Abstract
Adverse childhood experiences (ACEs) increase risk for depression at subsequent ages and have been linked to accelerated biological aging. We hypothesize that accelerated epigenetic aging may partially mediate the link between ACEs and depression. This study examines 3 three second-generation epigenetic aging measures (viz., GrimAge, PhenoAge, and DunedinPoAm38) as mediators of the link between ACEs and depressive symptoms in older adulthood. We utilize structural equation modeling to assess mediation in the Health and Retirement Study (N = 2672). Experiencing ACEs is significantly associated with an older GrimAge and a faster pace of aging via the DunedinPoAm38. Having an older GrimAge and faster DunedinPoAm38 pace of aging were also significantly associated with more depressive symptoms. PhenoAge was not significantly associated with depressive symptoms and was only associated with experiencing three ACEs. These associations were reduced by socioeconomic and lifestyle factors, including obesity and substance use. GrimAge explained between 9 and 14% of the association between ACEs and adult depressive symptoms, and DunedinPoAm38 explained between 2 and 7% of the association between ACEs and adult depressive symptoms. Findings indicate accelerated aging, as measured by GrimAge and DunedinPoAm38, is associated with ACEs and with depressive symptoms in older Americans. Findings also show these epigenetic aging measures mediate a portion of the association between ACEs and adult depressive symptoms. Epigenetic aging may represent a physiological mechanism underlying the link between early life adversity and adult depression. Weight maintenance and substance use are potentially important areas for intervention.
Keywords: Ageing, Depression, Adverse childhood events, Epigenetic aging
Abbreviations: ACEs, Adverse childhood events
Highlights
-
•
Accelerated aging (GrimAge and DunedinPoAm38) is associated with ACEs and with depressive symptoms in older Americans.
-
•
These associations were reduced by socioeconomic and lifestyle factors, including obesity and substance use.
-
•
GrimAge explained between 9 and 14% of the association between ACEs and adult depressive symptoms.
-
•
DunedinPoAm38 explained between 2 and 7% of the association between ACEs and adult depressive symptoms.
There is a wealth of evidence adverse childhood experiences (ACEs) have serious implications for mental health across the lifespan (Centers for Disease Contr, 2019). A large body of research investigating the “long arm of childhood” has pointed to a significant role of early life experiences raising lifetime risk for mood and anxiety disorders, as well as earlier onset of age related diseases (Kalmakis & Chandler, 2015). Experiencing more ACEs increases risk of major depressive disorder and depressive symptoms in adulthood (Merrick et al., 2017). This is important, as depression was recently shown to be the leading cause of disability worldwide (Friedrich, 2017), the estimated economic burden of adults with major depressive disorder in the US is $326.2 billion (Greenberg et al., 2021), and mental wellbeing is important for later life health (Moffitt & Caspi, 2019; Richmond-Rakerd et al., 2021). Emerging evidence suggests biological aging may be an important mediator linking ACEs and depression (Entringer & Epel, 2020; Mitchell et al., 2016; Sumner et al., 2019). Epigenetic aging—a methylation-based measure of aging—is thought to begin early in life and lead to health consequences later in life.
Accumulating evidence suggests exposure to ACEs accelerates aging, setting one on a trajectory for early onset of aging related deterioration of function and multi-morbidities, thus contributing to health disparities across the life course (Belsky et al., 2017), and potentially risk for psychopathology (Wolkowitz et al., 2008), although data supporting this link is limited (Epel & Prather, 2018). In this model, early social stress alters cellular processes (e.g., metabolic activity, cellular stress, damage accumulation) that are associated with epigenetic age acceleration (Miller et al., 2011) and contribute to elevated systemic inflammation, a key regulator of mood and proposed pathway to depression (Miller et al., 2009; Raison et al., 2006; Slavich & Irwin, 2014). There is limited evidence showing epigenetic aging may mediate the link between childhood adversity and depressive symptoms (Sumner et al., 2019). Additionally, lifestyle factors, including socioeconomic factors (e.g., education, wealth) and health behaviors (e.g., smoking, drinking), have been found to be associated with epigenetic aging (Oblak et al., 2021) and ACEs (Duffy et al., 2018). Thus, socioeconomic and lifestyle factors may explain some of the link between ACEs and accelerated epigenetic aging.
Past research has shown epigenetic age is accelerated in people with major depressive disorder (Ekaterina et al., 2021), depressive symptoms (Sumner et al., 2019), and PTSD (Yang et al., 2020); however, the role of early adversity in risk for psychiatric symptoms and the mechanisms that drive this are less clear. Epigenetic clocks summarize changes in DNAm-based markers associated with age and health outcomes common at older ages (Raffington et al., 2021). These clocks represent sets of DNAm sites that have been found to be differentially associated with chronological age and/or age-related biomarkers and diseases (Horvath & Raj, 2018). The first-generation clocks—HorvathAge and HannumAge—were trained to predict only chronological age and show relatively weak associations with clinical measures of physiological dysregulation and mortality (Horvath & Raj, 2018). In contrast, second generation clocks (e.g.., GrimAge, PhenoAge), trained on age and age related morbidity/mortality-relevant biomarkers, and pace of aging measures (e.g., DunedinPoAm38), trained on change in age-relevant biomarkers, have been shown to be associated with chronic disease morbidity and mortality in adults (Fransquet et al., 2019).
Past research on the association between childhood adversity and epigenetic aging measures has been inconsistent. Past research has reported both significant (Colich et al., 2020; Jovanovic et al., 2017; Lawn et al., 2018; Marini et al., 2020) and null (Austin et al., 2018; Fiorito et al., 2017; Simons et al., 2016; Verhoeven et al., 2018) results using the first generation clocks. Research using the second generation clocks has been more consistently supportive (Belsky et al., 2020; George et al., 2021; Hamlat et al., 2021); however, prior research using the Health and Retirement Study suggests childhood adversity only accounts for a small proportion of PhenoAge (Liu et al., 2019). Past investigations have been limited because they typically investigate only one epigenetic aging measure in a given analysis. These measures were trained in different populations, using different aging criteria (e.g., biomarkers, chronological age), and/or in different tissues. Though they theoretically all measure aging (or pace of aging), there is very little overlap in the probes utilized. Though they tend to agree on direction of effect, they vary greatly in magnitude of association with psychiatric conditions; therefore, some scholars have suggested the epigenetic aging measures may be capturing different elements of the aging process (Oblak et al., 2021). Thus, research comparing multiple clocks is needed to identify which best characterize the biological mechanisms underlying this association. This research could help clarify the complex biological processes underlying psychiatric conditions (Oblak et al., 2021).
