Abstract
Background
One of the global problems is to control the coronavirus epidemic, and the role of different medicines is still unknown to policymakers. This study was conducted to evaluate the effects of losartan on the mortality rate of COVID-19 in hypertensive patients.
Methods
The research sample of analytical study included 1458 patients presenting to COVID-19 diagnostic centers in Yazd that were examined in the first six months of 2020. Data were analyzed using descriptive statistics as well as chi-square, Fisher’s exact test, t test, and logistic regression.
Results
Of 1458 subjects that were studied, 280 were hypertensive of whom 179 tested positive for SARS-CoV-2 PCR. The results showed a lower chance of death by more than 5 times in hypertensive patients who used losartan (P = 0.003). Moreover, regarding the effect of losartan on the prevention of COVID-19 in hypertensive patients, it was found that this medicine played a protective role although this relationship was not statistically significant (P = 0.86).
Conclusions
The results showed that losartan reduced the chance of mortality in hypertensive patients. It is recommended that the effect of losartan and other blood pressure medicines on COVID-19 patients be investigated in larger studies as well as laboratory investigations.
Keywords: Blood pressure/hypertension, Losartan, Mortality, SARS-CoV-2
Background
Today, despite advances in medical and laboratory sciences, epidemics are one of the major problems of the medical society that threatens the lives of the general population. A series of unexplained cases of pneumonia were reported in Wuhan, China, in late December 2019. The virus was temporarily named 2019 Novel Coronavirus (2019-nCoV) by the World Health Organization on January 12, 2020. On February 11, 2020, the WHO named the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease caused by the virus coronavirus disease 2019 (COVID-19. The Organization considered COVID-19 a global concern on January 30, 2020.
This virus has a high infectivity and a long incubation period so that it takes 2 to 14 days for the symptoms to appear. This characteristic increases the outbreak rate and hampers prevention and control [1]. This virus is transmitted from one person to another. It can be transmitted through respiratory droplets, direct contact with secretions containing the virus, and through the mouth, nose, and eyes.
The mortality rate is extremely high in infected patients requiring mechanical ventilation (artificial respiration) due to the severe lung damage caused by coronavirus infection [2]. The results of a study conducted in Wuhan, China, showed that 28% of the patients with COVID died [3]. The mortality rate of patients with COVID in studies conducted by Shi [4] and Liu [5] was 10.5% and 6%, respectively.
Preliminary studies have indicated that people with underlying diseases are at higher risk for complications and mortality caused by COVID-19. Nearly 50% of the hospitalized patients suspected of COVID-19 have other chronic diseases, and almost 41% of the hospitalized patients with confirmed covid-19 suffer from cardiovascular or cerebrovascular disease [6]. Among patients with COVID-19, hypertension (HTN) is a significant risk factor and a common co-morbidity for acute respiratory failure, hospitalization, and mortality [7].
So far, there is no specific antiviral drug for COVID-19 and the main treatment is supportive care such as the.
Aside from ARDS and cytokine storm, COVID19 appears to be a mild disease. Nevertheless, the acute respiratory distress syndrome (ARDS) as the primary cause of significant morbidity and death in SARS-CoV2 needs further assessments [8]. Maintaining the vital signs, regulating oxygen and blood pressure, and reducing complications such as secondary infections or organ failure are the mainstay of supportive COVID-19 management [9]. According to the RECOVERY trial, after a month, dexamethasone reduced mortality in patients hospitalized with Covid-19 getting either invasive mechanical ventilation or oxygen alone at the time of randomization. However, it showed no signs of improvement in patients without respiratory support [10]. Antiviral medications such as remdesivir, and favipiravir impair viral propagation and infectivity. Similar to anti-inflammatory agents, they are associated with a variety of adverse effects and drug-drug interactions [11]. The SARS-CoV2 receptor on host cells is Angiotensin-converting enzyme 2 (ACE2), a renin-angiotensin system (RAS) component. The RAS’s systemic and local ligands and receptors control cellular growth, metabolic rate, and salt and electrolyte balance. Therefore, antihypertensive medications such as ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) might improve the COVID-19 outcome [7, 8, 12–14].
