Abstract
BACKGROUND AND AIMS:
Racial/ethnic minority children have worse liver transplant outcomes. We evaluated whether neighborhood socioeconomic deprivation affected associations between race/ethnicity and waitlist mortality.
APPROACH AND RESULTS:
We included children (age<18 years) listed 2005–2015 in the Scientific Registry of Transplant Recipients. We categorized patients as non-Hispanic White, Black, Hispanic, and other. We matched patient ZIP codes to a neighborhood socioeconomic deprivation index ([range, 0–1]; higher values indicate worse deprivation). Primary outcomes were waitlist mortality, defined as death/delisting for too sick, and receipt of living donor liver transplant (LDLT). Competing risk analyses modeled the association between race/ethnicity and waitlist mortality, with deceased donor liver transplant (DDLT) and LDLT as competing risks; and race/ethnicity and LDLT, with waitlist mortality and DDLT as competing risks. Of 7,716 children, 17% and 24% identified as Black and Hispanic, respectively. Compared to White children, Black and Hispanic children had increased unadjusted hazard of waitlist mortality (subhazard ratio [sHR], 1.44; 95% confidence interval [CI], 1.18, 1.75 and sHR, 1.48; 95% CI, 1.25, 1.76, respectively). After adjusting for neighborhood deprivation, insurance, and listing lab Model for End-Stage Liver Disease (MELD)/Pediatric End-Stage Liver Disease (PELD), Black and Hispanic children did not have increased hazard of waitlist mortality (sHR, 1.12; 95% CI, 0.91, 1.39 and sHR, 1.21; 95% CI, 1.00, 1.47, respectively). Similarly, Black and Hispanic children had decreased likelihood of LDLT (sHR, 0.58; 95% CI, 0.45, 0.75 and sHR, 0.61; 95% CI, 0.49, 0.75, respectively). Adjustment attenuated the effect of Black and Hispanic race/ethnicity on likelihood of LDLT (sHR, 0.79; 95% CI, 0.60, 1.02 and sHR, 0.89; 95% CI, 0.70, 1.11, respectively).
CONCLUSIONS:
Household and neighborhood socioeconomic factors and disease severity at waitlist entry help explain racial/ethnic disparities for children awaiting transplant. A nuanced understanding of how social adversity contributes to waitlist outcomes may inform strategies to improve outcomes.
INTRODUCTION:
The social determinants of health—where we live, learn, work, and play—are strongly associated with health outcomes and racial and ethnic disparities in outcomes. Conceptually, race and ethnicity are social constructs1; therefore, observed disparities for racial and ethnic minorities likely reflect differential exposure to underlying adversity driven by structural factors. Race and ethnicity are sociopolitical and not biological constructs. Racial and ethnic differences in health outcomes largely stem from inequitable positions within social hierarchies related to segregation, interpersonal and institutional racism, and discrimination. Resulting differences in conditions in which populations live their lives promulgate disparities in outcomes across conditions. Transplant-related outcomes have been shown to vary significantly by socioeconomic deprivation. So, too, have such outcomes varied by race and ethnicity.2–5
Previous work has demonstrated that Black children and children on public insurance are less likely to have nonstandard exception scores petitioned on their behalf while awaiting liver transplantation.5,6 These nonstandard exception scores are intended to enable adjustments to the Model for End-Stage Liver Disease (MELD)/Pediatric End-Stage Liver Disease (PELD) to better reflect excess mortality risk from variables outside those that comprise PELD/MELD (e.g., repeated episodes of ascending cholangitis). The decision to apply for nonstandard exception scores reflects subjective decision making, therefore these differences may be due to biases in such decision making. Furthermore, Black and Hispanic children are less likely to receive a living donor liver transplant (LDLT), which may further expose these children to longer wait list times.7
Previous research has shown that measures related to the social determinants, like neighborhood/community factors, are also relevant in transplant outcomes. One study of adults wait-listed for liver transplant found associations between a Community Health Score, a county-level metric of community health, and wait list mortality.8 We have previously found that increased neighborhood socioeconomic deprivation, captured via a composite measure derived from US Census Bureau data, is associated with increased risk of medication nonadherence, graft failure, and death for children following liver transplant.2,3 Such composite measures contextualize the neighborhoods in which people live and increase our understanding of the impacts of socioeconomic conditions on the health outcomes of vulnerable children. That said, to our knowledge, no studies to date have evaluated whether and how racial/ethnic disparities are associated with socioeconomic deprivation for children awaiting liver transplantation.
Therefore, our objective here was to evaluate ramifications of the social determinants on wait list mortality. Specifically, we sought to investigate the associations of race/ethnicity on wait list mortality and investigate how neighborhood socioeconomic deprivation modifies this effect using a validated index of neighborhood deprivation and the Scientific Registry of Transplant Recipients database. Building on previous work,7 we sought to determine whether neighborhood deprivation explains racial/ethnic disparities in LDLT. We hypothesized that Black and Hispanic children would have worse wait list mortality and decreased use of living donor transplant, but the magnitude of these relationships would be diminished after accounting for neighborhood socioeconomic deprivation.
