positions 130 to 132, glutamate-X-glutamate-leucine-asparagine (E-X-E-L-N) at positions 166 to 170, and lysine-threonine-glycine (K-T-G) at positions 234 to 236 (Fig. 2).
The comparison of FAR-1 indicated a relationship with several class A β-lactamases. The highest identities were found with the β-lactamases from B. cepacia (52%), S. lactamdurans (51%), Actinomadura sp. strain R39 (50%), M. tuberculosis (48%), S. clavuligerus (47%), S. badius (45%), B. amyloliquefaciens (44%), B. licheniformis (43%), B. cereus (42%), and K. oxytoca (43%). The amino acid identity with a β-lactamase of M. fortuitum was only 37%.
Biochemical properties of FAR-1 β-lactamase.
The specific activity of purified FAR-1 β-lactamase from E. coli JM109 was 6.5 μmol · min−1 · mg of protein−1, determined with 100 μM benzylpenicillin as the substrate. The overall recovery of FAR-1 β-lactamase was 90%, with a 30-fold purification. SDS-PAGE analysis revealed that FAR-1 β-lactamase was very weakly expressed in E. coli JM109 harboring pFAR-1. The kinetic parameters of the β-lactamase FAR-1 revealed its strong activity against penicillins and early-generation cephalosporins (Table 3). Aztreonam was a substrate for FAR-1 even if its affinity and hydrolysis rates were low.
TABLE 3.
Steady-state kinetic parameters of the purified FAR-1 β-lactamase
| Substrate | kcat (s−1) | Km (μM) | kcat/Km (μM · s−1) |
|---|---|---|---|
| Benzylpenicillin | 165 | 30 | 5.5 |
| Amoxicillin | 190 | 50 | 3.8 |
| Ticarcillin | 49.5 | 31 | 1.6 |
| Piperacillin | 412 | 45 | 9.2 |
| Cephalothin | 14 | 104 | 0.13 |
| Cephaloridine | 131 | >500 | <0.1 |
| Cefuroxime | 3.3 | >500 | <4 × 10−3 |
| Ceftazidime | NHa | NDb | ND |
| Cefotaxime | 5 | >500 | <6 × 10−3 |
| Aztreonam | 13.2 | 400 | <0.02 |
NH, not hydrolyzable.
ND, not detectable.
IC50 results with cephaloridine (100 μM) as the substrate showed that FAR-1 was less inhibited by the inhibitors than TEM-1 (Table 4). Its susceptibility to inhibitors was in the following decreasing order: clavulanic acid, tazobactam, and sulbactam.
TABLE 4.
Comparison of IC50 and Ki values of β-lactamase inhibitors for FAR-1 and TEM-1 β-lactamases
| β-Lactamase | Clavulanic acid
|
Sulbactam
|
Tazobactam
|
|||
|---|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | |
| FAR-1 | 0.3 | 1.3 | 600 | 180 | 20 | 4.3 |
| TEM-1 | 0.08 | 0.1 | 6.1 | 0.9 | 0.1 | 0.01 |
Distribution of β-lactamase FAR-1.
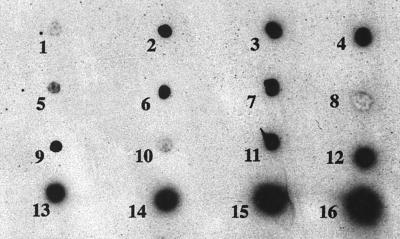
All the studied strains belonging to N. farcinica species showed the same β-lactam resistance profile except for amoxicillin-clavulanate, for which full susceptibility or intermediate levels of resistance were observed (data not shown). Moreover, N. farcinica CIP 96.0994 was resistant to imipenem (MIC, 64 μg/ml). These strains were β-lactamase positive as assessed by the positive results of the nitrocefin test. IEF results showed that all strains produced a single β-lactamase of pI 4.6 (data not shown). These strains showed positive dot blot hybridization results when the 700-bp HincII-SmaI fragment internal to blaFAR-1 was used as a probe (Fig. 3).
