Abstract
Purpose of review—
Calcific aortic stenosis (CAVS) is the most common form of valvular heart disease in developed countries, increasing in prevalence with the aging population. Surgical or transcatheter aortic valve replacement is the only treatment available for CAVS. However, these interventions are typically reserved for severe symptomatic AS. The purpose of this review is to summarize the recent literature in uncovering the underlying pathophysiology of CAVS in the setting of lipoprotein (a) [Lp(a)} and emerging therapies targeting Lp(a) which may help halt disease progression in CAVS.
Recent findings—
Pathophysiologic, epidemiological, and genetic studies over the past two decades have provided strong evidence that Lp(a) is an important mediator of calcific aortic valvular disease (CAVD). Studies suggest that Lp(a) is a key carrier of pro-calcifying oxidized phospholipids (OxPL). The metabolism of OxPL results in a pro-inflammatory state and subsequent valvular thickening and mineralization through pro-osteogenic signaling. The identification of Lp(a) as a causal mediator of CAVD has allowed for opportunities for emerging therapeutic agents which may slow the progression of CAVD (Figure 1).
Summary—
This review summarizes the current knowledge on the association of Lp(a) with CAVD and ongoing studies of potential Lp(a)-lowering therapies. Based on the rate-limiting and causal role of Lp(a) in progression of CAVS, these therapies may represent novel pharmacotherapies in AS and inform the developing role of Lp(a) in the clinical management of CAVD.
Keywords: Calcific aortic valvular disease, Calcific aortic stenosis, Elevated lipoprotein(a)
Introduction
Calcific aortic valve disease (CAVD), including calcific aortic valve stenosis (CAVS), is the most prevalent form of valvular heart disease in the Western World and is the leading cause of valve-related mortality in the United States1,2. CAVS affects nearly 3% of the population greater than 65 years of age with projections that the prevalence of CAVS is expected to triple in the next 40 years. CAVD is marked by thickening of the aortic valve leaflets with resultant progressive stenosis of the aortic valve. CAVD is an insidious disorder as the early stages of CAVD are predominantly asymptomatic. However, the eventual development of restricted aortic valve leaflet mobility ultimately leads to significant left ventricular outflow obstruction and subsequent symptoms of angina, heart failure, and syncope. Among those individuals who develop severe CAVS, the 2-year survival rate without surgical intervention approaches 50%3. At present, surgical or transcatheter aortic valve replacement (AVR) is the only treatment available for severe symptomatic CAVS. The number of individuals requiring surgical or transcatheter aortic valve replacement (AVR) is expected to double by 20504,5. Given the high cost and significant periprocedural and long-term morbidity and mortality associated with these procedures, there is a need for a better understanding of the pathogenesis of CAVD and for the development of effective pharmacologic therapies.
Pathogenesis of CAVS
Because its incidence increases with age, CAVD was traditionally considered a passive and degenerative disease caused by continuous wear and tear of the aortic valve leaflets. However, it is now well established that that CAVD is actually a multifactorial phenomenon characterized by active inflammation followed by highly regulated fibro-calcific remodeling of the valve6,7.
The pathogenesis of CAVD can be divided into two distinct phases: an early initiation phase marked by valvular endothelial injury, lipid deposition, and inflammation, and a later propagation phase driven by pro-calcific and pro-osteogenic factors8. The initiation phase is similar to the development of atherosclerosis, with both conditions sharing similar key risk factors of age, male sex, dyslipidemia, metabolic syndrome, hypertension, metabolic syndrome, diabetes mellitus9. This early stage is triggered by endothelial injury to the outer layer of valve endothelial cells (VECs) due to mechanical shear stress. Impaired integrity and activation of VECs ensues and allows for the infiltration of the same lipids implicated in atherosclerosis, in particular low density protein [LDL] and lipoprotein(a) [Lp(a)]10–12. Progressive endothelial injury and oxidation of these lipids stimulate an inflammatory response driven by macrophages, mast cells, and T cells which release pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α.
This inflammatory milieu induces the normally quiescent valvular interstitial cells (VICs) to undergo osteogenic differentiation during the propagation phase of CAVD. These activated VICs gain a myofibroblast-like phenotype and lay down a disorganized collagen matrix and release other bone-related proteins, such as bone morphogenetic protein 2 (BMP2) and Runt-related transcription factor 2 (RUNX2). These osteogenic markers lead to release of calcium- and phosphate-rich extracellular vesicles which aggregate and provide a disorganized scaffold upon which progressive dystrophic calcification of the aortic valve can develop13,14. Apoptosis of VICs also results in microcalcifications at sites of endothelial injury and lipid deposition15,16. Cell death and release of apoptotic bodies facilitates the formation of hydroxyapatite crystals which form nucleation sites for further calcium deposition. Hydroxyapatite deposition prompts further pro-inflammatory responses from macrophages, creating a positive feedback loop of calcification and inflammation13. The fibrotic remodeling, dystrophic calcification, and biomineralization of the valvular extracellular matrix during this propagation phase ultimately results in progressive fibrosis, thickening, and dysfunction of the aortic valve leaflets. Therefore, the self-perpetuating cycle of calcification and valvular injury is an important driver of disease progression in CAVD.