METHODS:
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration of the U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
This study was reviewed by and deemed exempt by the institutional review board at the University of California, San Francisco.
STUDY POPULATION:
We identified pediatric patients (<18 years of age) who were listed for liver transplantation between January 1, 2005 and December 31, 2015, in the United States (N = 8536). Patients (N = 820) were excluded if their home ZIP code could not be matched to the deprivation index. Therefore, for the present analyses, we had a total of 7,716 patients. Compared to included patients, excluded patients were more likely to be White race and less likely to be Black race, more likely to have other insurance, more likely to have metabolic disease, less likely to be listed as Status 1A/1B at time of transplant, have a lower allocation PELD/MELD at the time of listing, and less likely to die on the wait list (Supplemental Table S1).
PRIMARY EXPOSURES:
Our primary exposures were race/ethnicity and a validated index of neighborhood socioeconomic deprivation.2,3,9 For our race/ethnicity exposure, we classified patients as non-Hispanic White, non-Hispanic Black or African-American, Hispanic, and other. The deprivation index is a continuous variable and has a range of 0–1 with values closer to 1 indicating more deprived neighborhoods. The nationwide mean and standard error are 0.37 ± 0.0006. The deprivation index was derived using data from the U.S. Census Bureau’s 2015 American Community Survey. No adjustment was made for the year of transplant because previous research has demonstrated that when children move, it tends to be to neighborhoods with similar deprivation levels.10 This index is available at both the census tract and ZIP code levels. Neighborhood-level measures that comprise the index are described elsewhere.9,11 For the present analyses, we used the deprivation index at the ZIP code level because SRTR only collects home ZIP codes and not full addresses. The neighborhood deprivation index was matched to a patient by the home ZIP code reported to OPTN/United Network for Organ Sharing (UNOS) at the time of wait list enrollment.
PRIMARY OUTCOMES:
Our primary outcomes were wait list mortality and receipt of living donor liver transplant. We defined wait list mortality as wait list removal for medical unsuitability, for being too ill to transplant, or for death on the waiting list due to any cause. We applied administrative censoring on January 1, 2019. The outcomes were modeled as a time-to-event occurrence.
STATISTICAL ANALYSIS:
Descriptive statistics were calculated for patient demographic, allocation, and clinical characteristics. Patient characteristics were compared across race/ethnicity classifications using Kruskal-Wallis or chi-square test for continuous and categorical variables, respectively. The relationships between race/ethnicity, neighborhood deprivation, and time to wait list mortality were represented with a cumulative incidence function while accounting for the competing risk of DDLT or LDLT. To assess wait list mortality in the setting of a competing risk of transplant, we used the Fine and Gray method.12 The proportional hazards assumption was tested by checking the exposures for a time interaction. Since children waiting for transplant can be removed from the wait list for multiple reasons (e.g., transplantation vs. death) that directly compete with each other (i.e., one cannot die on the wait list and get transplanted), competing risk analysis quantifies the relative hazard of one cause of wait list exit (e.g., death) while accounting for the competing probability of another cause of wait list exit (e.g., deceased donor transplant). To better understand the determinants of LDLT, we again used competing risk analysis to quantify the likelihood of receiving a LDLT in the setting of competing risks (e.g., wait list mortality or DDLT). Risk estimates were described as subhazard ratios with 95% confidence intervals (CIs). To determine which variables to include in our multivariable model, we constructed a directed acyclic graph to determine the set of key covariables necessary to quantify the direct effect of race/ethnicity and neighborhood deprivation on wait list mortality (Figure 1). Because SRTR does not collect detailed individual socioeconomic status data on health-related social needs (e.g., transportation challenges, food insecurity), we could not include measures of health-related social needs in our multivariable models. We assumed no biological plausibility for race nor ethnicity to impact outcome through a biological pathway.13 Finally, we utilized initial laboratory MELD/PELD as a measure of disease severity at time of presentation; however, we recognize that this measure is an imperfect measure of acuity. All analyses were done in Stata version 16.1 (StataCorp, College Station, TX).
Figure 1.
