Introduction
Natural killer (NK) cells provide critical human host defense against malignancy and viral infections, particularly through the directed secretion of specialized secretory lysosomes termed lytic granules. This targeted lysis is a tightly regulated process as NK cells largely identify susceptible cells through germline encoded receptors. Therefore, their innate ability is harnessed and only released following passage through multiple regulatory checkpoints [1]. Key control of the cytotoxic process is contributed largely by cytoskeletal components, namely microtubules and F-actin. At each of the main stages of function, recognition, effector and termination, the cytoskeleton modulates NK cell responsiveness through the immunological synapse formed between it and the target cell.
The study of these processes, their regulation, and their contribution to disease has been driven largely by cell biological techniques. In particular, the use of high- and super-resolution microcopy to dissect the molecular contribution of cytoskeletal elements has been very productive. While detailed description of these techniques and their application is beyond the scope of this introductory targeted primer, perspectives on the use of microscopy to study the immunological synapse has been well reviewed elsewhere [2, 3]. This protocol will focus specifically on the application of confocal microscopy or STED microscopy to the study of an NK cell activated on glass coated with antibody to activating receptor.
The use of glass coated with antibody (or recombinant protein) has been shown to recapitulate the target cell interface while still allowing for high-resolution examination of the synapse. In addition, it allows for the selective ligation of receptors of interest. Under activating conditions, specifically the engagement of an adhesion receptor and an activating receptor, the NK cell will undergo degranulation at the plane of the glass, accompanied by the molecular events associated with exocytosis, including the expansion of the actin meshwork to enable or facilitate lytic granule passage, the exposure of CD107a (LAMP1) to the outer membrane and the release of lyosomal contents, namely perforin, granzymes and esterases [4, 5]. The choice of receptors to engage is dependent upon the cell origin (primary or cell line) and, if cell line, the expression of receptors on the cell surface. The primary adhesion molecule on all NK cells is LFA-1, the αLβ2 integrin (CD11a/CD18). Ligation of LFA-1 can occur through the use of a non-blocking anti-CD18 antibody, or through the use of recombinant ICAM-1, LFA-1’s most common physiological ligand. We use anti-CD18 (clone 1B4), which is commercially available in purified form from several suppliers. For activation of primary NK cells, we most commonly use anti-NKp30 in combination with anti-CD18, which is sufficient for NK cell activation and degranulation. Alternatively, anti-NKG2D may be used [4]. For cell lines, the choice is more dependent upon the line. For example, the NK-92 cell line [6] expresses the natural cytotoxicity receptor NKp30, and engagement of NKp30 in combination with LFA-1 results in robust activation and degranulation. Engagement of CD28 on YTS or YT cells has a similar effect, whereas for the NKL cell line NKG2D or CD16 engagement is commonly used [4].
Other methods to recapitulate the synapse include the use of supported lipid bilayers, which allows for imaging at the plane of the glass, yet is restricted to live cell imaging. The use of target cells has also been described, although it can be difficult to keep these oriented for imaging in the XY dimension at the synapse and the added depth of the target precludes imaging at the plane of the glass, a requirement for such techniques as total internal reflection microscopy. While all systems have their advantages and drawbacks, the recent report of similar behavior of T cells on lipid bilayer and glass lends confidence to the consistency of each system to study synaptic events [7].
Close examination of the NK cell lytic synapse has revealed some unexpected findings and underscored the importance of rigorous biophysical analysis. These findings include the interaction between activating and inhibitory receptors in microclusters, which shows that microclusters of inhibitory receptors at the NK cell synapse undergo reorganization following activating receptor ligation [8]. The undirected movement of lytic granules prior to degranulation at the synapse has been described by TIRF yet is incompletely understood [9]. Finally, our group and others recently described the presence of an actin mesh throughout the lytic synapse [4, 5]. This F-actin network creates granule-permissive clearances in response to activating signal, and its opening specifically requires the depolymerization of F-actin [10]. These findings were possible only because of the parallel use of multiple high- and super-resolution techniques and, again, underscore the importance for such type of analysis.