Lp(a) is a risk factor for CAVS
The pathogenesis of CAVS demonstrates an important link between lipid deposition, inflammation, and calcification. An improved understanding of the biology of CAVS has highlighted potential therapeutic targets to slow the progression of CAVD and possibly avoid or delay the need for valve replacement. In particular, the emergence of epidemiological and genetic studies over the past two decades has identified elevated plasma Lp(a) levels as an important mediator of CAVS and a predictor for faster CAVS disease progression.
Lp(a) is a low-density-like-lipoprotein-like particle which is covalently bound to an apolipoprotein(a) [apo(a)] tail, encoded by the LPA gene. Apo(a) is comprised of 10 unique subtypes of kringle 4 (KIV) domains, followed by a kringle 5-like (KV) domains, and an inactive protease domain. Of these KIV subtypes, only KIV2 is present at different copy numbers ranging from 1 to more than 40 on each allele. Only one copy of KIV1 and KIV3–10 are present, but the number of KIV2 repeats determines the apo(a) isoform size as well as the variability in plasma Lp(a) concentration between individuals21. In general, there is an inverse relationship between the number of KIV2 copies in apo(a) and plasma Lp(a) concentrations.
Elevated Lp(a) is highly prevalent, affecting at least 20% of the global population with likely an even higher incidence among individuals with atherosclerosis and CAVD17. A strong association between Lp(a) and CAVS was first described in 1995 by Gotoh et al. Amongst 748 men and women in a rural Japan, the prevalence of aortic valvular sclerosis on echocardiography was nearly threefold higher among individuals with Lp(a) levels greater than 30 mg/L compared with individuals who had lower Lp(a) levels, independent of other risk factors18.
Lp(a) levels have high heritability with an autosomal co-dominant pattern22. The study of Lp(a) genetics has been instrumental in establishing potential causality for Lp(a) in calcific AS. In 2013, the CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium) Consortium group published a landmark genome-wide association study which showed that the rs10455872 LPA single-nucleotide polymorphism is associated with CAVD. In addition, LPA genotype is also associated with increased incidence of aortic valve calcification and need for aortic valve replacement across multiple diverse cohorts and racial/ethnic groups19. No similar genome-wide associations have been noted in mitral valve calcification, highlighting the distinct specificity of this association with aortic valvular disease. Multiple studies, including the Copenhagen City Heart Study and the Copenhagen General Population Study, have corroborated these key findings, showing that LPA genotypes predicted elevated Lp(a) levels, and that elevated Lp(a) levels >90 mg/dL was associated with a 3-fold increased risk of AS20. These genetic studies have complemented prior observational studies in providing strong evidence of Lp(a) as a genetically determined and likely important causal risk factor for calcific AS.
Lp(a) mediates calcification in CAVS
At present, molecular studies have shown that Lp(a), not LDL, is the preferential lipoprotein carrier of oxidized phospholipids (OxPL). Lp(a) can infiltrate denuded valvular endothelium, accumulate in the valve, and subsequently deliver its cargo of OxPL24. OxPL transported by Lp(a) acts as a substrate for lipoprotein-associated phospholipase A2 (PLA-2), secreted largely by macrophages, to generate lysophophatidylcholine (LPC). LPC is a highly reactive metabolite with pro-osteogenic properties present in mineralized aortic valves. Autotaxin (ATX), a lysophopholipase D enzyme transported into the valvular endothelium by Lp(a), uses LPC as a substrate for the generation of lysophosphatidic acid (LPA) which has been shown to promote the microcalcification of the aortic valve through activation of NF-kB21. This induces the upregulation of genes involved in osteogenic differentiation including IL-6, BMP2, and RUNX222. ATX transported by Lp(a) can additionally induce ATX expression by VICs in a feed forward cycle. More recent research has additionally demonstrated that the protein apolipoprotein C-III (apoC-III) binds to Lp(a) to form ApoCIII-Lp(a) complexes which associate with progression of calcific aortic valve stenosis and are found in proximity to calcified regions of stenotic aortic valves23–25.
There are also ongoing animal studies to help further understand the mechanistic role of Lp(a) in CAVS. In an atherogenic mouse model (Ldlr−/−E06-scFv) that expressed a natural E06-derived antibody which binds to OxPL and thereby inhibits its pro-inflammatory properties, echocardiographic evaluation showed significant attenuation of transaortic mean gradients and histology showed decreased calcium content of the aortic valve when compared to Ldlr−/− mice26. In a transgenic mouse model of CAVD (Ldlr−/−/Apob100/100) fed a diet high in lysophosphatidic acid (the enzymatic product of ATX), findings demonstrated overexpression of ATX and lysophosphatidic acid-mediated promotion and acceleration of CAVS23. Though a Lp(a) mouse model does not yet exist, these current animal studies provide evidence for the central role of Lp(a)-associated OxPL and ATX in the progression of CAVD.