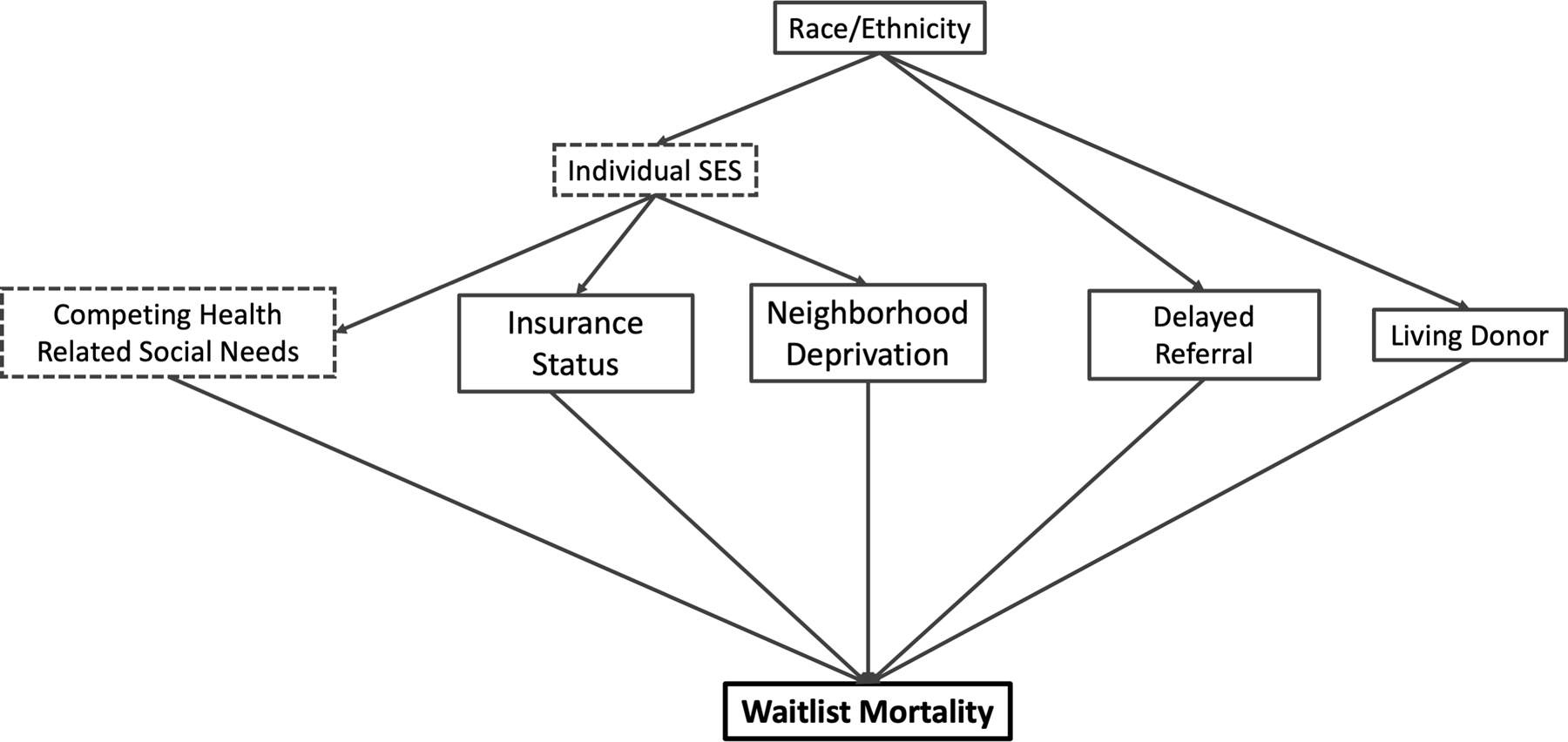
Hypothesized causal pathway for race/ethnicity impacting outcomes
SES, socio-economic status. Dashed boxes indicate unmeasurable variables within the SRTR dataset.
RESULTS:
STUDY POPULATION
A total of 7,716 patients met inclusion criteria for the study. About 17% of the cohort identified as Black and 24% identified as Hispanic. The median deprivation index for the study population was 0.38 (interquartile range (IQR), 0.30–0.46). Table 1 depicts patient characteristics by patient race/ethnicity. Compared to non-Hispanic White patients, Black patients had higher deprivation indices, were more likely to have public insurance, were less likely to have a living donor transplant, and have a higher initial and final lab PELD/MELD scores. Compared to non-Hispanic White patients, Hispanic children were more likely to live in higher deprivation neighborhoods (0.44 vs. 0.34), have public insurance, less likely to receive a living donor transplant, more likely to be listed as Status 1A/1B, and have higher lab PELD/MELD scores.
Table 1.
