Abstract
As a result of anthropogenic action, an increasing amount of toxic organic compounds has been released into the environment. These pollutants have adverse effects on human health and wildlife, which has motivated the development of different types of technologies for the treatment of effluents and contaminated environments. The electrochemical degradation of organic pollutants has attracted the interest of research centers around the world for its environmental compatibility, high efficiency, and affordable cost. In the present study, a bibliometric analysis was performed using the Web of Science database in order to assess the progress of publications related to electrochemical degradation of organic pollutants between the years 2001 and 2021. The data retrieved showed a significant increase in publications related to the topic in the last 20 years. Electrochimica Acta was the magazine responsible for the largest number of publications (291, 6.52%). The studies mainly included the areas of engineering, chemistry, and environmental science ecology. China with a total of 1472 (32.96%) publications dominated research in this area, followed by Spain (436, 9.76%) and Brazil (345, 7.72%). The institutions with the highest number of contributions were the University of Barcelona and the Chinese Academy of Sciences, and the most productive authors were Brillas E. and Oturan M. A. The results of this study provide important references and information on possible research directions for future investigations on electrochemical degradation of organic pollutants.
Keywords: Electrochemical oxidation, Organic contaminants, Electrode materials, Bibliometric study, Scientific production, Environment pollution
Introduction
The expansion of industry and agricultural production, together with the accelerated population growth, led the world to a critical scenario regarding the presence of organic pollutants in the environment. These contaminants include pesticides, pharmaceuticals, personal care products, endocrine disruptors, dyes, aromatic, phenolic compounds (Lu and Astruc 2020), flame retardants, perfluoroalkyl substances, and phthalates (Haines et al. 2017). Many of these substances represent a risk to ecosystems and human health due to their high toxicity (Alharbi et al. 2018). Thus, the development of efficient and economically viable technologies for the degradation of these pollutants becomes urgent.
Recently, many efforts have been made to develop technologies for removing organic pollutants. The techniques commonly employed involve physical adsorption (Zhang et al. 2021), biological degradation (Cheng et al. 2021), photocatalysis (Motora et al. 2021), and advanced oxidation processes (Ma et al. 2021). Among these techniques, electrochemical oxidation has stood out for its environmental compatibility, high efficiency, affordable cost, simple automation, and ability to degrade a wide variety of pollutants (Clematis and Panizza 2021; Jiang et al. 2021).
Electrochemical oxidation of organic compounds can occur in two different ways: direct and indirect electrochemical oxidation. In the case of direct oxidation, the transfer of charges between the anodic surface and the compound to be oxidized takes place directly, without the involvement of other substances. In indirect oxidation, organic compounds are degraded by strong oxidizing species (•OH, H2O2, O3, S2O82−, etc.) electrogenerated by an anodic or cathodic process (Martínez-Huitle and Panizza 2018; Qiao and Xiong, 2021). Among the anodically generated oxidizing species, active chlorine (Cl2, HOCl, or OCl−) is one of the most traditional and widely used for the electrochemical oxidation of organic pollutants. Among the cathode processes, the electro-Fenton (EF) technique is the most used; in this case H2O2 is formed from the reduction of O2 at the cathode while an iron catalyst is also regenerated at the cathode. The reaction between H2O2 and Fe2+ (Fenton’s reaction) generates homogeneous •OH responsible for the destruction of the organic compound (Brillas and Martínez-Huitle, 2015; Martínez-Huitle and Panizza 2018). The electrode materials used have a fundamental influence on the efficiency and selectivity of the electrochemical oxidation process. Thus, in recent years, many studies have focused on the preparation and application of different types of electrode materials for use in electrooxidation processes of organic pollutants, including Pt, metallic oxides (RuO2, IrO2, PbO2, SnO2, etc.), carbon-based materials, and boron-doped diamond (BDD) (He et al. 2019).
Some high-quality reviews have recently evaluated electrochemical oxidation processes for degradation of organic compounds (Clematis and Panizza 2021; Garcia-Segura et al. 2018; Hu et al. 2021; Jiang et al. 2021; Titchou et al. 2021). However, these reviews were not dedicated to analyzing the topic from a bibliometric perspective. Over the years, bibliometrics has become a popular means to classify bibliographic data and create representative summaries of the results obtained; this was driven by the rapid evolution of computers and the internet that allow for great ease in accessing and analyzing data (Cancino et al. 2017). Bibliometric analysis is a method used to analyze large amounts of scientific data. Through mathematical and statistical techniques, it allows you to follow the progress of publications on a given topic and simultaneously identify global trends and gaps in a research area. This provides researchers and organizations with guidance regarding the needs and objectives of future research (Donthu et al. 2021; Zhao et al. 2020).
In the present study, a bibliometric analysis of scientific publications on the electrochemical degradation of organic pollutants was carried out. The main objectives of this bibliometric analysis were to (1) evaluate the advances in the research of different materials used as catalysts; (2) analyze the historical and current growth of literature related to electrochemical degradation of organic pollutants; and (3) identify the main countries, authors, and journals that contribute to the advancement of the theme. This document contributes to the field of research concerning the electro-oxidation of organic pollutants in many respects, as it provides researchers with an understanding of the current state of research and development on the topic; helps to identify the authors, institutions, and countries with the greatest potential for conduct; and share research on degradation of organic pollutants. Furthermore, it allows researchers to be more aware of the main challenges in this area of research when making decisions about which topics will be studied.
Methodology
The main collection of the Web of Science (WoS) database was used to retrieve scientific publications related to the topic. The Web of Science is one of the most trusted and widely used bibliographic databases for bibliometric analysis (Singh et al. 2021), offering comprehensive coverage of excellent publications in general academic fields, ease of access, and advanced search and filtering features. The Web of Science database was chosen because, compared to other databases, it had the highest number of publications on the subject. The following keywords were used, in English, in the aforementioned database, related to the electrochemical degradation of organic pollutants: (“electrochemical degradation” OR “electrochemical oxidation” OR “electrocatalytic oxidation” OR “anodic oxidation” OR “electro-Fenton”) AND (“organic pollutant*” OR “volatile organic compound*” OR PAHs OR pesticide* OR phenol* OR “azo dye*”). The search was carried out on December 18, 2021, applying a chronological filter considering the publications in the timeframe of 2001 and 2021. Only publications in the English language were searched, the search field was limited to “title,” “abstract,” and “keyword.” VOSviewer 1.6.17 software was used for analysis of cooperation networks and keyword co-occurrences.
