Abstract
Background
Detection of the ROS1 rearrangement is mandatory in patients with advanced or metastatic non-small cell lung cancer (NSCLC) to allow targeted therapy with specific inhibitors. However, in Spanish clinical practice ROS1 determination is not yet fully widespread. The aim of this study is to determine the clinical and economic impact of sequentially testing ROS1 in addition to EGFR and ALK in Spain.
Methods
A joint model (decision-tree and Markov model) was developed to determine the cost-effectiveness of testing ROS1 strategy versus a no-ROS1 testing strategy in Spain. Distribution of ROS1 techniques, rates of testing, positivity, and invalidity of biomarkers included in the analysis (EGFR, ALK, ROS1 and PD-L1) were based on expert opinion and Lungpath real-world database. Treatment allocation depending on the molecular testing results was defined by expert opinion. For each treatment, a 3-states Markov model was developed, where progression-free survival (PFS) and overall survival (OS) curves were parameterized using exponential extrapolations to model transition of patients among health states. Only medical direct costs were included (€ 2021). A lifetime horizon was considered and a discount rate of 3% was applied for both costs and effects. Both deterministic and probabilistic sensitivity analyses were performed to address uncertainty.
Results
A target population of 8755 patients with advanced NSCLC (non-squamous or never smokers squamous) entered the model. Over a lifetime horizon, the ROS1 testing scenario produced additional 157.5 life years and 121.3 quality-adjusted life years (QALYs) compared with no-ROS1 testing scenario. Total direct costs were increased up to € 2,244,737 for ROS1 testing scenario. The incremental cost-utility ratio (ICUR) was 18,514 €/QALY. Robustness of the base-case results were confirmed by the sensitivity analysis.
Conclusions
Our study shows that ROS1 testing in addition to EGFR and ALK is a cost-effective strategy compared to no-ROS1 testing, and it generates more than 120 QALYs in Spain over a lifetime horizon. Despite the low prevalence of ROS1 rearrangements in NSCLC patients, the clinical and economic consequences of ROS1 testing should encourage centers to test all advanced or metastatic NSCLC (non-squamous and never-smoker squamous) patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09397-4.
Keywords: C-ros oncogene 1, Non-small cell lung cancer, Molecular testing, Biomarker guided selection, Cost-effectiveness analysis
Background
Lung cancer (LC) has a high incidence rate worldwide and is the main cause of cancer deaths (18.0% of all cancer deaths), so it represents a major health problem [1–4]. In Spain, according to the Spanish Society of Medical Oncology (SEOM), in 2020 lung cancer was responsible for the highest number of cancer deaths in Spain, causing 22,930 deaths (20.3% of all cancer deaths) [3].
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer cases and is classified into several histological subtypes, of which adenocarcinoma is the most common (55–60% of LC) [5]. In these histological subtypes, a wide variety of oncogenic driver alterations have been described, such as the presence of translocations or rearrangements of the anaplastic lymphoma kinase (ALK) gene, mutations in the epidermal growth factor receptor (EGFR) gene, rearrangements of the c-ros oncogene 1 (ROS1) gene, and also the presence of aberrant expression of programmed death-ligand 1 (PD-L1) [1]. Specifically, the ROS1 gene encodes a receptor with tyrosine kinase activity that is altered by chromosomal rearrangement in several tumor types, including LC where it can be detected in approximately 1% of NSCLC patients and appears to be associated with low tobacco exposure and adenocarcinoma histology [1, 6].
Patients with advanced LC generally have a poor prognosis; however, the advent of targeted therapy directed to oncogenic genetic alterations has created a new landscape, especially in NSCLC treatment, providing significant improvements in survival and quality of life [7, 8]. The growing number of targeted therapies to EGFR and ALK alterations has resulted in a rapid change in the prognostic of these subtype of NSCLC patients [9]. In particular, targeted therapy with specific inhibitors of ROS1 rearrangements in patients with advanced NSCLC has shown longer overall survival than patients treated with conventional chemotherapy. According to several studies, long-term disease control exerted by crizotinib in patients with ROS1 rearrangement is almost double that the control obtained in patients with ALK alterations [6, 10–13]. In addition, other drugs, such as entrectinib, brigatinib, lorlatinib and ceritinib, are being studied to treat patients harboring ROS1-positive cancers [1], but at the time of the analysis they are not yet available, although they are at different stages of the approval, pricing and reimbursement process.
In Spain, the SEOM and the Spanish Society of Pathology (SEAP) have published a clinical guideline to guide biomarker testing in patients with advanced NSCLC [1]. According to national and international recommendations for molecular diagnosis in advanced NSCLC patients, molecular testing of EGFR and BRAF mutations, ALK and ROS1 rearrangements and PD-L1 expression are considered mandatory [1, 14]. ROS1 rearrangement should be tested in patients with advanced stage (IIIB-IV) non-squamous NSCLC, regardless of its clinical characteristics and should not be tested in squamous cell carcinoma (except in the context of patients with no or low tobacco exposure and younger than 50 years) [1, 7, 14]. However, although the determination of ROS1 is mandatory according to guidelines, real-world evidence obtained from Lung Cancer Biomarker Testing Registry (LungPath) show that ROS1 fusions were not determined in almost half of the samples of patients with NSCLC (testing rate: 58.1%) [15]. According to the Thoracic Tumor Registry (TTR), an observational study also conducted in Spanish hospitals (up to the year 2018), showed even lower ROS1 tests (testing rate [with FISH]: 11.6%]) [16]. This low rate of ROS1 testing may be due to the low prevalence of ROS1 rearrangements in patients with NSCLC that could discourage its determination in some centers, also conditioned by limited diagnostic and/or sampling resources [1, 6, 15].
Essentially, there are three methodological approaches to detecting ROS1 rearrangements: immunohistochemistry (IHC), cytogenetic techniques (particularly fluorescent in situ hybridization [FISH], and molecular techniques such as real-time polymerase chain reaction (RT-PCR) or next-generation sequencing (NGS) [1, 17]. To determine ROS1 translocation in clinical specimens, national and international guidelines recommend IHC as the screening method and confirmation of positive cases with another orthogonal method (cytogenetic or molecular) like FISH [1, 7]. FISH is often considered the gold-standard in the detection of ROS1 rearrangement, although RT-PCR and NGS (DNA- or RNA-based) also show accurate results in most published studies [18–21].
Based on the clinical implications of ROS1 fusion detection in NSCLC patients, it is crucial to accurately identify ROS1 alterations while minimizing response time [1, 22]. The importance of testing for other biomarkers, such as ALK, has already been quantified in Spain by Nadal et al. [23], however, it has not been quantified for the determination of a less prevalent biomarker such as ROS1. For this reason, the main objective of this analysis was to quantify the clinical and economic impact of ROS1 determination in patients with advanced NSCLC in Spain, comparing a testing ROS1 strategy with sequentially testing ROS1 in addition to EGFR and ALK versus a no-testing ROS1 strategy.