Patient Characteristics by Race/Ethnicity
| Non-Hispanic White | Black | Hispanic | Other | P Value | |
|---|---|---|---|---|---|
| N (%) or median (interquartile range) | |||||
| N | 3926 | 1286 | 1837 | 667 | |
| Deprivation Index | 0.34 (0.28–0.41) | 0.44 (0.36–0.52) | 0.44 (0.36–0.53) | 0.34 (0.27–0.44) | <0.001 |
| Insurance | |||||
| Private | 2372 (60) | 356 (28) | 423 (23) | 347 (52) | <0.001 |
| Public | 1439 (37) | 908 (71) | 1378 (75) | 308 (46) | |
| Other | 113 (3) | 21 (2) | 36 (2) | 12 (2) | |
| Female gender | 1938 (49) | 670 (52) | 935 (51) | 336 (50) | 0.35 |
| Diagnosis | |||||
| Biliary atresia | 1031 (26) | 388 (30) | 470 (26) | 233 (35) | <0.001 |
| Cholestatic liver disease | 925 (24) | 291 (23) | 377 (21) | 116 (17) | |
| Acute liver failure | 402 (10) | 161 (13) | 324 (18) | 72 (11) | |
| Metabolic | 387 (10) | 65 (5) | 152 (8) | 56 (8) | |
| Tumor | 325 (8) | 53 (4) | 151 (8) | 49 (7) | |
| Autoimmune hepatitis | 198 (5) | 84 (7) | 64 (4) | 19 (3) | |
| Other | 658 (17) | 244 (19) | 299 (16) | 122 (18) | |
| Living donor transplant | 3558 (91) | 1214 (94) | 1726 (94) | 616 (92) | <0.001 |
| Time on waitlist (days) | 62 (13–203) | 65 (15–211) | 59 (12–190) | 54 (15–167) | 0.35 |
| Initial Status 1A/1B listing | 772 (20) | 249 (19) | 421 (23) | 140 (21) | 0.03 |
| Status 1A/1B at time of transplant | 1089 (28) | 310 (24) | 549 (30) | 195 (29) | 0.004 |
| Status 1A/1B at any time | 1234 (31) | 355 (28) | 648 (35) | 225 (34) | |
| Initial lab PELD/MELD | 13 (2–22) | 17 (8–25) | 15 (4–24) | 15 (5–25) | <0.001 |
| Final lab PELD/MELD | 13 (3–24) | 17 (7–25) | 15 (3–26) | 16 (5–27) | <0.001 |
| Initial allocation PELD/MELD | 15 (6–27) | 17 (10–27) | 16 (6–28) | 15.5 (7–28) | <0.001 |
| Final allocation PELD/MELD | 26 (16–34) | 24 (16–33) | 27 (16–33) | 25 (15–35) | 0.48 |
| Initial lab PELD/MELD quartile | |||||
| 1 | 1140 (29) | 230 (18) | 479 (26) | 166 (25) | <0.001 |
| 2 | 1003 (26) | 314 (24) | 426 (23) | 158 (24) | |
| 3 | 922 (24) | 380 (30) | 423 (23) | 160 (24) | |
| 4 | 860 (22) | 362 (28) | 509 (28) | 183 (27) | |
| Ascites | 562 (18) | 170 (16) | 300 (21) | 92 (17) | 0.01 |
| Encephalopathy | 264 (8.5%) | 109 (10.2%) | 172 (11.8%) | 50 (9.4%) | 0.01 |
| Waitlist outcome | |||||
| Censored | 65 (2) | 23 (2) | 49 (3) | 11 (2) | <0.001 |
| Death | 213 (5) | 100 (8) | 126 (7) | 37 (5) | |
| Too Sick | 119 (3) | 48 (4) | 93 (5) | 40 (6) | |
| DDLT | 2635 (67) | 870 (68) | 1182 (64) | 441 (66) | |
| Other removal | 526 (13) | 173 (14) | 276 (15) | 87 (13) | |
| LDLT | 368 (9) | 72 (6) | 111 (6) | 51 (8) | |
Abbreviations: DDLT, Deceased donor liver transplant; IQR, Interquartile range; LDLT, Living donor liver transplant; MELD, Model for End-Stage Liver Disease; PELD, Pediatric End-Stage Liver Disease
WAIT LIST MORTALITY (Table 2)
Table 2.
