Abstract
Background
Gastric ulcer is a major public health problem globally and associated with severe complications including hemorrhages, perforations, gastrointestinal obstruction, and malignancy. Urtica simensis is widely used for traditional management of gastric ulcer in different parts of Ethiopia. The present study was undertaken to evaluate the anti-gastric ulcer activity of aqueous and 80% methanol extracts of U.simensis in rats.
Methods
The leaf extracts were prepared using decoction (aqueous) and maceration (80% methanol) techniques and in vivo anti-gastric ulcer effects of various doses of U. simensis extracts and the effect were determined using the pylorus ligation, indomethacin and ethanol induced gastric ulcer models.
Results
In pylorus ligation induced gastric ulcer model, both aqueous and 80% methanol extracts at doses of 200 and 400 mg/kg were exhibited significant reduction in total acidity, volume of gastric secretion (p < 0.001) and substansial rise in pH (p˂0.05) of the gastric secretion. In indomethacin induced ulcer model, both aqueous and methanol extracts were exhibited dose dependent increment in gastric wall mucus compared to control (p < 0.001). In ethanol induced ulcer model, all doses of extract produced significant increment in gastric wall mucus from 46.66 ± 0.96 (AQ100) to 75.87 ± 1.52 (ME 400) μg alcian blue/g wet stomach. Five days pre-treatment with 200 mg/kg of both and aqueous and methanolic extracts exhibited significant (P < 0.001) ulcer inhibition in both indomethacin and ethanol-induced ulcer models.
Conclusion
Both extracts of U.simensis exhibited a promising anti-gastric ulcer activity in all of the three models and this findings supports for traditional claimed use of the leaf of U. simensis.
Keywords: Urtica simensis, Gastric ulcer, Pylorus ligation, Indomethacin, Ethanol
1. Introduction
Gastric ulcer, also called stomach ulcer, is a disruption in the normal integrity of gastric mucosa that extends through the muscularis mucosa into the sub mucosa or deeper. Peptic ulcer is caused by a lack of equilibrium between the gastric aggressive factors (acid, pepsin, H. pylori and non-steroidal anti-inflammatory agents) and the mucosal defensive factors (mucus bicarbonate, blood flow and prostaglandins) [1]. The incidence of peptic ulcer disease (PUD) varies with the age, gender, geographical location and is associated with severe complications including hemorrhages, perforations, gastrointestinal obstruction, and malignancy. Thus, this clinical condition represents a worldwide health problem because of its high morbidity, mortality and economic loss [2]. Worldwide, the prevalence of the disease is about 40% in the developed countries and 80% in the developing countries [2].An estimated of 15,000 deaths occur each year as a consequence of peptic ulcer. Annual incidence estimates of peptic ulcer hemorrhage and perforation were 19.4–57 and 3.8–14 per 100,000 individuals, respectively. The average 7-day recurrence of hemorrhage was 13.9% whereas the average long-term recurrence of perforation was 12.2% [3] the text we inserted here is not from our research (inserting Table 3, Table 4 is not appropriate), instead from other article as indecated, reference no. 3.
Table 3.
Effect of repeated (5days) treatment aqueous and 80% methanol extracts of U. simensis in indomethacin induced ulcer model.
| Mucin content (μg/g) | % of increment in mucin | Ulcer Index (UI) | % of protection | |
|---|---|---|---|---|
| Control | 32.68 ± 0.52 | 28 ± 1.97 | ||
| AQ 200 | 129.6 ± 1.94a3b3c2 | 74.8 | 7.5 ± 3.36 a3b2c2 | 73.1 |
| ME 200 | 131.2 ± 1.94a3b3c3 | 75.1 | 7.17 ± 3.22 a3b2c2 | 74.4 |
| C 100 mg/kg | 135.6 ± 2.38a3b3c3 | 75.9 | 6.83 ± 3.05 a3b2c2 | 75.6 |
AQ = Aqueous extract, ME = 80% methanol extract, Cimetidine = CI.
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey test; a compared to negative control b compared with single dose AQ200 and c compared with single dose ME 200 3: p < 0.001.
Table 4.
Effect of aqueous and 80% methanol extracts of U. simensis and cimetidine on mucin content (μg/g), % of increment in mucin, Ulcer index and % of protection ethanol induced ulcer.
| Mucin content | % of increment in mucin | UI | % of protection | |
|---|---|---|---|---|
| Control | 37.51 ± 0.89 | 34.33 ± 1.33 | ||
| AQ 100 | 46.66 ± 0.96 | 19.6 | 24.91 ± 1.47 | 27.44 |
| AQ 200 | 60.64 ± 2.17a2 | 38.1 | 18.33 ± 1.23a2 | 46.6 |
| AQ 400 | 75.08 ± 1.36a3 | 50 | 14.91 ± 3.13a3 | 56.5 |
| ME 100 | 47.34 ± 1.14a2 | 20.8 | 25 ± 1.07 | 27.2 |
| ME 200 | 61.27 ± 1.92a3 | 38.8 | 16.75 ± 1.37a2 | 51.2 |
| ME 400 | 75.87 ± 1.52a3 | 50.6 | 14.16 ± 4.50a3 | 58.7 |
| CI 100 mg/kg | 77.23 ± 0.64a3 | 51.43 | 13.16 ± 4.2a3 | 61.6 |
AQ = Aqueous extract, ME = 80% methanol extract, Cimetidine = CI.