Results
Annual publications and growth trend
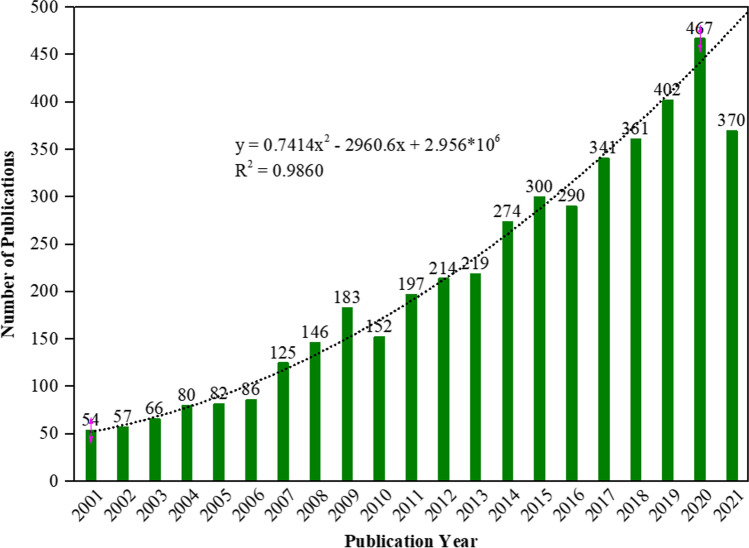
The search carried out in the Web of Science obtained a total of 4466 publications on the electrochemical degradation of organic pollutants with an average of 33.88 citations per document. Among these, the majority were research articles (4108, 91.98%) followed by event proceedings (259, 5.80%), reviews (234, 5.24%), early access (22, 0.493%), meeting summaries (11, 0.246%), book chapters (2, 0.045%), editorials (2, 0.045%), and retraction publication (1, 0.022%). As can be seen in Fig. 1, during the period evaluated there was a growing interest in research related to the electrochemical degradation of organic pollutants, initially with 54 publications in 2001 up to a significant number of 467 scientific studies published on the subject in 2020, which corresponds to an average growth rate of 11.39%. In general, few exceptions to this growth trend were observed, specifically in the years 2010 and 2016 the number of publications was lower compared to previous years. In the year 2021, up to the time of data research (December 18, 2021), 370 studies on the subject had already been published. The relatively low number of publications on the subject in 2021 may reflect the impacts caused by the COVID-19 pandemic. To assess the correlation between the number of documents and the year (data from 2021 were not included) the polynomial model was applied. A good fit of the polynomial curve to the increasing trend of publications was observed and a high coefficient of determination was obtained (R2 = 0.9860). This result suggests that, in the coming years, annual publications on the subject may continue to grow. The continued interest in the electrochemical degradation of organic contaminants, indicated by the increase in the number of publications, can be attributed to different factors, such as population growth and high demand, commitment to reduce environmental contamination, and importance of electrochemical methods as effective tools for removing organic pollutants.
Fig. 1.
Number of publications of research per year on electrochemical degradation of organic pollutants
Analysis of publications by subject area and source
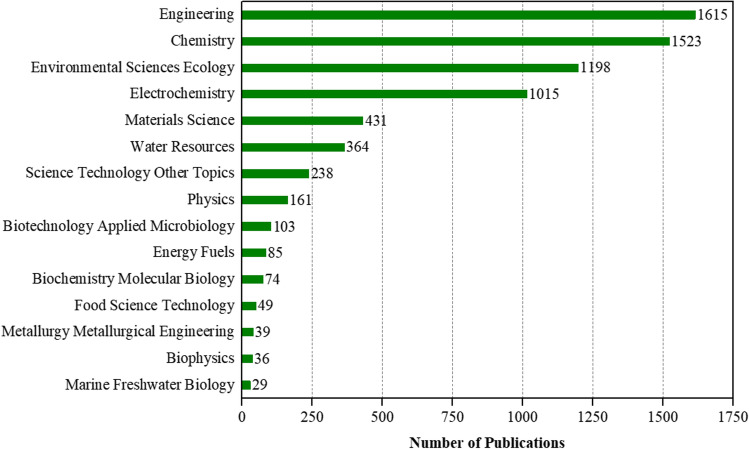
The electrochemical degradation of organic pollutants included 52 thematic areas of the Web of Science. As shown in Fig. 2, the analysis of the results showed that the recovered documents mainly belonged to the engineering area (1615, 36.16%). In the recovered literature, the areas of chemistry (1523, 34.10%), environmental sciences and ecology (1198, 26.82%), electrochemistry (1015, 22.73%), and material science (431, 9.65%) also stand out. Due to the assignment of journals to different subject categories, the total percentage of research areas is greater than 100%.
Fig. 2.
Fifteen main thematic areas of publications related to electrochemical degradation of organic pollutants
In total, 685 journals participated in research publications on electrochemical degradation of organic pollutants. Table 1 shows the 15 sources with the highest number of publications, representing 43.70% of all publications. The journal with the most publications was Electrochimica Acta (291, 6.52%), followed by Chemosphere (223, 4.99%), Journal of Hazardous Materials (209, 4.68%), Chemical Engineering Journal (169, 3.78%), and Journal of Electroanalytical Chemistry (162, 3.63%). The list of the top 15 sources includes five journals in the field of electrochemistry, while the remaining journals are in the areas of environmental chemistry, environmental science, catalysis, chemical engineering, and hydrology. Six of the leading journals are from the Netherlands, five from the UK, two from the USA, one from Germany, and one from Serbia. Furthermore, it is important to mention that among the journals on the list, 7 have an impact factor greater than 6.000, of which Applied Catalysis B Environmental has the highest impact factor (19.503).
Table 1.
Sources with more publications on electrochemical degradation of organic pollutants
| Position | Source title | Records | % | Impact factor |
|---|---|---|---|---|
| 1 | Electrochimica Acta | 291 | 6.52 | 6.901 |
| 2 | Chemosphere | 223 | 4.99 | 7.086 |
| 3 | Journal of Hazardous Materials | 209 | 4.68 | 10.588 |
| 4 | Chemical Engineering Journal | 169 | 3.78 | 13.273 |
| 5 | Journal of Electroanalytical Chemistry | 162 | 3.63 | 4.464 |
| 6 | Separation and Purification Technology | 146 | 3.27 | 7.312 |
| 7 | International Journal of Electrochemical Science | 107 | 2.40 | 1.765 |
| 8 | Applied Catalysis B Environmental | 102 | 2.28 | 19.503 |
| 9 | Environmental Science and Pollution Research | 90 | 2.02 | 4.223 |
| 10 | Journal of Applied Electrochemistry | 88 | 1.97 | 2.800 |
| 11 | Journal of the Electrochemical Society | 86 | 1.93 | 4.316 |
| 12 | Water Research | 84 | 1.88 | 11.236 |
| 13 | Desalination and Water Treatment | 77 | 1.72 | 1.254 |
| 14 | RSC Advances | 60 | 1.34 | 3.361 |
| 15 | Water Science and Technology | 58 | 1.30 | 1.915 |
Analysis of publications by country
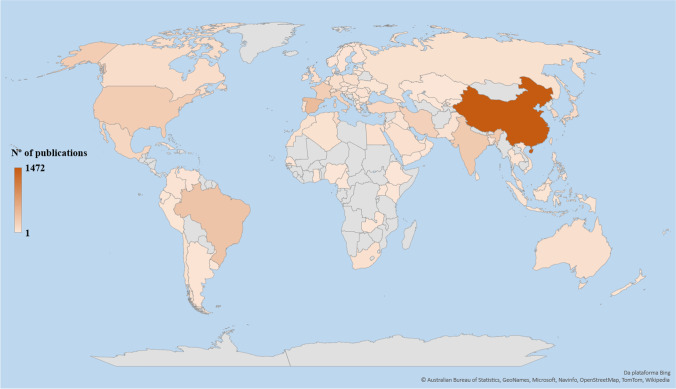
A total of 98 different countries participated in research publications on the electrochemical degradation of organic pollutants (the data from the countries that make up the United Kingdom were combined). The geographic distribution of the retrieved documents is shown in Fig. 3, and the 15 countries with the highest research production and the highest number of citations are shown in Table 2. In the period evaluated, China is notably the country with the highest number of publications (1472, 32.96%), followed by Spain (436, 9.76%), Brazil (345, 7.72%), India (291, 6.52%), and USA (258, 5.78%), which complete the top five. When evaluating the number of citations by country, some changes in ranking are observed. Among the top five, China (n = 41,778) and Spain (n = 29,381) still occupy the top two positions; however, Italy (n = 18,466) and France (n = 14,840) appear, respectively, as third and fourth place, followed by Brazil (n = 12,374). Switzerland, which occupies the tenth position in relation to the total number of citations (n = 5126), has the highest average of citations per document (142.39).
