Abstract
Mosquito borne diseases are on the rise because of their fast spread worldwide and the lack of effective treatments. Here we are focusing on the development of a novel anti-malarial and virucidal agent with biocidal effects also on its vectors. We have synthesized a new quinoline (4,7-dichloroquinoline) derivative which showed significant larvicidal and pupicidal properties against a malarial and a dengue vector and a lethal toxicity ranging from 4.408 µM/mL (first instar larvae) to 7.958 µM/mL (pupal populations) for Anopheles stephensi and 5.016 µM/mL (larva 1) to 10.669 µM/mL (pupae) for Aedes aegypti. In-vitro antiplasmodial efficacy of 4,7-dichloroquinoline revealed a significant growth inhibition of both sensitive strains of Plasmodium falciparum with IC50 values of 6.7 nM (CQ-s) and 8.5 nM (CQ-r). Chloroquine IC50 values, as control, were 23 nM (CQ-s), and 27.5 nM (CQ-r). In vivo antiplasmodial studies with P. falciparum infected mice showed an effect of 4,7-dichloroquinoline compared to chloroquine. The quinoline compound showed significant activity against the viral pathogen serotype 2 (DENV-2). In vitro conditions and the purified quinoline exhibited insignificant toxicity on the host system up to 100 µM/mL. Overall, 4,7-dichloroquinoline could provide a good anti-vectorial and anti-malarial agent.
Subject terms: Biochemistry, Biological techniques, Biotechnology
Introduction
Vector-borne maladies are providing a serious threat to the well-being and public health around the world. Malaria, or dschungle fever, a tropical parasitic illness caused by the eukaryotic protest Plasmodium spp., provides one of the most significant infections on the planet1. An assessed 3.3 billion of the world human population lives in areas with risk of Malaria infection2 is contaminated with its mosquito vector Anopheles spp. Despite being preventable and treatable, malaria continues to provide severe effects on public health and livelihood in the tropical world3,4.
According to the World Health Organization5, almost two million people in the Americas suffered from dengue virus infection in 2019, and more recent data showed that four billion people suffer from dengue and related viruses such as Zika and Chikungunya in 128 countries worldwide6.
Quinoline provides a well studied compound and shows potential biological activities against vector borne diseases7,8. Quinoline provided the first anti-malarial medicine. It is a special kind of alkaloid originating from the herbal tree Cinchona9. By altering the places of the chemical aldehyde groups, quinoline increases its pesticidal properties10. Chloroquine provides well known clinical uses because of its viability and its generally safe application11. Attributable to huge natural bioactivities, quinoline compounds have attracted increasingly more consideration in combinatorial and bioactivity research12,13.
Quinoline subsidiaries have widespread biopharmaceutical applications14 (Fig. 1). Analysts have just decided numerous helpful bioactivities of quinoline subordinates, including among others mitigative effects, against bacteria15,16, hostility to viruses17 and cell reinforcement18. Therapeutic scientists incorporated an assortment of quinoline compounds with various natural compounds by introducing different dynamic gatherings to the quinoline moiety, utilizing engineering techniques and the possible utilization of quinoline subsidiaries in different fields of science, pesticide development and biomedicine19–21. Since 2011, several quinoline compounds have shown Epidermal Growth Factor Receptor (EGFR) inhibition22.
Figure 1.
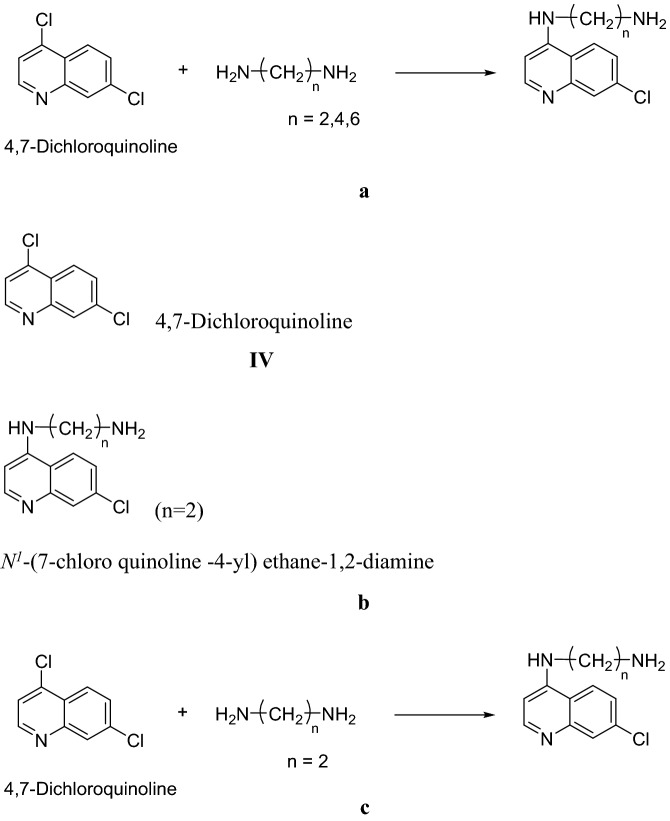
(a) 4,7-Dichloroquinoline design inspired by the natural molecule, chloroquine. (b) 4,7-Dichloroquinoline design inspired by the natural molecule, chloroquine. (c) 4,7-Dichloroquinoline design inspired by the natural molecule, chloroquine.
Among the heterocyclic compounds, 4,7-dichloroquinoline is a hydroxychloroquine intermediate for the treatment of different types of malaria23. Recently, numerous examinations are carried out with hydroxychloroquine for the therapeutic/forestall of pandemic COVID 1924. Likewise, the above said molecule was significant for the understanding of life performances25,26.
We synthesized a N1-(7-chloroquinoline-4-yl) ethane-1,2-diamine derivative by the method of Shafi et al.8 against A. stephensi providing no harmful impacts on the environment as well on non-target organisms (see Nyberg et al.27). As the plasmodium parasite becomes more resistant to quinoline based anti-malarial drugs, it becomes even more important to design a potent anti-malarial molecule28,29.
Hence, finding new compounds to treat malaria is urgently needed for the treatment of dangerous mosquito borne diseases30,31. This work provides a general overview of quinoline advantages for the discovery of more efficient compounds32,33. In continuation of the study for the preparation of a 4-diamine substituted-7-dichloroquinoline compounds against vector borne diseases34 we report herein the anti-malarial and anti-dengue potential of a novel quinoline compound.