To our knowledge, only one study has directly tested epigenetic aging as a mediator of the link between childhood adversity and depressive symptoms (Sumner et al., 2019). This study was limited to measures of epigenetic aging using first generation clocks and was conducted in children. The current study builds on this past research by utilizing second generation clocks and pace of aging in a nationally representative sample of older Americans to test the hypothesis that early life adversity is related to accelerated epigenetic aging and test whether epigenetic aging mediates the effect of ACEs on depressive symptoms. This study advances this past research by 1) examining the association between ACEs and three validated epigenetic aging measures that have been shown to be predictive of a variety of age-related health outcomes in a nationally representative sample of older adults in the US and 2) assessing epigenetic aging as a mediator of the association between ACEs and depressive symptoms. To our knowledge, this is the first study examining either of these issues in a nationally representative sample of older US adults.
1. Methods and materials
1.1. Sample
We utilize data from the DNA methylation subsample from the Health and Retirement Study (HRS) 2016 Venous Blood Study (N = 4018) (Health & Retirement Study, 2021). Detailed methods for this sample are published elsewhere (Crimmins et al., 2017). This sample was designed to be representative of the U.S. population when weighted. Items used to construct ACEs scores were assessed as part of the psychosocial leave behind questionnaire at various waves and the life history surveys in 2015 and 2017. Depressive symptoms were assessed in the core survey in 2016 and 2018. About 1000 participants did not participate in the life history mail surveys and about 550 did not participate in the psychosocial leave behind questionnaire from 2006 to 2012. 2672 participants were included in the current study due to missingness in independent variables described below.
1.2. Measures
Epigenetic aging. The epigenetic aging measures were developed using different tissues, were trained on different outcomes, and vary in how predictive they are of certain outcomes (Crimmins et al., 2021). GrimAge is trained on DNAm surrogates of 7 plasma proteins and smoking pack years in multiple tissues (Lu et al., 2019), DunedinPoAm38 was trained on changes in a variety of health-related biomarkers and is meant to capture the current pace of epigenetic aging (Belsky et al., 2020), and PhenoAge was developed to estimate mortality risk using 9 markers of tissue and immune function and age in whole blood (Levine et al., 2018). GrimAge and PhenoAge are expressed in years of epigenetic age. Because DunedinPoAm38 is designed to assess pace of aging, it is expressed in years of epigenetic aging per chronological year. We use the epigenetic aging measures derived from the 2016 HRS Venous Blood Study which are available as restricted health data at https://hrsdata.isr.umich.edu/data-products/epigenetic-clocks. More detailed information about the epigenetic aging measures is available elsewhere (Crimmins et al., 2020).
ACEs. An ACEs scale was constructed using 8 items that were included in the core survey and life history surveys. Items were selected that reflect the ACEs scale developed by the Centers for Disease Control and Prevention and childhood socioeconomic status (CDC, 2019). Specifically, participants reported on parental education (both parents < high school or one parent < high school if only one parent was reported), parental physical abuse (were you ever physically abused by either of your parents?), and parental alcohol and drug use (did either of your parents drink or use drugs so often that it caused problems in the family?) before age 18, as well as childhood separation from either parent (were you ever separated from your mother or father for 6 months or longer?), death of a parent (did one or both parents die?), living in a children's home or orphanage (did you ever live in a children's home or orphanage?), childhood poverty (would you say your family during that time was pretty well off financially, about average, or poor? poor = 1), parental separation or divorce (did your biological or adoptive parents separate or divorce?) before age 16. To address item nonresponse, participants were allowed to be missing on one item (coded as 0). Alternative coding schemes produced similar results. Because few participants reported more than 4 ACEs, this measure was top coded at 4 or more ACEs.
Consistent with past research on ACEs and health, this measure of ACEs was associated with greater physical limitations assessed using activities of daily life and instrumental activities of daily life and was associated with a greater chronic illness count after controlling for age, race, and gender (see Table S6; outcome measures were taken from the 2016 RAND data).
Depressive Symptoms were assessed in the 2016 and 2018 core survey using the Center for Epidemiologic Studies Depression Scale (CES-D)(Radloff, 1977). The HRS uses an 8 item subset of the CES-D in which respondents report whether or not they experience 8 depressive symptoms over the past two weeks. This measure has been shown to have similar psychometric properties to the original CES-D (Turvey et al., 1999). 2016 scores were used for participants without 2018 scores.
Socioeconomic Position. Socioeconomic position was assessed using self-reports of education (categorized: 0–11 years, 12 years, 13–15 years, and 16+ years as the reference) and wealth (natural log transformed to account for skewness) using the wealth measure available in the HRS RAND data including all assets less debt (Bugliari et al., 2016).
Lifestyle Factors. We assessed the role of lifestyle factors using self-reported smoking (categorized: current smoker, past smoker, and non-smoker as the reference), BMI (categorized: ≥ 25 to < 30 overweight, ≥ 30 to < 35 obese I, ≥ 35 obese II or morbidly obese, and <25 normal and underweight as the reference), and alcohol use (categorized: 1–4 drinks per day drinking, 5+ drinks per day drinking, and non-drinker as the reference). BMI is an imperfect measure of lifestyle factors, so we estimated the SEMs with lifestyle controls with BMI replaced by physical activity (using the mean of the three physical activity items in HRS in 2016 or 2014 if 2016 data were missing). Results were similar to the original results with a nearly identical pattern of significant results. The sole difference in significance pattern was that GrimAge was not significantly associated with depressive symptoms (this association was significant in the model with BMI). Thus, BMI appears to capture similar variance as this physical activity measure.
Controls. In all models we control for chronological age, race/ethnicity (non-Hispanic white as reference), and gender (male as reference).
