Abstract
Background
Real-world studies showed varying levels of effectiveness of CoronaVac vaccine against COVID-19 disease. This study aimed to assess the association between the vaccination with CoronaVac and the COVID-19 infections among the health care workers in a university hospital and to determine the vaccine effectiveness against COVID-19 in a period when alpha variant was dominant.
Methods
This retrospective cohort study was conducted in a university hospital in Istanbul, Turkey employs 4067 health care workers. The follow-up period was defined as starting 14 days after receiving the second dose for fully vaccinated group. Health care workers were censored when have a positive PCR test result or at the end of the study. Unvaccinated health care workers were censored if they receive any COVID-19 vaccine doses. The incidence rate ratio and Cox regression were used to estimate the unadjusted and adjusted effectiveness of the vaccine.
Findings: Seventy-one percent of the health care workers were fully vaccinated whereas 29% percent did not receive any doses. The incidence rate of SARS-CoV-2 infection was 133.7 vs 70.7 per 100.000 person-days in the unvaccinated and fully vaccinated groups, respectively. The unadjusted effectiveness against COVID-19 infection was 47% (95% CI 31–59%) whereas adjusted effectiveness was 39% (95% CI 20–64%).
Interpretation: This real life study conducted in health care workers demonstrated that the effectiveness of two doses of the CoronaVac vaccine (39%) was lower than that determined in clinical trials. Due to reduce in protection over time or against variants, booster doses may be needed.
Keywords: Inactivated vaccine, COVID-19 vaccine, Health care workers, Vaccine effectiveness
1. Introduction
The CoronaVac is an inactivated virus Coronavirus disease 2019 (COVID-19) vaccine developed by the Sinovac Biotech company. The Phase III clinical trials were conducted in different countries, including Brazil and Turkey, and showed efficacy at preventing symptomatic infections ranges between 50·7% and 83.5%.[1], [2] On 13 January 2021, the vaccine was given Emergency Use Authorization by the Turkish Medicines and Medical Devices Agency, and immediately health care workers (HCW) were eligible for vaccination.[3], [4] The vaccine was listed for Emergency Use Listing by the World Health Organization (WHO) on 1 June 2021.[5]
Real-world studies in different countries have shown varying levels of effectiveness of CoronaVac against symptomatic COVID-19, hospitalization, and mortality. In a study conducted in Serrana (Brazil), where 75% of the adult population was vaccinated, the results showed that symptomatic cases fell by 80%, admissions by 86%, and deaths by 95%.[6] A study in Chile presented that the adjusted effectiveness of CoronaVac among fully immunized (more than 14 days after the second dose) persons was 65.9% for COVID-19, 87.5% for admissions, and 86.3% for death.[7] Another study in Jakarta (Indonesia) monitored HCWs vaccinated with CoronaVac and found the effectiveness of vaccine against symptomatic disease was 94%.[8] Since mid-December 2020, several variants of the virus have been identified and these variants affected the effectiveness of the vaccine. A study conducted in Guangzhou city during the outbreak of the Delta variant demonstrated that the effectiveness of two doses of inactivated SARS-CoV-2 vaccines (CoronaVac or China National Biotec Group) were 59.0% against infection, which was lower than that previously reported ( ∼ 79.3%).[9]
This study aimed to assess the association between the vaccination with CoronaVac and the COVID-19 infections among the health care workers in a university hospital and to determine the vaccine effectiveness against COVID-19 in a period when 75% of cases were infected with alpha variant.[10]
2. Method
2.1. Study design
This retrospective cohort study was conducted between March 1 and May 31, 2021 in a university hospital in Istanbul, Turkey employing 4067 health care workers. The hospital has no screening policy for COVID-19 however highly recommends the routine PCR test to its employees with potential exposure such as working in the COVID-19 clinic. The hospital has a COVID-19 Diagnosis and Treatment Center conducted by the Department of Infectious Diseases and Clinical Microbiology. The follow-ups and records of HCWs with a positive result in polymerase chain reaction (PCR) tests are carried out by the Department of Public Health. Data consisting of demographic characteristics, vaccination status, and symptoms were retrieved from the hospital's medical records.