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey test; a compared to negative control; b compared to Aqueous 100 mg/kg c compared to methanol 100 mg/kg; 1: p < 0.05, 2: p < 0.01, 3: p < 0.001.
Proton pump inhibitors (PPIs), H2 receptor antagonists, Antacids, muscarinic (M1) receptor antagonist pirenzepine, Antimicrobial agents and PGE2 and its analog misoprostol were conventional drugs used in management of PUD nowadays [4]. Pharmacological treatments are the mainstay treatment for peptic ulcer for many centuries. Despite the fact, most of drugs are associated with adverse reactions (e.g. anaphylaxis reactions, gynecomastia, hematopoietic changes, thrombocytopenia, acute interstitial nephritis, nephrotoxicity and hepatotoxicity) requirement for multiple doses per day, large tablet size, and the need to separate the drug from meals and potentially interacting medications [5]. Due to those factors, there is a need to find new anti-ulcerogenic compound(s) with potentially less or no side effects and medicinal plants have always been the main sources of new drugs candidates for the treatment of gastric ulcer. The use of medicinal plants to treat major ailments has been utilized particularly in developing world because of easily afford ability and their belief of higher efficacy and relatively less toxic than synthetic drugs. In this modern era, about 75–80% of the world populations still use herbal medicines mainly in developing countries, for primary health care [6].
There are several models that are used to evaluate antiulcer medicines. Among those models pylorus-ligated-induced peptic ulcers, indomethacin induced gastric ulcers and ethanol-induced gastric ulcers were used in this study. In pylorus-ligated-induced peptic ulcers ligation of the pyloric end of the stomach causes accumulation of gastric acid in the stomach that produces ulcers and important to measure acidic content [7]. NSAID and ethanol induced ulcers are important in examining the potential usefulness of anti-secretory and cytoprotective agents since the fundamental pathophysiology involves gastric acid secretion and mucosal prostaglandin synthesis [8].
Urtica simensis (U.simensis) belongs to the family Urticaceae and the genus Urtica. Several species of the genus Urtica (especially the Urtica dioica L.) are used medicinally to treat variety of ailments. The U. dioica has been used for hundreds of years to treat rheumatism, arthritis, gout, eczema, anemia, urinary tract infections, kidney stones, hay fever and early stages of an enlarged prostate [9]. The plant U. simensis commonly known as Nattle (English), Sama (Amharic), Dobii (Oromifaa) and Ameie (Tigrigna) is one of species of Nettle and endemic in Ethiopia. As described in different ethno botanical studies the leaf of U. simensis has been frequently used as traditional medicine for various ailments like peptic ulcer disease, malaria [10], Rh factor, heart failure [11], gastritis, wound acute stomachache, body swelling and gonorrhea [12]. The aqueous fraction of U. simensis showed anti-diabetic activity in a dose dependent manner [13]. Butanol fraction of 80% methanol extracts of U. simensis exhibited greater activity against gram positive bacteria while ethyl acetate fraction revealed greater activity against gram negative bacteria and fungi [14]. This study attempted to validate the traditional claimed use of this endemic medicinal plant for its anti-gastric ulcer activity.
2. Materials and methods
Distilled water (Ethiopian Pharmaceutical Manufacturing Factory, Ethiopia), methanol and chloroform (Research Lab Fine Industries, India), glacial acetic acid, ammonia, hydrochloric acid and ferric chloride (BDH Laboratory Supplies Poole, England), acetic anhydride and Mayer‟s reagent (May and Baker LTD Dagenham, England), and Dragendroff‟s reagent and sulfuric acid (Fisher Scientific,UK), Cimetidine (Addis pharmaceutical company, Ethiopia), Ethanol (Dallul pharmaceutical, Ethiopia), Indomethacin (Leben, Laboratories, India). Phenophthalein indicator (Goenka chemical industry, India), sodium hydroxide (BDH, chemical lab, England), Alcian Blue (Arnish, Laborates, India), Sucrose (JHD, China), Magnesium chloride (Carbo Erba, Italy), Diethyl ether (Lobal, chemi, India) were used in the study.
2.1. Plant materials collections
The leaves of U. simensis were collected from Ayertena, Kolfe keranio sub city, Addis Ababa on March 2018. The plant was authenticated by a taxonomist and a voucher specimen (OA/001) was deposited at the National Herbarium of College of Natural and Computational Sciences, Addis Ababa University for future reference. After collection, the leaves were initially washed using running tap water to remove dirt or dust and dried under shade in pharmacology laboratory within the school of pharmacy. The leaves were then chopped into small pieces manually and ground into coarse powder mechanically using a clean mortar and pestle. The powder sample was weighed and stored in air tight containers until extraction.
2.2. Preparation of crude extract plant material
The extraction was carried out by maceration technique using 80% methanol as a solvent. Two hundred 50 g of the dried powder was weighed and soaked in 80% methanol (1:8 (w/v). The plant material was macerated for 72 h with occasional shaking using mini orbital shaker (Bibby Scientific Limited Stone Staffo Reshire, UK) at120 rpm. The extract was filtered through double layered muslin cloth followed by whatman No.1 filter paper (Maidstone, UK). The marc was then re-macerated for the second and third time using the same volume of fresh solvent. The resultant filtrates were then combined and concentrated using a rotary evaporator (Buchii model R-200,Switzerland) set at 40 °C. Finally, the concentrated extract was placed in deep freezer set at −20 °C to solidify and dried in a lyophilizer (Operan, Korea vacuum limited, Korea). Two hundred gram of the dried powder was weighed and boiled with 2000 ml of distilled water for 30 min in order to prepare the aqueous extract. The decoction was filtered twice with cotton gauze and aqueous filtrate was placed in deep freezer set at −20 °C to solidify and dried in a lyophilizer.