Methods
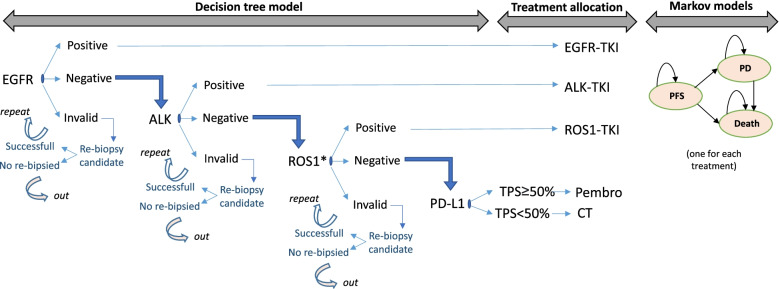
In line with the previous model developed by Nadal et al. [23], a joint model combining a decision-tree with Markov models was developed to determine long-term health results and associated costs of patients with NSCLC, but in this case by comparing a testing ROS1 strategy by comparison against a no-ROS1 testing strategy in Spain, using Microsoft Excel (Fig. 1).
Fig. 1.
Joint model diagram combining a decision-tree model with Markov model. * ROS1 determined by IHC, FISH, reflex or NGS in ‘ROS1-testing’ scenario. Not determined in ‘no-ROS1-testing’ scenario. EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; ROS1: c-ros oncogene 1; PD-L1: programmed death-ligand 1; pembro: pembrolizumab monotherapy; CT: Chemotherapy; TKI: Tyrosine kinase inhibitors; PFS: progression-free survival; PD: progression disease
The decision-tree models comprise the diagnostic phase, where the sequential determination of EGFR, ALK, ROS1 and PD-L1 were established. In case of a positive result for any of these biomarkers, the patient exits the model and receives the corresponding targeted treatment. In the model, in case of a negative result for EGFR, ALK and ROS1 (defined as wild type [WT] patients), the level of PD-L1 expression is determined and the result is categorized as Tumor Proportion Score (TPS) ≥ 50% or TPS < 50%. This threshold of PD-L1 expression was defined based on the indication of pembrolizumab monotherapy for patients with high PD-L1 expression without oncogenic alterations in EGFR and ALK. At some point, the results for EGFR, ALK and ROS1 can also be invalid, in which case patients will be direct candidates for re-biopsy. In the no-ROS1 testing strategy, the sequence shown in Fig. 1 excludes only ROS1, so in case of a negative result for EGFR and ALK, patients are directly considered as WT patients, and then the level of PD-L1 expression is determined.
Based on the determination results, a specific treatment is assigned (Fig. 1) and patients enter in the respective Markov model with different long-term clinical and economic outcomes. The Markov models are developed following an area under the curve structure with three health states: progression-free survival (PFS state), progressed-disease (PD state), and death state (absorbent state).
In line with the recommendations by the guidelines for the evaluation of health technologies in Spain, the duration of the Markov cycle was 1 month, the time horizon was 20-years (lifetime) and the discount rate for future costs and effects was 3% [24, 25].
The analysis was performed from the perspective of the Spanish National Health System (NHS), so only direct medical costs were considered (expressed in € 2021). The health consequences include life years (LY), progression-free life years (PF-LY) and quality-adjusted life years (QALYs).
The included parameters, the assumptions made as well as the clinical feasibility of the results were validated by a multidisciplinary group of oncologists and pathologists, who are also the authors of this article.
Target population
The definition of the target population was similar to the one used in the previous model developed by Nadal et al. (2021) [23]. A hypothetical cohort of patients with advanced or metastatic NSCLC, who were ‘theoretical’ candidates for the molecular diagnosis, was initially estimated. Therefore, both patients with non-squamous histology and those with squamous NSCLC who were never smokers were considered, following the current clinical guidelines for molecular diagnosis in advanced NSCLC [1].
The estimation of the target population is shown in Table 1.
Table 1.
Estimated target population
| % | Referencia | n | ||
|---|---|---|---|---|
| 1 | Patients with lung cancer in 2020 | [26] | 29,188 | |
| 2 | Patients with NSCLC | 85.0% | [14] | 24,810 |
| 3 | Patients with stage IV NSCLC with sample available | 54.5% | [27] | 13,521 |
| 4 | Patients with stage IV NSCLC non-squamous subtype | 66.9% | [16] | 9046 |
| 5 | Patients with stage IV NSCLC squamous subtype | 33.1% | [16] | 4476 |
| 6 | Patients with stage IV NSCLC squamous subtype, never smokers | 11.9% | [28] | 533 |
| 7 | Candidate for molecular diagnosis (steps 4 + 6) | 9579 | ||
| 8 | Patients finally tested for EGFR (testing rate) | 91.4% | [15] | 8755 |
NSCLC Non-small cell lung cancer, EGFR Epidermal growth factor receptor
As shown in Fig. 1, the diagnostic sequence starts with EGFR, so of the theoretical patients estimated in Table 1, only those finally tested for EGFR entered the model.
Decision-tree parameters
All the inputs that are used in the decision-tree sub-model are listed in Table 2.
Table 2.
Main decision-tree inputs
| Input | Reference | |
|---|---|---|
| Invalid results and positivity rate of selected biomarkers | ||
| EGFR (% positive / % invalid) | 13.6% / 1.70% | [15] |
| ALK (% positive / % invalid) | 3.4% / 2.60% | [15] |
| ROS1 (% positive / % invalid) | 1.0% / 3.30% | [15] |
| PD-L1 (TPS ≥ 50%) (% positive / % invalid) | 32.5% / 1.00% | Expert panel / [15] |
| Probability of re-biopsy | ||
| Percentage of invalid results re-biopsied | 33.3% | Expert panel |
| ROS1 determination strategies | ||
| IHC | 10.0% | Expert panel |
| FISH | 30.0% | Expert panel |
| REFLEX to FISH | 55.0% | Expert panel |
| NGS | 5.0% | Expert panel |
EGFR Epidermal growth factor receptor, ALK Anaplastic lymphoma kinase, ROS1 C-ros oncogene 1, PD-L1 Programmed death-ligand 1, IHC Immunohistochemistry, FISH Fluorescent in situ hybridization, NGS Next-generation sequencing
As shown in Fig. 1, the results of the biomarker determinations can be informative (positive or negative) or invalid, mainly due to insufficient sample. The positivity rates for EGFR, ALK and ROS1 determinations were obtained from Lungpath database while the PD-L1 positivity rate (considering TPS > 50% as the threshold for positivity) was agreed by the expert panel, given that the PD-L1 positivity rate obtained from Lungpath probably reflects a mixture of positivity rates with different thresholds depending on the center. Invalid rates for each biomarker were obtained from the Lungpath database. On the other hand, based on the experience of the experts, repeating invalid results does not usually give informative results, so it was assumed that invalid results would be direct candidates for re-biopsy (considered successful by experts in only 33.3% of cases). When re-biopsy is unsuccessful, patients receive doublet of chemotherapy if the molecular diagnosis of EGFR is unknown (due to an invalid result at the beginning of the sequential determination), or chemo-immunotherapy (the same received by patients with TPS < 50% or unknown PD-L1) if the invalid result was obtained for ALK or ROS1 but the diagnosis of EGFR is known and negative.