The quinoline skeleton is utilized for some important engineered agrochemicals and to plan manufactured mixtures providing several pharmacological effects. Quinoline and its related compounds belongs to a significant class of antimalarial sedates that affect the parasite’s hemoglobin breakdown pathway. Earlier studies reported that for some time this compound was utilizing quinoline to battle malaria35. Along these lines, it is significant to re-look into the antimalarial movement of existing quinoline libraries or blend some unique quinoline subsidiaries with improved action. A methodical and broad investigation is needed to find a compelling antimalarial compound structure 4-aminoquinoline based framework36. In the present research, we have orchestrated several analogs of 4,7-dichloroquinoline and screened against jungle fever parasites, dengue (DENV-2) and their respective mosquito vectors. Also, we reported the synthesis of N2-2-((7-chloroquinolin-4-yl) amino) ethyl)-N4, N6-bis(4-nitrophenyl)-1,3,5-triazine-2,4,6-triamine. Whose synthesis have been planned for the bi-substituted cyanuric chloride using p-nitroaniline incorporated N1-(7-chloroquinoline–4–yl) ethane-1,2–diamine. Synthesized molecules can be analyzed by IR, 1HNMR, 13C, mass and elemental analysis to characterize their molecular structure. This is a new compound that is easily synthesized by substituting cyanuric chloride to provide s-triazine derivatives. Substituted quinolines are historically among the most important antimalarial drugs and are expected to achieve a substantial reduction of malaria infections.
Materials and methods
Biogenesis of N1-(7-chloroquinoline -4-yl) ethane-1,2-diamine
A form of 4,7 dichloroquinoline (1.8 g, 0.01 mol) and ethylene diamine (0.06 g, 0.01 mol) was evaluated through thin layer chromatography (TLC) at the end of a chemical reaction. Filtration was used to remove the crystals of 4-substituted 7-chloroquinoline. After acetone treatment the end compound was recrystallized twice providing N1-(7-chloroquinoline-4-yl) ethane-1,2-diamine (CAS Number-5407-57-8).
N1-(7-chloroquinoline-4-yl) ethane-1,2-diamine in silico analysis
The synthesized compound N1-(7-chloroquinoline-4-yl) ethane-1,2-diamine was analyzed for its cytotoxic potential using the Osiris protocol from its official website (https://www.organic-chemistry.org/prog/peo/). Parts of the Lipinski rule of five important parameters were utilized for quantification in order to trace their biological functions.
Anopheles stephensi and Aedes aegypti cultures
Developmental instars of Anopheles stephensi and Aedes aegypti eggs were maintained at the following conditions of the laboratory: 27 ± 2 °C, 75–85% R.H. and 14 h:10 h (L:D) photoperiod.
Toxicity effects on developmental instars of Aedes aegypti and A. stephensi
The mosquitoes A. aegypti and A. stephensi were cultured and maintained following Murugan et al.37. For toxicology studies, 25 individuals of both A. stephensi and A. aegypti larva (1st, 2nd, 3rd, and 4th) and pupae were placed for a 24 h treatment in a tank filled with 500 mL of distilled water at concentrations of 4,7-dichloroquinoline (2, 4, 6, 8 and 10 ppm)38. In each treatment, 3 replications were carried out, in addition to negative controls. Mortality rate in percentage was studied applying the following formula:
Antiplasmodial cell culture assays on P. falciparum
CQ-sensitive strain 3D7 and CQ-resistant strain INDO of Plasmodium falciparum were used to test the antimalarial activity of 4,7-dichloroquinoline. They were maintained according to the method described by Murugan et al.39. Formulations of 4,7-dichloroquinoline in DMSO were evaluated by the procedure of Murugan et al.40, modified after Smilkstein et al.41. Microscopic examination of Giemsa stained smear samples of normal Plasmodium falciparum exposed to 4,7-dichloroquinoline was following Bagavan et al.42.
In vivo antiplasmodial assays on P. falciparum
Following the method of Murugan et al.43, male albino mice (weight 27–30 g) were tested. They were maintained as reported by Murugan et al.43. For each experiment, three albino mice were used to test the antimalarial potential of the synthesized compound, 4,7-dichloroquinoline following a four-day inhibition technique by Murugan et al.43. Chloroquine (Sigma-Aldrich, Germany) was used as a positive control drug with normal saline (0.9%) at 5 mg/kg, while the negative control group was treated with 1 mL deionized water. The parasites inoculated in mice were noticed after 4 days of infection through microscopic observations of the blood44. Chemosuppression (%) was analyzed for every concentration of the parasitemia following the method of Argotte et al.45.
Infection and toxicity towards cells
We procured Vero cells from the National Center for Cell Science (NCCS Maharashtra, India). The medium used for cultivation (EMEM) contained 10% fetal bovine serum and was incubated at 37 °C in a 5% CO2 atmosphere. We decreased the serum concentration to 2% when viral cultures were used. As described by Murugan et al.43 Dengue virus type-2 (DEN-2) New Guinea C strain was raised through adopting the cell line and were retrieved after the expression of cytopathic effects (CPE), commonly seven days after infection. Infected viral cells were stored at – 70 °C. Cytotoxicity assays and viral quantification assays were following Sujitha et al.46 with minor modification.
Statistical analysis
Data from Probit analysis allowed the analysis of the effective lethal concentrations of the mosquito larvicidal and pupicidal experiments47. From the drug concentration–response curves the IC50s of Plasmodium were calculated. In vivo antimalarial data were checked for normality and analysed using ANOVA with two factors (i.e. dose and treatment). DEN-2 PFU and cytotoxicity data were determined by ANOVA followed by the HSD test of Tukey with the following probabilities (P = 0.05). All analyses were commonly carried out with the SPSS software package version 16.0.
Results and discussion
N1-(7-chloroquinoline-4-yl) ethane-1, 2-diamine effects analyzed by in-silico approaches
The synthesized compound showed no tumorigenic, irritative, nor reproductively significant effects in silico. Besides, LogP and LogS values (Table 1) indicated that the synthesized compound was hydrophilic with a high probability of being distributed along with hydrophilic environments such as insect lymph or cellular cytosol. Molinspiration analysis indicated that the values regarding, GPCR ligand, kinase inhibitor, nuclear receptor ligand, ion channel modulator, protease inhibitor and enzyme inhibitor scores were high. Molinspiration analysis generally indicated that the larger the value of the score was, the more the compound would have biological effects. Therefore, according to in silico analysis, N1-(7-chloroquinoline-4-yl) ethane-1,2-diamine is likely to affect ion channels, kinases, and some important enzymes. The above results could be related to acute toxicity on young instars of A. aegypti and A. stephensi and highly increased the growth inhibition of Plasmodium falciparum48. The in silico study highlighted that quinoline derivatives (BT24) effectively inhibited all four dengue serotypes (1–4) of infected Vero cells by compound (BT24) binding to the active site of the DENV-2 protease. On the other hand, no cytotoxic in silico results could be corroborated by the effect of Vero cell line studies. The drug likeness value is similar to quinolineb (− 1.65, data not shown) as the compounds are closely related. As a result, the compound could be used for the above mentioned applications.
Table 1.