Fig. 3.
Geographical distribution of documents retrieved from the Web of Science (2001–2021) on electrochemical degradation of organic pollutants. Gray areas on the map represent regions with no documents retrieved
Table 2.
The 15 most productive countries in relation to the total number of publications and citations
| Most productive countries | Number of citations per country | ||||||
|---|---|---|---|---|---|---|---|
| Position | Country | Records | % | Position | Country | Total citations | Average citation |
| 1 | China | 1472 | 32.96 | 1 | China | 41,778 | 28.38 |
| 2 | Spain | 436 | 9.76 | 2 | Spain | 29,381 | 67.39 |
| 3 | Brazil | 345 | 7.72 | 3 | Italy | 18,466 | 97.70 |
| 4 | India | 291 | 6.52 | 4 | France | 14,840 | 61.83 |
| 5 | USA | 258 | 5.78 | 5 | Brazil | 12,374 | 35.87 |
| 6 | France | 240 | 5.37 | 6 | India | 9577 | 32.91 |
| 7 | Iran | 220 | 4.93 | 7 | USA | 8680 | 33.64 |
| 8 | Italy | 189 | 4.23 | 8 | Iran | 5404 | 24.56 |
| 9 | Mexico | 157 | 3.52 | 9 | Turkey | 5225 | 37.59 |
| 10 | Turkey | 139 | 3.11 | 10 | Switzerland | 5126 | 142.39 |
| 11 | South Korea | 130 | 2.91 | 11 | Mexico | 4939 | 31.46 |
| 12 | Tunisia | 113 | 2.53 | 12 | S. Korea | 4559 | 35.07 |
| 13 | Taiwan | 112 | 2.51 | 13 | Tunisia | 3883 | 34.36 |
| 14 | Japan | 103 | 2.31 | 14 | Japan | 3396 | 32.97 |
| 15 | Canada | 90 | 2.02 | 15 | Portugal | 3253 | 45.82 |
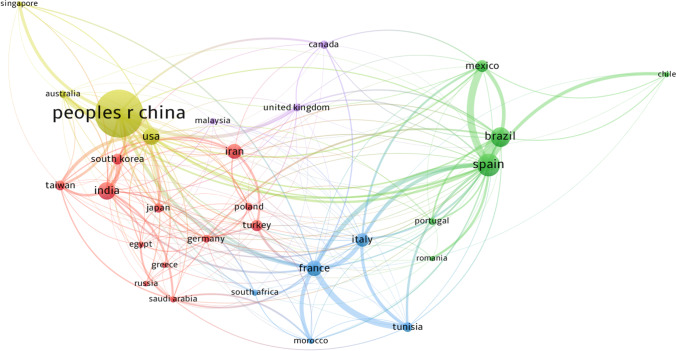
Academic cooperation between countries and institutions is a very important practice for the production and dissemination of scientific knowledge. Figure 4 shows the academic collaboration network among the 30 most productive countries. In this figure the nodes represent the different countries, the lines connecting the nodes indicate cooperation between countries, and the line thickness is proportional to the strength of the cooperation. China is the country with the highest number of publications in collaboration with other countries (n = 233), followed by Spain (n = 227), France (n = 170), USA (n = 163), and Brazil (n = 132). Despite occupying the first position, only 15.83% of all publications in China were in collaboration with other countries, which corresponds to the lowest rate among highly productive countries. For example, France cooperated with other countries in 70.83% of its publications, for the USA this percentage was 63.18%, for Spain 52.06%, and for Italy 50.26%. Therefore, it is important that China, which is the country with the most research in the area of electrochemical degradation of organic pollutants, intensifies its academic cooperation with other countries. The analysis of academic cooperation also revealed that the USA has the most diverse collaboration network, having collaborated on publications with 43 different countries. In this aspect, also stand out Spain (40), France (39), China (35), and Italy (34). Spain and Brazil are the countries that most cooperate with each other (68 publications), besides them an intense academic cooperation was verified between China and the USA (65 publications).
Fig. 4.
Map of the academic collaboration network among the 30 most productive countries
Analysis of publications by institutions and authors
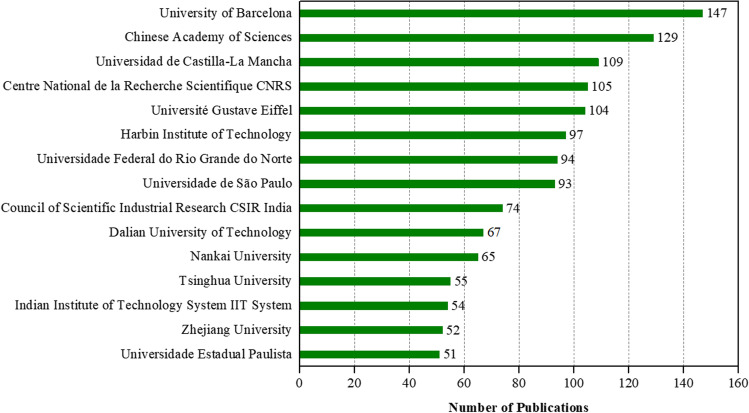
Figure 5 shows the 15 institutions with the most publications between 2001 and 2021. A total of 2387 institutions contributed to the 4466 publications retrieved. The most productive institution was the University of Barcelona, which published 147 documents, followed by the Chinese Academy of Sciences (129), Universidad de Castilla-La Mancha (109), Center National de la Recherche Scientifique CNRS (105), and Universite Gustave Eiffel (104). In addition, 6 of the 15 most productive institutions were from China, the other 9 institutions are distributed among Brazil (3), Spain (2), India (2), and France (2).
Fig. 5.
The 15 institutions with the most publications on electrochemical degradation of organic pollutants between 2001 and 2021
The authors with the most publications on electrochemical degradation of organic pollutants are shown in Table 3. A total of 10,648 researchers participated in publications on the subject between 2001 and 2021. Researcher Brillas E. appears with the highest number of publications (134, 3.000%), followed by Oturan M. A. (107, 2.396%), Rodrigo M. A. (107, 2.396%), Martinez-Huitle C. A. (102, 2.284%), and Oturan N. (87, 1.948%). Another important parameter to be considered is the number of citations; on this aspect Brillas E. leads the list with a total of 13,809 citations, Oturan M. A. is the second with the most citations (10,529), followed by Martinez-Huitle C. A., Panizza M., and Rodrigo M. A. who had a total of 8666, 8410, and 7888 citations, respectively.