The current distribution of ROS1 determination techniques in Spain included in the model was obtained from the panel of experts, as the data provided by Lungpath reflects clinical practice in 2008 and does not correspond with current guideline recommendations where reflex to FISH is mandatory for ROS1 determination. In the base case, the accuracy of the techniques is not taken into account, so that the specificity and sensitivity of all ROS1 determination techniques were assumed to be 100%. For this reason, in the base case, the distribution of ROS1 detection techniques only has an impact in terms of costs (not on health outcomes). However, an alternative scenario to the base case has been explored where the accuracy of the ROS1 determination techniques is considered. The parameter values for IHC were obtained from the values of the IHC clones from the ROSING study [21] and the distribution of clones agreed by the experts (70% SP384, 30% D4D6). The resulting sensitivity and specificity of IHC was 90.9 and 99.0%, respectively. For FISH, the specificity and sensitivity values agreed by the experts were 99 and 95%, respectively, and for NGS, the values of both parameters were assumed to be 100% despite that in rea-life testing the sensitivity and sensitivity of NGS would not be 100%.
The specific costs of the decision-tree were the cost of re-biopsy and the costs of the tests used for molecular diagnosis. For the re-biopsy, a cost of € 385.41 was considered. It was calculated by weighting the distribution of the type of biopsy performed (biopsy, cytology, blood: 65, 30, 5%, respectively) as reported by experts and the cost of each of the biopsy techniques (€ 555.70, € 58.70, € 131.84, respectively) [29]. The costs of the tests were agreed by the expert panel, taking into consideration the market price: € 70 for IHC; € 110 for FISH; € 455 for NGS; € 120 for the EGFR test; € 76 for the ALK test; and € 70 for the PD-L1 test [29].
As described in Table 3, thirteen treatments and inclusion in clinical trials were included in the analysis, and for each, costs and long-term outcomes are quantified using a specific Markov model. The expert panel established the distribution of all the most common first-line treatments in Spain for each molecular profile (Table 3).
Table 3.
Distribution of treatments according to molecular diagnosis
| Molecular diagnosis | Treatment | Distribution |
|---|---|---|
| ALK+ | Alectinib | 95% |
| Crizotinib | 5% | |
| EFGR+ | Erlotinib | 3,3% |
| Gefitinib | 6,7% | |
| Afatinib | 11.7% | |
| Osimertinib | 76.3% | |
| Dacomitinib | 2% | |
| ROS1+ | Crizotinib | 95% (40%a) |
| Clinical trials | 5% | |
| Entrectinib | 0% (55%a) | |
| WT | ||
| TPS ≥50% | Pembrolizumab monotherapy | 90% |
| Cisp+pmtrx | 5% | |
| Clinical trials | 5% | |
| TPS < 50% | Cisp+pmtrx | 25% |
| Carb+paclitx+beva | 5% | |
| Cisp+pmtrx+pembrolizumab | 60% | |
| Carb+ paclitx +beva+atezolizumab | 10% | |
EGFR Epidermal growth factor receptor, ALK Anaplastic lymphoma kinase, ROS1 C-ros oncogene 1, WT Wild-type, TPS Tumour proportion score, Cisp Cisplatin, Carb Carboplatin, pmtrx Pemetrexed, paclitx Paclitaxel, beva Bevacizumab
aDistribution considered in the alternative scenario in which entrectinib is a treatment alternative in ROS1-positive patients
An alternative scenario to the base case has been explored considering a potential treatment distribution scenario where ROS1-positive patients may also receive entrectinib, as although it is not yet commercially available for these patients in Spain, experts estimate that its use will increase in the future and could have some impact on the model results, unlike other upcoming ALK-targeted therapies that would have negligible effects on the results (Table 3).
Depending on the molecular diagnosis result, patients were assigned to a specific treatment and entered the respective Markov model developed, in which efficacy and costs associated with each treatment were considered.
Markov model parameters
To establish the transition of the hypothetical cohort between the health states of the Markov models and given that the time horizon of the analysis (lifetime) is longer than the observation periods of the clinical trials, it is necessary to extrapolate the Kaplan-Meier curves of PFS and OS to the long term. In the absence of individualized data for all treatments to explore different parametric distributions, the panel of experts assumed, in line with the previous model developed, the exponential models based on the median PFS and OS reported in the respective studies [23].
Median PFS and OS for ALK targeted therapies (alectinib, crizotinib) were obtained from the recent update of the ALEX study [30], except the median OS in the alectinib group was not reached and extrapolation curves were obtained from the alectinib cost-effectiveness model (data on file). Median PFS and OS for EGFR-targeted therapies were obtained from FLAURA study (assuming the same efficacy for afatinib as for erlotinib and gefitinib) [31, 32] and from ARCHER 1050 study for dacomitinib [33]. The PROFILE 1001 study was used for the median PFS and OS of crizotinib as a targeted therapy for ROS1 [10], and for the median PFS and OS of entrectinib, the entrectinib cost-effectiveness model (data on file) was used, given that in its STARTRK-2 study, the median OS has not yet been reached. For WT patients treated with pembrolizumab in monotherapy, median PFS and OS were obtained from KEYNOTE-024 [34, 35] and for WT patients treated with pembrolizumab in combination and cisplatin + pemetrexed, the medians of the parameters were obtained from the KEYNOTE-189 study (comparator and control arm, respectively) [36]. For WT patients with TPS < 50%, median PFS and OS for the remaining two treatment strategies considered in the model were obtained from Sandler et al. (2006) [37] and IMpower150 [38]. For patients entering a clinical trial, a small percentage of ROS1-positive and WT with TPS ≥50% patients, the experts assumed extrapolation with the longest medians of the corresponding therapeutic target (medians PFS and OS of crizotinib and pembrolizumab monotherapy, respectively).
In the alternative scenario considering the accuracy of the ROS1 determination techniques it was necessary to establish the clinical consequences of the false positive (FP) results for ROS1. In this regard, the expert panel agreed to keep the same assumptions made in the previous model developed. Thus, it was considered that most patients will have shown progression at the first follow-up visit and that all of them will have progressed at the second visit, assuming a median PFS of 2 months (considering that no patient exceeds 6 months of treatment -stop rule-) and a median OS of 18 months [23].
The costs of the Markov models included drug acquisition costs (first line and subsequent treatments) [see Additional file 1] and its associated cost of administration in case of intravenous drugs (€ 211) [29].
For the acquisition costs, all drug costs are expressed as the ex-factory price considering the corresponding deductions according to RDL 08/2010 [39, 40] where appropriate. For drugs where the dose depends on the patient’s characteristics, the same demographic characteristics of the hypothetical cohort from the previous model were assumed, that is, a mean body surface area of 1.81 and a mean weight of 72.885 kg [41]. The vial sharing was assumed for intravenous treatment in line also with previous model. For entrectinib, which was not yet priced at the time of analysis, an ex-factory price 10% higher than crizotinib was assumed. Clinical trials are assumed to have no cost to the Spanish NHS.
The efficacy of the treatments (in terms of median PFS and median OS) and the costs associated with them are shown in an additional file [see Additional file 1].