Multivariable competing risk models for waitlist mortality
| Univariable Analyses | Race + Deprivation | Race + Deprivation + Insurance | Race + Deprivation + Initial Lab MELD/PELD + Insurance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Variable | SHR | 95% CI | P Value | SHR | 95% CI | P Value | SHR | 95% CI | P Value | SHR | 95% CI | P Value |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | REF | REF | REF | REF | ||||||||
| Black | 1.44 | 1.18, 1.75 | <0.001 | 1.39 | 1.13, 1.71 | <0.01 | 1.28 | 1.04, 1.58 | 0.02 | 1.12 | 0.91, 1.39 | 0.29 |
| Hispanic | 1.48 | 1.25, 1.76 | <0.001 | 1.43 | 1.19, 1.72 | <0.001 | 1.30 | 1.08, 1.57 | 0.01 | 1.21 | 1.00, 1.47 | 0.05 |
| Other | 1.46 | 1.14, 1.88 | <0.01 | 1.46 | 1.14, 1.87 | <0.01 | 1.42 | 1.11, 1.82 | 0.01 | 1.31 | 1.02, 1.69 | 0.04 |
| Neighborhood deprivation | 1.09 | 1.02, 1.16 | 0.01 | 1.03 | 0.97, 1.11 | 0.31 | 0.99 | 0.92, 1.06 | 0.74 | 0.98 | 0.91, 1.05 | 0.52 |
| Insurance | ||||||||||||
| Private | REF | REF | REF | |||||||||
| Public | 1.58 | 1.36, 1.84 | <0.001 | 1.48 | 1.25, 1.76 | <0.001 | 1.55 | 1.30, 1.84 | <0.001 | |||
| Other | 1.52 | 0.96, 2.41 | 0.08 | 1.51 | 0.95, 2.40 | 0.08 | 1.65 | 1.03, 2.64 | 0.04 | |||
| Initial lab MELD/PELD | 1.05 | 1.04, 1.05 | <0.001 | 1.05 | 1.04, 1.05 | <0.001 | ||||||
Abbreviations: MELD, Model for End-stage Liver Disease; PELD, Pediatric End-stage Liver Disease; REF: Reference category; SHR: subhazard ratio
To better understand the effect of race/ethnicity on waitlist mortality, we used a hypothesized causal model (Figure 1) to select the set of covariates for the models. In these models, we included neighborhood deprivation, insurance status, and initial PELD/MELD (as a surrogate for disease severity at the time of listing).
In unadjusted analyses, Black race (sHR, 1.44; 95% CI, 1.18, 1.75), Hispanic ethnicity (sHR, 1.48; 95% CI, 1.25, 1.76), neighborhood deprivation (each 0.1 increase in the deprivation index was associated with sHR, 1.09; 95% CI, 1.02, 1.16), initial laboratory MELD/PELD (sHR, 1.05; 95% CI, 1.04, 1.05), and public insurance (sHR, 1.58; 95% CI, 1.36, 1.84) were each associated with increased hazard of wait list mortality. The effect size of Black race and Hispanic ethnicity decreased but remained statistically significant with the inclusion of neighborhood deprivation (sHR, 1.39; 95% CI, 1.13, 1.71 and sHR, 1.43; 95% CI, 1.19,1.72, respectively). With the addition of insurance as a surrogate of household socioeconomic status, the effect size of Black race and Hispanic ethnicity decreased but remained statistically significant (sHR, 1.28; 95% CI, 1.04, 1.58 and sHR, 1.30; 95% CI, 1.08, 1.57, respectively). Finally, with the addition of initial laboratory MELD/PELD, the effect size of Black race and Hispanic ethnicity both decreased, and neither were statistically significant (sHR, 1.12; 95% CI, 0.91, 1.39 and sHR, 1.21; 95% CI, 1.00, 1.47, respectively).
LIVING DONOR LIVER TRANSPLANT LIKELIHOOD (Table 3)
Table 3.
Multivariable competing risk models for likelihood of living donor liver transplant
| Univariable Effects | Race + Deprivation | Race + Deprivation + Insurance | Race + Deprivation + Initial Lab MELD/PELD + Insurance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Variable | SHR | 95% CI | P Value | SHR | 95% CI | P Value | SHR | 95% CI | P Value | SHR | 95% CI | P Value |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | REF | REF | REF | REF | ||||||||
| Black | 0.58 | 0.45, 0.75 | <0.001 | 0.70 | 0.54, 0.90 | 0.01 | 0.83 | 0.64, 1.07 | 0.15 | 0.79 | 0.60, 1.02 | 0.08 |
| Hispanic | 0.61 | 0.49, 0.75 | <0.001 | 0.73 | 0.59, 0.92 | 0.01 | 0.91 | 0.72, 1.14 | 0.41 | 0.89 | 0.70, 1.11 | 0.30 |
| Other | 0.80 | 0.60, 1.07 | 0.14 | 0.80 | 0.60, 1.08 | 0.14 | 0.85 | 0.63, 1.14 | 0.28 | 0.83 | 0.62, 1.11 | 0.21 |
| Neighborhood deprivation | 0.77 | 0.72, 0.84 | <0.001 | 0.81 | 0.75, 0.88 | <0.001 | 0.90 | 0.83, 0.98 | 0.02 | 0.90 | 0.83, 0.98 | 0.01 |
| Insurance | ||||||||||||
| Private | REF | REF | REF | |||||||||
| Public | 0.39 | 0.33, 0.47 | <0.001 | 0.45 | 0.37, 0.54 | <0.001 | 0.45 | 0.37, 0.55 | <0.001 | |||
| Other | 0.62 | 0.36, 1.08 | 0.09 | 0.65 | 0.37, 1.13 | 0.13 | 0.66 | 0.38, 1.16 | 0.15 | |||
| Initial Lab MELD/PELD | 1.01 | 1.01, 1.02 | <0.001 | 1.01 | 1.01, 1.02 | <0.001 | ||||||
Abbreviations: MELD, Model for End-Stage Liver Disease; PELD: Pediatric End-Stage Liver Disease; REF: Reference category; SHR: subhazard ratio
To better understand the effect of race/ethnicity on waitlist mortality, we used a hypothesized causal model (Figure 1) to select the set of co-variates for the models. In these models, we included neighborhood deprivation, insurance status, and initial PELD/MELD (as a surrogate for disease severity at the time of listing).
