Abstract
Introduction
Out-of-pocket (OOP) is among the payment methods in Iran’s health system. The present study aimed to examine the OOP treatment costs for patients with COVID-19 in Iran.
Methods
A descriptive-analytical, cross-sectional study was conducted in 2021. In this study, the cost records of 550 patients with COVID-19 hospitalized in a referral center of COVID-19 were selected using the stratified random sampling method. The required data were collected using a researcher-made questionnaire. Data were analyzed by t-test, ANOVA, and Pearson’s correlation coefficient in SPSS software version 23 at p = 0.05.
Results
The total direct costs were 1,037,992.15 US $. Moreover, the shares of patients (OOP), basic insurance, government subsidy, supplementary insurance, discounts, and out-of-government subsidy in the total direct costs were US $ 92,231.21, 746,932.99 US $, 155,127.08 US $, 39,690.25 US $ and 4010.61 US $, respectively. In addition, the results confirmed that there was a positive and significant relationship between the patients’ OOP payments and the length of stay. It also found that the patients’ OOP payments are subject to the type of insurance program and discharge method.
Conclusion
According to the results, 8.89% of the total direct costs were directly paid out of the patients’ pockets. The research findings confirm the urgent need to make decisions and implement effective interventions for COVID-19 disease by controlling risk factors and exploiting other countries’ successful experiences and international organizations’ recommendations to decrease the prevalence of the infected and consequently reduce the financial pressure of the disease on patients by approving the expansion of the insurance organizations’ role.
Keywords: Out-of-pocket payment, COVID-19, Coronavirus, Hospital
Introduction
In late 2019, the COVID-19 pandemic and the subsequent rapid epidemic trend worldwide have raised concerns in different countries [1]. The virus’s rapid spread has left governments with prominent infected individuals [2]. Millions of individuals worldwide are now hospitalized because of the COVID-19 pandemic [3]. In Iran, the first case of COVID-19 was formally announced on February 20, 2019. Given the rapid transmission of this disease, the reported prevalence and prevalence rates seem to be of great concern [4]. According to the World Health Organization (WHO), by August 10, 2021, more than 200 million positive cases have been confirmed, and more than four million deaths from COVID-19 are estimated worldwide. In this regard, Iran accounted for more than four million cases and 95,000 deaths from COVID-19 [5]. On this date, the daily incidence of new cases in Iran with 40,808 is ranked first among other countries [5].
In addition to stewardship, generating resources, and providing services, financing is one of the four main functions of the health system. The conventional techniques of financing health care services include taxes, social insurance, private health insurance, and out-of-pocket (OOP) payments [6]. If the government fails to finance health care services, the financial burden will be directly imposed on individuals who must have the OOP payment [7]. The direct cost paid by the household instead of a service is called OOP payment [8].
Although OOP is typical in both developed and developing countries, it is the most inefficient way of financing the health system and is a defective mechanism for risk aggregation [9]. OOP can have serious adverse effects, including access to health services, especially among the poor [10]. Increasing OOP for private and public health services has led many households towards poverty and intensified the poverty of the poor [11]. The OOP financing is often a declining payment method for health services, and many individuals are imposed by catastrophic costs [12]. With the high price of health care services, about 44 million households (above 150 million persons) worldwide face exorbitant costs annually [13]. Even with health insurance, patients’ OOP seems to increase the risk of poverty [14].
In low-income countries, lack of insurance poor coverage, and insufficient social support have raised the OOP payments for households [15]. In Asian countries, OOP payments are one of the main methods of financing the health system [16, 17]. In general, OOP accounts for 18.6% of total health system expenditures in the third world [18].
In addition to the physical and physiological burden, COVID-19 has imposed a substantial financial burden on the health systems in different countries. In a study on the economic burden of COVID-19 in China, Jin et al. reported the total health and social costs of COVID-19 to be 0.62 and 383.02 billion dollars, respectively [19]. In research on the consequences and economic burden of 173,942 patients with COVID-19 hospitalized in the United States, it is estimated that the average hospitalization cost is $ 12,046 [20]. Another research in the US conducted on a large sample of 4075 patients to assess the OOP for COVID-19 found that Medicare advantage patients and privately insured patients contributed to hospitalization costs 49.1% and 71.21%, respectively. In this study, the average OOP for both groups was about 2688$ [21].
Measuring and monitoring health expenditures would help the policymakers in the health system select appropriate policies to protect patients [22]. Households’ OOP rate and the consequent incurrence of exorbitant health care costs are two main factors to be considered in health care planning and policy-making [23]. Given the significance of OOP and its continuous evaluation to monitor justice in financing the health system, this study evaluated the OOP rate among patients with COVID-19 admitted to the referral hospital for treatment of COVID19. Therefore, the main research question in this study is how much the OOP is in patients with COVID19 hospitalized in the public sector.
The study setting
Financing the health system in Iran is underpinned by multiple models encompassing the public budget, social, private, supplementary insurance, and OOP. According to Iran’s National Health Accounts, OOP is usually above 50% and ranges from 50.4% in 2004 to 52% in 2013 [24–26]. In Gharibi (2013) and Keshavarz’ (2012) studies during 2011–2013, the OOP rates were 55 and 59.7%, respectively [27, 28]. Moreover, Amery (2013) and Kavosi (2009) revealed that the exorbitant costs were 8.3 and 14.2%, respectively [29, 30]. Hajizadeh and Nghiem (2011) considered the OOP rate above 50% in Iran as one barrier to access health services [31].