Concerning the costs of subsequent treatments administered once patients progress to first-line treatment, only the costs of second-line treatments were considered in order to simplify the model. Both the proportion of patients who would receive active second-line treatment and those who would receive best supportive care (BSC), as well as the distribution of the most representative second-line treatments (depending on the first-line received) was established by the experts. Median PFS of all subsequent treatments were obtained from the literature [11, 42–46]. The parameters related to the second-line treatments are shown in Table 4.
Table 4.
Definition of subsequent (second-line) treatments
| Molecular diagnosis | First-line treatment | Second-line treatment | |||
|---|---|---|---|---|---|
| Active treatment | Non-treatment | ||||
| % | Treatment | % | Treatment | ||
| ALK+ | Alectinib | 80%a | PbCT/lorlatinib | 20%a | BSC |
| Crizotinib | 80%a | Alectinib | 20%a | BSC | |
| EGFR+ | Osimertinib | 70%a | PbCT | 30%a | BSC |
| Erlotinib/Gefitinib/Afatinib/Dacomitinib | 70%a | Osimertinib | 30%a | BSC | |
| ROS1+ | Crizotinib/Clinical trial/Entrectinib | 80%a | PbCT | 20%a | BSC |
| WT | |||||
| TPS ≥50% | Pembrolizumab/Cisp+pmtrx/Clinical trial | 60.80%b | PbCT | 39.20%b | BSC |
| TPS < 50% | Cisp+pemetrexed | 46.60%c | immunotherapies/doce+nintedanib | 53.40%c | BSC |
| carb+paclitx+beva | 46.60%c | immunotherapies/doce+nintedanib | 53.40%c | BSC | |
| cisp+pmtrx+pembrolizumab | 30.50%c | Doce+nintedanib | 69.50%c | BSC | |
| carb+paclitx+beva+atezolizumab | 30.50%c | Doce+nintedanib | 69.50%c | BSC | |
For the grouping of PbCT/lorlatinib and immunotherapies/doce+nintedanib an arithmetic mean was considered
aexpert panel
b [47]
c [48]
EGFR Epidermal growth factor receptor, ALK Anaplastic lymphoma kinase, ROS1 C-ros oncogene 1, WT wild-type, Carb Carboplatin; pmtrx: pemetrexed, paclitx Paclitaxel, beva Bevacizumab, PbCT Platinum-based chemotherapy, doce Docetaxel, BSC Best supportive care
Regarding the utility values included, the experts decided to use the same values as those applied in the previously developed model (0.814 for the PFS state and 0.725 and 0.470 for the PD state with and without active treatment, respectively) [23, 49].
Sensitivity analysis
The variables used in the model have some uncertainty. To assess and determine the robustness of the results obtained, both deterministic (alternative scenarios to the base case and univariate analysis) and probabilistic sensitivity analyses were performed.
Alternatively to the main analysis (base case), two scenarios (described throughout the article) were also explored within the sensitivity analysis:
Considering a potential scenario in which entrectinib (not present in the base case scenario) is a treatment alternative in ROS1 positive patients (Table 3).
Considering the accuracy of the different techniques for ROS1 determination, where the specificity and sensitivity parameters of the different ROS1 determination techniques are included (detailed in 2.3 Decision-tree parameters section).
In the univariate deterministic analysis (one-way sensitivity analysis), some variables of the model were individually modified, depending on the degree of uncertainty associated with the variable, by 10% or 20% with respect to the base case value.
In the probabilistic sensitivity analysis (PSA), in line with recommendations in the literature [50], 1000 simulations were run by Monte-Carlo method simultaneously modifying all parameters with an established distribution. The biomarker prevalence variables, body weight and body surface area and the probability of re-biopsy in case of an invalid result, were modified by a normal distribution, utility values by a beta distribution, and unit costs were modified following a gamma distribution.
Results
Main analysis (base case)
The results of the base case are reported in Table 5 and are shown graphically in Fig. 2 (health outcomes) and Fig. 3 (cost outcomes).
Table 5.
Base case results: cost-effectiveness of testing ROS1 strategy vs. no-testing ROS1
| Testing ROS1 | No-testing ROS1 | Difference | |
|---|---|---|---|
| Cost of testing | € 2,843,608 | € 2,149,256 | € + 694,352 |
| Cost of re-biopsy | € 72,543 | € 45,451 | € + 27,093 |
| Cost of treatment | € 1,045,898,644 | € 1,044,375,352 | € + 1,523,292 |
| Total costs | € 1,048,814,795 | € 1,046,570,058 | € + 2,244,737 |
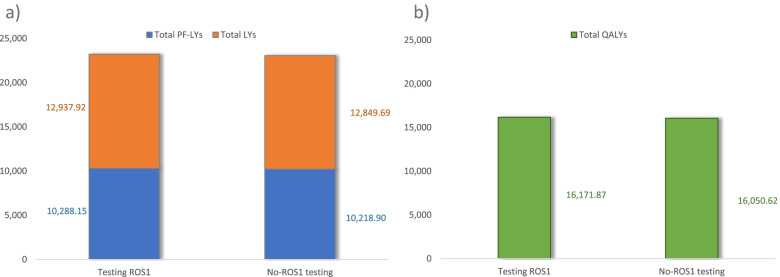
| PF-LYs | 10,288.15 | 10,218.90 | + 69.25 |
| LYs | 23,226.07 | 23,068.58 | + 157.49 |
| QALYs | 16,171.87 | 16,050.62 | + 121.25 |
| ICER (€/LY gained) | € 14,254/LY | ||
| ICUR (€/QALY gained) | € 18,514/QALY | ||
ROS1 C-ros oncogene 1, PF Progression-free, LY Life years, QALY Quality-adjusted life years, ICER Incremental cost-effectiveness ratio, ICUR Incremental cost-utility ratio
Fig. 2.
Long-term health outcomes associated to testing-ROS1 and to no-testing ROS1 strategies, base case. a Health outcomes expressed in total PF-LYs and total LYs; b Health outcomes expressed in total QALYs. ROS1: c-ros oncogene 1; PF:Progression-free life years; LY: life years; QALY: quality-adjusted life years
Fig. 3.
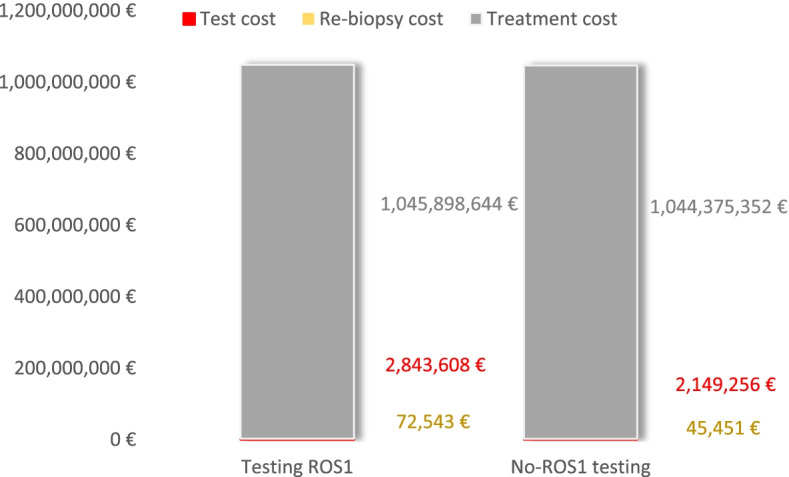
Long term cost outcomes associated to testing-ROS1 and to no-testing ROS1 strategies, base case. ROS1: c-ros oncogene 1
In the defined target population, the strategy of testing-ROS1 in patients with advanced NSCLC provided a gain of 121.25 QALYs compared with the no-testing ROS1 strategy over a 20-year time horizon. Testing ROS1 strategy in these patients also entailed higher costs, including those of the tests themselves and the re-biopsies, but mainly due to the cost of targeted treatments. The comparison of costs and health outcomes through the incremental cost-utility ratio (ICUR), shows that the testing ROS1 strategy in Spain is cost-effective (€ 18,514/QALYs), as it was below the cost-effectiveness thresholds commonly considered in Spain [51, 52].