In unadjusted analyses, Black race (sHR, 0.58; 95% CI, 0.45, 0.75), Hispanic ethnicity (sHR, 0.61; 95% CI, 0.49, 0.75), neighborhood deprivation (each 0.1 increase in the deprivation index was associated with sHR, 0.77; 95% CI, 0.72, 0.84), initial lab MELD/PELD (sHR, 1.01; 95% CI, 1.01, 1.02), and public insurance (sHR, 0.39; 95% CI, 0.33, 0.47) were each associated with decreased hazard of receiving LDLT. With the inclusion of neighborhood deprivation, the hazard of Black race and Hispanic ethnicity on LDLT increased, yet remained statistically significant (sHR, 0.70; 95% CI, 0.54, 0.90 and sHR, 0.73; 95% CI, 0.59, 0.92, respectively). With the inclusion of insurance, Black race and Hispanic ethnicity were no longer associated with decreased hazard of LDLT (sHR, 0.83; 95% CI, 0.64, 1.07 and sHR, 0.91; 95% CI, 0.72, 1.14, respectively). Finally, with the addition of lab MELD/PELD, the hazard of Black race and Hispanic ethnicity decreased but were still no longer statistically significant (sHR, 0.79; 95% CI, 0.60, 1.02 and sHR, 0.89; 95% CI, 0.70, 1.11, respectively).
DISCUSSION
This study evaluated how neighborhood socioeconomic deprivation affects racial and ethnic disparities in wait list mortality and likelihood of LDLT for children undergoing liver transplant. This study yielded several important observations. First, the increased hazard of wait list mortality experienced by Black and Hispanic children is not explained by neighborhood socioeconomic deprivation alone. However, incorporating insurance status and, in the case of wait list mortality, a measure of disease severity at the time of listing resulted in a decrease in the observed racial/ethnic disparities. Secondly, Black and Hispanic children were about half as likely to receive a LDLT. Similarly, this effect was not attenuated exclusively by neighborhood deprivation but did diminish after accounting for insurance status and initial lab MELD/PELD. Taken together, these results suggest that socioeconomic status and delayed listing until later in the disease course might explain racial/ethnic differences in wait list mortality. Finally, socioeconomic measures account for the decreased likelihood of LDLT for racial/ethnic minorities, and this decreased utilization may partly explain the disparity in wait list mortality.
Interestingly, Mogul, et al.(7) found that Black and Hispanic children were less likely to receive a LDLT even after adjusting for insurance status. Here, we find that the effect of race/ethnicity on LDLT diminishes when accounting for neighborhood socioeconomic deprivation and insurance status. Commonly used measures of socioeconomic status, such as insurance type and neighborhood-level measures of socioeconomic deprivation, each capture overlapping but distinct aspects of one’s household and neighborhood circumstances, and correlation among different measures of socioeconomic status can be low. Neither insurance nor neighborhood deprivation fully capture the breadth of health-related social needs or risks a family might face. Insurance status can be a particularly problematic measure of socioeconomic status because there is significant variability across states for Medicaid eligibility, and private insurance may have different out of pocket costs and coverage.14 On the other hand, neighborhood deprivation contextualizes the neighborhood of the patient and may capture different underlying social risks not captured by insurance. Such risks might account for the racial/ethnic disparities in LDLT that we observe and may include poor health literacy, threat of lost wages, or provider perception that the family would not be suitable donors. Indeed, a study in adults found that Black adult patients had reduced LDLT inquiries compared to White patients.15 Therefore, interventions that target the underlying social barriers to LDLT (e.g., covering lost wages during recovery from donation, policies that protect against loss of employment during the donation process) may lead to improved rates of LDLT among these minority groups. In kidney transplantation, it was found that patients with greater knowledge about the transplant process, access to improved educational material, and stronger motivation for transplant were more likely to receive a living donor transplant—suggesting that tailored health information may yield improvements in living donation rates.16 This study lays the groundwork for future studies to explore, in greater depth, why racial minorities and socioeconomically deprived patients do not receive LDLT at similar rates to less deprived, White patients to ultimately develop strategies that lead to improved LDLT rates for racial and ethnic minority children.