The study was approved by the Ethics Committee of Istanbul University - Cerrahpasa (Approval number: 103513 Approval date: 02.06.2021) and conducted in accordance with the Declaration of Helsinki. Permission was obtained from the Republic of Turkey’s Ministry of Health and Faculty administration for the use of HCWs' data in our study. The study was reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
2.2. Participants and definitions
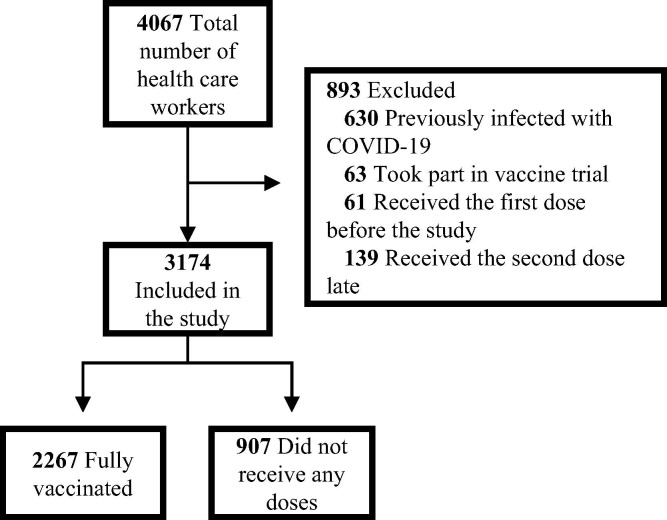
Eight hundred and ninety-three of 4067 health workers were excluded from the study for various reasons (Fig. 1 ).
Fig. 1.
The flowchart showing the included and excluded health care workers.
HCWs who had received two doses of CoronaVac vaccine and 14 days had passed after the second dose by March 31, 2021 were defined as fully the vaccinated group. HCWs who had no doses of any COVID-19 vaccine by March 1, 2021 were defined as the unvaccinated group.
The follow-up period was defined as starting either 14 days after receiving the second dose for the fully vaccinated group or on March 1, 2021, for the unvaccinated group. HCWs were censored when they received a positive PCR test result or on May 31, 2021. Also, the unvaccinated HCWs were censored if they received any COVID-19 vaccine doses.
HCWs with positive PCR tests but without symptoms were defined as asymptomatic.[11]
2.3. Statistical analysis
SPSS v21.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables were given as mean ± standard deviation, and categorical variables as frequency and percentage. The Chi-square test or Fisher Exact test, where appropriate, was used in the analysis of categorical variables. Kaplan-Meier analysis was performed to assess cumulative incidence of fully vaccinated and unvaccinated groups. The incidence rate ratio (IRR) was used to estimate unadjusted effectiveness of the vaccine.[12]
First, the incidence rates of fully vaccinated (IRFV) and unvaccinated (IRUV) groups were assessed as below:
Then, the incidence rates of two groups were used to calculate the unadjusted IRR (uIRR):
The number of COVID-19 positive cases in the vaccinated (nFV) and unvaccinated (nUV) groups were used for the standard error (SE), and the 95% CI of uIRR was calculated:
Cox regression was used to determine effectiveness of CoronaVac independent of covariates (adjusted effectiveness). Results were given as hazard ratio (HR) and 95% confidence interval (95% CI). A p-value < 0.05 was considered for statistical significance.
3. Results
Of the 4067 HCWs 3174 were included in the study. The flowchart showing the included and excluded health care workers was given in Fig. 1. Seventy-one percent of HCWs were fully vaccinated whereas 29% percent did not receive any doses. The median follow-up period was 90 days.
Forty-five percent of fully vaccinated HCWs were male while 39% percent of unvaccinated HCWs were male (p = 0.002). Fully vaccinated HCWs were older than unvaccinated HCWs (40 ± 11 vs 35 ± 10, p < 0.001) and also the frequency of HCWs older than 50 years were higher (22% vs 10%, p < 0.001). The percentage of having postgraduate or doctorate was higher in fully vaccinated HCWs (36% vs 19%, p < 0.001). Fully vaccinated HCWs were more frequently doctors (29% vs 14%) and less frequently nurses (22% vs 35%) while the frequency of administrative/technical personnel and other HCWs was similar (p < 0.001). The distribution of basic, internal, and surgical medical sciences of groups was similar however the frequency of administrative/technical department were higher in the unvaccinated HCWs (33% vs 24%, p < 0.001) (Table 1 ).
Table 1.