Sensitivity analysis
The cost and health results of the alternative scenarios are in line with those of the base case (Table 5). The modifications made in both analyses from the base case affect only the costs and health outcomes of the testing ROS1 strategy (no testing ROS1 strategy remained the same).
In the alternative scenario considering a potential future scenario in which entrectinib is available in ROS1-positive patients, the testing ROS1 vs. no-testing ROS1 strategy remains cost-effective, with an ICUR ratio of € 17,652/QALYs (slightly lower than in the base case). According to the results, although the inclusion of entrectinib in the treatment of ROS1-positive patients results in a slight increase in the treatment costs of testing ROS1 strategy compared to the corresponding base case strategy (€ 239,199 more € than in base case vs. € 1,044,375,352 in base case), it also results in an increase in QALYs gained (19.47 QALYs more than in base case; 140.72 QALYs in alternative scenario vs. 121.25 QALYs in base case).
In the alternative scenario that considers the accuracy of the techniques the testing ROS1 vs. no-testing ROS1 strategy is dominant, generating more QALYs at a lower cost. Although in this analysis there is a slight decrease in QALYs gained from the testing ROS1 strategy compared to the corresponding base case strategy (23.1 QALYS less than in base case; 98.15 QALYs in alternative scenario vs. 121.25 QALYs in base case), there is a more significant decrease in the costs of the strategy compared to the base case (€ 8,257,604 less than in base case vs. € 1,048,814,795 in base case), due mainly to the treatment costs.
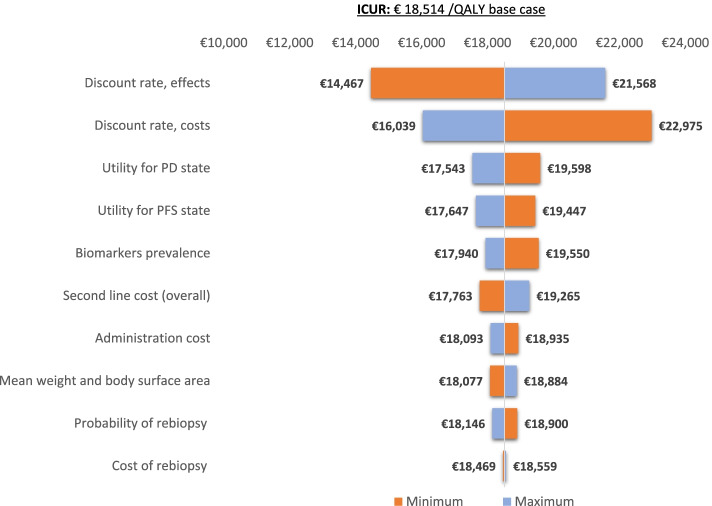
The results of the univariate analysis are represented by a tornado diagram in Fig. 4, showing how the variations of each variable analyzed modify the ICUR of the base case (€ 18,514 /QALYs). Discount rate (for both cost and effects), followed by utilities show the greatest impact on the ICUR of the base case.
Fig. 4.
Tornado diagram representing the results of the univariate analysis. ICUR: incremental cost-utility ratio; QALY: quality-adjusted life years; PD: progressed-disease; PFS: progression-free survival
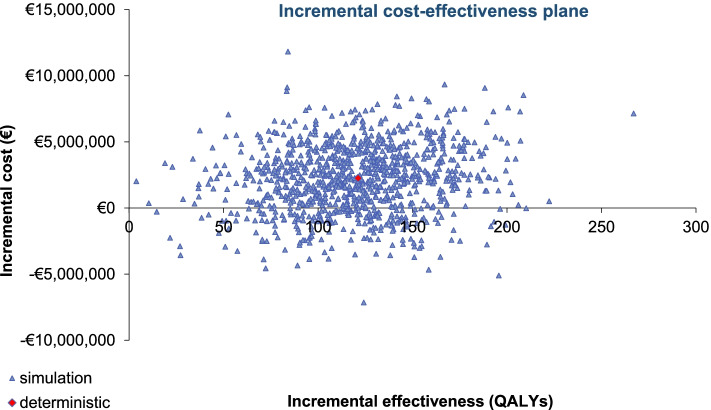
In the PSA, the means obtained from the 1000 simulations (€ + 2,209,967 and 18,456 QALYs gained with respect to no-testing ROS1 strategy) are in line with the deterministic results in Table 5. The graphical representation of the 1000 simulations is provided by an incremental cost-effectiveness plane (Fig. 5).
Fig. 5.
PSA results of 1000 simulations provided by incremental cost-effectiveness plane. QALY: quality-adjusted life years
Discussion
The effectiveness of targeted therapies with tyrosine kinase inhibitors in NSCLC depends on the accurate determination of the genomic status of tumor cells. For this reason, specific biomarker testing by molecular methods is widely recommended by guidelines in patients with advanced NSCLC [7, 53].
In particular, the identification of ROS1 rearrangement in NSCLC patients is mandatory to permit targeted therapy with specific inhibitors, demonstrating an improved overall survival when compared with conventional chemotherapy [1, 6, 7]. Treatment of ROS1-positive NSCLC patients with crizotinib has demonstrated a clinically significant benefit (with more than 19 and 51 months of PFS and OS, respectively), and the benefit of other targeted therapies, such as entrectinib are being studied [1, 10].
The availability of specific inhibitors of ROS1 and their clinical benefit should lead to simultaneous testing of ROS1 rearrangement with other recurrent biomarkers in NSCLC (e.g EGFR and ALK) in all advanced stage never/light smokers with squamous cell carcinoma and non-squamous NSCLC [6]. However, according to the analysis carried out by Salas et al. (2021) [15] in Spain in 2018, despite the relatively high testing rate reported in EGFR and ALK in NSCLC (91.4 and 80.1%, respectively), the real-world evidence obtained from the LungPath registry demonstrates that ROS1 and PD-L1 were not determined in a significant portion of patients (56.2 and 58.1%, respectively) [15].
.Given the incremental importance of ROS1 testing in guiding the treatment of patients with NSCLC and the fact that ROS1 is an under-analyzed biomarker in comparison to ALK or EGFR in Spain [15], the main objective of our study was to assess if testing ROS1 is a cost-effectiveness strategy in Spain and also to raise the awareness of testing ROS1 according the clinical guidelines.