2.3. Experimental animals and protocol
Healthy wistar rats of either sex, 12–16 weeks of age; weighing about 150–200 gm were used for this experiment. The Rats were obtained from animal house of School of Pharmacy, Addis Ababa University (AAU).The animals were kept in cages at room temperature and on a 12/12 h light/dark cycle with free access to pellet and water. Rats were acclimatized to laboratory condition for one week prior to the experiment. The care and handling was according to international guidelines for the use and maintenance of experimental animals [15] and approved by the Department of Pharmacology Research and Ethics Review Committee with reference number ERB/SOP/127/11/2019.
2.4. Acute oral toxicity test
Both aqueous extract (AQ) and 80% methanolic extract (ME) was evaluated for toxicity based on internationally accepted protocol drawn by OECD guidelines-425 [15].
2.5. Animal grouping and dosing
Rats were randomly divided into eight groups each comprising of six rats. Group I (negative control) was treated with distilled water, Group II (positive control) was treated with Cimetidine 100 mg/kg, p.o (CI 100), Group III- VIII were treated with three different doses (100,200 & 400 mg/kg) of AQ and 80% ME extracts. Five days pretreatment study was done in indomethacin and ethanol induced gasric ulcer models, rats were divided into three groups and treated with medium dose (200 mg/kg) of both extracts and CI100. The standard drugs and plant extracts were administered orally.
2.6. Pylorus ligation induced gastric ulcer
Animals were fasted for 48 h before started the study, but had free access to water. After 1 h of drug treatment, they were anesthetized with ether and the abdomen was opened by a small midline incision below the xiphoid process. Pyloric portion of the stomach was slightly lifted out and ligated and it was performed with caution to avoid traction to the pylorus or damage to its blood supply. The stomach was placed carefully and the abdominal wall was closed by interrupted sutures. Rats were sacrificed by ether after 6 h of pyloric ligation. The abdomen was opened, cardiac end of the stomach was dissected out, and the contents were drained into a glass tube and the volume, pH and total acidity of the gastric juice were evaluated. Each rat's stomach was examined for lesions and indexed according to severity [16].
2.7. Indomethacin-induced gastric ulcer
Gastric lesions were induced with indomethacin (40 mg/kg) administered to rats after fasting for 36 h. Animals were treated with either vehicle, extracts or standard Cimetidine orally 30 min prior to induction of gastric lesions. The animals were sacrificed 5 h after treatment with the ulcerogenic agent to assess the ulcer score and mucous producing activity [17].
2.8. Ethanol-induced gastric ulcer
All the animals were fasted for 48 h before administration of ethanol. The gastric ulcers were induced in rats by administering ethanol (90%) (1 ml/200 g) orally [18], after 60 min of extracts administration. The animals were anesthetized 1 h later with ether, and the stomach was incised along the greater curvature and ulceration was scored as for the pyloric ligation-induced ulcer model and gastric mucus was determined [19].
2.9. Determination of anti-ulcer activity
2.9.1. Evaluation of stomachs
The stomachs were opened along the greater curvature and rinsed with normal saline to remove gastric contents and blood clots and examined by a 10x magnifier lens to assess the formation of ulcers. The ulcer index can be measured using the following scores as described by Reddy et al. [16].
Normal colored stomach --------- (0) Red coloration-------------------- (0.5).
Spot ulcer --------------------------- (1) Hemorrhagic streak --------------- (1.5).
Deep ulcers------------------------- (2) Perforation------------------------- (3).
Ulcer index (UI) = UN + US + (UP/10).
Were UI = ulcer index, UN = average number of ulcer per animal, US = average of severity score.
And UP = percentage of animals with ulcer.
And percentage protective ratio was calculated as follow
| (1) |
2.9.2. Determination of volume and pH
The volume of gastric juice of each rat was measured after centrifugation with 1000 rpm for 10 min and analyzed. An aliquot of 1 ml of gastric juice was diluted with 1 ml of distilled water and pH of the solution was measured using pH meter [18].
2.9.3. Determination of total acidity
An aliquot of 1 ml of gastric juice was diluted with 1 ml of distilled water and was taken into a 50 ml conical flask and two drops of phenolphthalein indicator was added and titrated with 0.01 N NaOH until a permanent pink color was observed. The volume of 0.01 N NaOH consumed was noted. The total acidity was expressed as mEq/L and calculated by the following formula [18].