1.3. Plan of analysis
We began by estimating three structural equation models (SEMs) regressing each epigenetic clock first on ACEs and controls and regressing depressive symptoms on each epigenetic aging measure and controls. Mediation was tested using a bias corrected bootstrap procedure with 1000 draws. To assess the impact of lifestyle factors on these findings, we next estimated the same models controlling for lifestyle factors. Because all models were fully recursive, model fit statistics were all artificially perfect and therefore uninformative. Epigenetic aging measures and depressive symptoms were standardized in Mplus for the figures to ease comparison across measures using the STANDARDIZE command. Because the epigenetic measures all use meaningful scales, unstandardized results are discussed in the text below. All analyses were conducted in Mplus 8 (Muthén & Muthén, 1998).
We assessed gender differences by comparing models for men and women using the “GROUPING IS” command in Mplus. For each model in the study, we compared a model constraining all paths from ACEs to epigenetic measures and all paths from epigenetic measure to depressive symptoms to be equal for men and women with a model in which all these paths were freed. There was no significant χ2 change across all of these models suggesting that there is not a gender difference in these models.
2. Results
Descriptive Statistics. The weighted sample is 56.6% female and has a median age of 67 years. The weighted sample is 83.4% Non-Hispanic White, 8.4% Non-Hispanic Black, 5.7% Hispanic, and 2.5% Non-Hispanic Other Race. 10.4% of the weighted sample has less than 12 years of education, 31.5% has 12 years of education, 26.5% has 13–15 years of education, and 31.6% has 16+ years of education. The weighted sample had a median wealth of $232,119. 8.9% of the weighted sample are current smokers and 45.0% are former smokers. More than a third of the weighted sample is obese (35.4%), and 54.6% were non-drinkers. Descriptive statistics are shown in Table 1.
Table 1.
Descriptive Statistics.
| Variable | Mean/Proportion | SD | Range | Count |
|---|---|---|---|---|
| ACEs | ||||
| 0 ACEs | 0.34 | 775 | ||
| 1 ACE | 0.28 | 711 | ||
| 2 ACEs | 0.19 | 566 | ||
| 3 ACEs | 0.11 | 357 | ||
| 4+ ACEs | 0.09 | 263 | ||
| Depressive Symptoms | 1.25 | 1.86 | 0–8 | |
| Age | 68.98 | 9.20 | 50–97 | |
| Race/Ethnicity | ||||
| White, not Hispanic | 0.83 | 1978 | ||
| Black, not Hispanic | 0.08 | 378 | ||
| Hispanic | 0.06 | 257 | ||
| Other Race, not Hispanic | 0.03 | 59 | ||
| Gender | ||||
| Male | 0.43 | 1064 | ||
| Female | 0.57 | 1608 | ||
| Education | ||||
| Less than High School | 0.10 | 397 | ||
| High School | 0.32 | 876 | ||
| Some College | 0.27 | 680 | ||
| College or More | 0.32 | 719 | ||
| Wealth | 601,542 | 1,396,212 | −249,750–24,475,000 | |
| Smoker Status | ||||
| Never Smoked | 0.46 | 1216 | ||
| Current Smoker | 0.09 | 252 | ||
| Past Smoker | 0.45 | 1204 | ||
| BMI | ||||
| Under/Normal Weight | 0.27 | 725 | ||
| Overweight | 0.37 | 982 | ||
| Obese 1 | 0.22 | 581 | ||
| Obese 2 | 0.14 | 384 | ||
| Drinking | ||||
| Non-drinker | 0.55 | 1588 | ||
| 1 - 4 Drinks per Day Drinking | 0.43 | 1031 | ||
| 5+ Drinks per Day Drinking | 0.02 | 53 | ||
Note: means and standard deviations are weighted using survey weights provided by HRS. Counts are not weighted.
ACEs and Depressive Symptoms. The total effects of ACEs on depressive symptoms are shown in Table 2, Panel A. Results with epigenetic age and depressive symptoms standardized are shown in the tables and figure to ease comparison across measures, and unstandardized results are discussed in the text. The total effects of 2, 3, and 4 or more ACEs on depressive symptoms were statistically significant in the expected direction. Thus, in total, having 2 or more ACEs was associated with having more depressive symptoms compared to 0 ACEs. People who experienced 2 ACEs were expected to have 0.29 more depressive symptoms, and people who experienced 3 or 4+ ACEs were expected to experience 0.73 and 0.72 more depressive symptoms compared to similar peers who experienced 0 ACEs.
Table 2.
Direct and indirect effects from structrual equation models.
|
Panel A. Total Effect of ACEs on Depressive Symptoms | |||
|---|---|---|---|
| Total Effect | SE | p | |
| 1 ACE | 0.048 | 0.055 | 0.385 |
| 2 ACEs | 0.155 | 0.058 | 0.008 |
| 3 ACEs | 0.393 | 0.112 | <0.001 |
| 4+ ACEs |
0.387 |
0.108 |
<0.001 |
| Panel B. Indirect Effects of ACEs on Depressive Symptoms via GrimAge | |||
| Indirect Effect | 95% CI | ||
| 1 ACE | 0.020 | 0.011–0.035 | |
| 2 ACEs | 0.021 | 0.007–0.039 | |
| 3 ACEs | 0.034 | 0.019–0.058 | |
| 4+ ACEs |
0.051 |
0.023–0.090 |
|
| Panel C. Indirect Effects of ACEs on Depressive Symptoms via DunedinPoAm38 | |||
| Indirect Effect | 95% CI | ||
| 1 ACE | 0.008 | 0.002–0.020 | |
| 2 ACEs | 0.007 | 0.000–0.019 | |
| 3 ACEs | 0.009 | 0.001–0.024 | |
| 4+ ACEs |
0.027 |
0.006–0.058 |
|
| Panel D. Indirect Effects of ACEs on Depressive Symptoms via PhenoAge | |||
| Indirect Effect | 95% CI | ||
| 1 ACE | −0.001 | −0.009 - 0.003 | |
| 2 ACEs | 0.003 | −0.001 - 0.017 | |
| 3 ACEs | 0.007 | −0.001 - 0.024 | |
| 4+ ACEs | 0.002 | −0.003 - 0.011 | |
Note: CI = confidence interval. 95% CIs were estimated using a bias corrected bootstrap procedure with 1000 draws. Epigenetic aging and depressive symptoms, but not ACESs, are standardized using the “STANDARDIZE” command in Mplus.