The Iranian health system has faced quite a few challenges in terms of financing and the provision of healthcare, so much so that 3% of the people were faced with catastrophic health expenditure annually, leading to dissatisfaction among the citizens [32]. As a practical solution, within the last four decades, the Iranian health system has been initiating several reforms to pave the way towards universal health coverage (UHC) such as the establishment of an extensive Primary Health Care (PHC) network, the family physician program, and recently Health Transformation Plan (HTP) [33]. UHC that assures access to necessary health care services by all the population needs sustainable financing [34]. However, inefficient stewardship of the Iranian healthcare systems and the weak political support for investing in the healthcare sector, on the one hand, and the existence of the unfair international sanctions, on the other hand, can prevent the inflow of financial flows to Iran and consequently, it is hard to achieve the UHC [35]. That's why out-of-pocket payment is highlighted more than the other sustainable and fair financial resources.
According to a report by the WHO in 2000, countries and health policymakers were encouraged to provide equitable funding [36]. To this end, reforms in the health sector have been of interest to all health policymakers over the past decade, particularly in developing countries [37]. In this regard, the Ministry of Health in Iran implemented the health system transformation plan, which adopted three approaches (namely protecting individuals financially, creating justice in access to health services, and improving the quality of services in hospitals since May 2014. This plan was a stepping stone toward achieving all the ideals of the health system, especially the financial protection of patients, by providing basic health insurance for the uninsured and decreasing the payments for patients admitted to hospitals affiliated with the Ministry of Health [37]. The implementation of this plan aimed to protect citizens financially against health costs. According to the goal set in this plan, the OOP rate for inpatient services in public hospitals should be decreased by < 10% [38].
Methods
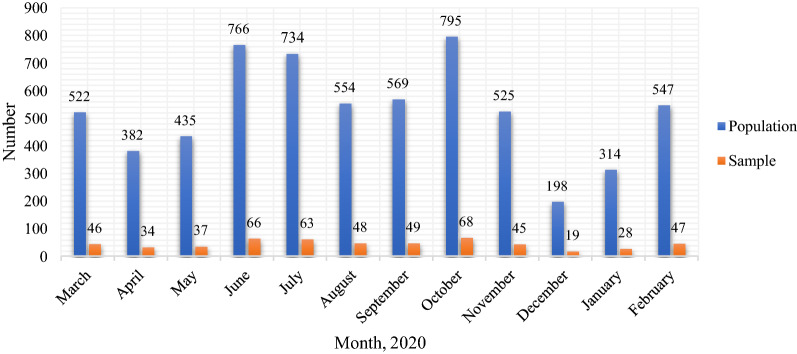
A cross-sectional descriptive-analytic study was conducted in 2021. The study’s statistical population encompassed the financial records of patients with COVID-19 hospitalized in a referral hospital in Shiraz, as COVID-19 Reference Hospital in southern Iran from April to late March 2020. Considering the population of patients with COVID-19 in 2021 (i.e., N = 6341) and the following formula, the sample size was 550 hospitalized patients. By dividing 550 by 6341 and multiplying the obtained number by the number of patients admitted per month, the desired sample size was obtained per month (stratified sampling proportional to the size). The random sampling method was adopted to select the sample based on patients' file numbers and the table of random numbers (Table 1, Fig. 1).
where, N = 6341; p = q = 0.5; z = 1.96; d = 0.04.
Table 1.
Population and a statistical sample of patients with COVID-19 admitted to the hospital
| Row | Month | Population | Sample |
|---|---|---|---|
| 1 | March | 522 | 46 |
| 2 | April | 382 | 34 |
| 3 | May | 435 | 37 |
| 4 | June | 766 | 66 |
| 5 | July | 734 | 63 |
| 6 | August | 554 | 48 |
| 7 | September | 569 | 49 |
| 8 | October | 795 | 68 |
| 9 | November | 525 | 45 |
| 10 | December | 198 | 19 |
| 11 | January | 314 | 28 |
| 12 | February | 547 | 47 |
| – | Total | 6341 | 550 |
Fig. 1.
Population and a statistical sample of patients with COVID-19 admitted to the Hospital
The data collection instrument was a researcher-made three-section form. The first section of the form addressed the patients' demographic information (age, gender, marital status, length of stay, discharge method, and type of insurance). The second and third sections dealt with the frequency of services and expenditure information, respectively. The former section encompassed the number of visit services, counseling, rehabilitation, CT scans, radiography, laboratory, dialysis, radiology, ultrasound, and echo. Expenditure information addressed total direct costs, patient share (OOP), basic insurance share, government subsidy, supplementary insurance share, discounts, and out of subsidy commitments from total direct costs by type of health care services.. For observing research ethics, all parts of the questionnaire were anonymous, and data concerning the costs have been kept confidential with one of researchers (ARY).
To collect the required data, after obtaining the approval of the Shiraz University of Medical Sciences, the researchers referred to the hospital's accounting unit, and the patients’ bills were received from the hospital’s health information system (HIS). Considering the ethical principles, all data collection forms were completed without mentioning patients’ names. After the data collection procedure, the data were analyzed using descriptive and inferential statistical methods, T-test, ANOVA, Pearson's correlation coefficient, and multiple linear regression with SPSS software version 23 at p = 0.05. We performed Pearson’s correlation to test the relationship between the OOP expenses with patients' age and the length of stay. T-test has been used to investigate the mean difference between the OOP expenses based on patients' gender and marital status. The ANOVA test has been applied to analyze any differences between the OOP expenses and variables such as type of insurance and discharge method. Further, we performed multiple linear regression to determine the simultaneous effect of background variables on the OOP expenses.
Results
Participants’ demographic information
The patients’ mean age was 56.29 ± 15.99 years, and most of the participants (23.09%) were in the age group of 51–60 years. Moreover, 54.2% of the patients were male, and most of the patients (93.1%) were married and had social security insurance (52.91%). The mean length of stay in the hospital for the patients was 6.64 ± 5.57 days, and most of these individuals (56.36%) were hospitalized for 5–10 days. Furthermore, 84.5% of the patients were discharged from the hospital in the “recovery” status. Table 2 shows the frequency distribution of the patients.