In this protocol we will describe the activation of NK cells on glass and the subsequent steps to the acquisition and preliminary processing of data obtained using confocal or STED microscopy (see example in Figure 1). In this case, the technique described utilizes a commercially available Leica SP8 laser scanning confocal microscope with STED capabilities. STED exploits the use of a high energy, torroidal shaped depletion beam that selectively darkens the photons produced around a single fluorophore and thus allows for sub-diffraction limited imaging [11]. In the case of the Leica system, this beam is positioned at 592 nm and/or 660 nm, potentially enabling depletion in the 488-630 nm range and allowing use of commercially available fluorophores. A further advantage of this system is the tunable white light laser and tunable PMTs or detectors. This allows for manipulation of both excitation and detection of emission. While this protocol describes the use of this system, the techniques described will be germane to the application of other confocal microscopes.
Figure 1. Representative image of the cytoskeleton of an NK cell activated on glass.
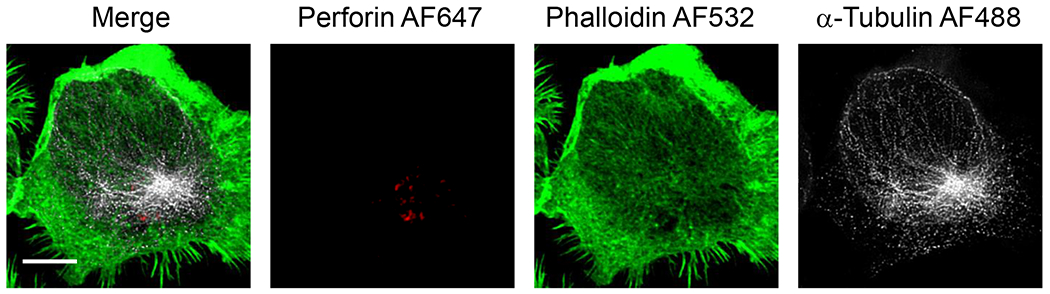
NK92 cells were activated on glass then fixed, permeabilized and stained for F-actin, a-tubulin and perforin as described in the protocol. Images were acquired on a Leica SP8 using both confocal (perforin) and STED (α-tubulin, F-actin) modules. Images were deconvolved with Huygens (SVI) software. Scale bar = 5 μm.
Materials
| Name of the Reagent | Company | Catalogue Number |
|---|---|---|
| Prolong anti-fade reagent | Life Technologies | P7481 |
| Saponin from Quillaja bark | Sigma | S4521 |
| Purified anti-NKp30 | Biolegend | 325202 |
| Purified anti-CD18 | Biolegend | 301202 |
| Bovine serum albumin | Sigma | A2153 |
| Phosphate Buffered Saline | Life Technologies | 14190250 |
| Cotton tipped applicator | Fisher Scientific | S450941 |
| Super PAP pen | Life Technologies | 008899 |
| BD Cytofix/Cytoperm | BD Biosciences | 554722 |
| Triton X-100 | Electron Microscopy Sciences | 22142 |
| Laboratory tissue wipers | VWR | 82003-820 |
| Thermo Scientific™ Colorfrost™ Plus Slides | Fisher Scientific | 99-910-02 |
| #1.5 cover slips | VWR | 48393-172 |
Methods
1. Coat slides with activating antibody
1.1. Prepare cells while coverslips are incubating (see Step 2 below).
1.2. Pre-warm 1 ml of BD Cytofix/Cytoperm buffer.
1.3. If necessary, cut #1.5 cover slips to 2 cm X 2 cm with a diamond pencil.
1.4. Using a hydrophobic pen, make a circle or square on each coverslip with approximately 1.5 cm diameter.
1.5. To coat coverslips with antibody, resuspend primary antibody against receptors of interest (i.e. CD18, NKp30, NKG2D—see note about antibody choice) at 5 μg/ml in Phosphate Buffered Saline (PBS). Prepare approximately 200 μl per condition. Vortex briefly to mix.