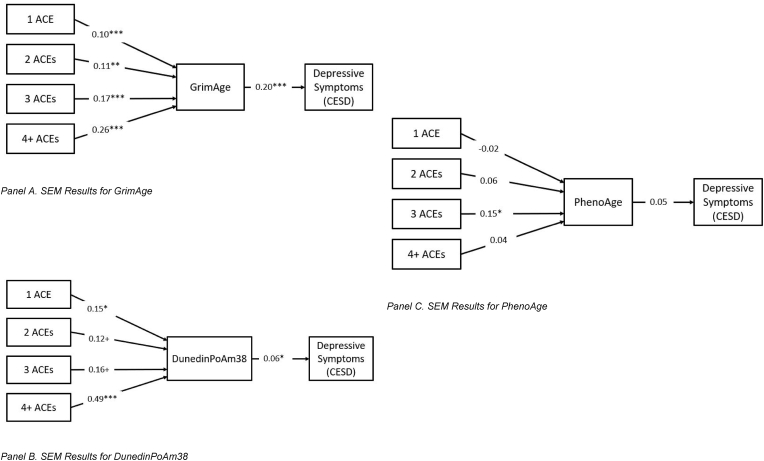
GrimAge SEM Results. SEM results for GrimAge are shown in Fig. 1, Panel A. Overall, having experienced childhood adversity was associated with an older GrimAge. Experiencing 1 or 2 ACEs was associated with having a GrimAge 0.87 and 0.88 years greater compared to those who experienced no ACEs. Having 3 ACEs was associated with a GrimAge 1.47 years older than those who experienced no ACEs. Finally, those exposed to 4 or more ACEs were expected to have a GrimAge 2.18 years older than those with no ACEs.
Fig. 1.
SEM Results. Note: N = 2672; all models are fully recursive, but not all paths are shown for ease of presentation. ACE = adverse childhood experience. Epigenetic aging and depressive symptoms, but not ACESs, are standardized using the “STANDARDIZE” command in Mplus. Unstandardized results are discussed in the text above.*p < .05.
GrimAge was significantly, positively associated with depressive symptoms. Increasing GrimAge was associated with increasing depressive symptoms (b = 0.04, p < .001). Thus, experiencing more ACEs was associated with greater epigenetic aging as measured by GrimAge, and more accelerated aging was associated with more depressive symptoms.
Indirect effects for the GrimAge model are shown in Table 2, Panel B. All four indirect effects that were tested were significant; however, because the total effect of 1 ACE was not significant, we focus on the other three indirect effects. In this model, GrimAge mediated about 14% of the effect of experiencing 2 ACEs on depressive symptoms, about 9% of the effect of 3 ACEs, and about 13% of the effect of 4+ ACEs on depressive symptoms.
DunedinPoAm38 SEM Results. SEM results for DunedinPoAm38 (Fig. 1, Panel B) were largely consistent with findings for GrimAge. People who experienced 1 ACE were expected to have a DunedinPoAm38 pace of aging about a tenth of year per chronological year faster than similar people who experienced no ACEs, and people who experienced 4 or more ACEs were expected to have a DunedinPoAm38 pace of aging about 4 tenths of year per chronological year faster than similar people who experienced no ACEs. The effects of experiencing 2 or 3 ACEs on DunedinPoAm38 were only marginally significant.
A faster pace of aging, as determined using the DunedinPoAm38, was significantly associated with more depressive symptoms. Having a DunedinPoAm38 pace of aging 1 year per chronological year greater than same-age peers was associated with a 1.18 higher score on this measure of depressive symptoms.
Indirect effects for DunedinPoAm38 are shown in Table 2, Panel C. Indirect effects for 1, 3, and 4 or more ACEs were significant; again, because the total effect of 1 ACE was not significant, we focus on the other indirect effects. DunedinPoAm38 appears to mediate about 2% of the effect of 3 ACEs and about 7% of the effect of 4+ ACEs on depressive symptoms.
PhenoAge SEM Results. Results for PhenoAge (Fig. 2, Panel C) were largely unsupportive. Having experienced 3 ACEs was associated with having a PhenoAge 1.46 years greater than similar peers who experienced 0 ACEs, but no other comparisons were significant. Additionally, PhenoAge was not significantly associated with depressive symptoms. Unsurprisingly given the non-significant main effects, there are no significant indirect effects of ACEs on depressive symptoms mediated by PhenoAge (shown in Table 2, Panel D).
Fig. 2.
Associations between ACEs and Epigenetic Aging Measures
Note: N = 2672. Epigenetic aging and depressive symptoms, but not ACESs, are standardized using the “STANDARDIZE” command in Mplus. Unstandardized results are discussed in the text above.
Socioeconomic and Lifestyle Factors. Finally, we estimated the same SEMs including socioeconomic and lifestyle factors as controls to assess how much these factors affect the pathways linking ACEs with epigenetic aging and depressive symptoms. Full results are included in the supplementary material.