Informatic analysis results using Osiris (https://www.organic-chemistry.org/prog/peo/) and Molinspiration (https://www.molinspiration.com, Slovensky Grob, Slovakia) software.
| Properties | Compound |
|---|---|
| N1-(7-chloro quinoline -4-yl) ethane-1,2-diamine | |
| Molecular weight (g/mol) | 221 |
| LogP | 1.31 |
| LogS | − 2.99 |
| TPSA | 50.94 |
| GPCR ligand | − 0.01 |
| Ion channel modulator | 0.42 |
| Kinase inhibitor | 0.41 |
| Nuclear receptor ligand | − 0.92 |
| Protease inhibitor | − 0.22 |
| Enzyme inhibitor | 0.24 |
| Mutagenic | 3 |
| Tumorigenic | 1 |
| Irritant | 1 |
| Reproductive effect | 1 |
| Druglikeness | − 1.35 |
The toxicity risk is expressed considering the following number code: (1) no risk (2) medium risk (3) high risk.
Toxicity effect of 4,7-dichloroquinoline on A. aegypti and A. stephensi
In agreement with the current research, Saini et al.49 studied the antimalarial potential of quinoline-pyrazolo pyridine derivatives. Mosquitocidal results revealed that the synthesized 4,7-dichloroquinoline was highly toxic to developmental stages of malarial and dengue vectors providing LC50 values ranging from 4.408 µM/mL (larva I) to 7.958 µM/mL (pupa) for the chosen malaria vector and 5.016 µM/mL (larva I) to 10.669 µM/mL (pupa) for the dengue vector (Table 2). Recently, Rueda et al.50 demonstrated both adulticidal and larvicidal activity of A. aegypti when exposed to synthesized α-amino nitriles. Shao et al.51 showed for hexahydroimidazo [1,2-α] pyridine derivatives that they had excellent pesticidal properties against aphid species. Furthermore, Sun et al.52 highlighted that piperazinedione derivatives were highly toxic on the root-knot nematode Meloidogyne incognita. The K1 strain being resistant against chloroquine (CQ) was shown by Gayam and Ravi53 and that cinnamoylated chloroquine hybrid analogues showed highest antimalarial activity. Lastly, Kondaparia et al.54 found that 4-aminoquinolines showed considerable antimalarial activity on Plasmodium falciparum. It was proposed that death rate caused by 4,7-dichloroquinoline for the different life stages of larval populations of both A. stephensi and A. aegypti may be due to the upregulation of electronegative ions which provided better biological activity on target pests55. Indeed, Rahuman et al.56 reported that Zingiber officinale derived molecules showed toxicity on the 4th larval stages of the dengue vectors belonging to Culex species.
Table 2.
Acute toxicity of synthesized 4,7-dichloroquinoline on young instars of Anopheles stephensi and Aedes aegypti.
| Species | Target | LC50 (LC90) (µM/mL) | 95% Confidence Limit LC50 (LC90) µM/mL | Regression equation | χ2 (df = 4) | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Anopheles stephensi | Larva I | 4.408 (8.145) | 3.001 (6.819) | 5.487 (11.046) | y = 1.512 + 0.343x | 8.699 n.s |
| Larva II | 4.916 (9.160) | 4.469 (8.507) | 5.333 (10.021) | y = 1.484 + 0.302x | 4.132 n.s | |
| Larva III | 5.572 (10.562) | 5.084 (9.726) | 6.043 (11.707) | y = 1.431 + 0.257x | 0.924 n.s | |
| Larva IV | 6.304 (12.102) | 5.767 (10.994) | 6.858 (13.699) | y = 1.393 + 0.221x | 1.586 n.s | |
| Pupa | 7.958 (15.159) | 7.264 (13.347) | 8.849 (18.060) | y = 1.416 + 0.178x | 2.832 n.s | |
| Aedes aegypti | Larva I | 5.016 (12.451) | 4.243 (11.018) | 5.684 (14.725) | y = 0.864 + 0.172x | 0.554 n.s |
| Larva II | 5.998 (14.198) | 5.244 (12.363) | 6.753(17.265) | y = 0.938 + 0.156x | 0.890 n.s | |
| Larva III | 7.838 (17.484) | 6.949 (14.712) | 9.074 (22.646) | y = 1.041 + 0.133x | 0.736 n.s | |
| Larva IV | 9.505 (19.900) | 8.339 (16.360) | 11.504 (26.984) | y = 1.172 + 0.123x | 1.691 n.s | |
| Pupa | 10.669 (20.355) | 9.353 (16.818) | 13.013 (27.294) | y = 1.412 + 0.132x | 0.710 n.s | |
Control no mortality, LC50 lethal concentration that kills 50% of the exposed organisms, LC90 lethal concentration that kills 90% of the exposed organisms, χ2 chi-square value, d.f. degrees of freedom, χ2 0.05 level of significance indicates homogeneity of results.
Antiplasmodial activities
As a result of antiplasmodial assays, when compared to chloroquine, the synthesized 4,7-dichloroquinoline expressed significant growth inhibition against both CQ-resistant (CQ-r) and CQ-sensitive (CQ-s) strains of P. falciparum (Fig. 2). Similarly, Kumawat et al.57 investigated 7-Chloro-4-aminoquinoline derivatives causing moderate growth inhibition on CQ-sensitive P. falciparum (RKL-2). Also, Faruk Khan58 noticed that the cyclen 4-Aminoquinoline anlog, bisquinoline, exhibited in vitro and in vivo antiplasmodial properties on D6 W2 chloroquine-sensitive and chloroquine-resistant strains of P. falciparum with IC50 values of 7.5 nM (D6 CQ-sensitive) and 19.2 nM (W2 CQ-resistance). Very recently, Pinheiro et al.59 showed that quinoline and non-quinoline derivatives were highly effective against both P. falciparum W2 chloroquine-resistant strains of P. falciparum in infected mice. Quinoline drugs exhibited potential inhibitory effect of proteolysis, DNA replication, RNA synthesis and heme polymerization in Plasmodium spp60,61. Additionally, Aboelnaga and El-Sayed62 reported that 7-chloroquinoline derivatives showed significant anticancer activity on cervical (Hela) cancer cell lines, human breast cancer (MCF-7) and colon carcinoma (HCT-116). Protein kinase inhibitors, topo isomerase inhibitors, carbonic anhydrase inhibitors, Hsp90 inhibitors are the anticancer mechanisms of quinoline derivatives63. Aderibigbe et al.64 found that polymer loaded aminoquinoline were highly potent against the strain of P. falciparum which was chloroquine-sensitive. A new quinoline derivative, thiazolyl hydrazone were synthesized as effective antifungal and anticancer agents by Erguc et al.65.
Figure 2.