After replication in the upper respiratory tract, the virus rapidly spreads to the lower airways and alveoli within a week. The pathophysiology of cytokine storm and ARDS in COVID-19 is explained precisely by hyperacute activation of AngII type 1 receptor (AT1R) by a rapid surge in intracellular Ang II due to ACE2 downregulation after SARS-CoV2 showering to the lungs. When the coronaviruses entering the body, it fuses their envelopes to the membranes of host cells and affected cells by transport their genetic material. This fusion is interposed by glycosylated spike proteins on the surface of the virion interacting with proper surface receptors on the membrane of the host cell. ACE2 receptor is a known human cell-surface protein to which CoV spike proteins specifically bind [15]. The conversion of AngI to AngII, a major effector or renin-angiotensin-aldosterone system (RAS), is mediated by ACE. ACE is a protein that is highly expressed on membranes of vascular endothelial cells, predominantly in the lung tissue [16]. Most of the physiological effects associated with RAS are mediated by the interaction of AngII with the G-protein receptor associated with AT1R, which activates the physiological pathway in various systems [15].
It is suggested that the use of losartan, an AT1R blocker with antihypertensive activity, may be beneficial in the treatment of ARDS in COVID-19. This is mainly due to the selective blockade of AT1 receptors and the reduction of the compressive effect of angiotensin II. Losartan also inhibits the development of dendritic cells and the T helper 1 immunological response; ultimately, reducing the inflammatory reactions caused by angiotensin II. Therefore, losartan could potentially protect the respiratory organs against COVID-19-induced damage [13]. It is among antihypertensive medications with the lowest side effects, nevertheless, blurry vision, dyspnea, dizziness, lightheadedness on rapid positional change, tachycardia, nausea or vomiting, and abdominal pain should be noted [17].
Several studies have revealed that losartan has an inhibitory effect on developing liver fibrosis and prevents aortic dilation in patients with Marfan syndrome [18, 19]. Moreover, it has been shown that losartan reduces the regulation of TGF-β1 and fibrogenic molecules in cells infected with cytomegalovirus [20]. Due to the lack of evidence regarding the effect of losartan on the COVID-19 mortality and morbidity, this study was conducted to evaluate the effect of losartan on COVID-19 mortality in hypertensive patients.
Methods
Study design, population and setting
All subjects suspected of COVID-19 who presented to COVID-19 laboratory and diagnostic centers were included in this analytical study. The hypertensive patients were divided to COVID- 19 positive (positive PCR test) and COVID-19 negative (negative PCR test) groups. Exclusion criteria were rejection of the general consent. The study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences with the ID of IR.SSU.REC.1399.101, located in Yazd, Iran.
Variables selection
The effect of losartan on the develoment and mortality of COVID-19 was the main variable and age, diabetes, cancer, liver disease, cardiovascular disease, chronic lung disease, chronic kidney disease, and chronic nervous disease were cobnsidered as confounding factors. The presence of a heart disease was confirmed by the cardiologist member of the COVID-19 treatment team in the ICU. The data collection tool was a researcher-made questionnaire based on the data available in the COVID-19 data dashboard of Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Statistical analysis
Frequency, percentage, mean, standard deviation, and median are used to describe the data. Chi-square, Fisher’s exact test, and t test were used to investigate the relationship between variables. Ultimately, logistic regression was applied for model building at a significance level of 5%.