Characteristics of unvaccinated and vaccinated health care workers.
| Unvaccined(n = 907) |
Vaccined (n = 2267) |
Total (n = 3174) |
p value |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | 0.002 | ||||||
| Male | 356 | 39.3 | 1024 | 45.2 | 1380 | 43.5 | |
| Female | 551 | 60.7 | 1243 | 54.8 | 1794 | 56.5 | |
| Age group (years) | <0.001 | ||||||
| <30 | 269 | 33.4 | 470 | 23.2 | 739 | 26.1 | |
| 30–39 | 271 | 33.6 | 550 | 27.1 | 821 | 29.0 | |
| 40–49 | 186 | 23.1 | 560 | 27.6 | 746 | 26.3 | |
| ≥50 | 80 | 9.9 | 449 | 22.1 | 529 | 18.7 | |
| Education status | <0.001 | ||||||
| Below high school | 94 | 11.9 | 251 | 12.7 | 345 | 12.5 | |
| High school | 199 | 25.2 | 358 | 18.1 | 557 | 20.2 | |
| Graduate | 348 | 44.1 | 662 | 33.5 | 1010 | 36.6 | |
| Post graduate / Doctorate | 148 | 18.8 | 703 | 35.6 | 851 | 30.8 | |
| Position | <0.001 | ||||||
| Medical doctor | 113 | 14.0 | 597 | 29.4 | 710 | 25.1 | |
| Nurse | 282 | 35.0 | 444 | 21.9 | 726 | 25.6 | |
| Administrative / Technical personnel | 272 | 33.7 | 623 | 30.7 | 895 | 31.6 | |
| Other health care workers | 139 | 17.2 | 364 | 17.9 | 503 | 17.7 | |
| Department | <0.001 | ||||||
| Basic medical sciences | 25 | 3.1 | 98 | 4.9 | 123 | 4.4 | |
| Internal medical sciences | 285 | 35.4 | 729 | 36.1 | 1014 | 35.9 | |
| Surgical medical sciences | 233 | 29.0 | 701 | 34.7 | 934 | 33.1 | |
| Administrative / Technical department | 261 | 32.5 | 490 | 24.3 | 751 | 26.6 | |
| COVID-19 status | <0.001 | ||||||
| Negative | 814 | 89.7 | 2130 | 94.0 | 2944 | 92.8 | |
| Positive | 93 | 10.3 | 137 | 6.0 | 230 | 7.2 | |
| Symptom | 0.790 | ||||||
| Asymptomatic | 19 | 20.4 | 30 | 21.9 | 49 | 21.3 | |
| Symptomatic | 74 | 79.6 | 107 | 78.1 | 181 | 78.7 | |
During follow-up, severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection was detected in ten percent of the unvaccinated HCWs and six percent of the fully vaccinated HCWs (p < 0.001). The percentage of symptomatic cases was similar between the groups (p = 0.790) (Table 1).
SARS-CoV-2 infection was detected in seven percent of both males and females (p = 0.890). As the age of the group decreased the frequency of COVID-19 positivity increased (p = 0.001). When assessed by education level, the highest positivity was among the high school graduates (p = 0.001). The frequency of COVID-19 positivity was higher in administrative / technical personnel and nurses (10% both) than the doctors and other HCWs (6% both) (p = 0.001). SARS-CoV-2 infection was detected in 3% of basic medical sciences, 8% of internal and surgical medical sciences, and 10% administrative/technical unit (p = 0.048) (Table 2 ).
Table 2.
Characteristics of COVID-19 negative and positive health care workers.