Table 2.
Participants’ demographic information (n = 550)
| Variable | Category | Number | Percentage |
|---|---|---|---|
| Gender | Female | 252 | 45.8 |
| Male | 298 | 54.2 | |
| Age (year) | < 20 | 2 | 0.37 |
| 20–30 | 20 | 3.64 | |
| 31–40 | 82 | 14.91 | |
| 41–50 | 91 | 16.54 | |
| 51–60 | 127 | 23.09 | |
| 61–70 | 118 | 21.45 | |
| > 70 | 110 | 20 | |
| Marital status | Single | 38 | 6.9 |
| Marital | 512 | 93.1 | |
| Length of stay | < 5 | 178 | 32.36 |
| 5–10 | 310 | 56.36 | |
| 11–15 | 33 | 6 | |
| 16–20 | 15 | 2.73 | |
| > 20 | 14 | 2.55 | |
| Type of insurance | Health Service | 192 | 34.91 |
| Social security | 291 | 52.91 | |
| Armed Forces | 51 | 9.27 | |
| Other | 16 | 2.91 | |
| Discharge | Recovery | 465 | 84.5 |
| Death | 50 | 9.1 | |
| Transfer | 28 | 5.1 | |
| Voluntary clearance | 7 | 1.3 |
Frequency of services provided to participants
According to the results, the highest frequency of services provided to the patients was for “laboratory” services with > 50,000 tests (average 107.70 tests per patient) (Table 3).
Table 3.
Frequency of services provided to patients (n = 550)
| Type of service | Number | Mean | Standard deviation |
|---|---|---|---|
| Visit | 4342 | 7.89 | 5.90 |
| Counseling | 433 | 0.79 | 1.05 |
| Rehabilitation | 524 | 0.95 | 3.56 |
| CT scans | 560 | 1.02 | 1.17 |
| Radiography | 1574 | 2.86 | 2.34 |
| Laboratory | 59,234 | 107.70 | 120.69 |
| Dialysis | 8 | 0.01 | 0.12 |
| Radiology | 182 | 0.33 | 1.20 |
| Ultrasound | 56 | 0.10 | 0.34 |
| Echo | 54 | 0.10 | 0.30 |
| Hoteling (Regular bed) | 2887 | 5.25 | 3.20 |
| Hoteling (Special bed) | 763 | 1.39 | 4.94 |
| Other services (Transport, Food) | 1326 | 2.41 | 1.93 |
Direct costs of all patients
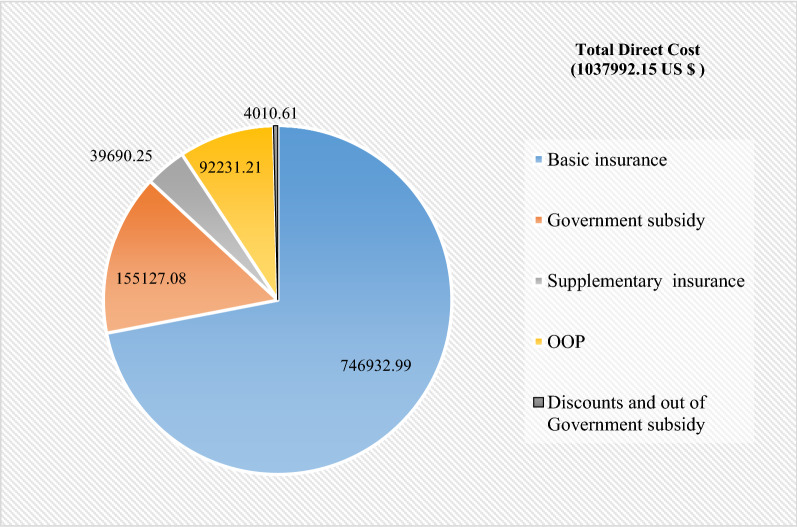
The study results indicated that the total direct cost was 1,037,992.15 US $, with 71.96% basic insurance share (746,932.99 US $,), 14.94% government subsidy share (155,127.08 US $), 3.82% supplementary insurance share (39,690.25 US $), 8.89% share of patients (OOP) (92,231.21 US $), and 0.39% discounts and out of subsidy commitments (4010.61 US $) (Table 4 and Fig. 2).
Table 4.
Shares of basic and supplementary insurance, government subsidy, patients (OOP), and discounts in the total direct cost of patients (US $)
| Category | Amount | Mean | Standard deviation | Percentage of total direct costs |
|---|---|---|---|---|
| Basic insurance | 746,932.99 | 1358.05 | 1910.03 | 71.96 |
| Government subsidy | 155,127.08 | 282.04 | 614.89 | 14.94 |
| Supplementary insurance | 39,690.25 | 72.16 | 348.69 | 3.82 |
| OOP | 92,231.21 | 167.69 | 271.21 | 8.89 |
| Discounts and Government subsidy | 4010.61 | 7.29 | 6.41 | 0.39 |
| Total direct cost | 1,037,992.15 | 1887.25 | 2562.55 | 100 |
*Values are expressed in US $
Fig. 2.