Results
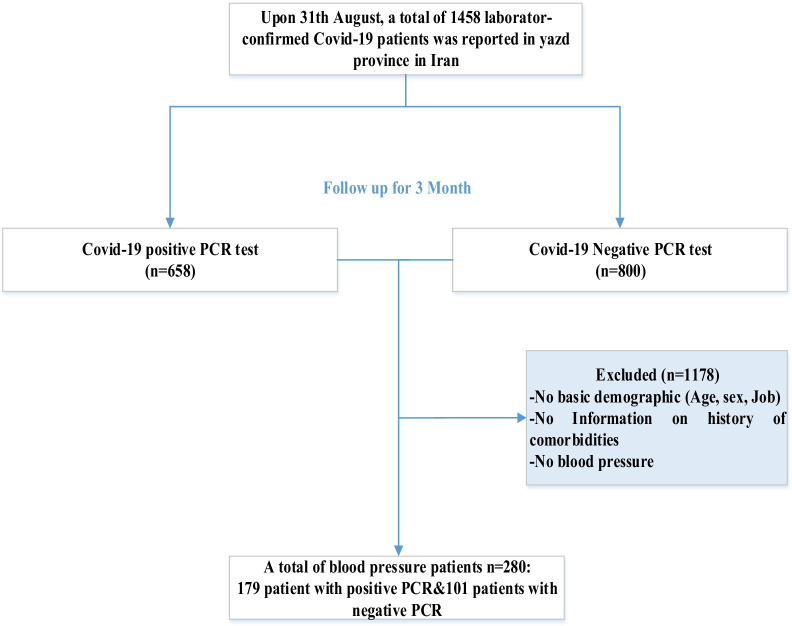
Of 1458 subjects included in this study, 658 tested positive for SARS-CoC-2 PCR and 800 tested negative. After applying the inclusion and exclusion criteria, 280 hypertensive patients were identified of whom 179 had a positive PCR test (Fig. 1]. Of 280 patients with hypertension, 134 (47.9%) were female and 146 (52.1%) were male. The mean age of the subjects was 64.60 ± 0.8 years old. Moreover, the mean, minimum and maximum age of the subjects was 66, 28, and 98 years, respectively. Table 1 provides details regarding the number of hospitalizations, respiratory failures, and ICU admissions.
Fig. 1.
Sample flow diagram detailing included subjects and exclusion criteria
Table 1.
Characteristics of subjects with hypertension and their COVID-19 test results
| Variables | Positive PCR | Negative PCR | P value |
|---|---|---|---|
| Losartan usage | 0.054** | ||
| yes | 59 | 102 | |
| no | 42 | 77 | |
| Age (Mean±SD) | 67.45±13.25 | 63.07±13.64 | 0.009* |
| LOS (Mean±SD) | 9.02±6.40 | 8.14±6.27 | 0.285* |
| Admission status | 0.593** | ||
| In-patient | 176 | 99 | |
| Out-patient | 3 | 2 | |
| ICU admission | 0.031** | ||
| Yes | 15 | 17 | |
| No | 152 | 79 | |
| Final outcome | 0.298** | ||
| Alive | 156 | 91 | |
| Dead | 23 | 10 | |
| Chronic liver disease | 0.639** | ||
| Yes | 0 | 1 | |
| No | 101 | 178 | |
| DM | 0.473** | ||
| Yes | 39 | 77 | |
| No | 62 | 102 | |
| CVD | 0.20** | ||
| Yes | 66 | 103 | |
| No | 35 | 76 | |
| CND | 0.693** | ||
| Yes | 4 | 4 | |
| No | 97 | 175 | |
| Chronic Lung Disease | 0.027** | ||
| Yes | 15 | 12 | |
| No | 86 | 167 | |
| CKD | 0.002** | ||
| Yes | 15 | 8 | |
| No | 86 | 171 | |
| Cancer | 0.681** | ||
| Yes | 1 | 1 | |
| No | 100 | 178 | |
Abbreviations: PCR: polymerase chain reaction, SD: standard deviation, LOS: length of stay, ICU: intensive care unit, DM: diabetes mellitus, CVD: cardiovascular diseases, CND: chronic neurological diseases, CKD: chronic kidney diseases. *Independent T test was used. **Exact test was used
Examining the effect of losartan on COVID-19 prevention in hypertensive subjects after adjustment for different variables indicated that this medicine had a non-significant protective role (P = 0.86), Table 2 presents more details. Examining the effect of losartan on the reduction of COVID-19 mortality in hypertensive patients revealed the protective role of this medicine. The results showed that patients who used this medicine had a more than 5-fold reduction in the chance of mortality, which was statistically significant (P = 0.003) (Table 3].
Table 2.