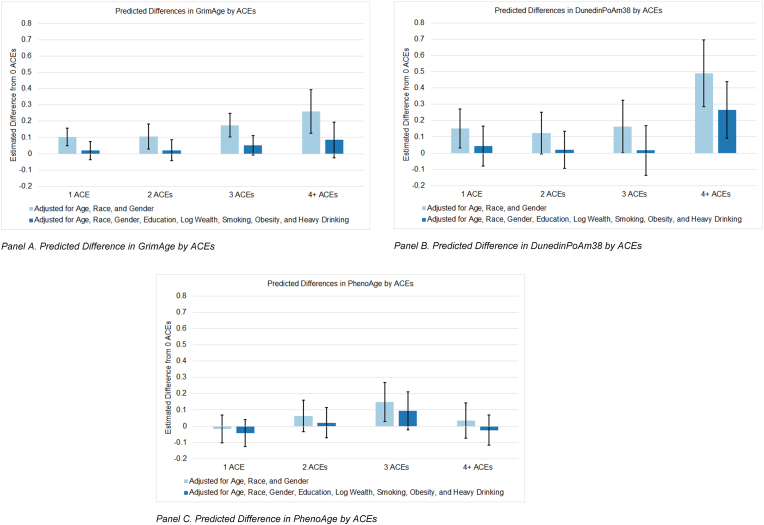
Controlling for socioeconomic and lifestyle factors reduced the effect of ACEs on GrimAge to non-significance compared to the model without socioeconomic and lifestyle factors because of the associations of GrimAge with years of education (0–11 years; b = 0.66, p = .04; 12 years; b = 0.91, p < .001; 13–15 years; b = 0.94, p < .001), wealth (b = −0.96, p = .001), high obesity (BMI ≥35; b = 1.43, p < .001), smoker status (current smoker; b = 6.96, p < .001; past smoker; b = 2.09, p < .001), and heavy alcohol use (5+ drinks per day drinking; b = 1.58, p = .02). Including lifestyle factors reduced the association between GrimAge and depressive symptoms (Table S1; b = 0.02, p = .04). Wealth (b = −0.48, p < .001) was significantly associated with depressive symptoms.
The pace of aging, as measured by DunedinPoAm38, of people who experienced 1, 2, or 3 ACEs were not significantly different from the pace of aging of similar peers who experienced no ACEs when controlling for socioeconomic and lifestyle factors. However, having 4 or more ACEs remained significantly associated with a more accelerated pace of aging compared to no ACEs, even after controlling for socioeconomic and lifestyle factors (b = 0.02, p = .003). Obesity (BMI ≥30 and < 35; b = 0.01, p = .03; BMI ≥35; b = 0.03, p < .001), and smoker status (current smoker; b = 0.12, p < .001; past smoker; b = 0.03, p < .001) were all significantly associated with faster DunedinPoAm38 in this model. For the DunedinPoAm38 model, pace of epigenetic aging was no longer significantly associated with depressive symptoms (b = 0.38, p = .45) after controlling for socioeconomic and lifestyle factors. Wealth (b = −0.50, p < .001) was significantly associated with depressive symptoms in this model.
For the PhenoAge model, the only significant effect of ACEs on PhenoAge (3 ACEs) was reduced to non-significance after including lifestyle factors. Wealth (b = −0.91, p = .03), obesity (BMI ≥35; b = 1.58, p = .002), and heavy alcohol use (5+ drinks per day drinking; b = 2.78, p = .011) were all associated with PhenoAge in this model.
2.1. First-generation clocks
As noted above, we focus on the second-generation clocks in the current study. As a supplemental analysis, we estimated the same models using the main first-generation clocks (viz., HorvathAge and HannumAge, see Fig. S3, Tables S4 and S5). Neither clock was significantly associated with ACEs or depressive symptoms before or after controlling for socioeconomic and lifestyle factors.
3. Discussion
This study builds on and addresses weakness of past research by investigating the association between early life adversity and 3 sthree second-generation DNAm clocks in a representative sample of Americans over age 50 and formally testing whether these clocks mediate the association between ACEs and depressive symptoms. Results indicate exposure to early life adversity is associated with accelerated epigenetic aging, as measured by GrimAge, and a faster pace of aging as measured by the DunedinPoAm38, compared to similar, same-aged peers who experienced no early life adverse events. Moreover, accelerated GrimAge and a faster pace of aging were significantly associated with elevated depressive symptoms. In mediation models, these clocks each mediated a portion of the total effect of ACEs on depressive symptoms. Controlling for socioeconomic and lifestyle factors such as smoking and obesity weakened all of these associations, suggesting these factors may play an important role in this biopsychological process.
This study builds on a large body of past research examining the association between ACEs and mental and physical health, and a few studies linking ACEs to epigenetic age acceleration. Our findings are consistent with past findings that suggest second generation clocks are more consistently associated with ACEs in adults (George et al., 2021), potentially because these clocks were trained to predict phenotypic indicators of accelerated aging and are more consistently associated with age-related health problems (Horvath & Raj, 2018). This study also builds on this past body of research examining the association between ACEs and epigenetic clocks by utilizing a representative sample of US adults over the age of 50. These findings suggest that the association between ACEs and epigenetic aging may be non-linear. That is, experiencing 1 or 4 or more ACEs is associated with DunedinPoAm38 before controlling for lifestyle factors and only with 4 or more ACEs after controlling for lifestyle factors. It is possible that experiencing a very high number of ACEs is particularly damaging and may directly affect aging independent of health behaviors. Further research is needed to assess these findings.
These results suggest epigenetic effects of ACEs may persist into later life. One proposed pathway may be inflammation, as epigenetic aging—and senescent cells in particular—is a source of inflammatory signaling. Steeper inflammatory trajectories have been observed in adults who were exposed to child abuse (Renna et al., 2021). Inflammatory signaling from accelerated epigenetic aging may be a key pathway by which exposure to ACEs affects psychiatric symptoms in adulthood. These paths may be bidirectional, as past research and theory suggests stress, epigenetic aging, psychiatric disorders may mutually reinforce one another (Epel & Prather, 2018). Further research investigating this pathway is needed.
Another potential mechanism linking ACEs to accelerated epigenetic aging is socioeconomic and lifestyle factors. We found the effect of fewer than 4 ACEs was no longer significant and the effect of 4+ ACEs on GrimAge and DunedinPoAm38 was reduced after controlling for socioeconomic and lifestyle effects. Specifically, GrimAge was affected by education, wealth, high obesity, smoking, and alcohol use; whereas, DunedinPoAm38 was consistently affected by BMI and smoking. Thus, a portion of the effects of ACEs on epigenetic aging may be mediated by socioeconomic and lifestyle consequences of childhood adversity. This is consistent with a large body of research linking childhood adversity with BMI (Chu et al., 2018; Pudrovska & Anikputa, 2014; Wickrama et al., 2014), health risk behaviors (Campbell et al., 2016; Duffy et al., 2018; Merrick et al., 2017), and socioeconomic attainment (Font & Maguire-Jack, 2016; Liu et al., 2013; Yang et al., 2017) and research suggesting the effect of ACEs on depressive symptoms is mediated by these socioeconomic and lifestyle factors (Hughes et al., 2017; Jones et al., 2018; Merrick et al., 2017; Wickrama et al., 2014). Additionally, BMI (Visser et al., 1999), health risk behaviors (Shiels et al., 2014), and socioeconomic attainment (Castagné et al., 2020; Chen & Miller, 2013) have all been linked to inflammation, a potential pathway linking ACEs to depressive symptoms (Howren et al., 2009). Thus, these lifestyle factors, especially health behaviors, may be a useful area for intervention.