Shares of basic and supplementary insurance, government subsidy, patients (OOP), and discounts in the total direct cost of patients (US $)
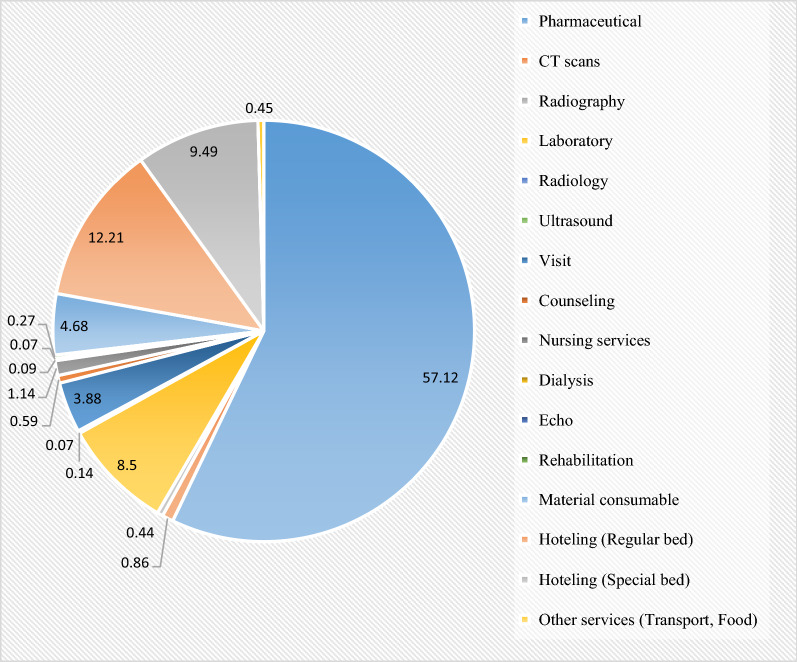
The results indicated that among the types of services, pharmaceutical services with 45.09% of the total direct costs (466,249.91 US $) and 57.12% of the patients’ share (52,681.39 US $) had the highest shares in OOP (Tables 5, 6 and Fig. 3).
Table 5.
Shares of basic and supplementary insurances, government subsidy, and patient in OOP by service type
| Type of service | Total Amount | Total basic insurance | Total government subsidy | Total Supplementary insurance | OOP | Percentage of total direct cost |
|---|---|---|---|---|---|---|
| Pharmaceutical | 466,249.91 | 247,798.40 | 133,512.24 | 31,269.61 | 52,681.39 | 45.09 |
| CT scans | 9319.98 | 8808.84 | 83.33 | 147.61 | 791.21 | 0.90 |
| Radiography | 4571.92 | 4162.26 | 13.51 | 64.88 | 402.38 | 0.44 |
| Laboratory | 80,625.58 | 67,429.97 | 6126.96 | 1356.29 | 7837.54 | 7.80 |
| Radiology | 1991.57 | 1739.82 | 128.92 | 12.93 | 127.36 | 0.19 |
| Ultrasound | 724.32 | 647.41 | 0 | 12.62 | 66.32 | 0.07 |
| Visit | 81,641.16 | 74,296.98 | 223.07 | 660.02 | 3580.52 | 7.89 |
| Counseling | 11,526.61 | 10,766.21 | 60.01 | 59.81 | 544.32 | 1.12 |
| Nursing services | 15,787.64 | 14,512.61 | 77.19 | 196.11 | 1055.37 | 1.53 |
| Dialysis | 2576.14 | 1957.40 | 180.45 | 4.98 | 86.09 | 0.25 |
| Echo | 1983.61 | 1819.93 | 6.53 | 24.52 | 62.67 | 0.19 |
| Rehabilitation | 4695.23 | 4365.43 | 45.53 | 44.77 | 244.45 | 0.45 |
| Material consumable | 28,340.71 | 9446.11 | 13,587.20 | 1658.83 | 4320.87 | 2.75 |
| Hoteling (Regular bed | 141,997.04 | 129,650.57 | 0 | 1488.76 | 11,260.55 | 13.73 |
| Hoteling (Special bed) | 169,856.59 | 158,927.37 | 0 | 2657.56 | 8755.83 | 16.43 |
| Other services (Transport, Food) | 12,093.46 | 10,603.62 | 1082.07 | 30.91 | 414.28 | 1.17 |
| Total | 1,033,981.55 | 746,932.99 | 155,127.08 | 39,690.25 | 92,231.21 | 100 |
*The total direct cost of services excluding "discounts and out of subsidy commitment”
**Values are expressed in US $
Table 6.
The mean and standard deviation of basic and supplementary insurance, government subsidy, and patient shares in OOP by service type
| Type of service | Mean (standard deviation) | ||||
|---|---|---|---|---|---|
| Total amount | Basic insurance | Government subsidy | Supplementary insurance | OOP | |
| Pharmaceutical | 847.72 (1163.86) | 450.54 (639.81) | 242.75 (552.61) | 56.85 (298.09) | 95.78 (193.09) |
| CT scans | 16.94 (18.01) | 16.01 (25.86) | 0.15 (1.49) | 0.26 (1.29) | 1.43 (3.49) |
| Radiography | 8.31 (6.86) | 7.56 (6.37) | 0.02 (0.24) | 0.11 (0.41) | 0.73 (1.33) |
| Laboratory | 146.59 (155.90) | 122.61 (134.49) | 11.13 (25.51) | 2.46 (8.78) | 14.25 (21.01) |
| Radiology | 3.62 (31.42) | 3.16 (24.15) | 0.23 (4.43) | 0.02 (0.22) | 0.23 (3.06) |
| Ultrasound | 1.31 (4.69) | 1.17 (4.22) | 0 (0) | 0.02 (0.18) | 0.12 (0.97) |
| Visit | 148.43 (100.02) | 135.08 (92.01) | 0.41 (2.49) | 1.21 (3.72) | 6.51 (7.52) |
| Counseling | 20.95 (28.49) | 19.57 (32.69) | 0.11 (1.23) | 0.11 (0.49) | 0.99 (2.46) |
| Nursing services | 28.70 (48.64) | 26.38 (45.25) | 0.14 (1.53) | 0.35 (1.49) | 1.91 (3.56) |
| Dialysis | 4.68 (35.04) | 3.55 (27.86) | 0.32 (7.56) | 0.009 (0.21) | 0.15 (2.26) |
| Echo | 3.60 (11.10) | 3.31 (10.18) | 0.01 (0.09) | 0.04 (0.31) | 0.11 (0.47) |
| Rehabilitation | 8.53 (34.21) | 7.93 (31.72) | 0.08 (1.13) | 0.08 (0.66) | 0.44 (2.11) |
| Material consumable | 51.52 (114.28) | 17.17 (48.82) | 24.71 (64.75) | 3.01 (13.24) | 7.85 (22.24) |
| Hoteling (regular bed | 258.17 (218.96) | 235.72 (144.75) | 0 (0) | 2.71 (5.91) | 20.47 (24.85) |
| Hoteling (special bed) | 308.83 (1124.51) | 288.95 (869.91) | 0 (0) | 4.83 (25.37) | 15.92 (63.44) |
| Other services (transport, Food) | 21.98 (25.38) | 19.27 (34.81) | 1.96 (1.01) | 0.05 (0.52) | 0.75 (2.71) |
*Values are expressed in US $
Fig. 3.