| Negative (n = 2944) |
Positive (n = 230) |
p value |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 0.890 | ||||
| Male | 1279 | 92.7 | 101 | 7.3 | |
| Female | 1665 | 92.8 | 129 | 7.2 | |
| Age group | 0.001 | ||||
| <30 | 662 | 89.6 | 77 | 10.4 | |
| 30–39 | 745 | 90.7 | 76 | 9.3 | |
| 40–49 | 699 | 93.7 | 47 | 6.3 | |
| ≥50 | 501 | 94.7 | 28 | 5.3 | |
| Education status | 0.001 | ||||
| Below high school | 320 | 92.8 | 25 | 7.2 | |
| High school | 490 | 88.0 | 67 | 12.0 | |
| Graduate | 918 | 90.9 | 92 | 9.1 | |
| Post graduate / Doctorate | 806 | 94.7 | 45 | 5.3 | |
| Position | 0.001 | ||||
| Medical doctor | 670 | 94.4 | 40 | 5.6 | |
| Nurse | 655 | 90.2 | 71 | 9.8 | |
| Administrative / Technical personnel | 806 | 90.1 | 89 | 9.9 | |
| Other health care workers | 473 | 94.0 | 30 | 6.0 | |
| Department | 0.048 | ||||
| Basic medical sciences | 119 | 96.7 | 4 | 3.3 | |
| Internal medical sciences | 933 | 92.0 | 81 | 8.0 | |
| Surgical medical sciences | 864 | 92.5 | 70 | 7.5 | |
| Administrative / Technical department | 676 | 90.0 | 75 | 10.0 | |
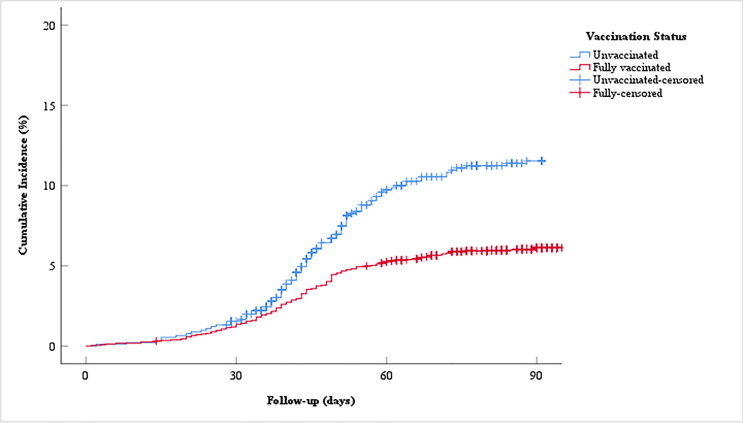
In Kaplan-Meier analysis age group, education status, position, department, and vaccination status were significant while sex was not significant (p = 0.001, p < 0.001, p = 0.001, p = 0.043, p < 0.001, p = 0.954). The results were given in Table 3 . During the median 90 days of follow-up the cumulative incidence of vaccinated HCWs was 6.1% whereas the cumulative incidence of unvaccinated HCWs was 11.4% (Fig. 2 ).
Table 3.
Results of Kaplan-Meier analysis.
| 95% CI |
|||||
|---|---|---|---|---|---|
| Mean | SE | Lower | Upper | p value | |
| Sex | 0.954 | ||||
| Male | 90.208 | 0.380 | 89.464 | 90.953 | |
| Female | 91.202 | 0.338 | 90.540 | 91.864 | |
| Age group | 0.001 | ||||
| <30 | 89.637 | 0.600 | 88.461 | 90.813 | |
| 30–39 | 89.237 | 0.549 | 88.160 | 90.313 | |
| 40–49 | 90.621 | 0.499 | 89.644 | 91.598 | |
| ≥50 | 91.218 | 0.543 | 90.153 | 92.283 | |
| Education status | <0.001 | ||||
| Below high school | 90.371 | 0.742 | 88.917 | 91.824 | |
| High school | 88.041 | 0.721 | 86.629 | 89.454 | |
| Graduate | 89.190 | 0.500 | 88.210 | 90.170 | |
| Post graduate / Doctorate | 92.088 | 0.439 | 91.228 | 92.948 | |
| Position | 0.001 | ||||
| Medical doctor | 91.976 | 0.485 | 91.026 | 92.926 | |
| Nurse | 89.069 | 0.585 | 87.922 | 90.216 | |
| Administrative / Technical personnel | 88.786 | 0.549 | 87.711 | 89.862 | |
| Other health care workers | 90.898 | 0.577 | 89.768 | 92.029 | |
| Department | 0.043 | ||||
| Basic medical sciences | 91.822 | 1.081 | 89.704 | 93.940 | |
| Internal medical sciences | 90.830 | 0.465 | 89.919 | 91.740 | |
| Surgical medical sciences | 90.360 | 0.438 | 89.501 | 91.219 | |
| Administrative / Technical department | 88.547 | 0.627 | 87.318 | 89.775 | |
| Vaccination status | <0.001 | ||||
| Unvaccinated | 85.837 | 0.528 | 84.802 | 86.872 | |
| Vaccinated | 91.808 | 0.278 | 91.264 | 92.352 | |
SE: Standard error, CI: Confidence interval
Fig. 2.