Our study is the first one that evaluate the efficiency of ROS1 determination in the management of patients with advanced NSCLC in Spain using an approach that integrates molecular diagnosis with subsequent pharmacological treatment.
Markov models allowed the calculation of long-term costs, LYs and QALYs for each treatment assigned according to the molecular diagnosis. In addition, the sequential decision-tree allowed comparison of the ROS1 testing strategy vs. no ROS1 testing strategy in Spain (EGFR, ALK and PD-L1 expression were tested in both strategies) and establish the subsequent treatment allocation according to the molecular diagnosis.
Thus, the ICUR obtained (for the base case and sensitivity analyses) demonstrates that the testing ROS1 strategy in patients with advanced NSCLC is a cost-effective strategy as it is below the cost-effectiveness thresholds usually considered in our country [51, 52].
Given that soon entrectinib will be available for ROS1-positive patients as a first-line targeted therapy (currently the only existing treatment is crizotinib), the sensitivity analysis also included entrectinib as a possible treatment for these patients. In this alternative scenario, the strategy of testing ROS1 vs. no testing is also remained as a cost-effective strategy (with a lower ICUR than in the base case). On the other hand, in a scenario that could be closer to real-world practice than the base case, which considers the accuracy of molecular diagnostic techniques, ROS1 testing strategy is shown to be dominant over no-ROS1 testing, with an increase in QALYs at a lower cost. However, given the uncertainty associated with the specificity and sensitivity parameters of the determination techniques, the results of this alternative scenario must be interpreted with caution.
Despite the low prevalence of ROS1 rearrangements in patients with advanced NSCLC (~ 1%), these results confirm that ROS1 testing is of crucial interest for the treatment of patients with advanced NSCLC [1, 6].
Prior to this joint model, the same group of experts developed a similar one to determine the efficiency of management of patients with advanced NSCLC in Spain but focusing on ALK detection [23]. Although both joint models have in common the integration of molecular diagnosis and subsequent treatment depending on the molecular results, they have also some methodological differences. First, the current work analyses the determination of ROS1 within the EGFR, ALK, ROS1 and PD-L1 sequence. In contrast, the previous study focused exclusively on the determination of ALK, and although it included other biomarkers, these were analyzed following ALK testing. In addition, the previous work considered the different ALK testing techniques and their accuracy (specificity and sensitivity), as the objective was to evaluate the current ALK testing scenario. In the present analysis, we focused on whether or not ROS1 was tested, so the accuracy of the ROS1 techniques was assessed only as an alternative scenario. According to the results of both studies, the strategies of testing ALK and ROS1 vs. no testing, not only generate more than 3000 QALYs and 120 QALYs, respectively, and remain cost-effective strategies in the Spanish context (€ 10,142 /QALY and € 18.514 /QALY, respectively) [23].
No other studies that analyze specifically the implications of ROS1 determination in our European context have been identified. A recent Canadian study has compared different diagnostic strategies for ROS1 testing using a decision model, obtaining that reflex IHC screening with FISH confirmation of positive cases yielded the best results for turnaround time, true-positives detection rate, and costs [54]. In a broader context, recent studies have reported on the factors that most affect the biomarker determination value such as that conducted by Safonov et al. (2016) in the United States, and also the cost-effectiveness of different NSCLC biomarker testing strategies including ROS1 among them, as the study carried out by Schluckebier et al. (2020) from the Brazilian health system perspective [55, 56].
Like all theoretical models, our study has several limitations. Firstly, the complexity of clinical practice cannot be fully captured by pharmacoeconomic models which are designs with a certain degree of intrinsic structural rigidity. In this particular case, capturing and reproducing all phases of pathological and molecular diagnostics is complicated by the fact that, according to the studies identified, they have a high degree of complexity and variation between centers. In line with the previous study focusing on ALK determination, to simplify the model the pre-analytical phase (e.g. response time from diagnosis to initiation of treatment) was not included as there is no evidence that it influences the results.
Certain assumptions have been made to model the treatment of invalid results and the probability of re-biopsy in the diagnostic phase. To simplify the model, it was assumed that after an invalid result there is no repetition of the technique using the same sample, but re-biopsy is considered directly. This assumption was made because, according to expert opinion, repeat testing on the same sample is rarely satisfactory and recoverable tests accounts for less than 10%. Regarding the success rate of re-biopsy, no reference was identified in the literature that according to the experts is in line with their clinical experience, where in 30–35% of cases an optimal re-biopsy specimen is achieved. Thirdly, since the main objective of the analysis was to determine the significance of ROS1 determination and not so much the validation of the testing techniques, a specificity and sensitivity of the techniques of 100% was assumed and validated by experts, which is far from real clinical practice. However, this limitation was evaluated in one of the alternative scenarios.
Fourth, Markov models have some limitations when reproducing at a long term the clinical management of NSCLC patients. On the one hand, as in all cost-effectiveness models where long-time horizons are used, extrapolations are necessary with the associated limitations. On the other hand, as described above, in the absence of individualized data for all treatments included in the analysis, it was assumed the use of exponential models for all treatments, as conducted in Nadal. et al. (2021) [23]. In the same way, no drug-specific utilities were found for all the treatments included in the model. Therefore, utility values associated with the patient’s condition (PFS, PD on-treatment and PD without active treatment) regardless of the treatment received were used, in line with previous economic evaluations [23, 49]. As our analysis focuses on molecular diagnosis and subsequent first-line treatment of NSCLC patients, the second-line treatment included in the analysis was entered into the model as a one-off cost and only shows its influence in economic terms.
Fifthly, and in relation to costs, it is difficult to establish the real price of the diagnostic tests and although these were validated by experts, there are large variations between centers and in many cases, as they are not reimbursed by the Spanish NHS, the cost of the tests is mainly assumed by the pharmaceutical companies and research funds in the vast majority of Spanish regions. On the other hand, the fact that the model assumed a sequential determination and that it focused on the determination of ROS1 did not allow us to capture the benefits of using NGS as a technique to evaluate multiple biomarkers simultaneously. An economic assessment of the use of NGS instead of sequential ROS1 testing in addition to EGFR and ALK will require a specifically designed model and is beyond the scope of this study.
Despite these limitations and the associated uncertainty, sensitivity analyses were carried out which confirmed the robustness of the assumptions and results obtained. It should also be noted that the panel of experts validated all the assumptions, parameters considered, and results obtained.
Conclusions
In conclusion, despite the low prevalence of ROS1 rearrangements in advanced NSCLC patients, our analysis shows that testing ROS1 in these patients in Spain is a strategy that would increase and improve patients’ lives and that it is a cost-effective strategy for the Spanish NHS. For this reason, this analysis should encourage that not even a single ROS1-positive patient goes undiagnosed.
Supplementary Information
Additional file 1: Table 1. Efficacy (median PFS and median OS) and costs associated with each first-line treatments.
Acknowledgements
Not applicable.