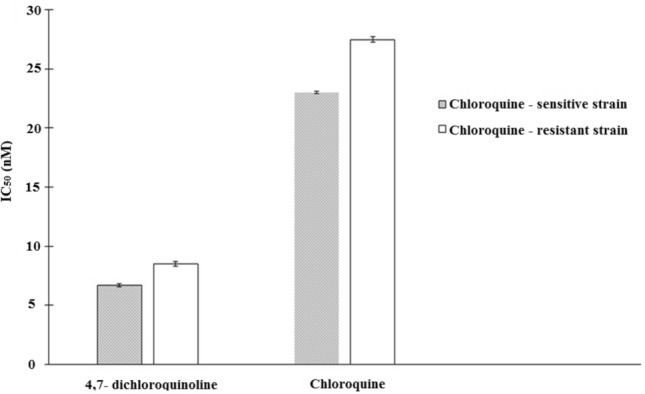
In vitro growth inhibition of chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum post-treatment with 4,7-dichloroquinoline and chloroquine. T-bars represent standard deviations.
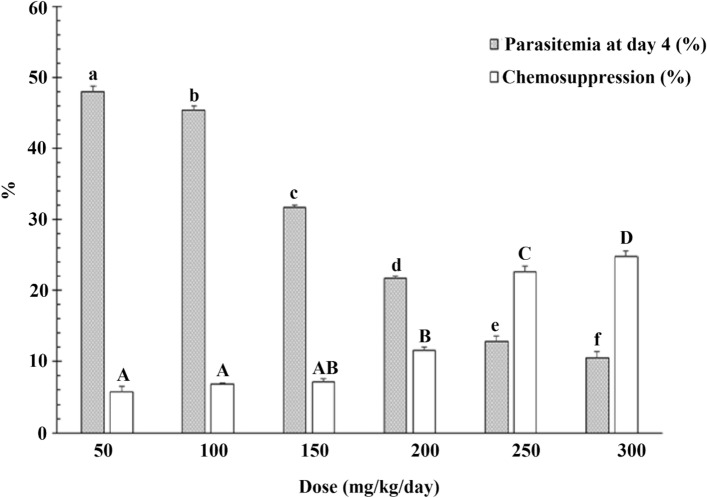
Dose-dependent chemosuppression against P. falciparum was demonstrated by Peters’ 4-day chemo-suppressive activity assay (Fig. 3). After 4 days of 4,7-dichloroquinoline treated groups exhibited the percentage of parasitemia 10.6 ± 0.8% at 300 mg/kg/day than that of the control drug chloroquine (CQ) 1.0 ± 0.0%37,66. Tang et al.67 showed antimalarial activities against the P. falciparum strain K173 with EC50 values ranging from 0.38 to 0.43 mg/kg. Manohar et al.68 found that 4-Aminoquinoline-pyrimidine hybrids exhibited 80% parasitemia suppression as compared to CQ (20%). Finally, Sahu et al.69 found that low doses of tigecycline (3.7 mg/kg) showed 77–91% of parasitaemia suppression. Inhibition of parasitaemia of 77–91% was provided by 3.7 mg/kg dose of tigecycline for 4 consecutive days. Furthermore, the authors reported that in vivo treatment with tigecycline in combination with sub-curative doses of CQ provided 100% mortality of P. falciparum in infected mice.
Figure 3.
In vivo growth inhibition of Plasmodium falciparum parasites infecting albino mice post-treatment with 4,7-dichloroquinoline. Positive control (chloroquine 5 mg/kg/day) led to mean parasitemia of 1.0 ± 0.0% at day 4. T-bars represent standard deviations. Above each column, different letters indicate significant differences (ANOVA, Tukey's HSD, P < 0.05).
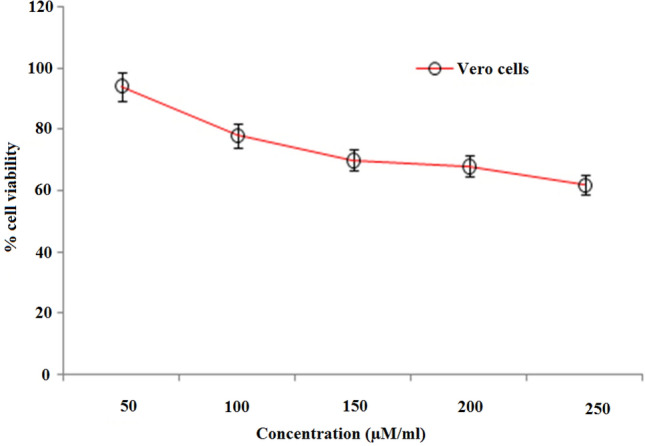
Cytotoxicity effect of 4,7-dichloroquinoline on Vero cells
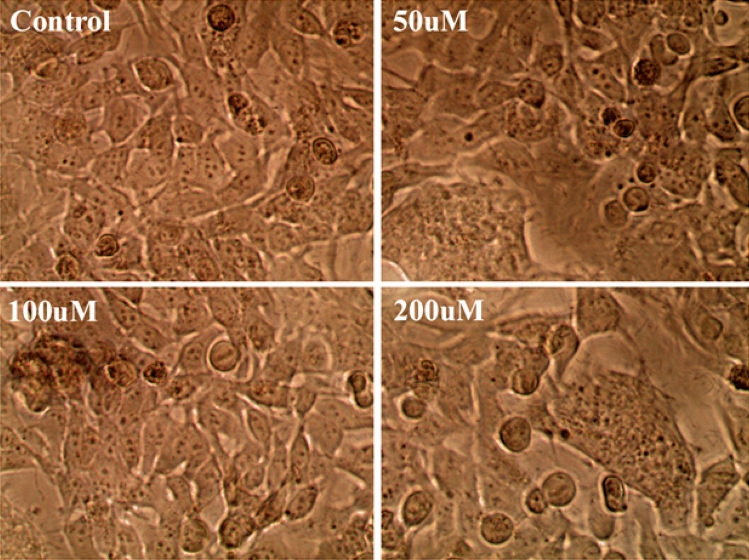
In the present study, the viability of Vero cells was incorporated in various concentrations of 4,7-dichloroquinoline70. We observed that there were no adverse morphological differences in the treated groups when compared to control Vero cells (Figs. 4, 5). For example, Tseng et al.71 studied that the new derivatives of synthesized 2-aroyl-3-arylquinoline compounds provided substantial cytotoxicity against Huh-7 cells with less than 20% viability at doses of 100 μM of 4,7-dichloroquinoline. Cell death above a concentration of 60 μM of 4-methyl pyrimido (5,4-c) quinoline-2,5(1H, 6H)-dione on MDCK cells were shown by Paulpandi et al.34. Recently, Beesetti et al.72 highlighted that quinoline derivatives, BT24 effectively inhibit DENV-2 protease with IC50 of 0.5 μM.
Figure 4.
Cytotoxic effects of 4,7-dichloroquinoline on Vero cells.
Figure 5.
Vero cell viability after the treatment with different concentrations of 4,7-dichloroquinoline.