Patients’ OOP percentage by service type
Relationship between demographic variables with total direct cost and OOP
Considering the results, there was a statistically significant relationship between the patients’ OOP payments and length of stay (r = 0.543, P < 0.001). As Table 7 shows that the patients’ OOP payments are subject to type of insurance program (F = 8.399, P < 0.001) and discharge method (F = 6.991, P < 0.001).
Table 7.
Relationship between patients’ demographic variables with their total cost and OOP payments
| Demographic variables | Main variables | Type of test and significance | |
|---|---|---|---|
| Pearson’s correlation coefficient | P value | ||
| Age | OOP | 0.052 | 0.225 |
| Total direct cost | 0.092 | 0.03 | |
| Length of stay | OOP | 0.543 | < 0.001 |
| Total direct cost | 0.888 | < 0.001 | |
| Main variables | T-test (t) | P value | |
|---|---|---|---|
| Gender | OOP | 0.74 | 0.45 |
| Total direct cost | 0.18 | 0.59 | |
| Marital status | OOP | 0.93 | 0.34 |
| Total direct cost | 1.12 | 0.26 | |
| Main variables | ANOVA (F) | P value | |
|---|---|---|---|
| Discharge | OOP | 6.991 | < 0.001 |
| Total direct cost | 27.44 | < 0.001 | |
| Type of insurance | OOP | 8.399 | < 0.001 |
| Total direct cost | 2.090 | 0.02 | |
For post hoc analysis, the Scheffe test indicated that average OOP was significantly different among death and two main groups: recovery (P = 0.001) and transfer group (P = 0.004). As such, the average OOP was 167.21 and 229.97 units larger in the death group than the recovery and transfer group, respectively. Also, the average OOP was significantly different between patients covered by armed forces insurance and health services insurance (P = 0.009) and patients covered by armed forces insurance and social security insurance (P = 0.04). The average OOP in patients covered by armed forces insurance decreased by 197.41 units compared to patients covered by health services and 101.93 units compared to patients covered by social security insurance.
The multivariable regression of the OOP is first influenced by the length of stay, followed by the type of insurance program. Adjusted R-Squared was calculated at 0.29 (Table 8).
Table 8.
Variables affecting OOP based on multiple linear regression
| Variable definition | Variable | Unstandardized coefficients | Standardized coefficient β | t | P-value* | |
|---|---|---|---|---|---|---|
| B | Std. error | |||||
| – | (Constant) | 11.12 | 14.324 | – | 0.776 | 0.238 |
| x1 | Gender | 0.199 | 2.932 | 0.003 | 0.068 | 0.646 |
| x2 | Age | 0.037 | 0.119 | 0.012 | 0.311 | 0.456 |
| x3 | Marital status | 3.008 | 7.367 | 0.016 | 0.408 | 0.383 |
| x4 | Length of stay | 4.636 | 0.308 | 0.544 | 15.054 | < 0.001 |
| x5 | Discharge | 1.489 | 3.451 | 0.016 | 0.432 | 0.366 |
| x6 | Type of insurance | 1.508 | 0.453 | 0.212 | 2.375 | 0.002 |
*P-value Correlation is significant at the 0.05 level, B unstandardized coefficients, Std. error standard error
Discussion
The present study aimed to determine OOP payment in the COVID-19 reference hospital in southern Iran. According to the current study’s findings, the OOP rate of hospitalized patients accounted for 8.89% of the total direct costs, with an average cost of 167.69 US $ per patient. Chua et al. averaged OOP for COVID-19 in 1,465 patients (according to the PharMetrics Plus for Academics database) over 90 days after discharge. They reported values from $534 for patients with private insurance and 680 US $ for patients covered by Medicare Advantage Plans [39]. Furthermore, Eisenberg et al. (2020) assessed the financial risk for COVID-19-like respiratory hospitalizations in the United States. They reported that the average OOP for patients admitted with respiratory infection in the two groups of consumer-directed health plans (CDHPs) and traditional plans were 1961 US $ and 1653 US $, respectively [40]. The findings of this study and previous studies indicate that COVID-19 disease has been associated with financial pressures imposed on patients because of hospitalization and the payment of a part of the treatment costs in the form of OOP. In addition to the need to pay for treatment, COVID-19 seems to have imposed financial pressure on patients by depriving them of some sources of income [41].