2.9.4. Determination of gastric mucus
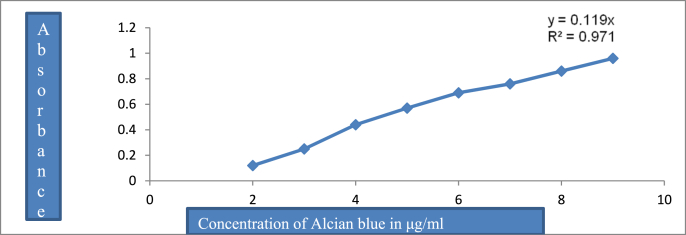
The glandular portion of gastric tissues was immediately transferred to 0.1% alcian blue solution prepared in 0.16 M sucrose and 0.05 M sodium acetate (pH 5.8) and stained for 2 h at room temperature. After the segments were rinsed twice with 0.25 M sucrose solution for 15 and 45 min, the dye complexed with the gastric mucus was extracted with 0.5 M magnesium chloride solution for 2 h (during this period the soaked stomach shaken for 1 min every 30 min). The extract was then mixed with equal volume of diethyl ether and centrifuged at 3000 rpm for 15 min. Absorbance was determined at 580 nm after decanting the lower layer, using spectrophotometer. Mucus amount were calculated using standard curves of alcian Blue (Fig. 1) [20]. And the fitted equation for the linear calibration curve was Y = 0.119 x+0.00 where represents the dependent variable (absorbance) and X stands for the independent variable (concentration of Alcian blue in microgram/ml). Thus, the absorbance measured in the different treatment groups was used to get the corresponding concentration of Alcian blue which was complexed with the mucin on the wall of the glandular portion of the stomach.
| (2) |
Fig. 1.
Calibration curve for alcian blue 8GX in aqueous solution. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.10. Data analysis
Results are expressed as mean ± standard error of the mean (SEM). The experimental results were analyzed using the Statistical Package for Social Sciences (SPSS), version 21. Statistical significance was determined by one way analysis of variance (ANOVA) followed by Turkey post Hoc test where P-value of less than 0.05 was considered statistically significant. Coefficient of determination (), using linear regression analysis, was determined where appropriate. Thedata were then presented using tables.
3. Results
3.1. Acute toxicity test
Acute toxicity study of both 80% ME and AQ extracts of the leaf of U.simensis revealed neither visible signs of behavioral (alertness, restlessness, irritability, and fearfulness), neurological, (spontaneous activity, reactivity, touch response, pain response, and gait) autonomic (defecation, and urination), or physical changes such as lacrimation, loss of appetite, tremors, hair erection, salivation, diarrhea, and for morbidity or mortality in rats nor death during the 14 days observation period following oral administration of a single dose of 2000 mg/kg. In addition, neither food nor water intake was found to be reduced during the two weeks period follow-up.
3.2. Effects of the leaf extract on pylorus ligation-induced ulcer
As shown in Table 1, both extracts at 100 mg/kg dose failed to show noticeable rise in pH of gastric secretion and reduction in total acidity compared to the negative control whereas AQ200 (p = 0.03) and ME200 (p = 0.02), AQ400 (P = 0.01) and ME400 and CI 100 mg/kg (p = 0.001) were exhibited significant rise in pH and reduction in total acidity. All doses of the extract were exhibited significant reduction in volume of gastric secretion (p < 0.001) compared to negative control whereas AQ 200 (p = 0.04) & ME 200 (p = 0.03), AQ 400, ME 400 and CI 100 (p = 0.001) showed significant reduction in the volume of gastric secretion compared to AQ100 and ME100 . All the above texts states about Table 1 (volume of gastric secretion) but Table 2 is different (about Mucin content & ulcer index).
Table 1.
The effect of aqueous and 80% methanol extracts of U. simensis on gastric pH, total acidity, volume, and percentage protection in pylorus ligation-induced ulcer.
| Groups PH | Ph | Total Acidity (mEq/l/100 g) | Volume (ml) | UI (mean ± SEM) | % protection |
|---|---|---|---|---|---|
| NC | 1.79 ± 0.14 | 83.16 ± 2.28 | 8.7 ± 0.25 | 23.5 ± 1.01 | |
| AQ100 | 2.56 ± 0.16 | 64.91 ± 3.05 | 6.5 ± 0.31 a3 | 15.58 ± 0.71 | 33.7 |
| AQ200 | 3.41 ± 0.20 a1 | 47.25 ± 4.56 a3 | 4.6 ± 0.45 a3b1c1 | 11.16 ± 2.25 a1 | 52.5 |
| AQ400 | 3.64 ± 0.32 a2 | 44.38 ± 6.32 a3 | 3.9 ± 0.57 a3b3c2 | 8.16 ± 2.59 a3 | 65.3 |
| M100 | 2.79 ± 0.15 | 64.25 ± 2.44 | 6.3 ± 0.16 a2 | 15.33 ± 0.81 | 34.8 |
| M200 | 3.50 ± 0.28 a1 | 45.4 ± 6.43 a3b1c1 | 4.4 ± 0.60 a3b1c1 | 10.5 ± 3.30 a2 | 55.3 |
| M400 | 3.80 ± 0.29 a3 | 43.8 ± 6.79 a3b1c1 | 3.8 ± 0.49 a3b3c3 | 7.75 ± 3.40 a3 | 67.0 |
| CI100 | 3.88 ± 0.31 a3 | 42.16 ± 6.29a3b1c | 3.5 ± 0.29 a3b3c3 | 7.16 ± 3.22 a3 | 69.5 |
AQ = Aqueous extract, ME = 80% methanol extract, Cimetidine = CI.
Data were expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey test; a compared to negative control; b compared to Aqueous 100 mg/kg c compared to methanol 100 mg/kg; 1: p < 0.05, 2: p < 0.01, 3: p < 0.001.
Table 2.