Antiviral effects of 4,7-dichloroquinoline on dengue and zika virus
Antiviral results showed that the synthesized compound, 4,7-dichloroquinoline tested at 10–40 μg/mL significantly inhibited dengue virus (DENV-2), with a reduction of PFU abundance73 (see also Fig. 6). Furthermore, a plaque assay displayed after an individual exposure and with a minimum dosis that 4,7-dichloroquinoline effectively inhibited the production of dengue viruses. Post 48 h treatment duration of the viral production was 91 PFU/mL in the control, whereas it was 19 PFU/mL, after the treatment of in 4,7-dichloroquinoline at a concentration of 40 μL/mL (Fig. 7). Similarly, Guardia et al.74 discovered that quinoline derivatives highly inhibited DENV-2 with IC50 values ranging from 3.03 to 0.49 μM, respectively. Very recently, Devaux et al.75 found that chloroquine/hydroxychloroquine significantly inhibited pandemic SARS-CoV-2. Furthermore, chloroquine highly inhibited HCoV-229E replication in epithelial lung cell cultures76. It became apparent that the Zika virus provided a regional threat for Latin America and the Caribbean77,78.
Figure 6.
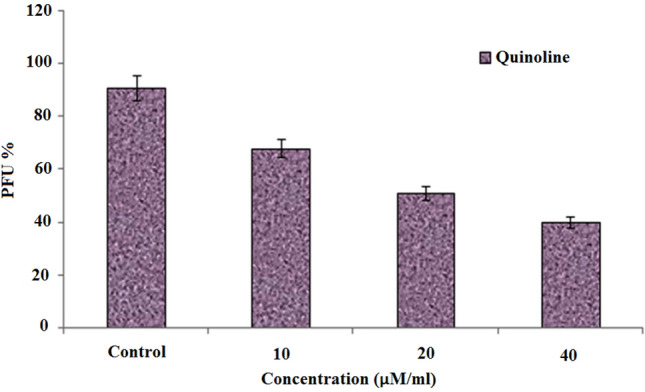
Inhibition of dengue virus (DEN-2) post-treatment with 4,7-dichloroquinoline.
Figure 7.
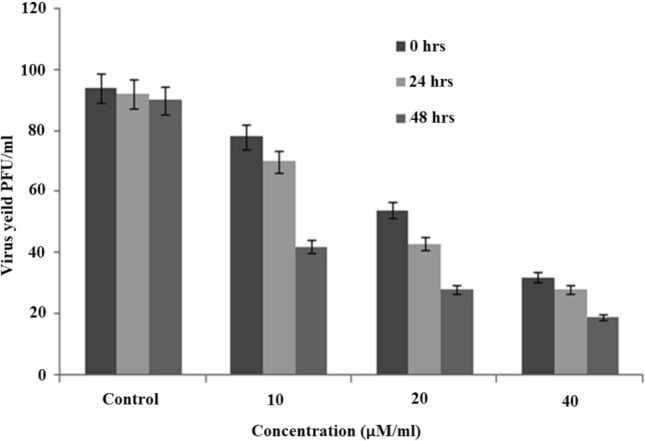
Post treatment reduction in DEN-2 viral yield with 4,7-dichloroquinoline at different time intervals.
Conclusion
It is clear from previous reports that resistance to the malaria vector continues to grow. This is increasingly limiting our ability to control malaria worldwide. Our present study demonstrated the mosquitocidal potential of 4,7-dichloroquinoline derivatives against the key mosquito vectors, An. stephensi and Ae. aegypti. This would be a promising advance in the development of clean, non-toxic, and environmentally acceptable quinoline compounds for their effect against mosquito vectors. Furthermore, 4,7-dichloroquinoline had a significant and promising anti-malarial potential to reduce the global threat malaria. Quinoline decreased virus proliferation and replication during protein synthesis at mRNA levels. No cell cytotoxicity was identified. A compound was recognized as a unique kind of structure different for additional improvement against DENV specialists. We have presented here novel quinoline subordinates that are fundamentally dynamic against dengue infection in a partially subordinate way. The discoveries presented here are significant as a starting point for additional clarification of the particular components of the antiviral action and to pick up the necessary information to additionally grow new, compelling, strong, and safe medications to lessen the risks from viral diseases.
Supplementary Information
Acknowledgements
Financial support from the Ministry of Science and Technology of Taiwan (Grant no. MOST 108-2621-M-019-003, MOST 109-2621-M-019-002, and MOST 110-2621-M-019-001) and the Center of Excellence for Ocean Engineering (Grant no. 109J13801-51 and 110J13801-51) to J.-S. Hwang is acknowledged.
Author contributions
K.M., J.S., J.S.H., R.R., S.P.: Wrote the original draft, conventionalization, experimentation, writing and editing. K.M., J.S.H., L.W., R.R., S.C.M.-S., J.E.D., A.T.A.: Project administration and funding acquisition. J.S., M.P., J.M., C.P., J.E.D., S.M.S., J.P.P., S.P.: Writing, revision, editing, and data curation and results interpretation. K.M., L.W., R.R., D.D., A.T.A., M.R., D.D., C.P., J.M., S.S.S., S.M.S.: Methodology and revision. M.V., B.C., J.M., M.R., J.S.P.-P., S.P.: Instruments, experimentation and synthesis, review and editing. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kadarkarai Murugan, Email: kmvvkg@gmail.com.
Jiang-Shiou Hwang, Email: jshwang@mail.ntou.edu.tw.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08397-5.
References
- 1.Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Mahesh Kumar P, Subramaniam J, Dinesh D, Chandramohan B. Tackling the growing threat of dengue. Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res. 2015;114:1551–1562. doi: 10.1007/s00436-015-4339-9. [DOI] [PubMed] [Google Scholar]
- 2.Somsak V, Polwiang N, Chachiyo S. In vivo anti-malarial activity of Annonamuricata leaf extract in mice infected with Plasmodium berghei. J. Pathogens. 2016;2016:3264070. doi: 10.1155/2016/3264070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karunamoorthi K, Sabesan S. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: A potential threat to global public health. Health Scope. 2013;2(1):4–18. doi: 10.5812/jhs.9840. [DOI] [Google Scholar]
- 4.Mir AH, Qamar A, Qadir I, et al. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 2020;10:1617. doi: 10.1038/s41598-020-58526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Number of Reported Cases of Dengue and Severe Dengue (SD) in the Americas by Country (retrieved on 23rd June 2019). http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 19 March 2017.