Table 3.
Authors with more publications on electrochemical degradation of organic pollutants
| Position | Author | Records | % | Total citations | Average citation |
|---|---|---|---|---|---|
| 1 | Brillas E | 134 | 3.000 | 13,809 | 103.05 |
| 2 | Oturan M. A | 107 | 2.396 | 10,529 | 98.4 |
| 3 | Rodrigo M. A | 107 | 2.396 | 7888 | 73.72 |
| 4 | Martinez-Huitle C. A | 102 | 2.284 | 8666 | 84.96 |
| 5 | Oturan N | 87 | 1.948 | 6208 | 71.36 |
| 6 | Canizares P | 86 | 1.926 | 3941 | 45.83 |
| 7 | Saez C | 76 | 1.702 | 3459 | 45.51 |
| 8 | Sires I | 76 | 1.702 | 6244 | 82.16 |
| 9 | Zhou M. H | 65 | 1.455 | 4079 | 62.75 |
| 10 | Pazos M | 47 | 1.052 | 1568 | 33.36 |
| 11 | Sanroman M. A | 47 | 1.052 | 1568 | 33.36 |
| 12 | Wang Y | 46 | 1.030 | 1088 | 23.65 |
| 13 | Garcia-Segura S | 45 | 1.008 | 2760 | 61.33 |
| 14 | Panizza M | 43 | 0.963 | 8410 | 195.58 |
| 15 | Wang H | 36 | 0.806 | 739 | 20.53 |
Most cited publications
Table 4 shows the most cited publications on electrochemical degradation of organic pollutants. Only review articles appear among the 10 most cited publications. Spain (6) and Italy (5) are the countries with the highest number of publications among the most cited. The journals with the most publications among the 10 most cited were Applied Catalysis B Environmental (n = 3) and Chemical Reviews (n = 3). The study by Brillas et al. (2009) entitled “Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry” was the most cited publication, with 2040 citations. This article presents an overview of electrochemical advanced oxidation processes (EAOPs) based on the Fenton reaction for the degradation of persistent organic pollutants (POPs), with special attention to electro-Fenton systems. The study by Martinez-Huitle and Brillas (2009), entitled “Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review,” was the second most cited publication, with 1684 citations. In this review, the authors present an overview of the main electrochemical technologies used to discolor and/or degrade synthetic organic dyes. Typical methods such as electrocoagulation, electrochemical reduction, electrochemical oxidation, and indirect electrooxidation with active chlorine species are discussed in terms of fundamentals, main applications, influence of electrode materials, and operating parameters.
Table 4.
Most cited publications on electrochemical degradation of organic pollutants
| Position | Title | Author/year | Citations |
|---|---|---|---|
| 1 | Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry | Brillas et al. (2009) | 2040 |
| 2 | Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review | Martinez-Huitle and Brillas (2009) | 1684 |
| 3 | Direct and mediated anodic oxidation of organic pollutants | Panizza and Cerisola (2009) | 1493 |
| 4 | Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review | Brillas and Martinez-Huitle (2015) | 1178 |
| 5 | Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes | Martinez-Huitle and Ferro (2006) | 1174 |
| 6 | Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes | Bokare and Choi (2014) | 1096 |
| 7 | Electrochemical advanced oxidation processes: today and tomorrow. A review | Sires et al. (2014) | 1064 |
| 8 | Advanced oxidation processes in water/wastewater treatment: principles and applications. A review | Oturan and Aaron (2014) | 1037 |
| 9 | Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters | Moreira et al. (2017) | 965 |
| 10 | Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review | Martinez-Huitle et al. (2015) | 843 |
Keyword analysis
The keywords of a scientific article reflect the general themes of the study. Thus, by analyzing the frequency and co-occurrence of keywords, it is possible to identify important topics and trends in a particular field of research (Li et al. 2009a, b; Zhang et al. 2010). In this study, the VOSviewer 1.6.17 software was used to assess the frequency and co-occurrence of keywords. Terms with similar meaning and different spelling were combined, such as “electrooxidation” and “electro-oxidation,” “hydroxyl radical,” and “hydroxyl radicals.” In all, 7463 keywords were found in publications related to electrochemical degradation of organic pollutants. The vast majority of these keywords had only one (5585, 74.8%) or two (845, 11.3%) occurrences, while 424 (5.7%) keywords had more than 5 occurrences. Table 5 shows the keywords with the highest number of occurrences in different periods. Between 2001 and 2021, the most frequent keywords were “electrochemical oxidation” (580), “electro-Fenton” (406), “phenol(s)” (288), “hydroxyl radical(s)” (259), and “wastewater treatment” (233). By analyzing the ranking of the most frequent keywords in different periods, search trends can be noticed. For example, the keyword “electro-Fenton” between the years 2001 and 2007 has the 11th highest frequency; between 2008 and 2014, it appears in the 3rd position; and between 2015 and 2021, it appears in the 2nd position, which indicates a growing interest in the process electro-Fenton for degradation of organic pollutants. An important increase in occurrences was also observed for the term “water treatment,” which occupied the 21st position in 2001–2007 and rose to the 11th position in 2015–2021. “Advanced oxidation processes” also appears as an emerging topic moving from 25th position in 2001–2007 to 8th position in 2015–2021.
Table 5.