Effect of the aqueous and 80% methanol extracts of U. simensis on mucin content (μg/g), % of increment in mucin, Ulcer index and % of protection Indomethacin induced ulcer.
| Extract/control | Mucin content (μg/g) | % of increment in mucin | UI | % of protection |
|---|---|---|---|---|
| DW | 32.68 ± 0.52 | 28 ± 1.97 | ||
| AQ 100 | 41.16 ± 1.37a1 | 20.6 | 18.91 ± 0.89a1 | 32.5 |
| AQ 200 | 67.9 ± 0.69 a3b3c3 | 51.8 | 14.83 ± 0.9 a3 | 47 |
| AQ 400 | 87.23 ± 2.11 a3b3c3 | 62.5 | 11.08 ± 3.18 a3 | 60.4 |
| ME 100 | 45.38 ± 1.18 a3 | 28 | 18.41 ± 0.59 a1 | 34.25 |
| ME 200 | 73.46 ± 1.07a3b3c3 | 55.5 | 13.41 ± 0.3a3 | 52.1 |
| ME 400 | 90.94 ± 1.31 a3b3c3 | 64 | 10.58 ± 3.41a3 | 62.2 |
| C100 mg/kg | 93.87 ± 1.58 a3b3c3 | 65 | 2 9.75 ± 3.14a3 | 65.1 |
AQ = Aqueous extract, ME = 80% methanol extract, Cimetidine = CI.
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey test; a compared to negative control; b compared to Aqueous 100 mg/kg c compared to methanol 100 mg/kg; 1: p < 0.05, 2: p < 0.01, 3: p < 0.001.
Concerning to ulcer index and percentage of protection, the AQ200 (p = 0.03), ME200 (p = 0.01), AQ 400 and CI 100 (p = 0.001) showed significant reduction in ulcer index compared to control group. The protection index were 33.7% (AQ100), 52.5% (AQ200), 65.3% (AQ400), 34.8% (ME100), 55.3% (ME200), 67% (ME400) and 69.5%(CI 100) (Table 1).
3.3. Effects of the leaf extract on indomethacin induced ulcer
Indomethacin produced massive gastric ulcers in all rats, ulcers were mostly superficial and few were penetrating. Ulcer index (UI) was 28 ± 1.97 in ulcerative rat. Pretreatment of rats with AQ ( =0.999, p = 0.001) and ME ( =0.98, p = 0.001) extracts of U. simensis exhibited dose dependent reduction in ulcer index. AQ extract at different doses exhibited reduction in UI such as 100 (18.91 ± 0.89), 200 (14.83 ± 0.9), 400 (11.08 ± 3.18) and ME extracts were exhibited an UI of ME 100 (18.41 ± 0.59), ME 200 (13.41 ± 0.3) and ME 400 (10.58 ± 3.41) and CI 100 produced an UI 9.75 ± 3.14. Percentage of protection for different doses of extracts were AQ 100(32.5%), AQ 200(47%), AQ 400(60.4%), ME100 (34.25%), ME 200(52.1%), ME400 (62.2%) and CI 100(65.1%).
All doses of extract produced a statistically significant (P < 0.01) alteration in mucin content compared to negative control groups and the percentage of increment in mucin content were 20.6 (AQ100), 51.8 (AQ200), 62.5(AQ400), 28(ME100), 55.5(ME200) and 64 (ME400) and the standard drug CI100 (65.2) was comparable to the maximal dose (400 mg/kg) of both extracts and there was a dose-dependent increment in gastric wall mucus in AQ ( =0.93, p = 0.001) and ME ( =0.92; p = 0.001).
In five days pretreatment study, there were remarkable changes in the gastric parameters of extract-treated group compared to ulcerated control. Significant reduction in ulcer index and increment in mucin content were exhibited in both AQ and ME extracts as well as the standard drug CI (P = 0.001). The percentage of increment in mucin content for AQ 200 and ME 200 were 74.8% and 75.1% respectively and it was comparable with the standard CI 100(75.9%).
3.4. Effects of the leaf extract on ethanol-induced gastric ulcer
Oral administration of ethanol significantly decreased gastric wall mucus in the control rats compared to different doses of extract (P = 0.001).All doses of extracts showed significant increment of gastric wall mucus from 46.66 ± 0.96 to 75.87 ± 1.52 and the standard drug CI100showed mucus content of 77.23 ± 0.64 μg alcian blue/g wet stomach. Dose dependent increment in mucin content was found with both AQ (R2 = 1, P = 0.001) and ME (R2 = 1, P = 0.001) extracts.
Administration of 90% ethanol (1 ml/200 g) produced superficial or deep erosions and bleeding as examined in ulcerative rats, at which the ulcer index was 34.33 ± 1.33, whereas pretreatment with extracts produced reduction in ulcer index ranges from 24.91 ± 1.47 with AQ100 to 14.16 ± 4.5 with ME 400. Dose-dependent ulcer inhibition was examined with AQ ( = 0.968, P = 0.001) and ME ( = 0.916, P = 0.001) extracts against ethanol-induced ulcers in rat. The percentage of reduction in ulcer index was found to be 19.6% (p = 0.001), 38.1% (p = 0.001), and 50% (p = 0.001) at doses of 100 mg/kg, 200 mg/kg, 400 mg/kg of AQ extract, respectively while ME extracts produced significant reduction in ulcer index (P = 0.001) at doses of 100 mg/kg (20.8%), 200 mg/kg (38.8%) and 400 mg/kg (50.6%) doses.