1.6. Gently pipette coating antibody on to coverslip and incubate at 37°C 5% CO2 for 30 minutes. (Note: alternatively, coverslips can be coated overnight at 4 degrees but should be pre-warmed at 37°C prior to use).
1.7. Following incubation, when cells are ready, wash coverslips by gently dipping them in a 50 ml conical tube of PBS.
2. Prepare NK cells and activate on cover slips
2.1. Upon harvesting, NK cells (primary or cell lines) should be washed once (centrifuge 1200 rpm) with pre-warmed media and resuspended in the same at a density of 106/ml.
2.2. Following resuspension, gently pipette cells onto coverslips and incubate at 37°C 5% CO2 for desired time (see note regarding incubation time).
3. Fix and permeabilize immobilized cells
3.1. During incubation, add 1 μl of Triton X-100 to 1 ml of pre-warmed Cytofix/Cytoperm buffer. Vortex well to mix.
3.2. Gently dip cells in 50 ml conical tube of PBS to remove unbound cells
3.3. Fix/perm cells on coverslips by gently pipetting 200 μl of Cytofix/Cytoperm buffer from Step 3.1.
3.4. Incubate 10-30 minutes in humidified chamber (slide box with moist paper towel) in the dark.
4. Detect cytoskeletal components by antibody and phalloidin staining
4.1. Prepare staining buffer: 1% BSA 0.1% Saponin in PBS.
4.2. Following fix/perm, wash cells gently in staining buffer (by either gently dipping coverslips in 50 ml conical or pipetting).
4.3. Prepare antibodies for staining in staining buffer (see note regarding antibody concentration and sequence of staining).
4.4. Incubate each primary and secondary antibody for 30-60 minutes at room temperature in a humidified chamber.
4.5. Between each sequential antibody stain rinse gently in staining buffer and dab edges of hydrophobic region with cotton swab. Maintain humidity during staining by performing all stains in humidified chamber.
5. Mount coverslips on slides
5.1. Use mounting media compatible with microscope and imaging technique. Gently pipette approximately 10-20 ml of mounting media, avoiding bubbles, on each slide and gently lay coverslip down. Ensure that the coverslip is oriented so that the surface on which the cells are fixed is in contact with the mounting media.
5.2. Allow coverslips to set if necessary (see note regarding choice of mounting media) and then seal edges of coverslips with nail polish.
6. Acquire images
6.1. Allow lasers to warm up for 30-60 minutes prior to initiation of imaging.
6.2. Place slide on microscope and adjust focus using eyepieces.
6.3. Acquire first channel and set laser power for optimal signal without pixel saturation. Adjust gain and, if necessary, exposure time (see note about file format and pixel size).
6.4. Repeat set-up for each channel to be imaged. Acquire single stained controls using each setting and adjust as necessary to reduce spectral overlap into other channels.
6.5. Acquire images. For quantitative imaging it is recommended to acquire at least 20 cells per condition, although this can be best dictated by the experiment in question and using traditional appropriately powered statistical sample size calculations. To preserve fluorescence during sequential scan it is best to acquire those longer wavelengths first. For best resolution of those components at the synapse, focus finely to ensure imaging at the plane of the glass.
6.6. Should the application of STED be desired, align the depletion laser prior to beginning STED imaging.
6.7. Apply STED laser and determine conditions that give best resolution. Variables to be adjusted at this stage may include laser power, gain, STED laser power and time gating (where applicable). A visual stepwise demonstration of this process has been published [12].
6.8. Save file and export.
7. Process and analyze images
7.1. Determine threshold based on background fluorescence (see note about image processing and deconvolution). In general, it is best to analyze data in its most unmanipulated form possible.
7.2. The analysis performed will be dependent upon the experiment. However, common measurements may include mean fluorescent intensity and area.
7.3. Choose representative images for display. These should reflect the mean of values obtained and be processed in the same way as during analysis. If possible, each channel should be shown separately and together as a merge. Scale bars should be shown. It is recommended that in an aggregate data summary that the representative image be specifically denoted as to where it resides within the aggregate.