FIG. 3.
Dot blot hybridizations of different strains with the radiolabelled 700-bp HincII-SmaI internal probe for blaFAR-1. Blots: 1, E. coli JM109 (negative control); 2, N. farcinica 94.0250; 3, N. farcinica 94.0664; 4, N. farcinica 95.0288; 5, N. farcinica 95.0684; 6, N. farcinica 96.0027; 7, N. farcinica 96.0087; 8, N. asteroides; 9, N. farcinica 96.0624; 10, R. equi ATCC 6939 (negative control); 11, N. farcinica 96.0691; 12, N. farcinica 96.0994; 13, N. farcinica 96.1087; 14, N. farcinica 97.0244; 15, N. farcinica VIC; and 16, E. coli JM109(pFAR-1).
DISCUSSION
N. farcinica VIC expresses a novel class A β-lactamase named FAR-1. The GC content and codon usage of its gene corresponded to those of actinomycetes or taxonomically related species. The determined molecular mass of 32 kDa corresponds to that of class A β-lactamases. A protein comparison with other class A β-lactamases showed that FAR-1 had the greatest percentage of identity with β-lactamases from actinomycetes (S. clavuligerus [47%], Actinomadura sp. strain R39 [50%], S. lactamdurans [51%], S. badius [45%], and M. tuberculosis [48%]). It was also related to class A β-lactamases from Bacillus sp. and, surprisingly, from B. cepacia and K. oxytoca.
The comparison of inhibitor properties of FAR-1 to TEM-1 underlines that clavulanic acid was less active against FAR-1 than against TEM-1 and that sulbactam was the weakest inhibitor of FAR-1 activity. Analysis of the three-dimensional structure of some inhibitor-resistant TEM derivatives highlights that some amino acids, such as Met69, Met182, Arg244, Arg275, or Asn276, are important for inhibitor activity (9, 10, 12, 13, 18, 28). Therefore, the amino acid changes found in FAR-1 compared to TEM-1, i.e., Met69Arg (Met69 changed to Arg), Met182Thr, Arg244Asn, or Arg275Asp, may explain its low susceptibility to tazobactam and sulbactam (Fig. 2). Very few studies show that β-lactamase activity is observed for all N. farcinica isolates (2, 52). According to Ambaye et al. (2), N. farcinica strains are always resistant to amoxicillin and susceptible to amoxicillin-clavulanate. Although the amoxicillin-clavulanate combination may be used for treating nocardiosis due to N. farcinica, any other β-lactam combination with tazobactam or sulbactam should be excluded. Sulbactam and tazobactam weak inhibitor activities against FAR-1 are similar to those found against a β-lactamase from M. fortuitum (3, 19). However, both of these enzymes are distantly related to one another (55).
FAR-1 has specific hydrolysis activity toward aztreonam (at a low level), while none was found towards ceftazidime, cefotaxime, and imipenem. This characteristic is rather specific to FAR-1 compared to the previously published gram-positive class A β-lactamases (38, 44). A few substitutions on TEM-1-derived class A β-lactamases, especially Glu104Lys, Ala238Gly, and Glu240Lys, have been found to increase hydrolytic activity toward aztreonam, ceftazidime, or cefotaxime (14, 39, 50). FAR-1 contains identical or similar amino acid substitutions (Glu104Ser, Ala238Gly, and Glu240Ser), which may explain, in part, the extension of the FAR-1 substrate profile (Fig. 2). Activity toward aztreonam and not toward ceftazidime is also found for KOXY from K. oxytoca, with both enzymes being weakly related (22).