- 6.Tavares M, da Silva MR, de Oliveira M, de Siqueira LB, Rodrigues RAS, Bodjolle-d’Almeira L, dos Santos EP, Ricci-Júnior E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018;539(1–2):190–209. doi: 10.1016/j.ijpharm.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Priyanka G, Mandhane Ratnadeep S, Joshi Pravin S, Mahajan MD, Deepak RN, Charansingh HG. Synthesis, characterization, and antimicrobial screening of substituted quiazolinone derivatives. Arab. J. Chem. 2015;8:474–479. doi: 10.1016/j.arabjc.2011.01.025. [DOI] [Google Scholar]
- 8.Shafi S, Kavitha N, Karthi A, Arun A. Synthesis, characterisation, and antimicrobial activity of some novel s-triazine derivatives incorporating a quinoline moiety. Acta. Chim. Pharm. Indica. 2016;6(2):53–56. doi: 10.1016/j.jscs.2015.01.004. [DOI] [Google Scholar]
- 9.Shang XF, Morris-Natschke SL, Liu YQ, Guo X, Xu XS, Goto M, Li JC, Yang GZ, Lee KH. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018;38(3):775–828. doi: 10.1002/med.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon JH, Kim MG, Lee HS. Insecticidal activities of Rutachalepensis leaves isolated constituent and structure-relationships of its analogues against Sitophilus oryzae. J. Korean Soc. Appl. Biol. Chem. 2013;56(5):591–596. doi: 10.1007/s13765-013-3215-5. [DOI] [Google Scholar]
- 11.Sondos M, Bedoui A, Bensalah N. Efficient degradation of chloroquine drug by electro-fenton oxidation: Effects of operating conditions and degradation mechanisms. Chemosphere. 2020;260:127558. doi: 10.1016/j.chemosphere.2020.127558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Chen L, Li X, Gao L, Hao Y, Li B, Yan Y. Novel 4-arylaminoquinazolines bearing N,N-diethyl (aminoethyl) amino moiety with antitumour activity as EGFRwt-TK inhibitor. J. Enzyme Inhib. Med. Chem. 2019;34(1):1668–1677. doi: 10.1080/14756366.2019.1667341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel DB, Rajani DP, Rajani SD, Patel HD. A green synthesis of quinoline-4-carboxylic derivatives using p-toluenesulfonic acid as an efficient organocatalyst under microwave irradiation and their docking, molecular dynamics, ADME-Tox and biological evaluation. J. Heterocycl. Chem. 2020;57(4):1524–1544. doi: 10.1002/jhet.3848. [DOI] [Google Scholar]
- 14.Alagarsamy V, Solomon VR, Dhanabal K. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bio-org. Med. Chem. 2007;15:235–241. doi: 10.1002/ardp.200600189. [DOI] [PubMed] [Google Scholar]
- 15.Antipenko L, Karpenko A, Kovalenko S, Katsev A, Komarovska-Porokhnyavets E, Novikov V, Chekotilo A. Synthesis of new 2-thio-[1,2,4]-triazolo[1,5-c] quinazoline derivatives and its antimicrobial activity. Chem. Pharm. Bull. 2009;57:580–585. doi: 10.1248/cpb.57.580. [DOI] [PubMed] [Google Scholar]
- 16.Rohini R, Muralidhar Reddy P, Shanker K, Hu A, Ravinder V. Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c] quinazolines. Eur. J. Med. Chem. 2010;45:1200–1205. doi: 10.1016/j.ejmech.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Hou X, Wang G, Pan W, Yang X, Zhang Y, Fang H. Design, synthesis and biological evaluation of quinoline derivatives as HDAC class I inhibitors. Eur. J. Med. Chem. 2017;133:11–23. doi: 10.1016/j.ejmech.2017.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Saravanan G, Alagarsamy V, Prakash CR. Synthesis and evaluation of antioxidant activities of novel quinazoline derivatives. Int. J. Pharm. Sci. 2010;2:83–86. doi: 10.1056/NEJMp2009457. [DOI] [Google Scholar]
- 19.Jadhav AG, Halikar NK. Synthesis and biological activity of pyrimido [1, 2- a] quinoline moiety and its 2-substituted derivatives. J. Phys. Conf. Ser. 2013;423:012007. doi: 10.1088/1742-6596/423/1/012007. [DOI] [Google Scholar]
- 20.Li Y, Zhang X, Niu S, et al. Synthesis and biological activity of imidazo [4,5-c] quinoline derivatives as PI3K/mTOR inhibitors. Chem. Res. Chin. Univ. 2017;33:895–902. doi: 10.1007/s40242-017-7074-1. [DOI] [Google Scholar]
- 21.Martinez PDG, Krake SH, Poggi ML, Campbell SF, Willis PA, Dias LC. 2,3,8-Trisubstituted quinolines with antimalarial activity. An Acad. Bras. Cienc. 2018 doi: 10.1590/0001-3765201820170820. [DOI] [PubMed] [Google Scholar]
- 22.Pogrmic-Majkic K, et al. BPA activates EGFR and ERK1/2 through PPARγ to increase expression of steroidogenic acute regulatory protein in human cumulus granulosa cells. Chemosphere. 2019;229:60–67. doi: 10.1016/j.chemosphere.2019.04.174. [DOI] [PubMed] [Google Scholar]
- 23.Yu E, et al. High-yielding continuous-flow synthesis of antimalarial drug hydroxychloroquine. Beilstein J. Org. Chem. 2018;14:583–592. doi: 10.3762/bjoc.14.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rome BN, Avorn J. Drug evaluation during the Covid-19 pandemic. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
- 25.Shaikh AR, Farooqui M, Satpute RH, Abed S. Overview on nitrogen containing compounds and their assessment based on ‘International Regulatory Standards’. J. Drug Deliv. Ther. 2018;8(6):424–428. doi: 10.22270/jddt.v8i6-s.2156. [DOI] [Google Scholar]
- 26.Kerru N, Gummidi L, Maddila S, Gangu KK, Jonnalagadda SB. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules. 2020;25(8):1–42. doi: 10.3390/molecules25081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg HJ, Muto K. Acoustic tracheal rupture provides insights into larval mosquito respiration. Sci. Rep. 2020;10:2378. doi: 10.1038/s41598-020-59321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yung MM, Fougères PA, Leung YH, Liu F, Djurišić AB, Giesy JP, Leung KM. Physicochemical characteristics and toxicity of surface-modified zinc oxide nanoparticles to freshwater and marine microalgae. Sci. Rep. 2017;7(1):15909. doi: 10.1038/s41598-017-15988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efferth T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018;36(6):1730–1737. doi: 10.1016/j.biotechadv.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Mazumder JA, Khan E, Perwez M, et al. Exposure of biosynthesized nanoscale ZnO to Brassica juncea crop plant: Morphological, biochemical, and molecular aspects. Sci. Rep. 2020;10:8531. doi: 10.1038/s41598-020-65271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naseer M, Aslam U, Khalid B, Chen B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020;10:9055. doi: 10.1038/s41598-020-65949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selim YA, Azb MA, Ragab I, Abd El-Azim MH. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020;10:3445. doi: 10.1038/s41598-020-60541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan A, Verma R, Kumari S, et al. Photocatalytic dye degradation and antimicrobial activities of pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020;10:7881. doi: 10.1038/s41598-020-64419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulpandi M, Thangam R, Kavithaa K, Sumathi S, Sankaran M, Mohan PS, Gunasekaran P, Kannan S. Pyrimido quinolin derivative: A potential inhibitor for pandemic influenza A (H1N1) viral growth and its replication. J. Pharm. Res. 2013;6(5):532–537. doi: 10.1038/s41598-020-60541-1. [DOI] [Google Scholar]
- 35.Blake, L. D. Antimalarial Exoerythrocytic Stage Drug Discovery and Resistance Studies. ProQuest Dissertations and Theseses. 172. (2016).