We also found GrimAge mediates about 8–13% of the association between 2 or more ACEs and depressive symptoms in later life. This finding is consistent with past research that found DNAm age significantly mediated the association between childhood threat exposure and depressive symptoms in children (Sumner et al., 2019). Further research is needed to examine the mechanisms linking ACEs, DNAm age, and psychiatric symptoms across the life course. The association between GrimAge and depressive symptoms was substantially reduced and the association between DunedinPoAm38 was reduced to non-significance after controlling for socioeconomic and lifestyle factors. Obesity and smoking were relevant for both clocks, suggesting interventions targeting these factors may be useful for breaking the link between ACEs and depressive symptoms in later life.
Similar to a past study using the HRS, PhenoAge was not significantly associated with childhood adversity. This may be a result of the ways in which the different clocks were trained. PhenoAge was trained to predict mortality risk using markers of tissue and immune function (albumin, creatinine, serum glucose, CRP, lymphocyte percent, mean (red) cell volume, red cell distribution width, alkaline phosphatase, white blood cell count) (Levine et al., 2018). These indicators may be less sensitive to childhood conditions or may be more affected by recent stress and adversity compared to the indicators used to train GrimAge (DNAm surrogate measures of adrenomedullin, cystatin c, leptin, TIMP-1, pack years, plasminogen activation inhibitor 1 (PAI-1), growth differentiation factor 15 (GDF15), and beta 2 microglobulin (B2M) regressed on time to death)(Levine et al., 2018) and DunedinPoAm38 (rates of change in 18 biomarkers associated with aging) (Belsky et al., 2020). Similarly, past research using the HRS has shown that GrimAge and DunedinPoAm38 are more consistently associated with smoking and educational attainment (Crimmins et al., 2021), two important mechanisms identified in past literature linking ACEs to depressive symptoms (discussed above). The use of different individual clocks—each of which has different strengths and weaknesses—in individual studies may be a cause of the mixed findings in past research. More research like the current study comparing multiple clocks is needed to clarify what clocks are most responsive to certain exposures and are most predictive of psychiatric outcomes.
The current study has some key limitations. This is a study of US older adults. Further research using international samples is needed. ACEs were assessed using a retrospective self-report and may be biased by recall and social desirability; however, past research has established the validity and reliability of retrospective recall of childhood adversity (Brewin et al., 1993). Not all major ACEs, as defined by the CDC, were available. Particularly, we do not have a measure of neglect, which has been shown to be associated with adult mental health (Horwitz et al., 2001). Future research using the complete CDC ACEs scale is needed to validate these findings. We are not able to control for early life epigenetic age or psychiatric symptoms before ACEs exposure. We focused on BMI as a mediator of the effect of ACEs on epigenetic aging. Future studies should investigate whether modifiable health behaviors that affect BMI (e.g., diet, physical activity) mediate this association. Our measure of depressive symptoms does not capture clinically diagnosed depressive disorder. Because this was a population study, obtaining clinical diagnoses for all participants would be impractical and would substantially increase subject burden. Future work should validate our results in a well characterized clinical psychiatric population.
The current findings have important implications for biological psychiatric research, providing novel evidence accelerated DNAm aging may be a plausible biological mechanism explaining how childhood adversity may lead to elevated risk for depression. Moreover, these results suggest interventions aimed at healthy weight maintenance, smoking cessation, and alcohol abuse may be an important target for reducing the mental health risk and epigenetic aging risk associated with ACEs.
Author statement
Eric T. Klopack: Conceptualization, Formal analysis, Writing - Original Draft, Eileen M. Crimmins: Writing - Review & Editing, Supervision, Steve W. Cole: Writing - Review & Editing, Teresa E. Seeman: Writing - Review & Editing, Judith E. Carroll: Conceptualization, Writing - Review & Editing, Supervision.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
Support was provided by the USC/UCLA Center on Biodemography and Population Health through a grant from NIA (P30AG017265) and R25AG053227.