According to the results of this study, 57.12% of the OOP rates for the hospitalized patients were associated with pharmaceutical services, and the pharmaceutical cost had the largest share of OOP. Darab et al. in their study on the economic burden of COVID-19 in a reference hospital in southern Iran, reported that the costs of medicines and consumables devices with 28% of the total OOP were ranked second [42]. Medicines are one of the main expenditure items in the treatment of diseases, which, in line with the present study, are reported as the largest share of direct costs in many studies [43, 44].
The present results revealed a significant relationship between the OOP of hospitalized patients and the length of stay. Patients’ OOP rates also increased with the increasing length of stay. Hajizadeh and Nghiem conducted a study to promote an understanding of inequality and determinants of the OOP payments and exorbitant health costs for hospital services in Iran using national health services statistics. They concluded that the length of stay were significantly associated with patients’ OOP and increased likelihood of exorbitant health costs [31]. Taheri et al. studied all discharged patients (above 12,000 patients) from a teaching hospital in the United States. They found out that reducing the patients’ length of stay by a day decreases the total care cost by an average of three percent or less [45]. Riascos and Serna in Colombia conducted a study to predict the patients’ length of stay and estimate its impact on the health costs of about one million persons who had received at least one service during the last year and had not changed their insurance company during 2009–2011. They showed that the patients’ long stay in hospitals was costly for service providers, insurers, and patients due to increased demand for health services and the likelihood of serious risks during the stay [46]. Increasing the number of hospitalization days would also affect the cost and quality of the provided care [47]. Prolonged hospitalization increases the use of limited resources and the depreciation of hospital functions [48]. On the other hand, with an increase in length of stay, the costs of patients and the hospital increase, and the patients’ recovery and rehabilitation time also increase, thereby increasing costs [49]. To decrease costs, the length of unnecessary stay for patients should be reduced; hence, different interventions such as discharge planning, care strategies, periodic audits to detect and act against care delays, the use of checklists to plan admissions, the detection of motivated reference physicians can be implemented. Such interventions not only can reduce the hospitalization cost but also the incidence of infections and limited access to resources available to patients can be decreased [50, 51].
The present study suggested a statistically significant relationship between OOP for the COVID-19 patients and the discharge method in the death group. In their research, El-Khatib et al. reported a positive relationship between the mortality rate of the COVID-19 disease and patients’ OOP [52]. Yang et al. also revealed that the OOP rate in patients increases significantly with approaching death [53]. The significant relationship between OOP and mortality rates can be because patients with worse conditions need to spend more resources to regain their health, increasing hospital costs and patient payments.
Finally, the findings also indicated a significant relationship between patients' OOP and the type of insurance program. In their study in China, You and Kobayashi reported that some types of insurance programs were associated with increased patients’ OOP rates [54]. In Mexico, Galárraga et al. found out that voluntary public insurance for the unemployed and self-employed individuals was associated with a 54% decrease in catastrophic health expenditures [55]. Finkelstein and McKnight (2008) also reported a 40 percent decrease in patients’ OOP following the introduction of Medicare in the United States in 1965 [56].
Conclusions
The present study reported the hospitalized patients’ OOP rate of 8.89%. The findings of this study confirm the urgent need to make decisions and implement effective interventions against COVID-19 by controlling risk factors and using other countries’ successful experiences and international organizations’ recommendations to reduce the prevalence and consequently the economic burden of the disease on patients and expand the role of insurance organizations.
The main limitation of the present study was that the OOP rate was estimated based on the documents and financial records of patients registered in HIS. Some patients, however, may have other OOP payments, such as informal payments and payments for drugs not available in the hospital. Such costs are likely to be reflected in official records.
Acknowledgements
This article was extracted from a research project approved by the Higher Institute of Social Security Research in Tehran (Code: 20992026). The researchers would like to express their gratitude to the esteemed President of the Higher Institute of Social Security Research in Tehran and also the Vice-Chancellor for Research and Technology of the Shiraz University of Medical Sciences for their material and spiritual cooperation.
Authors’ contributions
Substantial contribution to study conception and design: ARY, GM, AKH; substantial contribution to analysis and interpretation of the data: ARY, RA, FV, FH; drafting the article or revising it: ARY, GM, AKH, RA; final approval of the version of the article to be published: ARY, GM, PB. All authors read and approved the final manuscript.
Funding
There was no funding.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Reza Yusefi, Email: alirezayusefi67@gmail.com.
Gholamhossein Mehralian, Email: gmehralian@gmail.com.
Abdolvahed Khodamoradi, Email: khodamoradi.av@ssor.ir.
Roghaye Abbasi, Email: nikmaneshp.n@gmail.com.
Fatemeh Vatankhah, Email: fatemehvatankhah1377@gmail.com.
Fatemeh Heaidari, Email: fateme.heydari.gharaei@gmail.com.
Peivand Bastani, Email: peivandbastani@hotmail.com, Email: p.bastani@uq.edu.au.
References
- 1.Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Yang J, Yang W, Wang C, Bärnighausen T. COVID-19 control in China during mass population movements at New Year. The Lancet. 2020;395(10226):764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doshmangir L, Ahari AM, Qolipour K, Azami S, Kalankesh L, Doshmangir P, et al. East Asia’s strategies for effective response to COVID-19: lessons learned for Iran. Quarter J Manag Strat Health Syst. 2019 doi: 10.18502/mshsj.v4i4.2542. [DOI] [Google Scholar]
- 5.World Health Organization. https://covid19.who.int/. Accessed 10 Aug 2021.