Fifteen most used author keywords in different periods
| 2001–2007 | 2008–2014 | 2015–2021 | 2001–2021 | |||||
|---|---|---|---|---|---|---|---|---|
| P | Keywords | KO | Keywords | KO | Keywords | KO | Keywords | KO |
| 1 | Electrochemical oxidation | 77 | Electrochemical oxidation | 201 | Electrochemical oxidation | 302 | Electrochemical oxidation | 580 |
| 2 | Phenol(s) | 55 | Phenol(s) | 126 | Electro-Fenton | 279 | Electro-Fenton | 406 |
| 3 | Wastewater treatment | 41 | Electro-Fenton | 108 | Hydroxyl radical(s) | 143 | Phenol(s) | 288 |
| 4 | Anodic oxidation | 35 | Hydroxyl radical(s) | 90 | Wastewater treatment | 134 | Hydroxyl radical(s) | 259 |
| 5 | Oxidation | 31 | Boron-doped diamond | 74 | Electrooxidation | 117 | Wastewater treatment | 233 |
| 6 | Hydroxyl radical(s) | 26 | Anodic oxidation | 67 | Phenol(s) | 107 | Electrooxidation | 207 |
| 7 | Cyclic voltammetry | 26 | Electrooxidation | 67 | Anodic oxidation | 104 | Anodic oxidation | 206 |
| 8 | Electrooxidation | 23 | Wastewater treatment | 58 | Advanced oxidation process(es) | 89 | Boron-doped diamond | 181 |
| 9 | Electrocatalysis | 22 | Electrochemical degradation | 58 | Boron-doped diamond | 88 | Degradation | 144 |
| 10 | Electrolysis | 20 | Cyclic voltammetry | 48 | Degradation | 88 | Electrochemical degradation | 134 |
| 11 | Electro-Fenton | 19 | Mineralization | 46 | Water treatment | 84 | Advanced oxidation process(es) | 133 |
| 12 | Boron-doped diamond | 19 | Degradation | 46 | Mineralization | 71 | Water treatment | 129 |
| 13 | Wastewater | 17 | Wastewater | 38 | Electrochemical degradation | 67 | Mineralization | 127 |
| 14 | Hydrogen peroxide | 15 | Oxidation | 37 | Hydrogen peroxide | 57 | Cyclic voltammetry | 118 |
| 15 | Electrochemistry | 14 | Advanced oxidation process(es) | 36 | Azo dye(s) | 53 | Oxidation | 102 |
P position, KO keyword occurrence
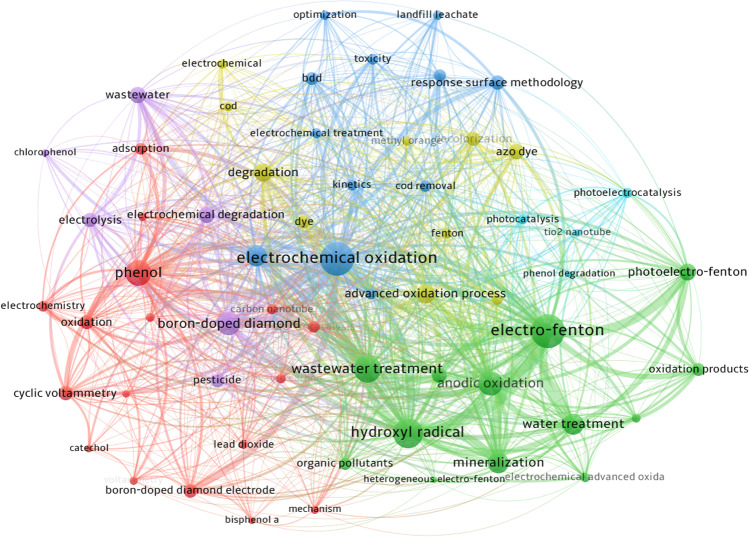
Figure 6 shows the co-occurrence network map of the most frequent keywords in publications related to the topic. Each keyword is represented by a node and the lines connecting the nodes indicate co-occurrence between the keywords. Node size is proportional to the number of links keywords have, and line thickness is proportional to the number of co-occurrences between two keywords. For the construction of the map, keywords with at least 30 occurrences were selected, which resulted in 62 keywords. “Electrochemical oxidation” has the highest number of links with other keywords as it is the most frequent word, which reflects its central position in this research field. Thicker lines are observed between the words “electro-Fenton” and “hydroxyl radical(s)” (65 co-occurrences), “electrochemical oxidation” and “wastewater treatment” (59 co-occurrences), “electrochemical oxidation” and “phenol(s)” (50 co-occurrences), “electro-Fenton,” and “mineralization” (50 co-occurrences), which indicates that these themes are frequently addressed in the studies together. There is also a relevant co-occurrence between the keywords “electrochemical oxidation” and “boron-doped diamond,” “electron-Fenton” and “photoelectro-Fenton,” “electrochemical oxidation,” and “hydroxyl radical”; therefore, these combinations are focused in research on the degradation of organic pollutants.
Fig. 6.
Co-occurrence network of the most frequent author keywords
Author keywords with strong correlation were grouped into six clusters indicated by different colors. Among these clusters, three main groups were observed, defined by the number of keywords contained. Cluster 1 (Fig. 6, red color) is the largest group and has 17 keywords, focusing on the electrochemical oxidation applied to the degradation of phenolic compounds, adding the terms “electrocatalytic oxidation,” “oxidation,” “phenol,” “phenolic compounds,” “bisphenol A,” “boron-doped diamond electrode,” “lead dioxide,” etc. Cluster 2 (Fig. 6, green color) has 13 keywords, mainly related to the application of combined techniques and advanced oxidative processes for water and wastewater treatment; this group includes the keywords “anodic oxidation,” “mineralization,” “electro-Fenton,” “photoelectro-Fenton,” “advanced oxidation process,” “hydroxyl radical,” etc. Cluster 3 (Fig. 6, blue color) has 12 keywords and mainly involves electrochemical techniques used for the degradation of organic pollutants. Among the keywords belonging to this group are “electrochemical oxidation,” “electrooxidation,” “electrocoagulation,” “electrochemical treatment,” and “bdd,” among others.
Discussion
The retrieved data point to a considerable expansion of technologies for the electrochemical oxidation of organic pollutants in the last 20 years. The analysis of the publications found shows that a great deal of attention was given to the investigation of efficient electrode materials for the degradation of organic pollutants.
Electrode materials used in the electrochemical degradation process
Active anodes (anodes with low oxygen evolution potential), mainly Pt, graphite, IrO2, and RuO2, have been widely used in studies on the oxidation of organic compounds due to their high electrocatalytic activity and good chemical stability (Li et al. 2009a, b; Mahmoudi et al. 2020). However, these materials generally allow only partial oxidation of organic compounds. Thus, non-active anodes (anodes with high potential for oxygen evolution) have been appointed as ideal anodes because they favor the complete oxidation of organic pollutants to CO2 (Panizza and Cerisola 2009).
Among the non-active anodes, the most used are boron-doped diamond (BDD), SnO2, and PbO2. On the other hand, these materials also have their disadvantages; for example, the BDD electrode has a high cost, which limits its practical application. Pure SnO2 electrodes have high resistance to charge transfer and the use of the PbO2 electrode presents the risk of lead leaching, which raises environmental concerns considering the high toxicity and bioaccumulation of lead (Jiang et al. 2021). In view of these issues, alternative materials in addition to different types of modifications and preparation methods were evaluated in order to obtain better performance.
The results obtained in different studies showed that a very effective way to improve the degradation performance is through the application of materials on a nanometric scale. For example, nanostructured BDD electrodes (nanocones, nanowires) showed improved electrocatalytic activity compared to conventional BDD electrodes (Lee et al. 2017; Shi et al. 2020). Several types of nanocomposites and metallic nanoparticles have shown to be promising materials for the electrochemical oxidation of organic pollutants. In the study by Espinoza et al. (2020) a mixture of RuO2 and IrO2 nanoparticles was successfully applied for oxamic acid mineralization. MnFe2O4 nanoparticles used for tetracycline degradation were able to remove 86.23% of the pollutant after 60 min of treatment (Tang et al. 2021a, b). The nanocomposite formed by poly(vinylidene fluoride-co-hexafluoropropylene) airgel decorated with RuO2 nanoparticles (PVDF HFP_RuO2), produced by supercritical drying, showed a relevant purification performance in the treatment of effluents (Sarno et al. 2021). The benefit of using nanomaterials is mainly associated with their high surface area and high catalytic activity.
It was demonstrated that the material used as a support has a fundamental influence on the performance of the electrode, as the use of an adequate support can provide better stability, improvement of the active sites on the surface, lower resistance to charge transfer, and greater surface area. Ti is a material commonly used as a substrate for the electrodeposition of metallic elements and metallic oxides due to its high chemical stability, high mechanical strength, wide electrochemical potential windows, and low cost (Ansari and Nematollahi 2020; Tang et al. 2021a, b). Furthermore, TiO2 nanotubes used as an intermediate layer can improve the adhesion between the substrate and the catalytic layer, and thus improve the electrocatalytic activity and stability of the electrodes (Wu et al. 2019; Xu et al. 2020). The development of anodes based on carbonaceous materials such as carbon fibers (Pereira et al. 2020), single-walled carbon nanotubes (CNTs) (Liu et al. 2021), multi-walled carbon nanotubes (MWCNTs) (Zhu and Chen 2021), graphene (Savić et al. 2020), and reduced graphene oxide (rGO) (Hamous et al. 2021) provided very promising results. In the study conducted by Chen et al. (2021) rGO@Ti/SnO2-Sb composite electrodes showed significantly improved electrocatalytic oxidation activity for degradation of the fluoroquinolone antibiotic norfloxacin due to rGO coupling. Xia et al. (2021) showed that the multilayer CNT-PbO2 anode has better efficiency for isoniazid removal compared to the pure PbO2 electrode.