Adjusted odds ratio for relationship between losartan usage and COVID-19 development
| Factors | OR (95% CI) | P value |
|---|---|---|
| Losartan usage | 0.95 (0.56, 1.61) | 0.86 |
| Age | 0.97 (0.95, 0.99) | 0.23 |
| DM | 1.62 (0.92, 2.84) | 0.09 |
| CVD | 0.73 (0.42, 1.26) | 0.256 |
| CND | 0.43 (0.10, 1.85) | 0.257 |
| Chronic Lung Diseases | 0.44 (0.18, 1.02) | 0.058 |
| Kidney Diseases | 0.20 (0.07, 0.55) | 0.002 |
| Cancers | 0.55 (0.03, 9.29) | 0.683 |
OR: odds ratio, CI: confidence interval, DM: diabetes mellitus, CVD: cardiovascular disorders, CND: chronic neurological diseases
Table 3.
Adjusted odds ratio for relationship between losartan usage and COVID-19 mortality
| Factors | OR (95% CI) | P value |
|---|---|---|
| Losartan usage | 0.17 (0.05, 0.55) | 0.003 |
| Age | 1.04 (1.00, 1.09) | 0.032 |
| DM | 3.42 (1.13, 10.4) | 0.030 |
| CVD | 0.44 (0.14, 1.34) | 0.15 |
| CND | 1.17 (0.07, 18.54) | 0.91 |
| Chronic Lung Diseases | 4.13 (0.93, 18.3) | 0.063 |
| Kidney Diseases | 2.57 (0.29, 22.74) | 0.394 |
| Cancers | 546 (0, Infinity) | 1 |
OR: odds ratio, CI: confidence interval, DM: diabetes mellitus, CVD: cardiovascular disorders, CND: chronic neurological diseases
Discussion
The global spread of the novel coronavirus infection has posed a serious and important threat to human health. Some studies have reported that some protease inhibitors, such as remdesivir and chloroquine, are effective in COVID-19 infection but they have not proved effective in reality [12]. Accordingly, there is now growing interest in the effect of FDA-approved medicines on COVID-19 treatment.
This study examined the effect of on the mortality rate of COVID-19 losartan in hypertensive patients. Losartan is an angiotensin II receptor antagonist that is also used to treat hypertension and reduce kidney damage in the long term in type 2 diabetic patients with hypertension [21]. According to the results of the present study, losartan has a protective role against COVID-19 mortality in patients with high blood pressure, so that the patients who used this drug had a lower chance of mortality by more than five times. Several studies have found that ACE inhibitors such as losartan may have a protective role against tissue damage. Losartan has anti-platelet aggregation and anti-diabetic properties and prevents organ damage and fibrosis [22]. Additionally, one study found that losartan prevented liver fibrosis [18], and another study reported that losartan reduced the regulation of TGF-β1 and fibrogenic molecules in cells infected with cytomegalovirus [20]. Losartan has also been recently recommended for Marfan syndrome treatment [19]. A study conducted by Salari et al. showed that losartan had a protective effect in patients with COVID-19 [12]. Another study revealed that losartan could be beneficial for COVID-19-infected patients experiencing pneumonia [23]. Nevertheless, more clinical trial studies are required in this regard.
According to the results, the COVID-19 mortality rate increased with age. In a study by Liu et al. In Hainan, China, the mortality rate was higher in the elderly versus young and middle-aged patients [5]. The results of a study conducted in the United States revealed that 80% of COVID-19 mortality occurred in patients over 65 years old [24]. These results are consistent with the results of a study conducted in China in which 80% of the mortality occurred in subjects aged 60 and over [25]. Elderly people usually develop a more severe COVID-infection and also suffer from other underlying diseases. Therefore, they require more care.
The results showed that diabetes increased the chance of death.