- 36.Manohar S, Tripathi M, Rawat DS. 4-Aminoquinoline based molecular hybrids as antimalarials: An overview. Curr. Top. Med. Chem. 2014;14:1706–1733. doi: 10.2174/1568026614666140808125728. [DOI] [PubMed] [Google Scholar]
- 37.Murugan K, Madhavan J, Samidoss CM, Panneerselvam C, Malathi A, Rajasekar A, Pandiyan A, Kumar S, Alarfaj AA, Higuchi A, Benelli G. Bismuth oxyiodide nanoflakes showed toxicity against the malaria vector Anopheles stephensi and in vivo antiplasmodial activity. J. Clust. Sci. 2018;29(2):337–344. doi: 10.1007/s10876-018-1332-3. [DOI] [Google Scholar]
- 38.Kovendan K, Murugan K, Vincent S, Barnard DR. Studies on larvicidal and pupicidal activity of Leucas aspera Willd (Lamiaceae) and bacterial insecticide, Bacillus sphaericus against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae) Parasitol. Res. 2012;110:195–203. doi: 10.1007/s00436-011-2469-2. [DOI] [PubMed] [Google Scholar]
- 39.Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, Chandramohan B, Subramaniam J, Madhiyazhagan P, Dinesh D, Rajaganesh R, Alarfaj AA, Nicoletti M, Kumar S, Wei H, Canale A, Mehlhorn H, Benelli G. Seaweed synthesized silver nanoparticles: An eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol. Res. 2015;11:4087–4097. doi: 10.1007/s00436-015-4638-1. [DOI] [PubMed] [Google Scholar]
- 40.Murugan K, Dinesh D, Paulpandi M, Althbyani AD, Subramaniam J, Madhiyazhagan P, Wang L, Suresh U, Kumar PM, Mohan J, Rajaganesh R, Wei H, Kalimuthu K, Parajulee MN, Mehlhorn H, Benelli G. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res. 2015;114:4349–4361. doi: 10.1016/j.arabjc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48(5):1803–1806. doi: 10.1128/aac.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagavan A, Rahuman AA, Kaushik NK, Sahal D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol. Res. 2011;108(1):15–22. doi: 10.1155/2020/5041919. [DOI] [PubMed] [Google Scholar]
- 43.Murugan K, Panneerselvam C, Samidoss CM, Madhiyazhagan P, Suresh U, Roni M, Chandramohan B, Subramaniam J, Dinesh D, Rajaganesh R, Paulpandi M, Wei H, Aziz AT, Saleh AM, Devanesan S, Nicoletti M, Pavela R, Canale A, Benelli G. In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res. Vet. Sci. 2016;106:14–22. doi: 10.1016/j.rvsc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Ene AC, Ameh DA, Kwanashie HO, Agomo PU, Atawodi SE. Preliminary in vivo antimalarial screening of petroleum ether, chloroform and methanol extracts of fifteen plants grown in Nigeria. J. Pharmacol. Toxicol. 2008;3(4):254–260. doi: 10.3923/jpt.2008.254.260. [DOI] [Google Scholar]
- 45.Argotte-Ramos R, Ramírez-Avila G, Rodríguez-Gutiérrez MC, Ovilla-Muñoz M, Lanz-Mendoza H, Rodríguez MH, Manasés G-C, Álvarez L. Antimalarial 4-phenylcoumarins from the stem bark of Hintonia latiflora. J. Nat. Prod. 2006;69(10):1442–1444. doi: 10.1021/np060233p. [DOI] [PubMed] [Google Scholar]
- 46.Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, Nicoletti M, Higuchi A, Madhiyazhagan P, Subramaniam J, Dinesh D, Vadivalagan C, Chandramohan B, Alarfaj AA, Munusamy MA, Barnard DR, Benelli G. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015;114(9):3313–3325. doi: 10.1007/s00436-015-4556-2. [DOI] [PubMed] [Google Scholar]
- 47.Finney DJ. Probit Analysis. Cambridge University Press; 1971. pp. 68–78. [Google Scholar]
- 48.Singh A, Kalamuddin MD, Mohmmed A, Malhotra P, Hoda N. Quinoline-triazole hybrids inhibit falcipain-2 and arrest the development of Plasmodium falciparum at the trophozoite stage. RSC Adv. 2019;9:39410. doi: 10.1039/c9ra06571grsc.li/rsc-advances. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saini D, Jain S, Kumar A, Jain N. Synthesis and anti-malarial potential of some novel quinoline-pyrazolopyridine derivatives. EXCLI J. 2016;15:730–737. doi: 10.17179/excli2016-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rueda AG, Carreno Otero AL, Duque JE, Kouznetsov VV. Synthesis of new α-amino nitriles with insecticidal action on Aedes aegypti (Diptera: Culicidae) Revista Brasileira de Entomologia. 2018;62:112–118. doi: 10.1016/j.rbe.2018.01.004. [DOI] [Google Scholar]
- 51.Shao X, Xu Z, Zhao X, Xu X, Tao L, Li Z, Qian X. Synthesis, crystal structure, and insecticidal activities of highly congested hexahydroimidazo [1,2-a] pyridine derivatives: Effect of conformation on activities. J. Agric. Food Chem. 2010;58(5):2690–2695. doi: 10.1021/jf902513t. [DOI] [PubMed] [Google Scholar]
- 52.Sun H, Li H, Wang J, Song G. Synthesis and nematocidal activity of piperazinedione derivatives based on the natural product Barettin. Chin. Chem. Lett. 2017;29(6):977–980. doi: 10.1016/j.cclet.2017.10.015. [DOI] [Google Scholar]
- 53.Gayam V, Ravi S. Cinnamoylated chloroquine analogues: A new structural class of antimalarial agents. Eur. J. Med. Chem. 2017;135:382–391. doi: 10.1016/j.ejmech.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 54.Kondaparia S, Soni A, Manhas A, Srivastava K, Puri SK, Katti SB. Antimalarial activity of novel 4-aminoquinolines active against drug resistant strains. Bioorg. Chem. 2017;70:74–85. doi: 10.1016/s1383-5769(99)00023-9. [DOI] [PubMed] [Google Scholar]
- 55.Cai M, Li Z, Fan F, Huang Q, Shao X, Song G. Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl) ethyl]-4-[3-(trifluoromethyl)phenyl] piperazine (PAPP) J. Agric. Food Chem. 2010;58(5):2624–2629. doi: 10.1021/jf902640u. [DOI] [PubMed] [Google Scholar]
- 56.Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K, Bagavan A. Mosquito larvicidal activity of isolated compounds from the rhizome of Zingiber officinale. Phytother. Res. 2008;22(8):1035–1039. doi: 10.1002/ptr.2423. [DOI] [PubMed] [Google Scholar]