Among the strategies used to improve electrocatalytic performance is the introduction of doping elements. In the case of PbO2 and SnO2 electrodes, doping with F, Bi, Fe, Ni, Co, Sb, Al, Pd, Yb, Ce, La, and Gd, among other elements (Ferreira et al. 2020; Yao et al. 2019), managed to further improve the performance for electrochemical degradation of organic pollutants. For example, the addition of nickel metal to the Ti/SnO2-Sb-Ni electrode generated a more compact and uniform surface compared to the Ti/SnO2-Sb electrode, in addition to improving the oxidation performance for mineralization of the chloramphenicol compound (Li et al. 2021). The Cu-doped PbO2 electrode showed 98.4% combustion efficiency for amoxicillin degradation in wastewater, while for the non-doped PbO2 electrode this value was 65.6% (Hu et al., 2020).
Important strategies to improve the degradation process
In addition to the nature of the electrode material, another crucial factor for the efficiency of electrochemical degradation is the operating conditions. To obtain better mineralization efficiency, the influence of factors such as temperature, pH, type and concentration of the supporting electrolyte, current density, and concentration of organic pollutants must be carefully analyzed and the optimal conditions for carrying out the degradation process must be determined (Jiang et al. 2021).
The use of hybrid and sequential processes, in which electrochemical oxidation is combined with other techniques for a more efficient removal of pollutants, emerges as a very promising field and presents remarkable results (Hu et al. 2021). Among the methods usually applied in conjunction with electrocatalysis are Fenton’s reaction, photocatalysis, ozonization, membrane filtration, adsorption, ultrasound, UV irradiation, and biological treatment (Chen et al. 2019). In general, the combination of these methods with electrochemical oxidation has a synergistic effect causing an increase in the oxidative capacity of the process (Dewil et al. 2017). For example, Ren et al. (2021) obtained a degradation rate of 95.03% of the malachite green organic dye using an electrochemical system combined with ultrasound, while using only the electrochemical method the degradation rate was 83.91%. In the research carried out by Barrera et al. (2021) a sequential treatment combining electro-oxidation and gamma irradiation achieved 100% efficiency for degradation of nonylphenol ethoxylate 10 (NP10EO), in addition to reducing treatment time by 80%.
Conclusions and perspectives
In this article a historical mapping and current trends related to the process of electrochemical degradation of organic pollutants were carried out. From 2001 to 2021, a total of 4466 documents related to the topic were published in scientific journals indexed in the Web of Science database. A significant increase in publications was observed in the last 20 years and the interest in this area should keep growing due to the efficiency of electrochemical methods and high demand. China dominated this field of research and presented a remarkably higher number of publications compared to other countries. It is important for other countries to be more interested in the subject considering that organic pollutants are a global problem. Bibliometric analysis also indicated that the most productive authors were Brillas E., Oturan M. A., and Martinez-Huitle C. A. The authors’ favorite journals were Electrochimica Acta, Chemosphere, and Journal of Hazardous Materials. Electrochemical oxidation, electro-Fenton, phenol(s), hydroxyl radical(s), and wastewater treatment are the most frequent keywords in publications and indicate the main focus and direction of current research in this research area.
Following the content analysis, it was possible to verify that the electrochemical degradation process of organic pollutants presents itself as a promising technique for mineralization of pollutants. Electrode materials are critical to process efficiency and great strides have been made in the construction of high-performance catalysts. However, further development is still needed to obtain more stable electrodes with better electrocatalytic performance. The adoption of strategies such as doping, surface modification, and nanostructured construction can effectively improve anode efficiency. Another factor to be considered is the cost; investigations involving simple fabrication techniques and with non-precious metals can help to enable large-scale application.
The experimental parameters of the degradation process (pH, temperature, supporting electrolyte, etc.) need to be carefully evaluated to ensure the best performance of the electrocatalysts. The by-products generated during the electrochemical degradation process must be considered, considering that the incomplete oxidation of organic compounds can lead to the formation of toxic compounds, which is highly undesirable. It is also important that future investigations consider the combined use of electrochemical methods with renewable energies, such as solar energy, wind energy, and geothermal energy, in order to obtain a cleaner process at a reduced cost.
Author contribution
JRNS: performed the investigation, methodology, writing, and editing and was a major contributor in writing the manuscript.
ICBA: performed the investigation, methodology, writing, and editing and gave important contribution in writing the manuscript.
EPM: held scientific orientation, writing, and editing.
MALB: provided funding and held scientific orientation, writing, and editing.
All authors read and approved the final manuscript.
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior — CAPES-PROCAD AM (Edital 2018 - Line 2; Number SCBA: 88887.200615/2018–00), Conselho Nacional de Desenvolvimento Científico e Tecnológico — CNPq (PQ 2017, Proc. 310664/2017–9), Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão — FAPEMA (Edital UNIVERSAL-01136/17, Grant 015759/2017), and Agência Nacional do Petróleo, Gás Natural e Biocombustíveis — ANP (Research Project PMQC/QUALIPETRO, No 1.028/2021; CONSEPE-UFMA No 2.460/2022).
Data availability
Data will be made available upon request.