Abbreviations
- ALK
Anaplastic lymphoma kinase
- Beva
Bevacizumab
- BSC
Best supportive care
- Carb
Carboplatin
- Cisp
Cisplatin
- CT
Chemotherapy
- Doce
Docetaxel
- EGFR
Epidermal growth factor receptor
- FISH
Fluorescent in situ hybridization
- FP
False positive
- ICER
Incremental cost-effectiveness ratio
- ICUR
Incremental cost-utility ratio
- IHC
Immunohistochemistry
- LC
Lung cancer
- LF
Life years
- LungPath
Lung cancer biomarker testing registry
- NGS
Next-generation sequencing
- NHS
National health system
- NSCLC
Non-small cell lung cancer
- Paclitx
Paclitaxel
- PbCT
Platinum-based chemotherapy
- PD
Progression disease
- PD-L1
Programmed death-ligand 1
- PF-LY
Progression-free life years
- PFS
Progression-free survival
- Pmtrx
Pemetrexed
- PSA
Probabilistic sensitivity analysis
- QALYs
Quality-adjusted life years
- ROS1
C-ros oncogene 1
- RT-PCR
Real-time polymerase chain reaction
- SEAP
Spanish society of pathology
- SEOM
Spanish society of medical oncology
- TKI
Tyrosine kinase inhibitors
- TPS
Tumor proportion score
- TTR
Thoracic tumor registry
- WT
Wild type
Authors’ contributions
All authors contributed substantially to the development of the study. NA, JFG and DC designed the analysis. DC developed the model and wrote the first draft of the manuscript. FR, EC, HT, LC-G, DB, IR and EN conformed the panel of experts that collected and validated data inputs and contributed to results interpretation. All the authors read and approved the final manuscript.
Authors’ information
Not applicable.
Funding
This study was funded by Roche Farma S.A.
Availability of data and materials
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
FR received research grant support from Pfizer and Roche and received speaker or consulting fees from Roche, BMS, MSD, Merck, Novartis, Pfizer, AstraZeneca, Genomic Health, Bayer. EC received research grant support from Lilly, Roche and Thermo Fischer, and received speaker or consulting fees from Astra Zeneca, BMS, Lilly, MSD, Pfizer, Roche and Takeda. HT received grants from Roche and AstraZeneca. LCG received speaker or consulting fees from Angelini, Grunenthal, Kyowa Kirin, Mudipharma, Pfizer, Roche, Rovi, Leo Pharma, Merck Serono, Ipsen Pharma, Lilly, Amgen, Boehringer Ingelheim, and AstraZeneca. DB received research support from Roche and AstraZeneca, participated in advisory boards for MSD, Roche, AstraZeneca and Abbie, and received speaker fees from Roche, MSD and AstraZeneca. IR declared that she has no competing interest. EN received research grant support from Pfizer, Roche, Bristol Myers Squibb and Merck Serono and participated in advisory boards or gave lectures for BMS, MSD, Lilly, Roche, Pfizer, Takeda, Boehringer Ingelheim, Amgen, Merck Serono, Sanofi, Bayer and AstraZeneca. NA, JFG and RG are employees of Roche. DC is employee of Hygeia Consulting which received funding from Roche to conduct the analysis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garrido P, Conde E, de Castro J, Gómez-Román JJ, Felip E, Pijuan L, et al. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2020;22(7):989–1003. doi: 10.1007/s12094-019-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felip E, Concha A, de Castro J, Gómez-Román J, Garrido P, Ramírez J, et al. Biomarker testing in advanced non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2015;17(2):103–112. doi: 10.1007/s12094-014-1248-9. [DOI] [PubMed] [Google Scholar]
- 3.SEOM . Sociedad española de oncologia médica. Cifras del cancer en españa. 2020. [Google Scholar]
- 4.Remon J, Reguart N, García-Campelo R, Conde E, Lucena C-M, Persiva O, et al. Lung Cancer in Spain. J Thorac Oncol. 2021;16:197–204. doi: 10.1016/j.jtho.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398(10299):535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 6.Rossi G, Jocollé G, Conti A, Tiseo M, Zito Marino F, Donati G, et al. Detection of ROS1 rearrangement in non-small cell lung cancer: current and future perspectives. Lung Cancer (Auckl) 2017;8:45–55. doi: 10.2147/LCTT.S120172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung Cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the. Arch Pathol Lab Med. 2018;142(3):321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 8.Jonna S, Subramaniam DS. Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med. 2019;27(148):167–170. [PubMed] [Google Scholar]
- 9.Kerr KM, Bibeau F, Thunnissen E, Botling J, Ryška A, Wolf J, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Riely GJ, Bang Y-J, Kim D-W, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(7):1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Jiang T, Zhao C, Li W, Li X, Zhao S, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–75154. doi: 10.18632/oncotarget.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YF, Hsieh MS, Wu SG, Chang YL, Yu CJ, Yang JCH, et al. Efficacy of pemetrexed-based chemotherapy in patients with ROS1 fusion-positive lung adenocarcinoma compared with in patients harboring other driver mutations in east Asian populations. J Thorac Oncol. 2016;11(7):1140–1152. doi: 10.1016/j.jtho.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Song Z, Su H, Zhang Y. Patients with ROS1 rearrangement-positive non-small-cell lung cancer benefit from pemetrexed-based chemotherapy. Cancer Med. 2016;5(10):2688–2693. doi: 10.1002/cam4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 15.Salas C, Martín-López J, Martínez-Pozo A, Hernández-Iglesias T, Carcedo D, Ruiz De Alda L, et al. Real-world biomarker testing rate and positivity rate in NSCLC in Spain: prospective central lung Cancer biomarker testing registry (LungPath) from the Spanish Society of Pathology (SEAP) J Clin Pathol. 2021;75:1–8. doi: 10.1136/jclinpath-2020-207280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provencio M, Carcereny E, Rodríguez-Abreu D, López-Castro R, Guirado M, Camps C, et al. Lung cancer in Spain: information from the thoracic tumors registry (TTR study) Transl Lung Cancer Res. 2019;8(4):461–475. doi: 10.21037/tlcr.2019.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde E, Hernandez S, Benito A, Caminoa A, Garrido P, Lopez-Rios F. Screening for ROS1 fusions in patients with advanced non-small cell lung carcinomas using the VENTANA ROS1 (SP384) rabbit monoclonal primary antibody. Expert Rev Mol Diagn. 2021;21(5):437–444. doi: 10.1080/14737159.2021.1919512. [DOI] [PubMed] [Google Scholar]
- 18.Reguart N, Teixidó C, Giménez-Capitán A, Paré L, Galván P, Viteri S, et al. Identification of ALK, ROS1, and RET fusions by a multiplexed mRNA-based assay in formalin-fixed, paraffin-embedded samples from advanced non-small-cell lung Cancer patients. Clin Chem. 2017;63(3):751–760. doi: 10.1373/clinchem.2016.265314. [DOI] [PubMed] [Google Scholar]
- 19.Davies KD, Le AT, Sheren J, Nijmeh H, Gowan K, Kenneth L, et al. ROS1 rearrangements in a cohort of positive patient samples. J Thorac Oncol. 2019;13(10):1474–1482. doi: 10.1016/j.jtho.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y-L, Yang JC-H, Kim D-W, Lu S, Zhou J, Seto T, et al. Phase II study of Crizotinib in east Asian patients with ROS1-positive advanced non-small-cell lung Cancer. J Clin Oncol off J am Soc. Clin Oncol. 2018;36(14):1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 21.Conde E, Hernandez S, Martinez R, Angulo B, De Castro J, Collazo-Lorduy A, et al. Assessment of a new ROS1 immunohistochemistry clone (SP384) for the identification of ROS1 rearrangements in patients with non–small cell lung carcinoma: the ROSING study. J Thorac Oncol. 2019;14(12):2120–2132. doi: 10.1016/j.jtho.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Doshi S, Ray D, Stein K, Zhang J, Koduru P, Fogt F, et al. Economic analysis of alternative strategies for detection of ALK rearrangements in non small cell lung cancer. Diagnostics. 2016;6(1):1–11. doi: 10.3390/diagnostics6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadal E, Bautista D, Cabezón-Gutiérrez L, Ortega AL, Torres H, Carcedo D, et al. Vol. 21, BMC cancer. Catalan Institute of Oncology. L’Hospitalet de Llobregat: Hospital Duran i Reynals, IDIBELL; 2021. Clinical and economic impact of current ALK rearrangement testing in Spain compared with a hypothetical no-testing scenario; p. 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit. 2010;24(2):154–170. doi: 10.1016/j.gaceta.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Puig-Junoy. Guía y recomendaciones para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut: General Catalunya Dep Salut Serv Català la Salut Barcelona; 2014. p. 9–11. https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/farmaeconomica/caeip/gaeip_publica_castellano_octubre2014_catsalut.pdf.