The cumulative incidence of vaccinated and unvaccinated HCWs for COVID-19 during the median 90 days of follow-up.
The incidence rate of SARS-CoV-2 infection was 133.7 vs 70.7 per 100.000 person-days in the unvaccinated and vaccinated HCWs, respectively. The unadjusted IRR was 0.53 (95% CI 0.41–0.69). The unadjusted effectiveness (1-uIRR) of CoronaVac for COVID-19 infection was 47% (95% CI 31–59%). The unadjusted IRR for symptomatic disease was similar. The incidence rate of symptomatic SARS-CoV-2 infection was 106.4 per 100.000 person-days for unvaccinated HCWs and 55.3 per 100.000 person-days for fully vaccinated HCWs, so the unadjusted IRR was 0.52 (95% CI 0.39–0.71). The unadjusted effectiveness of CoronaVac for symptomatic infection was 48% (95% CI 29–61%).
The adjusted effectiveness of the vaccine was estimated by using Cox regression. The covariates determined in the model 1 were analyzed in model 2 and none of the parameters in model 2 except vaccination status were significant. The adjusted effectiveness was 39% (HR 0.610, 95% CI 0.463–0.804) (Table 4 ).
Table 4.
Results of Cox regression analysis.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR |
95% CI |
p value |
HR |
95% CI |
p value |
|||
| Lower | Upper | Lower | Upper | |||||
| Vaccination status (Ref. Unvaccinated) | .. | .. | .. | .. | 0.610 | 0.463 | 0.804 | <0.001 |
| Sex (Ref. Male) | 0.960 | 0.740 | 1.246 | 0.760 | ||||
| Age group (Ref. ≥ 50) | 0.019 | 0.076 | ||||||
| <30 | 1.751 | 1.129 | 2.716 | 0.012 | 1.565 | 0.976 | 2.511 | 0.063 |
| 30–39 | 1.589 | 1.026 | 2.462 | 0.038 | 1.502 | 0.958 | 2.355 | 0.076 |
| 40–49 | 1.123 | 0.702 | 1.795 | 0.629 | 1.045 | 0.650 | 1.681 | 0.856 |
| Education status (Ref. Post grad. / Doct.) | 0.002 | 0.175 | ||||||
| Below high school | 1.290 | 0.790 | 2.106 | 0.308 | 1.909 | 0.783 | 4.655 | 0.155 |
| High school | 2.096 | 1.429 | 3.072 | <0.001 | 2.451 | 1.065 | 5.638 | 0.035 |
| Graduate | 1.592 | 1.110 | 2.285 | 0.012 | 1.991 | 0.907 | 4.370 | 0.086 |
| Position (Ref. Medical doctor) | 0.012 | 0.109 | ||||||
| Nurse | 1.534 | 1.033 | 2.278 | 0.034 | 0.722 | 0.318 | 1.641 | 0.437 |
| Other health care workers | 0.969 | 0.602 | 1.560 | 0.898 | 0.464 | 0.187 | 1.155 | 0.099 |
| Administrative / Technical personnel | 1.635 | 1.122 | 2.383 | 0.010 | 0.764 | 0.314 | 1.854 | 0.551 |
| Department (Ref. Basic medical sci.) | 0.127 | 0.317 | ||||||
| Internal medical sciences | 2.364 | 0.866 | 6.453 | 0.093 | 1.782 | 0.638 | 4.974 | 0.270 |
| Surgical medical sciences | 2.237 | 0.816 | 6.127 | 0.117 | 1.585 | 0.563 | 4.462 | 0.383 |
| Administrative / Technical department | 2.866 | 1.047 | 7.847 | 0.040 | 2.086 | 0.729 | 5.970 | 0.171 |
HR: Hazard ratio, CI: Confidence interval, Ref: Reference, Post grad: Post graduate, Doct: Doctorate, Sci: Sciences
Model 1: Parameters were adjusted by vaccination status
Model 2: Parameters that have p ≤ 0.2 in model 1 were analyzed in multivariate analysis
4. Discussion
This retrospective cohort study conducted with HCWs found that unadjusted effectiveness of CoronaVac for COVID-19 infection was 47% (95% CI 31–59%) whereas the effectiveness independent of covariates was 39% (95% CI 20–64%). The vaccine has protected 884 health care workers against COVID-19 infection. On the other hand, the unadjusted effectiveness of the vaccine for symptomatic infection was 48% (95% CI 29–61%).