According to the results of other studies, patients with cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer have had the highest sensitivity to the disease [1, 2, 6]. In a study conducted by Zhang et al., the mortality rate was 41% higher in patients with a history of respiratory disease and 13% higher in patients with a history of heart disease [26]. I Shi et al. also found that the mortality rate was 9% higher in patients with a history of heart disease and 11% higher in patients with a history of lung disease [4]. In COVID-19 patients, pre-existing cardiovascular illness may increase the risk of myocardial damage and death [27]. Also, Nuzzi et al. reported that the occurrence of cardiac damage during hospitalization significantly raised the likelihood of poor outcome. Since most participants experiencing myocardial injury 2 days after admission had the history of hypertension, it can be concluded that optimal blood pressure regulation may lower the incidence of myocardial damage in admitted COVID-19 patients [28].
However, some studies argue that ACEIs and ARBs may enhance ACE2 receptor expression in animals, and recommend these drug classes as a COVID-19 therapeutic adjunct. Therefore, more studies regarding the mechanism of action of ARBs in the management of inflammation and cardiovascular complications in COVID-19 patients are required [13, 29]. Only 280 of the 1458 individuals studied had hypertension, with 161 of those on losartan and the rest taking other medications. Due to the small number of patients using different antihypertensive drugs, only the effect of losartan was investigated in the statistical analysis. Therefore, investigating the effects of other antihypertensive medicines in COVID-19 patients is suggested.
Conclusions
According to the results of this study, losartan has a protective role against COVID-19 mortality in hypertensive patients. Furthermore, the mortality rate was higher in the elderly patients with underlying diseases compared to other patients. Losartan may activate intracellular defense against COVID-19 virus by producing interferon-gamma. It is recommended that the effect of losartan and other blood pressure medicines on COVID-19 patients be investigated in larger studies as well as laboratory investigations.
Acknowledgements
Authors would like to thank Yazd University of Medical Sciences in partially funding the study.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- PCR
Polymerase chain reaction
- ACE2
Angiotensin-converting enzyme 2
- Ang I
Angiotensin I
- RAS
Renin–angiotensin system
Authors’ contributions
MM and AD have designed the study and supervised the thesis. MR collected the data and analyzed it. They also prepared the first draft of the manuscript. MTS has edited and finalized the manuscript. All authors read the manuscript and approved it.
Funding
This Study Was Part of Research Project Supported by Yazd University of Medical Sciences, Grant NO: 7867. In this project, part of the research costs were paid to the researchers.
Availability of data and materials
The data-sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The article’s proposal was approved by the ethics committee of Shahid Sadoughi University of Medical Sciences with the ID of IR.SSU.REC.1399.101. Due to the retrospective nature of the study, no study specific consent form was used. However, patients admitted to our hospital are asked to sign a general consent upon admission, which covers the collection of patient data and publication of these results. Therefore, the individual’s informed consent was waived by the above ethics committee. We received administrative permission from (Secretary of University/Regional Research Ethics Committee Shahid Sadoughi University of Medical Sciences) to access and use the data. Data used in the study were anonymized. The ethics committee approved this procedure with the above ethical code. The present study was conducted in terms of the principles of the revised Declaration of Helsinki, which waived requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of interest regarding this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alshahrani MS, Sindi A, Alshamsi F, Al-Omari A, El Tahan M, Alahmadi B, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1):1–10. doi: 10.12659/msm.925364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes care. 2020;43(7):1382–91. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infection. 2020;80(6):e14-e8. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Puskarich MA, Ingraham NE, Merck LH, Driver BE, Wacker DA, Black LP, et al. Effect of losartan on hospitalized patients with COVID-19-induced lung injury: A randomized clinical trial. medRxiv. 2021. 10.1101/2021.08.25.21262623. [DOI] [PMC free article] [PubMed]