Five days repeated dose administration revealed that mucin contents were increased from 37.51 ± 0.89 for negative control to AQ 200 (101.95 ± 1.64), ME 200 (106.85 ± 1.87) and CI 100 (108 ± 2.11) and UI was reduced from 34.33 ± 1.33 (negative control) to 9.5 ± 3.01(AQ200), 9.33 ± 2.95 (ME 200) and 9.16 ± 2.9(CI 100).
3.5. Phytochemical screening
The qualitative photochemical investigations of AQ extracts of leaf of U. simensis were carried out using standard tests. As shown in Table 6, alkaloids, terpenioids, tannins, saponins, phenols, and flavonoids were present in AQ extracts of U.simensis. Phytochemical screening is on Table 5 rather than Table 6 (about 5-days treatment outcome).
Table 6.
Preliminary phytochemical screening of aqueous extracts of U. simensis leaf.
| Chemical constituent | Aqueous extract (AQ) |
|---|---|
| Alkaloid | + |
| Flavonoids | + |
| Tannins | + |
| Phenols | + |
| Saponins | + |
| Steroids | – |
| Terpenioids | + |
| Anthroquinones | – |
Symbol indicates (+) present and (−) absence.
Table 5.
Effect of repeated (5days) treatment aqueous and 80% methanol extracts of U. simensis in ethanol induced ulcer model.
| Mucin content | % of increment in mucin | Ulcer Index (UI) | % of protection | |
|---|---|---|---|---|
| Control | 37.51 ± 0.89 | 34.33 ± 1.33 | ||
| AQ 200 | 101.95 ± 1.64 a3b2c2 | 63.2 | 9.5 ± 3.01 a3b2b2 | 72.3 |
| ME 200 | 106.85 ± 1.87a3b3c2 | 64.9 | 9.33 ± 2.95 a3b2b2 | 72.8 |
| CI100 mg/kg | 108 ± 2.11 a3b2c2 | 65.3 | 9.16 ± 2.90 a3b2b2 | 73.3 |
AQ = Aqueous extract, ME = 80% methanol extract, Cimetidine = CI.
Data are expressed as mean ± SEM (n = 6); analysis was performed with One-Way ANOVA followed by Tukey test; a compared to negative control b compared with single dose AQ200 and c compared with single dose ME 200 3: p < 0.001.
3.6. Limitation of the study
This study is conducted in animal models, which may not transitional to human subjects. Even though, the study was conducted on different models to assess the designed pharmacological activity, the study has its own shortcoming because of its letdown to explain the precise mechanism (s) how the extract exhibited the tested biological activity.
4. Discussion
Medicinal plants, although assumed to be safe, are potentially toxic which necessitates investigation of their safety status [22]. It is therefore important to properly evaluate their safety and efficacy profile of plants that are under use in traditional medicines. Acute toxicity study revealed that both aqueous and methanol extracts at the dose of 2000 mg/kg didn't exhibit any sign of toxicity or mortality up to 14 days and this finding suggested that the experimental plant had a wider safety margin and LD50 value greater than 2000 mg/kg in rats since any test substance that is not toxic at 2000 mg/kg is considered relatively safe [23].
Pylorus ligation is an important procedure that shows the possible changes of the parameters for gastric content e.g. volume of gastric juice, total acidity and pH [24]. Ulcers caused by pyloric ligation are due to increased accumulation of gastric acid and pepsin, leading to the auto digestion of gastric mucosa [25]. Inhibition of elevated gastric acidity is one of the important protective factors, since overwhelming of the mucosal defense mechanisms by acid level leads to ulcer formation [26]. This model was important to assess the potential parameter important to measure an overall anti gastric ulcer activity of plant extracts such as pH, total acidity, volume, ulcer index and percentage of protection, in which all of them were examined in this study. In this model, AQ100 andME100 failed to show significant rise in pH of gastric juice and reduction in total acidity (P > 0.05) compared to the negative control, while the AQ200 and ME200 exhibit significant rise in pH and reduction of total acidity and the maximal dose of extracts (AQ400 & ME400) exhibited better neutralizing capacity (P < 0.001) and is comparable with standard CI100.This indicates that the low dose of the extract is in adequate to neutralize the acid compared to medium and maximal dose.
Both AQ ( = 0.998, P < 0.001) and ME ( = 0.976, P < 0.001) extracts revealed dose dependent reduction in ulcer index. These results suggest that the extracts interfered with digestive effect of accumulated gastric juice and also possessed reduction of both gastric acidity and gastric secretory volume which could be partly attributed to its flavonoid component which decrease histamine secretion from mast cells by inhibition of histidine decarboxylase and blocked acid formation in parietal cells in response to histamine, H+/K + ATPase, the gastric proton pump are also inhibited by flavonoids [27]. Alkaloidal constituents present in both types of extract might be responsible to anti-secretory effect through H2-receptor antagonism and anti-cholinergic action [28].
Indomethacin is a commonly used type of NSAIDs in animal experiment to induce gastric ulcer, dose dependent and repeated dose study were employed to examine gastric cytoprotective effect of extracts and the results of single dose study showed doses dependent decrease in ulcer index and increase in content of mucin in both extracts (AQ and ME p < 0.001) Compared to ulcerated group.