Expectedly the real-world effectiveness of the vaccine differed from efficacy in clinical trials.[13] The results of phase III trials in Turkey found that efficacy of CoronaVac for preventing symptomatic COVID-19 at least 14 days after the second dose was 83.5% relative to placebo which is notably higher than the effectiveness in present study. The fact that the majority of cases in Turkey during the study period was infected with alpha variant and only participants in a certain age group (from 18 to 59 years) were included in the phase III trial might be possible reasons for the lower effectiveness in the real-world data.[10], [2] Similar drop in effectiveness (from 79.3% to 59.0%) was found in a study conducted in Guangzhou, China during the outbreak of the Delta variant.[9] In a Brazilian test-negative case-control study, a significant wane in vaccine effectiveness with increasing age was observed (59.0% in adults 70–74 years old, 56.2% in adults 75–79 years old and 32.7% in adults ≥ 80 years old).[14]
Vaccine performance is affected by the risk of infection and disease in the population.[15] Hence, effectiveness in healthcare workers at high risk of exposure to SARS-CoV-2 could be low as it was estimated in our study. In a study conducted with HCWs in the setting of high SARS-CoV-2 Gamma variant transmission, estimated effectiveness of two doses of CoronaVac against SARS-CoV-2 during the period ≥ 14 days after receiving the second dose was 37.9%, which was similar to our findings.[16]
The evidence on the need for additional vaccine doses has been reviewing by the WHO and several reasons such as waning protection over time or against variants shows booster doses may be needed.[17] Turkish Society of Intensive Care demonstrated that 51.4% of the 952 COVID-19 intensive care patients hospitalized in 60 centers across Turkey were unvaccinated, 39.4% had two doses of, and 1.9% had three doses of the CoronaVac vaccine.[18] The results of our study showing the effectiveness of the vaccine against COVID-19 was low (39%) supported this evidence and showed the need for a booster dose with same vaccine or another type of vaccine that may have stronger immunogenicity (e.g. mRNA vaccine).
In our study, the rate of vaccination was higher in participants who were male, aged 50 and over, were doctors, had a higher education level, and were working in areas other than the administrative/technical department. These results were consistent with previous studies in literature. In a study examining the vaccination status and acceptance of the COVID-19 vaccine among healthcare workers in China, it was shown that vaccine acceptance is lower in females, nurses, and those with no contact with COVID-19 cases, similar to our study. In the same study, it was shown that if the education level is high, the vaccination rates are also high.[19] In another study investigating COVID-19 vaccine acceptance among healthcare workers after the vaccination campaign in Canada, male gender, over 50 years of age, and occupational exposure to patients infected with COVID-19 were found to be significantly associated with vaccine acceptance.[20]
The present study has several limitations. First, this is a single-center, retrospective cohort study so the findings might not be generalized to the whole population. Second, the hospital has no screening policy for COVID-19 therefore the number of asymptomatic cases might have been underestimated in our cohort. This limitation might cause that unadjusted effectiveness of the CoronaVac for COVID-19 infection to be estimated at 47%, while it was estimated 48% for symptomatic infection. Third, no data was available about comorbidity of health care workers therefore the association between comorbidity and vaccination status or vaccine effectiveness could not be estimated. Fourth, the effect of variants could not be assessed since the cases were not confirmed in our hospital in terms of variants. However, the alpha variant was dominant in Turkey during our study period and it might be affected the effectiveness we found in our study. Finally, the protective effect of the vaccine from severe infection (hospitalization, intensive care admission, and death) could not be demonstrated, since the health care workers included in the study were a cohort with a relatively low risk of severe disease in terms of mean age, and did not develop a severe disease requiring hospitalization during the follow-up period.
In conclusion, this real life study conducted in health care workers demonstrated that the effectiveness of two doses of the CoronaVac vaccine (39%) was lower than that determined in clinical trials. Due to a reduction in protection over time or against other variants, future studies should be concerned with booster doses and their effectiveness.