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2022.101071.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Austin M.K., Chen E., Ross K.M., McEwen L.M., Maclsaac J.L., Kobor M.S., Miller G.E. Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology. 2018;97:131–134. doi: 10.1016/j.psyneuen.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Belsky D.W., Caspi A., Arseneault L., Baccarelli A., Corcoran D.L., Gao X., Hannon E., Harrington H.L., Rasmussen L.J., Houts R., Huffman K., Kraus W.E., Kwon D., Mill J., Pieper C.F., Prinz J.A., Poulton R., Schwartz J., Sugden K., Moffitt T.E. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9 doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D.W., Caspi A., Cohen H.J., Kraus W.E., Ramrakha S., Poulton R., Moffitt T.E. Impact of early personal-history characteristics on the Pace of Aging: Implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell. 2017;16(4):644–651. doi: 10.1111/acel.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C.R., Andrews B., Gotlib I.H. Psychopathology and early experience: A reappraisal of retrospective reports. Psychological Bulletin. 1993;113(1):82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Bugliari D., Carroll J., Hayden O., Hayes J., Hurd M., Karabatakis A., Main R., Marks J., McCullough C., Meijer E., Moldoff M., Pantoja P., Rohwedder S., StClair P. 2016. RAND HRS longitudinal file 2016 (V2) documentation.https://hrsdata.isr.umich.edu/data-products/2016-rand-hrs-fat-file [Google Scholar]
- Campbell J.A., Walker R.J., Egede L.E. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. American Journal of Preventive Medicine. 2016;50(3):344–352. doi: 10.1016/j.amepre.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné R., Kelly-Irving M., Krogh V., Palli D., Panico S., Sacerdote C., Tumino R., Hebels D.G., Kleinjans J.C., de Kok T.M., Georgiadis P., Kyrtopoulos S.A., Vermeulen R., Stringhini S., Vineis P., Chadeau-Hyam M., Delpierre C. A multi-omics approach to investigate the inflammatory response to life course socioeconomic position. Epigenomics. 2020;12(15):1287–1302. doi: 10.2217/epi-2019-0261. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2019. Preventing adverse childhood experiences: Leveraging the best available evidence. [Google Scholar]
- Chen E., Miller G.E. Socioeconomic status and health: Mediating and moderating factors. Annual Review of Clinical Psychology. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- Chu S.H., Loucks E.B., Kelsey K.T., Gilman S.E., Agha G., Eaton C.B., Buka S.L., Huang Y.-T. Sex-specific epigenetic mediators between early life social disadvantage and adulthood BMI. Epigenomics. 2018;10(6):707–722. doi: 10.2217/epi-2017-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin. 2020;146(9):721–764. doi: 10.1037/bul0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E.M., Faul J.D., Thyagarajan B., Weir D.R. 2017. Venous blood collection and assay protocol in the 2016 health and retirement study 2016 venous blood study (VBS)https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/HRS2016VBSDD.pdf [Google Scholar]
- Crimmins E.M., Kim J.K., Fisher J., Faul J.D. University of Michigan Survey Research Center; 2020. HRS epigenetic clocks.https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/EPICLOCKS_DD.pdf [Google Scholar]
- Crimmins E.M., Thyagarajan B., Levine M.E., Weir D.R., Faul J. Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative U.S. Sample: The health and retirement study. Journal of Gerontology: Series A, glab016. 2021 doi: 10.1093/gerona/glab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy K.A., McLaughlin K.A., Green P.A. Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Annals of the New York Academy of Sciences. 2018;1428(1):151–169. doi: 10.1111/nyas.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekaterina P., Ruoting Y., Brent N., Reus V., Rasha H., Rampersaud R., Y W.G.W., Hough C.M., Elissa E., Prather A.A., Marti J., Aarti G., Mellon S.H., Wolkowitz O.M., Aarti G., Mellon S.H., Wolkowitz O.M. GrimAge,” an epigenetic predictor of mortality, is accelerated in major depressive disorder. Translational Psychiatry. 2021;11(1) doi: 10.1038/s41398/021/013020. & Link to external site, this link will open in a new window. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Epel E.S. The stress field ages: A close look into cellular aging processes. Psychoneuroendocrinology. 2020;113:104537. doi: 10.1016/j.psyneuen.2019.104537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E.S., Prather A.A. Stress, telomeres, and psychopathology: Toward a deeper understanding of a triad of early aging. Annual Review of Clinical Psychology. 2018;14:371–397. doi: 10.1146/annurev-clinpsy-032816-045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G., Polidoro S., Dugué P.-A., Kivimaki M., Ponzi E., Matullo G., Guarrera S., Assumma M.B., Georgiadis P., Kyrtopoulos S.A., Krogh V., Palli D., Panico S., Sacerdote C., Tumino R., Chadeau-Hyam M., Stringhini S., Severi G., Hodge A.M., Vineis P. Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Scientific Reports. 2017;7(1):16266. doi: 10.1038/s41598-017-16391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font S.A., Maguire-Jack K. Pathways from childhood abuse and other adversities to adult health risks: The role of adult socioeconomic conditions. Child Abuse & Neglect. 2016;51:390–399. doi: 10.1016/j.chiabu.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransquet P.D., Wrigglesworth J., Woods R.L., Ernst M.E., Ryan J. The epigenetic clock as a predictor of disease and mortality risk: A systematic review and meta-analysis. Clinical Epigenetics. 2019;11:62–79. doi: 10.1186/s13148-019-0656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M.J. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- George A., Hardy R., Fernandez J.C., Kelly Y., Maddock J. Life course socioeconomic position and DNA methylation age acceleration in mid-life. Journal of Epidemiology & Community Health. 2021 doi: 10.1136/jech-2020-215608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P.E., Fournier A.-A., Sisitsky T., Simes M., Berman R., Koenigsberg S.H., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018) PharmacoEconomics. 2021;39(6):653–665. doi: 10.1007/s40273-021-01019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat E.J., Prather A.A., Horvath S., Belsky J., Epel E.S. Developmental Psychobiology; 2021. Early life adversity, pubertal timing, and epigenetic age acceleration in adulthood. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health & Retirement Study . 2021. Produced and distributed by the university of Michigan with funding from the national Institute on aging. (grant number U01AG009740), Ann Arbor, MI. [Google Scholar]
- Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics. 2018;19(6):371–374. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- Horwitz A.V., Widom C.S., McLaughlin J., White H.R. The impact of childhood abuse and neglect on adult mental health: A prospective study. Journal of Health and Social Behavior. 2001;42(2):184–201. doi: 10.2307/3090177. [DOI] [PubMed] [Google Scholar]
- Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hughes K., Bellis M.A., Hardcastle K.A., Sethi D., Butchart A., Mikton C., Jones L., Dunne M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health. 2017;2(8):e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- Jones T.M., Nurius P., Song C., Fleming C.M. Modeling life course pathways from adverse childhood experiences to adult mental health. Child Abuse & Neglect. 2018;80(1):32–40. doi: 10.1016/j.chiabu.2018.03.005. edselp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Vance L.A., Cross D., Knight A.K., Kilaru V., Michopoulos V., Klengel T., Smith A.K. Exposure to violence accelerates epigenetic aging in children. Scientific Reports. 2017;7(1):8962. doi: 10.1038/s41598-017-09235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmakis K.A., Chandler G.E. Health consequences of adverse childhood experiences: A systematic review. Journal of the American Association of Nurse Practitioners. 2015;27(8):457–465. doi: 10.1002/2327-6924.12215. [DOI] [PubMed] [Google Scholar]
- Lawn R.B., Anderson E.L., Suderman M., Simpkin A.J., Gaunt T.R., Teschendorff A.E., Widschwendter M., Hardy R., Kuh D., Relton C.L., Howe L.D. Psychosocial adversity and socioeconomic position during childhood and epigenetic age: Analysis of two prospective cohort studies. Human Molecular Genetics. 2018;27(7):1301–1308. doi: 10.1093/hmg/ddy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.E., Lu A.T., Quach A., Horvath S., Chen B.H., Ferrucci L., Assimes T.L., Bandinelli S., Hou L., Baccarelli A.A., Stewart J.D., Whitsel E.A., Li Y., Wilson J.G., Reiner A.P., Aviv A., Lohman K., Liu Y. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Chen X., Gill T.M., Ma C., Crimmins E.M., Levine M.E. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: Evidence from the Health and Retirement Study. PLoS Medicine. 2019;16(6) doi: 10.1371/journal.pmed.1002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Croft J.B., Chapman D.P., Perry G.S., Greenlund K.J., Zhao G., Edwards V.J. Relationship between adverse childhood experiences and unemployment among adults from five US states. Social Psychiatry and Psychiatric Epidemiology. 2013;48(3):357–369. doi: 10.1007/s00127-012-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A.T., Quach A., Wilson J.G., Reiner A.P., Aviv A., Raj K., Hou L., Baccarelli A.A., Li Y., Stewart J.D., Whitsel E.A., Assimes T.L., Ferrucci L., Horvath S. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11(2):303–327. doi: 10.18632/aging.101684. edswsc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S., Davis K.A., Soare T.W., Zhu Y., Suderman M.J., Simpkin A.J., Smith A.D.A.C., Wolf E.J., Relton C.L., Dunn E.C. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology. 2020;113:104484. doi: 10.1016/j.psyneuen.2019.104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M.T., Ports K.A., Ford D.C., Afifi T.O., Gershoff E.T., Grogan-Kaylor A. Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse & Neglect. 2017;69(1):10–19. doi: 10.1016/j.chiabu.2017.03.016. edselp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Parker K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Schneper L.M., Notterman D.A. DNA methylation, early life environment, and health outcomes. Pediatric Research. 2016;79(1):212–219. doi: 10.1038/pr.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Caspi A. Psychiatry's opportunity to prevent the rising burden of age-related disease. JAMA Psychiatry. 2019;76(5):461–462. doi: 10.1001/jamapsychiatry.2019.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. 7th ed. Muthén & Muthén; 1998. Mplus user's guide. [Google Scholar]
- Oblak L., van der Zaag J., Higgins-Chen A.T., Levine M.E., Boks M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Research Reviews. 2021;69:101348. doi: 10.1016/j.arr.2021.101348. [DOI] [PubMed] [Google Scholar]
- Pudrovska T., Anikputa B. Early-life socioeconomic status and mortality in later life: An integration of four life-course mechanisms. Journal of Gerontology: Serie Bibliographique. 2014;69(3):451–460. doi: 10.1093/geronb/gbt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raffington L., Belsky D.W., Kothari M., Malanchini M., Tucker-Drob E.M., Harden K.P. 2021. Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M.E., Peng J., Shrout M.R., Madison A.A., Andridge R., Alfano C.M., Povoski S.P., Lipari A.M., Malarkey W.B., Kiecolt-Glaser J.K. Childhood abuse histories predict steeper inflammatory trajectories across time. Brain, Behavior, and Immunity. 2021;91:541–545. doi: 10.1016/j.bbi.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd L.S., D'Souza S., Milne B.J., Caspi A., Moffitt T.E. Longitudinal associations of mental disorders with physical diseases and mortality among 2.3 million New Zealand citizens. JAMA Network Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.33448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels M.S., Katki H.A., Freedman N.D., Purdue M.P., Wentzensen N., Trabert B., Kitahara C.M., Furr M., Li Y., Kemp T.J., Goedert J.J., Chang C.M., Engels E.A., Caporaso N.E., Pinto L.A., Hildesheim A., Chaturvedi A.K. Cigarette smoking and variations in systemic immune and inflammation markers. Journal of the National Cancer Institute: 2014;106(11) doi: 10.1093/jnci/dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.L., Lei M.K., Beach S.R.H., Philibert R.A., Cutrona C.E., Gibbons F.X., Barr A.B. Economic hardship and biological weathering: The epigenetics of aging in a US sample of black women. Social Science & Medicine. 2016;150(1):192–200. doi: 10.1016/j.socscimed.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140(3):774–815. doi: 10.1037/a0035302. & Link to external site, this link will open in a new window. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J.A., Colich N.L., Uddin M., Armstrong D., McLaughlin K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry. 2019;85(3):268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey C.L., Wallace R.B., Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics. 1999;11(2):139–148. doi: 10.1017/S1041610299005694. [DOI] [PubMed] [Google Scholar]
- Verhoeven J.E., Yang R., Wolkowitz O.M., Bersani F.S., Lindqvist D., Mellon S.H., Yehuda R., Flory J.D., Lin J., Abu-Amara D., Makotkine I., Marmar C., Jett M., Hammamieh R. Epigenetic age in male combat-exposed war veterans: Associations with posttraumatic stress disorder status. Complex Psychiatry. 2018;4(2):90–99. doi: 10.1159/000491431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wickrama K.A.S., Kwon J.A., Oshri A., Lee T.K. Early socioeconomic adversity and young adult physical illness: The role of body mass index and depressive symptoms. Journal of Adolescent Health. 2014;55(4):556–563. doi: 10.1016/j.jadohealth.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz O.M., Epel E.S., Mellon S. When blue turns to grey: Do stress and depression accelerate cell aging? World Journal of Biological Psychiatry. 2008;9(1):2–5. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- Yang Y., Gerken K., Schorpp K., Boen C., Harris K.M. Early-life socioeconomic status and adult physiological functioning: A life course examination of biosocial mechanisms. Biodemography and Social Biology. 2017;63(2):87–103. doi: 10.1080/19485565.2017.1279536. sih. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Wu G.W.Y., Verhoeven J.E., Gautam A., Reus V.I., Kang J.I., Flory J.D., Abu-Amara D., Hood L., Doyle F.J., Yehuda R., Marmar C.R., Jett M., Hammamieh R., Mellon S.H., Wolkowitz O.M. A DNA methylation clock associated with age-related illnesses and mortality is accelerated in men with combat PTSD. Molecular Psychiatry. 2020:1–11. doi: 10.1038/s41380-020-0755-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.