This specific hydrolytic activity of FAR-1 toward the aztreonam remains intriguing, however. It is known that soil actinomycetes produce both β-lactams and β-lactamases (16, 31, 39, 53), allowing them to survive in the presence of these antibiotics (53). The first example of naturally produced monobactams, named nocardicin A, was extracted from a fermentation broth of a Nocardia strain (25, 30). Therefore, it may be expected that N. farcinica VIC produces both FAR-1 and a monobactam similar to aztreonam.
Additionally, if FAR-1 is cell wall bound and poorly excreted, it may be a convenient protection tool against β-lactams. Indeed, FAR-1 structure analysis reveals a membrane lipoprotein lipid attachment motif (LLGGC) (26). This agrees with the findings of Steingrube et al., who have studied β-lactamase preparations of 31 N. farcinica strains (52). These culture supernatants possessed a β-lactamase activity 25-fold lower than those from cell extracts. Similar observations were recently reported for the β-lactamases of M. fortuitum (58) and N. asteroides (48).
FAR-1 activity does not, however, explain the entire β-lactam resistance profile of N. farcinica strains, such as the resistance to extended-spectrum cephalosporins (ceftriaxone or cefotaxime). An undetected second β-lactamase could not be excluded, although only one β-lactamase pI was identified from N. farcinica cultures. Most likely, as for other gram-positive organisms, this resistance profile may be due to different penicillin-binding protein affinities.
Our hybridization and IEF analysis showed that all of the tested N. farcinica isolates possessed a blaFAR-1-like gene (one of them, N. farcinica 95.06.84, hybridized only weakly), thus confirming the homogeneity of the antimicrobial susceptibility pattern of N. farcinica.
Based on the isolates tested by Provost et al. (47), the incidence of plasmid-bearing strains is significantly higher among N. farcinica strains than among N. asteroides strains without a relationship between plasmid presence and any specific antibiotic resistance phenotypes. The chromosomal or plasmid location of blaFAR-1 remains to be determined.
Although different β-lactamases have been characterized by IEF for isolates of N. asteroides sensu stricto (48) and N. brasiliensis (51, 59), much remains to be known about this β-lactamase research field. None of these β-lactamases seems to correspond to FAR-1. Therefore, further work should be directed toward the identification of the molecular structure of β-lactamases from other Nocardia spp. and in order to elucidate their relation in respect to their β-lactam resistance profile.
ACKNOWLEDGMENT
This work was financed by a grant from the Ministère de la Recherche et de l’Education Nationale (UPRES-JE 2227), Université Paris XI, Paris, France.
REFERENCES
- 1.Ambler R P. The structure of β-lactamases. Philos Trans R Soc Lond Biol. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Ambye A, Khoner P C, Wollan P C, Roberts K L, Roberts G D, Cockerill F R., III Comparison of agar dilution, broth microdilution, disk diffusion, E-Test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol. 1997;35:847–852. doi: 10.1128/jcm.35.4.847-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amicosante G, Franceschini N, Segatore B, Oratore A, Fattorini L, Orefici G, Van Beeumen J, Frère J M. Characterization of a beta-lactamase produced in Mycobacterium fortuitum D316. Biochem J. 1990;271:729–734. doi: 10.1042/bj2710729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman B L, Boiron P, Beaman L, Brownell G H, Schaal K, Gombert M E. Nocardia and nocardiosis. J Med Vet Mycol. 1992;30:317–331. [PubMed] [Google Scholar]
- 5.Bjellqvist B, Basse B, Olsen E, Celis J E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis. 1994;15:529–539. doi: 10.1002/elps.1150150171. [DOI] [PubMed] [Google Scholar]
- 6.Bjellqvist B, Hughes G J, Pasquali C, Paquet N, Ravier F, Sanchez J C, Frutiger S, Hochstrasser D F. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–1031. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- 7.Boiron, P. Unpublished data.