One of the barriers to addressing racial and socioeconomic disparities in pediatric liver transplantation is that we lack a nuanced understanding of the specific social risk factors that account for racial disparities in outcomes. Conceptually, race/ethnicity are social constructs and social inequities across racial groups stem from the relative location of one’s race within a society’s social ladder. Therefore, racial inequities may be due to underlying social determinants like interpersonal and institutional racism, increased adversity over time, residential segregation, and decreased trust in the health care system. Indeed, some authors have argued that instead of “race,” a better taxonomic descriptor might be “racism.”1,13 Similarly, ethnicity refers to the “sharing of a common culture, including shared origin, shared psychological characteristics and attitudes, shared language, religion, and cultural traditions.”1 Ethnic disparities may be due to similar underlying constructs and also include constructs like differing health beliefs, language barriers, and degree of acculturation. While neighborhood deprivation might more closely approximate social and environmental conditions, evidence suggests that such measures may fail to capture patients with social risks associated with adverse health outcomes17—further evidence that more granular data related to life experiences associated with race and ethnicity are needed.
This study adds to a growing body of literature suggesting that social and environmental context are relevant and important for transplant outcomes.2,3,8,11,18,19 Taken together, these studies highlight that adverse social determinants of health are important predictors of morbidity and mortality for children undergoing liver transplantation. Disparities begin prior to wait list entry and extend through the long-term posttransplant period. Therefore, the sum risk of adverse social determinants on a particular child’s outcomes is compounded—she is not only at increased risk of death before transplant but also afterwards. In this study, we demonstrated that disease severity at the time of listing partially accounts for the observed racial/ethnic disparities. Critically, because SRTR only starts collecting data at wait list entry, SRTR data cannot help to elucidate why these children are listed later in their disease course. Based on work with adult liver transplant candidates,20 we would hypothesize that racial/ethnic minorities and socioeconomically deprived children with end-stage liver disease are further disadvantaged by having delayed referral to a liver transplant center. This study lays the groundwork for future work to evaluate disparities in referral and listing practices for these children.
As wait list and short-term outcomes for children after liver transplant improve, the transplant community must see addressing social adversity as a moral imperative21 and start to address the persistent socioeconomic and racial inequities in outcomes for these children. To do so, regulatory bodies (e.g., OPTN) might consider publicly reporting these disparities in outcomes to incentivize institutions to prioritize equitable care for their patients. Of course, in conjunction with any such monitoring, efforts will be needed to ensure that this does not incentivize transplant programs from withholding transplant for children who experience adverse social determinants. However, the OPTN/SRTR data system does not yet collect detailed household level or individual level data on these topics. In order to address inequities, we need a more nuanced understanding of how specific underlying constructs impact outcomes for these children. Specifically, we need to move past describing racial, ethnic, and socioeconomic disparities and identify strategies to intervene. Future work should therefore evaluate how specific constructs such as material economic hardship, health literacy, and discrimination impact outcomes for these children. Such data should be enriched with qualitative data that sheds light on the mechanisms in which these constructs impact outcomes. In tandem, it will be essential to engage key stakeholders, including patients, caregivers, and medical team members, to collaboratively develop solutions to address these persistent disparities. Interventions that intervene on these specific adverse social determinants may ultimately lead to improved outcomes for racial and ethnic minorities. Novel programs, such as the African American Transplant Access Program at Northwestern University, are already being developed to deliver culturally competent care and may ultimately prove useful in narrowing the racial and ethnic disparities we observe.
We acknowledge the following limitations to our study. First, the pretransplant period is characterized by three distinct phases: access (e.g., being referred for transplant), evaluation/listing, and waiting for an organ. Each of these phases has a risk of mortality. However, in the present study, we only evaluate mortality for those who ultimately made it on the wait list. This would bias our results toward the null because we do not measure potential disparities in access and evaluation/listing. Our findings that racial/ethnic minorities were listed with higher PELD/MELDs suggests that there are indeed disparities prior to wait list entry. Second, the geographic resolution of our deprivation index was at the ZIP code level. In contrast to census tracts, which are fixed in time and drawn for the explicit purpose of population level studies, ZIP codes are drawn by the U.S. Postal Service to ensure efficient mail delivery. As such, they can be redrawn to optimize efficiency. Therefore, there are drawbacks to using ZIP code spatial resolution. However, the ZIP codes are available and collected by SRTR and allow us to group patients within relatively small geographic units. Third, common limitations to registry studies, such as data completeness and quality, apply to this work. However, the SRTR database is the most robust data source for transplant recipients. Finally, there have been recent policy changes to the liver organ allocation system, yet it remains unknown whether these changes will lead to more equitable outcomes for racial and ethnic minorities.
Pediatric liver transplantation is a lifesaving procedure for children with end-stage liver disease, but transplants are not equitably distributed. Understanding the factors associated with wait list outcomes can lead to targeted interventions to improve liver transplant equity.