Footnotes
- The duration of activation should be chosen based on the function of interest. For example, F-actin accumulation will occur relatively quickly (5-10 minutes), where as complete polarization of lytic granules may take 25-30 minutes.
- Ensure that if primary-secondary antibody combinations are used, they were not raised in the same species as the antibody used to coat the cover slip.
- For STED, avoid streptavidin-biotin combinations.
- We recommend staining each antibody sequentially, although phalloidin may be combined with the final secondary antibody.
- Antibody concentration will require titration depending on the sample and the antibody. For secondary antibodies, generally 1:100 or 1:200 are suitable.
- Recommended secondary antibodies for STED include Alexa Fluor 488, tetramethylrhodamine, Alexa Fluor 532, and Horizon V500.
- Generally, 30 minutes staining time is sufficient. In the case of low avidity antibodies staining time may be increased to one hour.
- The use of hard set medias is preferred, namely Prolong, Prolong Gold or Prolong Diamond. Vectashield is not compatible with STED. Mowiol is acceptable. 2, 2 thiodioethanol cannot be used in combination with phalloidin.
- Pixel size is a function of several variables on a laser scanning confocal microscope. These include the image format (number of pixels contained in the image), the amount of zoom used and the objective.
- Data collected as part of a single experiment should be obtained with the same pixel size throughout, particularly if the images are to be analyzed.
- For STED, a pixel size of less than 40 nm is highly recommended.
- For STED images, deconvolution can dramatically improve image quality and enable more accurate analysis. Deconvolution recovers information lost through imaging and can be performed mathematically through software. Huygens (SVI) is provided with Leica LASAF software and uses the theoretical point spread function estimated by the parameters used in your experiment to deconvolve both confocal and STED data. We generally use default parameters for deconvolution although optimizing the signal to noise ratio may be necessary.
- In general, image processing should be limited to the applicaltion of a threshold to remove background signal. All forms of nonlinear thresholding (gamma) should be avoided. The threshold should be chosen based on fluorescent intensity of the background and should generally be fixed at this intensity for all images and analyses.
References
- 1.Mace EM, et al. , Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol, 2014. 92(3): p. 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagrue K, et al. , The central role of the cytoskeleton in mechanisms and functions of the NK cell immune synapse. Immunol Rev, 2013. 256(1): p. 203–21. [DOI] [PubMed] [Google Scholar]
- 3.Mace EM and Orange JS, New views of the human NK cell immunological synapse: recent advances enabled by super- and high-resolution imaging techniques. Front Immunol, 2012. 3: p. 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AC, et al. , Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol, 2011. 9(9): p. e1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rak GD, et al. , Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol, 2011. 9(9): p. e1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong JH, Maki G, and Klingemann HG, Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia, 1994. 8(4): p. 652–8. [PubMed] [Google Scholar]
- 7.Comrie WA, Babich A, and Burkhardt JK, F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J Cell Biol, 2015. 208(4): p. 475–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pageon SV, et al. , Superresolution microscopy reveals nanometer-scale reorganization of inhibitory natural killer cell receptors upon activation of NKG2D. Sci Signal, 2013. 6(285): p. ra62. [DOI] [PubMed] [Google Scholar]
- 9.Mace EM, et al. , NK cell lytic granules are highly motile at the immunological synapse and require F-actin for post-degranulation persistence. J Immunol, 2012. 189(10): p. 4870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mace EM and Orange JS, Lytic immune synapse function requires filamentous actin deconstruction by Coronin 1A. Proc Natl Acad Sci U S A, 2014. 111(18): p. 6708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toomre D and Bewersdorf J, A new wave of cellular imaging. Annu Rev Cell Dev Biol, 2010. 26: p. 285–314. [DOI] [PubMed] [Google Scholar]
- 12.Mace EM and Orange JS, Visualization of the immunological synapse by dual color time-gated stimulated emission depletion (STED) nanoscopy. J Vis Exp, 2014(85). [DOI] [PMC free article] [PubMed] [Google Scholar]