- 8.Boiron P, Provost F, Chevrier G, Dupont B. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709–714. doi: 10.1007/BF01989975. [DOI] [PubMed] [Google Scholar]
- 9.Bonomo R A, Dawes C G, Knox J R, Shlaes D M. Beta-lactamase mutations far from the active site influence inhibitor binding. Biochim Biophys Acta. 1995;1247:121–125. doi: 10.1016/0167-4838(94)00188-m. [DOI] [PubMed] [Google Scholar]
- 10.Bush K, Jacoby G A. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1997;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canica M M, Barthélémy M, Gilly L, Labia R, Krishnamoorthy R, Paul G. Properties of IRT-14 (TEM-45), a newly characterized mutant of TEM-type beta-lactamases. Antimicrob Agents Chemother. 1997;41:374–378. doi: 10.1128/aac.41.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canica M M, Lu C Y, Krishnamoorthy R, Paul G C. Molecular diversity and evolution of bla TEM genes encoding beta-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol. 1997;44:57–65. doi: 10.1007/pl00006121. [DOI] [PubMed] [Google Scholar]
- 14.Cantu C, Huang W, Palzkill T. Selection and characterization of amino acid substitutions at residues 237-240 of TEM-1 beta-lactamase with altered substrate specificity for aztreonam and ceftazidime. J Biol Chem. 1996;271:22538–22545. doi: 10.1074/jbc.271.37.22538. [DOI] [PubMed] [Google Scholar]
- 15.Chan P T. Nucleotide sequence of the Staphylococcus aureus PC1 beta-lactamase gene. Nucleic Acids Res. 1986;14:5940. doi: 10.1093/nar/14.14.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coque J J R, Liras P, Martin F. Genes for a β-lactamase, a penicillin-binding protein and a transmembrane protein are clustered with the cephamycin biosynthetic genes in Nocardia lactamdurans. EMBO J. 1993;12:631–639. doi: 10.1002/j.1460-2075.1993.tb05696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornish-Bowden A. Fundamentals of enzyme kinetics. Seattle, Wash: Portland Press, Inc.; 1995. Graphs of the Michaelis-Menten equation; pp. 30–37. [Google Scholar]
- 18.Farzaneh S, Chaibi E B, Peduzzi J, Barthélémy M, Labia R, Blazquez J, Baquero F. Implication of Ile-69 and Thr-182 residues in kinetic characteristics of IRT-3 (TEM-32) beta-lactamase. Antimicrob Agents Chemother. 1996;40:2434–2436. doi: 10.1128/aac.40.10.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattorini L, Amicosante G, Fiorentino D, Franceschini N, Di Marzio L, Oratore A, Orefici G. Inhibitors and inactivators of beta-lactamase from Mycobacterium fortuitum. J Chemother. 1989;1:293–297. doi: 10.1080/1120009x.1989.11738911. [DOI] [PubMed] [Google Scholar]
- 20.Filice G A, Simpson G L. Management of Nocardia infections. In: Remington J S, Swartz M N, editors. Current clinical topics in infectious diseases. New York, N.Y: McGraw-Hill Book Co.; 1984. pp. 49–64. [Google Scholar]
- 21.Forsman M, Haggstrom B, Lindgren L, Jaurin B. Molecular analysis of β-lactamases from four species of Streptomyces: comparison of amino acid sequences with those of other β-lactamases. J Gen Microbiol. 1990;136:589–598. doi: 10.1099/00221287-136-3-589. [DOI] [PubMed] [Google Scholar]
- 22.Fournier B, Lagrange P H, Philippon A. Beta-lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca. Antimicrob Agents Chemother. 1996;39:1365–1368. doi: 10.1128/aac.40.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gombert M E, Berkowitz L B, Aulicino T M, Dubouchet L. Therapy of pulmonary nocardiosis in immunocompromised mice. Antimicrob Agents Chemother. 1990;34:1766–1768. doi: 10.1128/aac.34.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackbarth C, Unsal I, Chambers H F. Cloning and sequence analysis of a class A β-lactamase from Mycobacterium tuberculosis H37Ra. Antimicrob Agents Chemother. 