Supplementary Material
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR001870 (PI Bauer, support for SIW). The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
AASLD Advanced/Transplant Hepatology Award (SIW). NIH T32 DK 7727-24 (PI Lee Denson, support for SIW). NIH T32 DK 060414-17 (PI Jacquelyn Maher, support for JG).
Abbreviations:
- DDLT
deceased donor liver transplant
- CI
confidence interval
- LDLT
living donor liver transplant
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-Stage Liver Disease
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
REFERENCES:
- 1.Ford ME, Kelly PA. Conceptualizing and Categorizing Race and Ethnicity in Health Services Research. Health Serv Res. 2005;40(5 Pt 2):1658–1675. doi: 10.1111/j.1475-6773.2005.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwani SI, Bucuvalas JC, Brokamp C, et al. Association between neighborhood-level socioeconomic deprivation and the Medication Level Variability Index for children following liver transplantation. Transplantation Published online January 19, 2020. doi: 10.1097/TP.0000000000003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant Published online January 20, 2020. doi: 10.1111/ajt.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadhwani SI, Hsu EK, Shaffer ML, Anand R, Ng VL, Bucuvalas JC. Predicting ideal outcome after pediatric liver transplantation: An exploratory study using machine learning analyses to leverage Studies of Pediatric Liver Transplantation Data. Pediatr Transplant. Published online July 22, 2019. doi: 10.1111/petr.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transpl. 2015;15(2):436–444. doi: 10.1111/ajt.13089 [DOI] [PubMed] [Google Scholar]
- 6.Braun HJ, Perito ER, Dodge JL, Rhee S, Roberts JP. Nonstandard Exception Requests Impact Outcomes for Pediatric Liver Transplant Candidates. Am J Transpl. 2016;16(11):3181–3191. doi: 10.1111/ajt.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogul DB, Luo X, Chow EK, et al. Impact of Race and Ethnicity on Outcomes for Children Waitlisted for Pediatric Liver Transplantation. J Pediatr Gastroenterol Nutr. 2018;66(3):436–441. doi: 10.1097/MPG.0000000000001793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross K, Patzer RE, Goldberg DS, Lynch RJ. Sociodemographic Determinants of Waitlist and Posttransplant Survival Among End-Stage Liver Disease Patients. Am J Transplant. 2017;17(11):2879–2889. doi: 10.1111/ajt.14421 [DOI] [PubMed] [Google Scholar]
- 9.Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Ann Epidemiol Published online November 29, 2018. doi: 10.1016/j.annepidem.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brokamp C, LeMasters GK, Ryan PH. Residential mobility impacts exposure assessment and community socioeconomic characteristics in longitudinal epidemiology studies. J Expo Sci Env Epidemiol. 2016;26(4):428–434. doi: 10.1038/jes.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhwani SI, Brokamp C, Rasnick E, Bucuvalas JC, Lai JC, Beck AF. Neighborhood socioeconomic deprivation, racial segregation, and organ donation across 5 states. Am J Transplant. Published online July 12, 2020. doi: 10.1111/ajt.16186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 13.Boyd R, Lindo E, Weeks L, McLemore M. On Racism: A new standard for publishing on racial health inequities. Health Affairs Blog. doi: 10.1377/hblog20200630.939347 [DOI] [Google Scholar]
- 14.Kachmar AG, Connolly CA, Wolf S, Curley MAQ. Socioeconomic Status in Pediatric Health Research: A Scoping Review. J Pediatr. 2019;213:163–170. doi: 10.1016/j.jpeds.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 15.Nobel YR, Forde KA, Wood L, et al. Racial and ethnic disparities in access to and utilization of living donor liver transplants. Liver Transpl. 2015;21(7):904–913. doi: 10.1002/lt.24147 [DOI] [PubMed] [Google Scholar]
- 16.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J. Modifiable Patient Characteristics and Racial Disparities in Evaluation Completion and Living Donor Transplant. Clin J Am Soc Nephrol. 2013;8(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottrell EK, Hendricks M, Dambrun K, et al. Comparison of Community-Level and Patient-Level Social Risk Data in a Network of Community Health Centers. JAMA Netw Open. 2020;3(10). doi: 10.1001/jamanetworkopen.2020.16852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler JT, Bababekov YJ, Markmann JF, Chang DC, Yeh H. Distance is associated with mortality on the waitlist in pediatric liver transplantation. Pediatr Transpl. 2017;21(2). doi: 10.1111/petr.12842 [DOI] [PubMed] [Google Scholar]
- 19.Thammana RV, Knechtle SJ, Romero R, Heffron TG, Daniels CT, Patzer RE. Racial and socioeconomic disparities in pediatric and young adult liver transplant outcomes. Liver Transpl. 2014;20(1):100–115. doi: 10.1002/lt.23769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234–1243. doi: 10.1001/jama.2014.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berwick DM. The Moral Determinants of Health. JAMA. 2020;324(3):225. doi: 10.1001/jama.2020.11129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