Declarations
Ethics approval and consent to participate
The article does not contain any studies with human participants or animal subjects and all the authors named in a manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Consent for publication
The article is an original research which has neither been published previously nor submitted to more than one journal for simultaneous consideration.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alharbi OML, Basheer AA, Khattab RA, Ali I. Health and environmental effects of persistent organic pollutants. J Mol Liq. 2018;263:442–453. doi: 10.1016/j.molliq.2018.05.029. [DOI] [Google Scholar]
- Ansari A, Nematollahi D. Convergent paired electrocatalytic degradation of p-dinitrobenzene by Ti/SnO2-Sb/β-PbO2 anode A new insight into the electrochemical degradation mechanism. Appl Catal B Environ. 2020;261:118226. doi: 10.1016/j.apcatb.2019.118226. [DOI] [Google Scholar]
- Barrera H, Ureña-Nuñez F, Barrios JA, Becerril E, Frontana-Uribe BA, Barrera-Díaz CE. Degradation of nonylphenol ethoxylate 10 (NP10EO) in a synthetic aqueous solution using a combined treatment: electrooxidation-gamma irradiation. Fuel. 2021;283:118929. doi: 10.1016/j.fuel.2020.118929. [DOI] [Google Scholar]
- Bokare AD, Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater. 2014;275:121–135. doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Brillas E, Martínez-Huitle CA. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ. 2015;166:603–643. doi: 10.1016/j.apcatb.2014.11.016. [DOI] [Google Scholar]
- Brillas E, Sirés I, Oturan MA. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev. 2009;109:6570–6631. doi: 10.1021/cr900136g. [DOI] [PubMed] [Google Scholar]
- Cancino C, Merigó JM, Coronado F, Dessouky Y, Dessouky M. Forty years of computers & industrial engineering: a bibliometric analysis. Comput Ind Eng. 2017;113:614–629. doi: 10.1016/j.cie.2017.08.033. [DOI] [Google Scholar]
- Chen Z, Liu Y, Wei W, Ni BJ. Recent advances in electrocatalysts for halogenated organic pollutant degradation. Environ Sci Nano. 2019;6(8):2332–2366. doi: 10.1039/C9EN00411D. [DOI] [Google Scholar]
- Chen Y, Li F, Dong X, Guo D, Huang Y, Li S. Construction of rGO@Ti/SnO2–Sb composite electrode for electrochemical degradation of fluoroquinolone antibiotic. J Alloys Compd. 2021;869:159258. doi: 10.1016/j.jallcom.2021.159258. [DOI] [Google Scholar]
- Cheng Y, Chon K, Ren X, Kou Y, Hwang MH, Chae KJ. Bioaugmentation treatment of a novel microbial consortium for degradation of organic pollutants in tannery wastewater under a full-scale oxic process. Biochem Eng J. 2021;175:108131. doi: 10.1016/j.bej.2021.108131. [DOI] [Google Scholar]
- Clematis D, Panizza M. Electrochemical oxidation of organic pollutants in low conductive solutions. Curr Opin Electrochem. 2021;26:100665. doi: 10.1016/j.coelec.2020.100665. [DOI] [Google Scholar]
- Dewil R, Mantzavinos D, Poulios I, Rodrigo MA. New perspectives for advanced oxidation processes. J Environ Manage. 2017;195:93–99. doi: 10.1016/j.jenvman.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. 2021;133:285–296. doi: 10.1016/j.jbusres.2021.04.070. [DOI] [Google Scholar]
- Espinoza LC, Sepúlveda P, García A, de Godoi DM, Salazar R. Degradation of oxamic acid using dimensionally stable anodes (DSA) based on a mixture of RuO2 and IrO2 nanoparticles. Chemosphere. 2020;251:126674. doi: 10.1016/j.chemosphere.2020.126674. [DOI] [PubMed] [Google Scholar]
- Ferreira MB, Solano AMS, dos Santos EV, Martínez-Huitle CA, Ganiyu SO. Coupling of anodic oxidation and soil remediation processes: a review. Materials. 2020;13(19):4309. doi: 10.3390/ma13194309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura S, Ocon JD, Chong MN. Electrochemical oxidation remediation of real wastewater effluents—a review. Process Saf Environ Prot. 2018;113:48–67. doi: 10.1016/j.psep.2017.09.014. [DOI] [Google Scholar]
- Haines DA, Saravanabhavan G, Werry K, Khoury C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int J Hyg Environ Health. 2017;220(2):13–28. doi: 10.1016/j.ijheh.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Hamous H, Khenifi A, Orts F, Bonastre J, Cases F. Carbon textiles electrodes modified with RGO and Pt nanoparticles used for electrochemical treatment of azo dye. J Electroanal Chem. 2021;887:115154. doi: 10.1016/j.jelechem.2021.115154. [DOI] [Google Scholar]
- He Y, Lin H, Guo Z, Zhang W, Li H, Huang W. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep Purif Technol. 2019;212:802–821. doi: 10.1016/j.seppur.2018.11.056. [DOI] [Google Scholar]
- Hu J, Bian X, Xia Y, Weng M, Zhou W, Dai Q. Application of response surface methodology in electrochemical degradation of amoxicillin with Cu-PbO2 electrode: optimization and mechanism. Sep Purif Technol. 2020;250:117109. doi: 10.1016/j.seppur.2020.117109. [DOI] [Google Scholar]
- Hu Z, Cai J, Song G, Tian Y, Zhou M. Anodic oxidation of organic pollutants: anode fabrication, process hybrid and environmental applications. Curr Opin Electrochem. 2021;26:100659. doi: 10.1016/j.coelec.2020.100659. [DOI] [Google Scholar]
- Jiang Y, Zhao H, Liang J, Yue L, Li T, Luo Y, Liu Q, Lu S, Asiri AB, Gong Z, Sun X. Anodic oxidation for the degradation of organic pollutants: anode materials, operating conditions and mechanisms. A Mini Review Electrochem Commun. 2021;123:106912. doi: 10.1016/j.elecom.2020.106912. [DOI] [Google Scholar]
- Lee CH, Lee ES, Lim YK, Park KH, Park HD, Lim DS. Enhanced electrochemical oxidation of phenol by boron-doped diamond nanowire electrode. RSC Adv. 2017;7(11):6229–6235. doi: 10.1039/c6ra26287b. [DOI] [Google Scholar]
- Li LL, Ding G, Feng N, Wang MH, Ho YS. Global stem cell research trend: bibliometric analysis as a tool for mapping of trends from 1991 to 2006. Scientometrics. 2009;80(1):39–58. doi: 10.1007/s11192-008-1939-5. [DOI] [Google Scholar]
- Li M, Feng C, Hu W, Zhang Z, Sugiura N. Electrochemical degradation of phenol using electrodes of Ti/RuO2–Pt and Ti/IrO2–Pt. J Hazard Mater. 2009;162(1):455–462. doi: 10.1016/j.jhazmat.2008.05.063. [DOI] [PubMed] [Google Scholar]
- Li D, Zhang L, Gao W, Meng J, Guan Y, Liang J, Shen X. Electrochemical degradation of chloramphenicol using Ti-based SnO2-Sb-Ni electrode. Water Sci Technol. 2021;84(3):512–523. doi: 10.2166/wst.2021.226. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Wang J. Activation of peroxydisulfate by a novel Cu0-Cu2O@CNTs composite for 2, 4-dichlorophenol degradation. Sci Total Environ. 2021;754:141883. doi: 10.1016/j.scitotenv.2020.141883. [DOI] [PubMed] [Google Scholar]
- Lu F, Astruc D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord Chem Rev. 2020;408:213180. doi: 10.1016/j.ccr.2020.213180. [DOI] [Google Scholar]