1997;41:1182–1185. doi: 10.1128/aac.41.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto M, Komori T, Kamiya T. Nocardicin A and B, novel monocyclic beta-lactam antibiotics from a Nocardia species. J Am Chem Soc. 1976;12:3023–3025. doi: 10.1021/ja00426a063. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 27.Houba S, Willem S, Duez C, Molitor C, Dusart J, Frère J M, Ghuysen J M. Nucleotide sequence of the gene encoding the active-site serine β-lactamase from Actinomadura R39. FEMS Microbiol Lett. 1989;65:241–246. doi: 10.1016/0378-1097(89)90224-3. [DOI] [PubMed] [Google Scholar]
- 28.Imtiaz U, Billings E, Knox J R, Manavathu E K, Lerner S A, Mobashery S. Inactivation of class-A β-lactamases by clavulanic acid: the role of arginine-244 in a proposed nonconcerted sequence events. J Am Chem Soc. 1993;115:4435–4442. [Google Scholar]
- 29.Joris B, Ledent P, Dideberg O, Fonze E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frère J M. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojo H, Mine Y, Nishida M, Goto S, Kuwahara S. Nature of monocyclic beta-lactam antibiotic nocardicin A to beta-lactamases. Microbiol Immunol. 1988;32:119–130. doi: 10.1111/j.1348-0421.1988.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 31.Kurai S, Urabe H, Ogawara H. Cloning, sequencing and site-directed mutagenesis of β-lactamase gene from Streptomyces fradiae Y59. Antimicrob Agents Chemother. 1995;39:260–263. doi: 10.1128/aac.39.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Laurent F, Carlotti A, Boiron P, Villard J, Freney J. Ribotyping: a tool for taxonomy and identification of the Nocardia asteroides complex species. J Clin Microbiol. 1996;34:1079–1082. doi: 10.1128/jcm.34.5.1079-1082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent, F., F. Provost, and P. Boiron. Rapid identification of clinically relevant Nocardia to the genus level with 16S rDNA polymerase chain reaction. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 35.Lechevalier M. Nocardioforms actinomycetes. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. 9th ed. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 2348–2404. [Google Scholar]
- 36.Maggwick P J, Waley S G. Beta-lactamase I from Bacillus cereus. Structure and site-directed mutagenesis. Biochem J. 1987;248:657–662. doi: 10.1042/bj2480657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massida O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamase. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeil M M, Brown J M. The medically important aerobic actinomycete: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 40.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 41.National Center of Biotechnology Information Website. 11 December 1998, revision date. Software. [Online.] http://www.ncbi.nlm.nih.gov. [8 March 1999, last date accessed.]
- 42.Neugebauer K, Sprengel R, Schaller H. Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a gram-positive bacterium. Nucleic Acids Res. 1981;9:2577–2588. doi: 10.1093/nar/9.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum beta-lactamase from Pseudomonas aeruginosa and comparison with class A beta-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastor N, Pinero D, Valdes A M, Soberon X. Molecular evolution of class A β-lactamases: phylogeny and patterns of sequence conservation. Mol Microbiol. 1990;4:1957–1965. doi: 10.1111/j.1365-2958.1990.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 45.Pedro’s BioMolecular Research Tools Website. 16 June 1995, revision date. Software. [Online.] http://www.fmi.ch/biology/research_tools.html. [8 March 1999, last date accessed.]