- 6.Yu CP, Whynes DK, Sach TH. Equity in health care financing: the case of Malaysia. Int J Equity Health. 2008;7(1):1–4. doi: 10.1186/1475-9276-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolasa K, Kowalczyk M. Does cost sharing do more harm or more good?—a systematic literature review. BMC Public Health. 2016;16(1):992. doi: 10.1186/s12889-016-3624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanjani H, Alizadeh M, Fazaeli AA. The equity in the financing of the health system in Iran. Soc Walfare Quarterly. 2005;5(19):279–300. [Google Scholar]
- 9.Correa-Burrows P. Out-of-pocket health care spending by the chronically ill in Chile. Proc Econ Finance. 2012;1:88–97. [Google Scholar]
- 10.Hotchkiss DR, Hutchinson PL, Malaj A, Berruti AA. Out-of-pocket payments and utilization of health care services in Albania: evidence from three districts. Health Policy. 2005;75(1):18–39. doi: 10.1016/j.healthpol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead M, Dahlgren G, Evans T. Equity and health sector reforms: can low-income countries escape the medical poverty trap? Lancet. 2001;358(9284):833–836. doi: 10.1016/S0140-6736(01)05975-X. [DOI] [PubMed] [Google Scholar]
- 12.Krůtilová V, Yaya S. Unexpected impact of changes in out-of-pocket payments for health care on Czech household budgets. Health Policy. 2012;107(2):276–288. doi: 10.1016/j.healthpol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Moghadam MN, Banshi M, Javar MA, Amiresmaili M, Ganjavi S. Iranian household financial protection against catastrophic health care expenditures. Iran J Public Health. 2012;41(9):62. [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen XT. The injury poverty trap in rural Vietnam: causes, consequences and possible solutions. PhD thesis, Department of Public Health and Clinical Medicine, Umea University, Umea, Sweden; 2005.
- 15.Roy K, Howard DH. Equity in out-of-pocket payments for hospital care: evidence from India. Health Policy. 2007;80(2):297–307. doi: 10.1016/j.healthpol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Limwattananon S, Vongmongkol V, Prakongsai P, Patcharanarumol W, Hanson K, Tangcharoensathien V, et al. The equity impact of universal coverage: health care finance, catastrophic health expenditure, utilization and government subsidies in Thailand, London: consortium for research on equitable health systems; 2011. http://www.crehs.lshtm.ac.uk/thai_biafia_19jul.pdf. Accessed 15 Jan 2016.
- 17.Skordis-Worrall J, Pace N, Bapat U, Das S, More NS, Joshi W, et al. Maternal and neonatal health expenditure in Mumbai slums (India): a cross sectional study. BMC Public Health. 2011;11(1):1–2. doi: 10.1186/1471-2458-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Health financing. 2015. http://www.who.int/gho/health_financing/en/. Accessed 30 Oct 2015.
- 19.Jin H, Wang H, Li X, Zheng W, Ye S, Zhang S, et al. Economic burden of COVID-19, China, January–March, 2020: a cost-of-illness study. Bull World Health Organ. 2021;99(2):112–124. doi: 10.2471/BLT.20.267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Fusco M, Shea KM, Lin J, Nguyen JL, Angulo FJ, Benigno M, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–317. doi: 10.1080/13696998.2021.1886109. [DOI] [PubMed] [Google Scholar]
- 21.Chua KP, Conti RM, Becker NV. Assessment of out-of-pocket spending for COVID-19 hospitalizations in the US in 2020. JAMA Netw Open. 2021;4(10):e2129894. doi: 10.1001/jamanetworkopen.2021.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jame SZB, Markazi-Moghaddam N, Ebrahim Z, Kavosi Z, Marzaleh MA, Yusefi AR. The comparison of out of pocket payments for coronary artery bypass graft surgery before and after the health sector evolution plan in the South of Iran. Shiraz E Med J. 2019;20(10):e87097. [Google Scholar]
- 23.Asefzadeh S, Alijanzadeh M, Peyravian F. Out of pocket expenditures for outpatient clinics in teaching hospitals. Payesh. 2014;13(3):267–276. [Google Scholar]
- 24.Ahmadi A, Nikravan A, Naseri A, Asari A. Effective determinants in household out of packet payments in health system of Iran, using two part regression model. JHA. 2014;17(56):7–18. [Google Scholar]
- 25.Tabibi M, Davoodi A. Economic analysis of national health accounts in Iran during 2002–2011. Econ J. 2015;15(56):41–64. [Google Scholar]
- 26.Raghfar H, Khezri M, Vaez Mahdavi Z, Sangari MK. Impact of health insurance inefficiency on poverty among Iranian households. Hakim Res J. 2013;16(1):9–19. [Google Scholar]
- 27.Gharibi F, Heydari A, Zarei M. Percent out of pocket for health services by people in Kurdistan province. Sci J Kurdistan Univ Med Sci. 2013;18(2):20–28. [Google Scholar]
- 28.Keshavarz A, Kalhor R, Javadi A, Asefzadeh S. Estimating Out Of Pocket payments (oop) for medical cares in Qazvin province in 2009. Hospital. 2012;10(4):71–77. [Google Scholar]
- 29.Amery H, Jafari A, Panahi M. Determining the rate of catastrophic health expenditure and its influential factors on families in Yazd Province. J Health Adm. 2013;16(52):51–60. [Google Scholar]
- 30.Kavosi Z, Rashidian A, Pourmalek F, Majdzadeh R, Pourreza A, Mohammad K, et al. Measuring household exposure to catastrophic health care expenditures: a longitudinal study in zone 17 of Tehran. Hakim Res J. 2009;12(2):38–47. [Google Scholar]
- 31.Hajizadeh M, Nghiem HS. Out-of-pocket expenditures for hospital care in Iran: who is at risk of incurring catastrophic payments? Int J Health Care Finance Econ. 2011;11(4):267–285. doi: 10.1007/s10754-011-9099-1. [DOI] [PubMed] [Google Scholar]
- 32.Ghiasi G, Rashidian A, Kebriaeezadeh A, Salamzadeh J. The impact of the sanctions made against Iran on availability to asthma medicines in Tehran. Iran J Pharm Res. 2016;15(3):567–571. [PMC free article] [PubMed] [Google Scholar]
- 33.Sajadi HS, Goodarzi Z, Takian A, Mohamadi E, Olyaeemanesh A, et al. Assessing the efficiency of Iran health system in making progress towards universal health coverage: a comparative panel data analysis. Cost Eff Resour Alloc. 2020;18(1):1–11. doi: 10.1186/s12962-020-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadeghi A, Barati O, Bastani P, Jafari DD, Etemadian M. Experiences of selected countries in the use of public-private partnership in hospital services provision. J Pak Med Assoc. 2016;66(11):1401–1406. [PubMed] [Google Scholar]
- 35.Nouhi M, Olyaeemanesh A, Teymourzadeh E, Bahadori M, Hakimzadeh SM, Babaei M. Rouhani-care and the joint comprehensive plan of action: a nightmare scenario. Health Policy Technol. 2019;8(1):5–6. [Google Scholar]
- 36.World Health Organization . The world health report 2000: health systems: improving performance. Geneva: World Health Oraganization; 2000. [Google Scholar]
- 37.Asefzadeh S. Health sector reform in view. J Qazvin Univ Med Sci. 2006;10(3):5–6. [Google Scholar]
- 38.Ministry of Health and Medical Education (Iran). Health Sector Evolution.2015. http://tahavol.behdasht.gov.ir/. Accessed 13 Oct 2015.