- 26.GLOBOCAN Global Cancer Observatory. Available from: https://gco.iarc.fr/. [cited 2021 Feb 28]
- 27.Vidal J, Clavé S, De Muga S, González I, Pijuan L, Gimeno J, et al. Assessment of ALK status by FISH on 1000 Spanish non-small cell lung Cancer patients. J Thorac Oncol. 2014;9(12):1816–1820. doi: 10.1097/JTO.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 28.Ruano-Raviña A, Provencio M, Calvo de Juan V, Carcereny E, Moran T, Rodriguez-Abreu D, et al. Lung cancer symptoms at diagnosis: results of a nationwide registry study. ESMO Open. 2020;5(6):e001021. doi: 10.1136/esmoopen-2020-001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisbert R, Brosa M. Healthcare cost database eSalud. Barcelona: Oblikue consulting, S.L. Available from: http://esalud.oblikue.com/. [cited 2021 Feb 28].
- 30.Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim D-W, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(8):1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 31.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung Cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 32.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y-L, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 34.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 35.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with Pembrolizumab versus chemotherapy for metastatic non-small-cell lung Cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(21):2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 37.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 38.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 39.CGCOF. Botplusweb.Base de datos del Consejo General de Colegios Oficiales de Farmacéuticos. Available at: www.portalfarma.com. Available from: https://botplusweb.portalfarma.com/. [cited 2021 Feb 28]
- 40.BOE . Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. 2010. [Google Scholar]
- 41.INE. Instituto Nacional de Estadística. Índice de masa corporal según grupos de edad y periodo. Available from: https://www.ine.es/jaxi/Tabla.htm?path=/t00/mujeres_hombres/tablas_1/l0/&file=d06001.px&L=0. [cited 2021 Feb 28]
- 42.Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, Helland A, et al. Alectinib versus chemotherapy in crizotinibpretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda T, Imai H, Kuwako T, Miura Y, Yoshino R, Kaira K, et al. Efficacy of platinum combination chemotherapy after first-line gefitinib treatment in non-small cell lung cancer patients harboring sensitive EGFR mutations. Clin Transl Oncol. 2015;17(9):702–709. doi: 10.1007/s12094-015-1297-8. [DOI] [PubMed] [Google Scholar]
- 44.Ahn MJ, Tsai CM, Shepherd FA, Bazhenova L, Sequist LV, Hida T, et al. Osimertinib in patients with T790M mutation-positive, advanced non–small cell lung cancer: long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125(6):892–901. doi: 10.1002/cncr.31891. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Piperdi B, Walsh WV, Kim M, Beckett LA, Gucalp R, et al. Randomized phase 2 trial of Pharmacodynamic separation of Pemetrexed and intercalated Erlotinib versus Pemetrexed alone for advanced nonsquamous, Non-small-cell Lung Cancer. Clin Lung Cancer. 2017;18(1):60–67. doi: 10.1016/j.cllc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen R, Tao Y, Xu X, Shan L, Jiang H, Yin Q, et al. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov Med. 2018;26(143):155–166. [PubMed] [Google Scholar]
- 47.Chouaid C, Bensimon L, Clay E, Millier A, Levy-Bachelot L, Huang M, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. 2019;127:44–52. doi: 10.1016/j.lungcan.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Zeng X, Wan X, Peng L, Peng Y, Ma F, Liu Q, et al. Cost-effectiveness analysis of pembrolizumab plus chemotherapy for previously untreated metastatic non-small cell lung cancer in the USA. BMJ Open. 2019;9(12):e031019. doi: 10.1136/bmjopen-2019-031019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of Alectinib vs. Crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung Cancer. Pharmacoeconomics. 2018;36(4):495–504. doi: 10.1007/s40273-018-0625-6. [DOI] [PubMed] [Google Scholar]
- 50.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 51.Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–761. doi: 10.1002/hec.3633. [DOI] [PubMed] [Google Scholar]
- 52.Sacristán JA, Oliva J, Campillo-Artero C, Puig-Junoy J, Pinto-Prades JL, Dilla T, et al. What is an efficient health intervention in Spain in 2020? Gac Sanit. 2020;34(2):189–193. doi: 10.1016/j.gaceta.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Conde E, Rojo F, Gómez J, Enguita AB, Abdulkader I, González A, et al. Molecular diagnosis in non-small-cell lung cancer: expert opinion on ALK and ROS1 testing. J Clin Pathol. 2022;75(3):145–53. 10.1136/jclinpath-2021-207490. Epub 2021 Apr 19. PMID: 33875457. [DOI] [PMC free article] [PubMed]
- 54.Makarem M, Ezeife DA, Smith AC, Li JJN, Law JH, Tsao MS, et al. Reflex ROS1 IHC screening with FISH confirmation for advanced non-small cell lung cancer—a cost-efficient strategy in a public healthcare system. Curr Oncol. 2021;28(5):3268–3279. doi: 10.3390/curroncol28050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safonov A, Wang S, Gross CP, Agarwal D, Bianchini G, Pusztai L, et al. Assessing cost-utility of predictive biomarkers in oncology: a streamlined approach. Breast Cancer Res Treat. 2016;155(2):223–234. doi: 10.1007/s10549-016-3677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schluckebier L, Caetano R, Garay OU, Montenegro GT, Custodio M, Aran V, et al. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer. 2020;20(1):875. doi: 10.1186/s12885-020-07240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Efficacy (median PFS and median OS) and costs associated with each first-line treatments.
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).