The repeated-dose study showed a significant reduction in ulcer index (P < 0.001) and increments in mucin contents (P < 0.001) compared to single dose of AQ200 andME200. Percentage of ulcer inhibition for repeated AQ 200 (72.3%) and ME 200 (72.8%) exhibited significant reduction compared to the single-dose study for AQ200 (46.6%) and ME200 (51.2%). This suggests that the cumulative ulcer healing effect of the extract is better than that of the single dose. This finding signifies that the extract possesses a gastro protective effect in both dose dependent and time dependent manners.
Flavonoids, tannins, terpenoids and saponins were responsible for gastro protective effects of plant extracts [28]. Flavonoids are effective to stimulate PGE2 production in gastric mucosal cells. Exogenous prostaglandins particularly E series protects gastrointestinal (GI) mucosa from the damage induced by a wide range of GI irritants and evidence suggested that endogenous prostaglandins are important in maintaining gastro duodenal integrity [29]. Saponins and triterpenoids have been reported to have antiulcer activity in several experimental models through enhancingthe formation of protective mucus on the gastric mucosa and also protect the mucosa from gastric acid by selectively inhibiting prostaglandin [30]. Tannins also possessedantiulcer effectsby it’s a stringent property and vasoconstriction effects [31]. Due to precipitation of micro proteins on the ulcer site, a protective layer was formed which hinders gut secretions and protects the mucosa from toxins and other irritants while alkaloids have been reported to have antiulcer properties although the mechanism is not yet known. However, it was suggested that the possible antiulcer effect of alkaloides might be viathe activation of cyclooxygenase enzyme, which subsequently stimulates PG synthesis and increasing bicarbonate level in the GI lumen [28].
Ethanol is readily penetrates the gastric mucosa due to its ability to solubilize the protective mucous and expose the mucosa to the proteolytic and hydrolytic actions of hydrochloric acid and pepsin [5] and also cause profound micro-vascular changes with strong vasoconstriction accompanied by arteriolar dilatation responsible for engorgement of mucosal capillaries [8]. The pathogenesis of mucosal damage in the stomach is associated with reduction of bicarbonates secretion and generation of ROS that seem to play a vital role in the formation of lipid peroxides, accompanied by impairment of anti-oxidative enzyme activity of cells [32].
In ethanol induced ulcer model, both extracts (AQ and ME) at dose of200 mg/kg (p < 0.01) and 400 mg/kg (p < 0.001) possessed significant inhibition of ulcer index compared toulcerative control.This suggestedthat protective effect of extracts in ethanol induced ulceration needs Increment in dose since ethanol produced more ulceration than other two models. The repeated dose (5 days) study for ethanol-induced ulcer model showed a significant reduction in ulcer index (P < 0.001) and increment in mucin content compared tosingle dose study. These phytocompounds (flavonoids, tannins, Polyphenols and terpenoids) had ability to stimulate mucus, bicarbonate and prostaglandin secretion and neutralize the deteriorating effects of reactive oxidants in gastrointestinal lumen [33].
5. Conclusion
This study revealed that both aqueous and 80% methanol leaf extracts of U.simensis possessed promising anti-gastric ulcer activity in pylorus ligation, indomethacine and ethanol induced gastric ulcer models. The antiulcer activities may be related to anti-secretory as well as cytoprotective activities of one or more of phytoconstituents presented in U.simensis. These findings provide a scientific support for folkloric use of U.simensis leaves as treatment of gastric ulcer and this plant might b apotential source for discovery of novel anti-gastric ulcer agent.
CRediT authorship contribution statement
Ousman Ahmed: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Teshome Nedi: Supervision, Validation, Visualization, Writing – review & editing. Ebrahim M. Yimer: Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Digambar Ambikar for his constructive guidance on experimental procedure and Woldia University for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2022.100172.
Contributor Information
Ousman Ahmed, Email: ousmanjena@gmail.com.
Teshome Nedi, Email: teshome.nedi@aau.edu.et.