- Ma S, Lee S, Kim K, Im J, Jeon H. Purification of organic pollutants in cationic thiazine and azo dye solutions using plasma-based advanced oxidation process via submerged multi-hole dielectric barrier discharge. Sep Purif Technol. 2021;255:117715. doi: 10.1016/j.seppur.2020.117715. [DOI] [Google Scholar]
- Mahmoudi MM, Khaghani R, Dargahi A, Tehrani GM (2020) Electrochemical degradation of diazinon from aqueous media using graphite anode: effect of parameters, mineralisation, reaction kinetic, degradation pathway and optimisation using central composite design. Int J Environ Anal Chem 1–26.10.1080/03067319.2020.1742893
- Martínez-Huitle CA, Brillas E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ. 2009;87(3–4):105–145. doi: 10.1016/j.apcatb.2008.09.017. [DOI] [Google Scholar]
- Martínez-Huitle CA, Ferro S. Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev. 2006;35(12):1324–1340. doi: 10.1039/B517632H. [DOI] [PubMed] [Google Scholar]
- Martínez-Huitle CA, Panizza M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr Opin Electrochem. 2018;11:62–71. doi: 10.1016/j.coelec.2018.07.010. [DOI] [Google Scholar]
- Martínez-Huitle CA, Rodrigo MA, Sires I, Scialdone O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev. 2015;115(24):13362–13407. doi: 10.1021/acs.chemrev.5b00361. [DOI] [PubMed] [Google Scholar]
- Moreira FC, Boaventura RAR, Brillas E, Vilar VJP. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ. 2017;202:217–261. doi: 10.1016/j.apcatb.2016.08.037. [DOI] [Google Scholar]
- Motora KG, Wu CM, Naseem S. Magnetic recyclable self-floating solar light-driven WO2.72/FE3O4 nanocomposites immobilized by Janus membrane for photocatalysis of inorganic and organic pollutants. J Ind Eng Chem. 2021;102:25–34. doi: 10.1016/j.jiec.2021.06.025. [DOI] [Google Scholar]
- Oturan MA, Aaron JJ. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review Crit Rev Environ Sci. 2014;44(23):2577–2641. doi: 10.1080/10643389.2013.829765. [DOI] [Google Scholar]
- Panizza M, Cerisola G. Direct and mediated anodic oxidation of organic pollutants. Chem Rev. 2009;109(12):6541–6569. doi: 10.1021/cr9001319. [DOI] [PubMed] [Google Scholar]
- Pereira LA, Couto AB, Almeida DAL, Ferreira NG. Singular properties of boron-doped diamond/carbon fiber composite as anode in Brilliant Green dye electrochemical degradation. Diam Relat Mater. 2020;103:107708. doi: 10.1016/j.diamond.2020.107708. [DOI] [Google Scholar]
- Qiao J, Xiong Y. Electrochemical oxidation technology: a review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. J Water Process Eng. 2021;44:102308. doi: 10.1016/j.jwpe.2021.102308. [DOI] [Google Scholar]
- Ren Q, Kong C, Chen Z, Zhou J, Li W, Li D, Cui Z, Xue Y, Lu Y. Ultrasonic assisted electrochemical degradation of malachite green in wastewater. Microchem J. 2021;164:106059. doi: 10.1016/j.microc.2021.106059. [DOI] [Google Scholar]
- Sarno M, Scudieri C, Ponticorvo E, Baldino L, Cardea S, Reverchon E. PVDF HFP_RuO2 nanocomposite aerogels produced by supercritical drying for electrochemical oxidation of model tannery wastewaters. Nanomaterials. 2021;11(6):1436. doi: 10.3390/nano11061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić BG, Stanković DM, Živković SM, Ognjanović MR, Tasić GS, Mihajlović IJ, Brdarić TP. Electrochemical oxidation of a complex mixture of phenolic compounds in the base media using PbO2-GNRs anodes. Appl Surf Sci. 2020;529:147120. doi: 10.1016/j.apsusc.2020.147120. [DOI] [Google Scholar]
- Shi L, Xu F, Gao J, Yuen M, Sun S, Xu J, Jia K, Zuo D. Nanostructured boron-doped diamond electrode for degradation of the simulation wastewater of phenol. Diam Relat Mater. 2020;109:108098. doi: 10.1016/j.diamond.2020.108098. [DOI] [Google Scholar]
- Singh VK, Singh P, Karmakar M, Leta J, Mayr P. The journal coverage of Web of Science, Scopus and Dimensions: a comparative analysis. Scientometrics. 2021;126(6):5113–5142. doi: 10.1007/s11192-021-03948-5. [DOI] [Google Scholar]
- Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M. Electrochemical advanced oxidation processes: today and tomorrow. A Review Environ Sci Pollut Res. 2014;21(14):8336–8367. doi: 10.1007/s11356-014-2783-1. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu Z, Lu W, Wang L, Zhang C, Su P. Electrochemical degradation of perfluorinated compounds by Ag coated Ti (Ti/Ag) anode: electrode preparation, characterization and application. Environ Sci Water Res Technol. 2021;7(2):455–467. doi: 10.1039/D0EW00785D. [DOI] [Google Scholar]
- Tang S, Zhao M, Yuan D, Li X, Zhang X, Wang Z, Jiao T, Wang K. MnFe2O4 nanoparticles promoted electrochemical oxidation coupling with persulfate activation for tetracycline degradation. Sep Purif Technol. 2021;255:117690. doi: 10.1016/j.seppur.2020.117690. [DOI] [Google Scholar]
- Titchou FE, Zazou H, Afanga H, El Gaayda J, Akbour RA, Nidheesh PV, Hamdani M. An overview on the elimination of organic contaminants from aqueous systems using electrochemical advanced oxidation processes. J Water Process Eng. 2021;41:102040. doi: 10.1016/j.jwpe.2021.102040. [DOI] [Google Scholar]
- Wu J, Zhu K, Xu H, Yan W. Electrochemical oxidation of rhodamine B by PbO2/Sb-SnO2/TiO2 nanotube arrays electrode. Chinese J Catal. 2019;40(6):917–927. doi: 10.1016/S1872-2067(19)63342-5. [DOI] [Google Scholar]
- Xia Y, Feng J, Fan S, Zhou W, Dai Q. Fabrication of a multi-layer CNT-PbO2 anode for the degradation of isoniazid: kinetics and mechanism. Chemosphere. 2021;263:128069. doi: 10.1016/j.chemosphere.2020.128069. [DOI] [PubMed] [Google Scholar]
- Xu L, Yi Y, Liang G, Zhang W. Antimony doped tin oxide nanoparticles deposited onto Nb−TiO2 nanotubes for electrochemical degradation of bio-refractory pollutions. Electroanalysis. 2020;32(6):1370–1378. doi: 10.1002/elan.201900775. [DOI] [Google Scholar]
- Yao Y, Teng G, Yang Y, Huang C, Liu B, Guo L. Electrochemical oxidation of acetamiprid using Yb-doped PbO2 electrodes: electrode characterization, influencing factors and degradation pathways. Sep Purif Technol. 2019;211:456–466. doi: 10.1016/j.seppur.2018.10.021. [DOI] [Google Scholar]
- Zhang G, Xie S, Ho YS. A bibliometric analysis of world volatile organic compounds research trends. Scientometrics. 2010;83(2):477–492. doi: 10.1007/s11192-009-0065-3. [DOI] [Google Scholar]
- Zhang Y, Xu S, Zhou H, Qi H, Wang H. Adsorption of organic pollutants and heavy metal by Co-doped core-shell MoO2/Mo2C adsorbent. J Solid State Chem. 2021;293:121801. doi: 10.1016/j.jssc.2020.121801. [DOI] [Google Scholar]
- Zhao N, Liang D, Meng S, Li X. Bibliometric and content analysis on emerging technologies of hydrogen production using microbial electrolysis cells. Int J Hydrog Energy. 2020;45(28):33310–33324. doi: 10.1016/j.ijhydene.2020.09.104. [DOI] [Google Scholar]
- Zhu H, Chen Z (2021) Preparation of MWCNT-MnO2/Ni foam composite electrode for electrochemical degradation of Congo red wastewater. Int J Electrochem Sci 16(5):1–12. 10.20964/2021.05.19
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.