- 46.Perez-Llarena F, Martin J F, Gallieni M, Coque J J, Fuente J L, Frère J M, Liras P. The bla gene of the cephamycin cluster of Streptomyces clavuligerus encodes a class A beta-lactamase of low enzymatic activity. J Bacteriol. 1997;179:6035–6040. doi: 10.1128/jb.179.19.6035-6040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provost F, Blanc M V, Beaman B L, Boiron P. Occurrence of plasmids in pathogenic strains of Nocardia. J Med Microbiol. 1996;45:344–348. doi: 10.1099/00222615-45-5-344. [DOI] [PubMed] [Google Scholar]
- 48.Scopetti F, Fattorini L, Franceschini N, Amicosante G, Orefici G. Non-inducible, mainly cell associated β-lactamase from Nocardia asteroides strain 108. J Antimicrob Chemother. 1997;40:5–11. doi: 10.1093/jac/40.1.5. [DOI] [PubMed] [Google Scholar]
- 49.Smego R A, Moeller M B, Gallis H A. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med. 1983;143:711–718. [PubMed] [Google Scholar]
- 50.Sowek J A, Singer S B, Ohringer S, Malley M F, Dougherty T J, Gougoutas J Z, Bush K. Substitution of lysine at position 104 or 204 of TEM-1 β-lactamase enhances the effect of serine-164 substitution on hydrolysis or affinity for cephalosporins and the monobactam aztreonam. Biochemistry. 1991;30:3179–3188. doi: 10.1021/bi00227a004. [DOI] [PubMed] [Google Scholar]
- 51.Steingrube V A, Wallace R J, Jr, Brown B A, Pang Y, Zeluff B, Steele L C, Zhang Y. Acquired resistance of Nocardia brasiliensis to clavulanic acid related to a change in β-lactamase following therapy with amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991;35:524–528. doi: 10.1128/aac.35.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steingrube V A, Wallace R J, Jr, Brown B A, Zhang Y, Steele L C, Young G, Nash D R. Partial characterization of Nocardia farcinica β-lactamases. Antimicrob Agents Chemother. 1993;37:1850–1855. doi: 10.1128/aac.37.9.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strynadka N J C, Jensen S E, Johns K. Structural and kinetic characterization of a β-lactamase-inhibitor protein. Nature. 1994;368:657–660. doi: 10.1038/368657a0. [DOI] [PubMed] [Google Scholar]
- 54.Sugar A M, Chahal R S, Stevens D A. A cephalosporin active in vivo against Nocardia: efficacy of cefotaxime in murine model of acute pulmonary nocardiosis. J Hyg. 1983;91:421–427. doi: 10.1017/s0022172400060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Timm J, Perilli M G, Duez C, Trias J, Orefici G, Fattorini L, Amicosante G, Oratore A, Joris B, Frère J M, Pugsley A P, Gicquel B. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitum beta-lactamase genes cloned from a natural isolate and a high-level beta-lactamase producer. Mol Microbiol. 1994;12:491–504. doi: 10.1111/j.1365-2958.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 56.Trepanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its Lys-R-Type transcriptional regulator. Antimicrob Agents Chemother. 1997;41:2399–2405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Dijl, J. M., A. De Jong, A. Nauta, G. Venema, and S. Bron. GenBank accession number Z35653). Unpublished data.
- 58.Wagner B, Fattorini L, Wagner M, Jin S H, Stracke R, Amicosante G, Franceschini N, Orefici G. Antigenic properties and immunoelectron microscopic localization of Mycobacterium fortuitum beta-lactamase. Antimicrob Agents Chemother. 1995;39:739–745. doi: 10.1128/AAC.39.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace R J, Jr, Nash D R, Johnson W K, Steele L C, Steingrube V A. Beta-lactamase in Nocardia brasiliensis is mediated by beta-lactamase and reversed in the presence of clavulanic acid. J Infect Dis. 1987;156:959–966. doi: 10.1093/infdis/156.6.959. [DOI] [PubMed] [Google Scholar]
- 60.Wallace R J, Jr, Septimus E J, Williams T W, Conklin R H, Satterwhite T K, Bushby M B, Hollowell D C. Use of trimethoprim-sulfamethoxazole for treatment of infections due to Nocardia. Rev Infect Dis. 1982;4:315–325. doi: 10.1093/clinids/4.2.315. [DOI] [PubMed] [Google Scholar]