- 57.Kumawat MK, Singh UP, Singh B, Prakash A, Chetia D. Synthesis and antimalarial activity evaluation of 3-(3-(7-chloroquinolin-4-ylamino) propyl)-1,3-thiazinan-4-one derivatives. Arab. J. Chem. 2016;9:S643–S647. doi: 10.1016/j.arabjc.2011.07.007. [DOI] [Google Scholar]
- 58.Faruk Khan MO, Levi MS, Tekwani BL, Khan SI, Kimura E, Borne RF. Synthesis and antimalarial activities of cyclen 4-aminoquinoline analogs. Antimicrob. Agents Chemother. 2009;53(4):1320–1324. doi: 10.1128/AAC.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinheiro LCS, Feitosa LM, Gandi MO, Silveira FF, Boechat N. The development of novel compounds against malaria: Quinolines, triazolpyridines, pyrazolopyridines and pyrazolopyrimidines. Molecules. 2019;24:1–20. doi: 10.3390/molecules24224095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foley M, Tiley L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998;79(1):55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 61.Herraiz T, Guillen H, Gonzalez-Pena D, Aran VJ. Antimalarial quinoline drugs inhibits Hematin and increase free hemin catalyzing peroxidative reactions and inhibition of cysteine proteases. Sci. Rep. 2019;9:15398. doi: 10.1038/s41598-019-51604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aboelnaga A, El-Sayed TH. Click synthesis of new 7-chloroquinoline derivatives by using ultrasound irradiation and evaluation of their biological activity. Green Chem. Lett. Rev. 2018;11(3):254–263. doi: 10.1080/17518253.2018.1473505. [DOI] [Google Scholar]
- 63.Afzal O, Kumar S, Haider MR, Ali MR, Kumar R, Jaggi M, Bawa S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015;97:871–910. doi: 10.1016/j.ejmech.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 64.Aderibigbe BA, Neuse EW, Sadiku ER, Ray SS, Smith PJ. Synthesis, characterization, and antiplasmodial activity of polymer-incorporated aminoquinolines. J. Biomed. Mater. Res. Part A. 2013;102(6):1941–1949. doi: 10.1002/jbm.a.34866. [DOI] [PubMed] [Google Scholar]
- 65.Erguc A, Altintop MD, Atli O, Sever B, Iscan G, Gormus G, Ozdemir A. Synthesis and biological evaluation of new quinoline-based Thiazolyl hydrazone derivatives as potent antifungal and anticancer agents. Lett. Drug Des. Discovery. 2018;15(2):193–202. doi: 10.2174/1570180814666171003145227. [DOI] [Google Scholar]
- 66.Theerthagiri J, Madhavan J, Murugan K, Samidoss CM, Kumar S, Higuchi A, Benelli G. Flower-like copper sulphide nanocrystals are highly effective against chloroquine-resistant Plasmodium falciparum and the malaria vector Anopheles stephensi. J. Cluster Sci. 2017;28(1):581–594. doi: 10.1007/s10876-016-1128-2. [DOI] [Google Scholar]
- 67.Tang L, Bei Z, Song Y, Xu L, Wang H, Zhang D, Dou Y, Ly K, Wang H. Synthesis and in vivo antimalarial activity of novel naphthoquine derivatives with linear/cyclic structured pendants. Future Med. Chem. 2017;9:11. doi: 10.4155/fmc-2017-0058. [DOI] [PubMed] [Google Scholar]
- 68.Manohar S, Rajesh UC, Khan SI, Tekwani BL, Rawat DS. Novel 4-Aminoquinoline-pyrimidine based hybrids with improved in vitro and in vivo antimalarial activity. ACS Med. Chem. Lett. 2012;3:555–559. doi: 10.1021/ml3000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahu R, Walker LA, Tekwani BL. In vitro and in vivo anti-malarial activity of tigecycline, a glycylcycline antibiotic, in combination with chloroquine. Malar. J. 2014;13(1):414. doi: 10.1186/1475-2875-13-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murugan K, Aruna P, Panneerselvam C, Madhiyazhagan P, Paulpandi M, Subramaniam J, Rajaganesh R, Wei H, Alsalhi MS, Devanesan S, Nicoletti M, Syuhei B, Canale A, Benelli G. Fighting arboviral diseases: Low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol. Res. 2016;115:651–662. doi: 10.1007/s00436-015-4783-6. [DOI] [PubMed] [Google Scholar]
- 71.Tseng CH, Lin CK, Chen YL, Hsu CY, Wu HN, Tseng CK, Lee CJ. Synthesis, antiproliferative and anti-dengue virus evaluations of 2-aroyl-3-arylquinoline derivatives. Eur. J. Med. Chem. 2014;79:66–76. doi: 10.1016/j.ejmech.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 72.Beesetti H, Tyagi P, Medapi B, Siva Krishna V, Sriram D, Khanna N, Swaminathan S. A quinoline compound inhibits the replication of dengue virus serotypes 1–4 in vero cells. Antivir. Ther. 2018;23(5):385–394. doi: 10.3851/IMP3231. [DOI] [PubMed] [Google Scholar]
- 73.Santos VS, Vieira JEL, Pereira BB. Association of low concentrations of pyriproxyfen and spinosad as an environment-friendly strategy to rationalize Aedes aegypti control programs. Chemosphere. 2020;247:125795. doi: 10.1016/j.chemosphere.2019.125795. [DOI] [PubMed] [Google Scholar]
- 74.Guardia GDL, Stephens DE, Dang HT, Quijada M, Larionov OV, Lleonart R. Antiviral activity of novel quinoline derivatives against dengue virus serotype 2. Molecules. 2018;23:672. doi: 10.3390/molecules23030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colón-González FJ, Peres CA, Steiner São Bernardo C, Hunter PR, Lake IR. After the epidemic: Zika virus projections for Latin America and the Caribbean. PLoS Negl. Trop. Dis. 2017;11(11):1–19. doi: 10.1371/journal.pntd.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amuthavalli P, Hwang JS, Dahms HU, Wang L, Anitha J, Vasanthakumaran M, Gandhi DM, Murugan K, Subramaniam J, Paulpandi M, Chandramohan B, Singh S. Zinc oxide nanoparticles using plant Lawsonia inermis and their mosquitocidal, antimicrobial, anticancer applications showing moderate side effects. Sci. Rep. 2021;11:8837. doi: 10.1038/s41598-021-88164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.