- 8.Nejat R, Sadr AS. Are losartan and imatinib effective against SARS-CoV2 pathogenesis? A pathophysiologic-based in silico study. In silico Pharmacol. 2021;9(1):1–22. doi: 10.1007/s40203-020-00058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:1–13. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L. Dexamethasone in Hospitalized Patients with Covid-19. 2021;384(8):693–704. https://dx.doi.org/10.1056%2FNEJMoa2021436. [DOI] [PMC free article] [PubMed]
- 11.Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, et al. Current pharmacological treatments for COVID-19: What’s next? Br J Pharmacol. 2020;177(21):4813–24. doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jazzi AS, Mahnam K, Hejazi SH, Damavandi MS, Sadeghi P, Zeinalian M, et al. Inhibition of viral macrodomain of covid-19 and human trpm2 by losartan. Preprint. 2020 doi: 10.20944/preprints202003.0457.v1. [DOI] [Google Scholar]
- 13.Zeinalian M, Salari-Jazi A, Jannesari A, Khanahmad H. A potential protective role of losartan against coronavirus-induced lung damage. Infection Control Hosp Epidemiol. 2020;41(6):752–3. doi: 10.1017/ice.2020.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flacco ME, Martellucci CA, Bravi F, Parruti G, Cappadona R, Mascitelli A, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID-19: a meta-analysis. Heart. 2020;106(19):1519–24. doi: 10.1136/heartjnl-2020-317336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wevers BA. Hoek Lvd. Renin–angiotensin system in human coronavirus pathogenesis. Future Virol. 2010;5(2):145–61. doi: 10.2217/fvl.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke NE, Turner AJ. Angiotensin-converting enzyme 2: the first decade. Int J Hypertens. 2012;2012. 10.1155/2012/307315. [DOI] [PMC free article] [PubMed]
- 17.McIntyre M, Caffe S, Michalak R, Reid J. Losartan, an orally active angiotensin (AT1) receptor antagonist: a review of its efficacy and safety in essential hypertension. Pharmacol Therap. 1997;74(2):181–94. doi: 10.1016/S0163-7258(97)82002-5. [DOI] [PubMed] [Google Scholar]
- 18.Salama ZA, Sadek A, Abdelhady AM, Darweesh SK, Morsy SA, Esmat G. Losartan may inhibit the progression of liver fibrosis in chronic HCV patients. Hepatob Surg Nutr. 2016;5(3):249. doi: 10.21037/hbsn.2016.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellers SL, Milad N, Chan R, Mielnik M, Jermilova U, Huang PL, et al. Inhibition of Marfan Syndrome Aortic Root Dilation by Losartan: Role of Angiotensin II Receptor Type 1–Independent Activation of Endothelial Function. Am J Pathol. 2018;188(3):574–85. doi: 10.1016/j.ajpath.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Choi JA, Kim J-E, Ju H-h, Lee J, Jee D, Park CK, et al. The effects of losartan on cytomegalovirus infection in human trabecular meshwork cells. Plos one. 2019;14(6):e0218471. doi: 10.1371/journal.pone.0218471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21(5):730. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov M, Mihailović-Stanojević N, Grujić Milanović J, Jovović Đ, Marković-Lipkovski J, Ćirović S, et al. Losartan improved antioxidant defense, renal function and structure of postischemic hypertensive kidney. PloS one. 2014;9(5):e96353. doi: 10.1371/journal.pone.0096353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouhpayeh S, Shariati L, Boshtam M, Rahimmanesh I, Mirian M, Zeinalian M, et al. The molecular story of COVID-19; NAD+ depletion addresses all questions in this infection. 2020. Kouhpayeh, S.; Shariati, L.; Boshtam, M.; Rahimmanesh, I.; Mirian, M.; Zeinalian, M.; Salari-jazi, A.; Khanahmad, N.; Damavandi, M.S.; Sadeghi, P.; Khanahmad, H. The Molecular Story of COVID-19; NAD+ Depletion Addresses All Questions in this Infection. Preprints 2020, 2020030346 10.20944/preprints202003.0346.v1.
- 24.Covid C, Team R, COVID C, Team R, COVID C, Team R, et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. Morbidity and mortality weekly report. 2020;69(12):343. https://dx.doi.org/10.15585%2Fmmwr.mm6912e2. [DOI] [PMC free article] [PubMed]
- 25.TNCPERE T. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19)-China, 2020. 2020. [PMC free article] [PubMed]
- 26.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–72. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuzzi V, Merlo M, Specchia C, Lombardi CM, Carubelli V, Iorio A, et al. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Failure. 2021;8(5):3504–11. doi: 10.1002/ehf2.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microb Infect. 2020;9(1):757–60. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sets used and/or analyzed during the current study available from the corresponding author on reasonable request.