- 39.Chua KP, Conti RM, Becker NV. Out-of-pocket spending within 90 days of discharge from COVID-19 hospitalization. MedRxiv. 2021 doi: 10.1101/2021.06.11.21258766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg MD, Barry CL, Schilling CL, Kennedy-Hendricks A. Financial risk for COVID-19—like respiratory hospitalizations in consumer-directed health plans. Am J Prev Med. 2020;59(3):445–448. doi: 10.1016/j.amepre.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leightner JE. Covid-19, out-of-pocket medical expenses and consumption. J Financ Econ Policy. 2021;13(4):462–478. [Google Scholar]
- 42.Darab MG, Keshavarz K, Sadeghi E, Shahmohamadi J, Kavosi Z. The economic burden of coronavirus disease 2019 (COVID-19): evidence from Iran. BMC Health Serv Res. 2021;21(1):1–7. doi: 10.1186/s12913-021-06126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotstein Z, Hazan R, Barak Y, Achiron A. Perspectives in multiple sclerosis health care: special focus on the costs of multiple sclerosis. Autoimmun Rev. 2006;5(8):511–516. doi: 10.1016/j.autrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Miltenburger C, Kobelt G. Quality of life and cost of multiple sclerosis. Clin Neurol Neurosurg. 2002;104(3):272–75. doi: 10.1016/s0303-8467(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 45.Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191(2):123–130. doi: 10.1016/s1072-7515(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 46.Riascos AJ, Serna N. Predicting annual length-of-stay and its impact on health costs: the case of the colombian health care system. In: proceedings of the first workshop medical informatics and healthcare held with the 23rd SIGKDD conference on knowledge discovery and data mining, PMLR. 2017;69:27–34.
- 47.Gruenberg DA, Shelton W, Rose SL, Rutter AE, Socaris S, McGee G. Factors influencing length of stay in the intensive care unit. Am J Crit Care. 2006;15(5):502–509. [PubMed] [Google Scholar]
- 48.Nasiripour AA, Riyahi L, Gholamipour M. The effect of the presence of full time gynecologist on length of stay among inpatient of gynecology ward in yazd social security hospital, 2008. J Med Counc Islam Repub Iran. 2010;28(2):169–175. [Google Scholar]
- 49.Arab M, Zarei A, Rahimi A, Rezaiean F, Akbari F. Analysis of factors affecting length of stay in public hospitals in Lorestan Province, Iran. Hakim Health Sys Res. 2010;12(4):27–32. [Google Scholar]
- 50.Haghgoshaei E, Narimani M, Modir Shahla A, Takbiri A, Abolghasem GH. Day clinic: a model for reducing the length of stay in hospitals. JHA. 2012;14(46):21–30. [Google Scholar]
- 51.Caminiti C, Meschi T, Braglia L, Diodati F, Iezzi E, Marcomini B, et al. Reducing unnecessary hospital days to improve quality of care through physician accountability: a cluster randomized trial. BMC Health Serv Res. 2013;13(1):14. doi: 10.1186/1472-6963-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Khatib Z, Otu A, Neogi U, Yaya S. The association between out-of-pocket expenditure and COVID-19 mortality globally. J Epidemiol Glob Health. 2020;10(3):192–193. doi: 10.2991/jegh.k.200725.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z, Norton EC, Stearns SC. Longevity and health care expenditures: the real reasons older people spend more. J Gerontol B Psychol Sci Soc Sci. 2003;58(1):S2–10. doi: 10.1093/geronb/58.1.s2. [DOI] [PubMed] [Google Scholar]
- 54.You X, Kobayashi Y. Determinants of out-of-pocket health expenditure in China. Appl Health Econ Health Policy. 2011;9(1):39–49. doi: 10.2165/11530730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Galárraga O, Sosa-Rubí SG, Salinas-Rodríguez A, Sesma-Vázquez S. Health insurance for the poor: impact on catastrophic and out-of-pocket health expenditures in Mexico. Eur J Health Econ. 2010;11(5):437–447. doi: 10.1007/s10198-009-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finkelstein A, McKnight R. What did Medicare do? The initial impact of Medicare on mortality and out of pocket medical spending. J Public Econ. 2008;92(7):1644–1668. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.