5. Contributors
HCA, SNA, NS, and GC conceived and designed the study. HCA, SNA, IIB, RK, and BB contributed to the literature search. RK, NS, and BB contributed to data collection. BB, NS, and GC verified the data. HCA, SNA, and GC contributed to the analysis. HCA, IIB, and GC contributed to interpretation of the data. HCA, SNA, IIB, and BB drafted the manuscript. RK, NS, and GC reviewed and edited the manuscript. HCA and SNA contributed to the figures and tables. All authors critically reviewed the manuscript and approved the final version. All authors confirmed that they had full access to all the data in the study and accepted responsibility to submit for publication.
Data sharing
The data analyzed during the current study are not publicly available due to Turkish Personal Data Protection Law no 6698 but anonymized data are available from the corresponding author on reasonable request.
Patient and other consents
The requirement for written informed consent was waived due to the retrospective nature of the study, and data used in this study was anonymized before its use. The study was approved by the Ethics Committee of Istanbul University - Cerrahpasa (Approval number: 103,513 Approval date: 02.06.2021). Permission was obtained from the Republic of Turkey Ministry of Health and Faculty administration for the use of patient data in our study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the health care workers of Cerrahpasa Faculty of Medicine for their devoted work during the pandemic period.
References
- 1.Palacios R, Batista AP, Albuquerque CSN, et al. Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: the PROFISCOV Study. SSRN 2021; published online April 14. https://ssrn.com/abstract=3822780 (preprint).
- 2.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkish Medicines and Medical Devices Agency. https://www.titck.gov.tr/haber/kamuoyunun-dikkatine-13012021185623 (accessed August 20, 2021)
- 4.Republic of Turkey, Ministry of Health. https://covid19asi.saglik.gov.tr/TR-77707/asi-uygulanacak-grup-siralamasi.html (accessed August 20, 2021)
- 5.WHO. Coronavirus disease (COVID-19): Vaccines. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (accessed August 20, 2021)
- 6.Instituto Butantan. https://butantan.gov.br/noticias/projeto-s-imunizacao-em-serrana-faz-casos-de-covid-19-despencarem-80-e-mortes-95 (accessed August 20, 2021)
- 7.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahendradhata Y., Andayani NLPE., Hasri E.T., et al. The Capacity of the Indonesian Healthcare System to Respond to COVID-19. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.649819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X.N., Huang Y., Wang W., et al. Efficacy of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case-control real-world study. Emerg Microbes Infect. 2021;10(1):1751–1759. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.dailysabah.com. https://www.dailysabah.com/turkey/covid-19-variants-dominate-new-cases-but-no-peak-yet-in-turkey/news (accessed February 20, 2021)
- 11.WHO. Coronavirus disease (COVID-19). https://www.who.int/health-topics/coronavirus#tab=tab_3 (accessed August 20, 2021)
- 12.Angel Y., Spitzer A., Henig O., Saiag E., Sprecher E., Padova H., et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopalco P.L., DeStefano F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine. 2021;33(13):1541–1548. doi: 10.1016/j.vaccine.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranzani OT, Hitchings MD, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021, 374: n2015 [DOI] [PMC free article] [PubMed]
- 15.Hungerford D., Cunliffe N.A. Real world effectiveness of covid-19 vaccines. BMJ. 2021;374 [Google Scholar]
- 16.Hitchings MD, Ranzani OT, Scaramuzzini Torres MS, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. medRxiv 2021; published online May 1. https://doi.org/10.1101/2021.04.07.21255081 (preprint). [DOI] [PMC free article] [PubMed]
- 17.WHO. Interim statement on COVID-19 vaccine booster doses. https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccine-booster-doses (accessed September 3, 2021)
- 18.Turkish Society of Clinical Microbiology and Infectious Diseases. https://www.klimik.org.tr/koronavirus/covid-19-yogun-bakim-arastirmasi-hastalarin-yuzde-98i-asisiz-veya-eksik-asili/ (accessed September 3, 2021)
- 19.Li X.-H., Chen L., Pan Q.-N., Liu J., Zhang X., Yi J.-J., et al. Vaccination status, acceptance, and knowledge toward a COVID-19 vaccine among healthcare workers: a cross-sectional survey in China. Hum Vaccin Immunother. 2021;17(11):4065–4073. doi: 10.1080/21645515.2021.1957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzieciolowska S., Hamel D., Gadio S., Dionne M., Gagnon D., Robitaille L., et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: A multicenter survey. Am J Infect Control. 2021;49(9):1152–1157. doi: 10.1016/j.ajic.2021.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]