Ebrahim M. Yimer, Email: ebrahim99muhammed@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zatorski H. Pathophysiology and risk factors in peptic ulcer disease. Introd Gastrointest Dis. 2017;2:7–20. Springer. [Google Scholar]
- 2.Adinortey M.B., Ansah C., Galyuon I. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013:1–12. 2013. [Google Scholar]
- 3.Dongo A.E., Uhunmwagho O., Kesieme E.B. A five-year review of perforated peptic ulcer disease in irrua, Nigeria. Int Sch Res Not. 2017;(10):1155. doi: 10.1155/2017/8375398. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardman J., Limbird L. McGraw-Hill; New York: 2001. Goodman and gilman's the pharmacological basis of therapeutics. [Google Scholar]
- 5.Srikanth J., Muralidharan P. Antiulcer activity of Morinda citrifolia Linn fruit extract. J Sci Res. 2009;1(2):345–352. [Google Scholar]
- 6.Kamboj. harbal. current scince. 2000;78 [Google Scholar]
- 7.Lakshmi V., Singh N., Shrivastva S., Mishra S., Dharmani P., Mishra V., et al. Gedunin and photogedunin of Xylocarpus granatum show significant anti-secretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine. 2010;17(8–9):569–574. doi: 10.1016/j.phymed.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Ulucan A. Etiopathogenesis of peptic ulcers and prostaglandin relationship. Van Tıp Dergisi. 2020;27(2):238–245. [Google Scholar]
- 9.Gülçin I., Küfrevioǧlu İ., Oktay M., Büyükokuroǧlu M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90(2):205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Wubetu Ma T., Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern Ethiopia. BMC Res Notes. 2017;10(1):157. doi: 10.1186/s13104-017-2482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molla A.E., Asfaw Z. Ethnobotanical study of traditional medicinal plants in and around fiche district, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. [Google Scholar]
- 12.Chekole G., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi: 10.1186/1746-4269-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsegaye W., Urga K., Asres K. Antidiabetic activity of samma (Urtica simensis hochst. Ex. A. Rich.) in streptozotocin-induced diabetic mice. Ethiop Pharmaceut J. 2008;27:75–82. [Google Scholar]
- 14.Kassa F. Addis Ababa University Addis Ababa; Ethiopia: 2016. Evaluation of the antimicrobial activity of 80% methanol extract and solvent fractions of the leaves of Urtica simensis Hochst. ex. A. Rich.(Urticaceae) [Google Scholar]
- 15.Oecd T.N. 425: acute oral toxicity: up-and-down procedure. OECD Guidelines for the Testing of Chemicals, Section. 2008;4:1–27. [Google Scholar]
- 16.Prasanth reddy V., Sudheshna G., Afsar S., Sai saran S., Nelson kumar C., Raja ram C., et al. Evaluation of anti-ulcer activity of Citrullus colocynthis fruit against pylorus ligation induced ulcers in male wistar rats. Int J Pharm Pharmaceut Sci. 2012;4(2):446–451. [Google Scholar]
- 17.Yesiladaa E., Gurbuz I. Evaluation of the antiulcerogenic activity profile of a flavonol diglucoside from Equisetum palustre L. J Ethnopharmacol. 2010;131(1):17–21. doi: 10.1016/j.jep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Abebaw M., Mishra B., Gelayee D. Evaluation of anti-ulcer activity of the leaf extract of Osyris quadripartita Decne. (Santalaceae) in rats. J Exp Pharmacol. 2017;9:1–11. doi: 10.2147/JEP.S125383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayakumar M.E., Ojha M.B., Rao Ch S.K., Rawat V., Antiulcer A.K. Activity of hydroalchol extract of momordica dioica roxb. Fruit. Indian J Pharmaceut Sci. 2011;73(5):572–577. doi: 10.4103/0250-474X.99018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baggio C.H., Freitas C.S., Otofuji Gde M., Cipriani T.R., Souza L.M., Sassaki G.L., et al. Flavonoid-rich fraction of Maytenus ilicifolia Mart. ex. Reiss protects the gastric mucosa of rodents through inhibition of both H+,K+ -ATPase activity and formation of nitric oxide. J Ethnopharmacol. 2007;113(3):433–440. doi: 10.1016/j.jep.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Ifeoma O., Oluwakanyinsola S. intechopen; 2013. Screening of herbal medicines for potential toxicities. [Google Scholar]
- 23.Erhirhie E.O., Ihekwereme C.P., Ilodigwe E.E. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdiscipl Toxicol. 2018;11(1):5. doi: 10.2478/intox-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakshmi V., Singh N., Shrivastva S., Mishra S.K., Dharmani P., Mishra V., et al. Gedunin and photogedunin of Xylocarpus granatum show significant anti-secretory effects and protect the gastric mucosa of peptic ulcer in rats. Phytomedicine : Int J Phytother Phytopharmacol. 2010;17(8–9):569–574. doi: 10.1016/j.phymed.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Eswaran M.B., Surendran S., Vijayakumar M., Ojha S.K., Rawat A.K., Rao Ch V. Gastroprotective activity of Cinnamomum tamala leaves on experimental gastric ulcers in rats. J Ethnopharmacol. 2010;128(2):537–540. doi: 10.1016/j.jep.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Arawwawala L.D., Thabrew M.I., Arambewela L.S. Gastroprotective activity of Trichosanthes cucumerina in rats. J Ethnopharmacol. 2010;127(3):750–754. doi: 10.1016/j.jep.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Borrelli F., Izzo A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14 doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Mossadic Khandker, Faisal The anti-ulcerogenic effect of an alkaloidal fraction from mikania cordata on diclofenac sodium-induced gastrointestinal lesions in rats. J Pharmacol. 2000;52:1157–1162. doi: 10.1211/0022357001774930. [DOI] [PubMed] [Google Scholar]
- 29.Okokon J.E., Nwafor P.A. Antiulcer and anticonvulsant activity of Croton zambesicus. Pak J Pharm Sci. 2009;22(4):384–390. [PubMed] [Google Scholar]
- 30.Sharifi-Rad M., Fokou P.V.T., Sharopov F., Martorell M., Ademiluyi A.O., Rajkovic J., et al. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23(7):1751. doi: 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesus NZTd, Lima G., Gomes I.F., Filho J.M.B. Tannins, peptic ulcers and related mechanisms. Int J Mol Sci. 2012;13:3203–3228. doi: 10.3390/ijms13033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzmán-Gómez O., García-Rodríguez R.V., Quevedo-Corona L., Pérez-Pastén-Borja R., Rivero-Ramírez N.L., Ríos-Castro E., et al. Amelioration of ethanol-induced gastric ulcers in rats pretreated with phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients. 2018;10(6):763. doi: 10.3390/nu10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga RSaIS. Total antioxidant power in some species of Labiatae (Adaptation of FRAP method) Curr Topics Biophys. 2000;24(2):219–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.