The findings from these in vitro and animal studies have been borne out in clinical investigations as well. In recent years, 18F-sodium fluoride (NaF) uptake on positron emission tomography computed tomography (PET/CT) has emerged as an important measure of micro-calcification predictive of CAVS progression. Studies have shown that individuals with elevated Lp(a) > 75 nmol/L or > 35 mg/dL develop increased aortic valve micro-calcifications on 18F-NaF PET/CT imaging; these micro-calcifications are predictive of developing CAVD, manifesting before the development of clinically significant CAVS28,29. Compared with persons with Lp(a) < 35 mg/dL, those with elevated Lp(a) > 35 mg/dL additionally experienced increased progression on serial valvular computed tomography calcium score and faster hemodynamic progression on serial echocardiography based on peak transaortic velocity when compared with persons with Lp(a) levels < 35 mg/dL. Moreover, individuals with elevated OxPL-apoB levels, the predominant contributor to OxPL content of Lp(a) and reflective of the biological activity of Lp(a), also had increased valvular 18F-NaF uptake29. In a secondary analysis of the ASTRONOMER study, patients with preexisting mild to moderate CAVS with elevated Lp(a) levels in the top tertile exhibited faster disease progression of CAVD with a linear relationship between Lp(a) levels and the annual rate of peak transaortic velocity29. More importantly, elevated Lp(a) levels were associated with increased risk for aortic valve replacement or cardiovascular death in the ASTRONOMER, SALTIRE, Ring of Fire, and SAFEHEART studies29,31,32. Overall, these basic, translational and clinical studies support the important roles that Lp(a) and its associated OxPL play in the development of CAVS.
Emerging Lp(a) lowering therapies
The current data present important clinical implications regarding the monitoring and management of patients with CAVD. First, for patients with severe asymptomatic CAVS, Lp(a) and OxPL-apoB might serve as biomarkers to help guide timing of valve intervention and timing of imaging surveillance. Second, in the absence of any pharmacologic treatments for CAVD, Lp(a)-lowering therapeutics are an attractive strategy to halt the progression of CAVD.
Several existing lipid-lowering therapies and their potential in attenuating Lp(a) levels and CAVD have been studied. Statins have been widely investigated in CAVS with several clinical trials showing that statins are not only unable to reduce progression or induce regression of CAVS in patients with mild to moderate disease, but actually also increase Lp(a) levels by ~20%33–36. The mechanism by which this occurs is not understood but is felt to be attributed to increased apo(a) expression.
Niacin therapy lowers Lp(a) by ~ 20% to 30%37–39. Despite studies showing favorable reduction in Lp(a) with niacin treatment, niacin is not included in current guidelines due to the lack of clinical benefit in patients with atherosclerotic cardiovascular disease and increased risk of serious adverse events39. However, niacin therapy in patients with early CAVD and high Lp(a) ≥ 50 mg/dL is under ongoing investigation in the Early Aortic Valve Lipoprotein(a) Lowering (EAVaLL) randomized trial40.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can similarly lower Lp(a) levels by ~20–30% though the mechanism is currently unclear41,42. Nevertheless, there is growing evidence that PCSK9 may be involved in CAVD. In one prospective study, increased plasma PCSK9 levels were a predictor of CAVD; however, unlike Lp(a), PCSK9 levels positively correlated with the presence of CAVD, but not its severity43. From a large cohort of Danish patients, PCSK9 loss of function mutation R46L was associated with lower levels of Lp(a) as well as reduced risk of CAVS44. One study of a PCSK9 knockout mouse model has shown that PCSK9 deficient mice had lower aortic valve calcification compared to wildtype mice45. In vitro data from this study additionally showed that PCSK9 is highly expressed in calcified aortic valves and that aortic valve calcification might be caused by VIC-related PCSK9 expression. Sub-analyses of cardiovascular outcomes of studies of evolocumab (FOURIER trial) and alirocumab (ODYSSEY OUTCOMES) showed that the greatest absolute Lp(a) reductions were observed in those patients in the high quartile of baseline Lp(a) values. These patients additionally derived greater cardiovascular benefit from PCSK9 inhibitor treatment. An exploratory analysis of PCSK9 inhibition and aortic stenosis in the FOURIER trial revealed Lp(a) concentration was associated with future AS events of new or worsening CAVS or aortic valve replacement. More interestingly, this study showed that long-term therapy with evolocumab beyond 1 year may reduce AS events46. However, this post hoc analysis only encompassed a small number of patients and AS events, and similarly to the ODYSSEY OUTCOMES trial, the FOURIER trial was not designed to evaluate the effect of PCSK9 inhibitors on CAVD or the impact of Lp(a) on this disease. Furthermore, the modest reduction in Lp(a) levels brought about by niacin or PCSK9 inhibitor treatment may be inadequate to provide a clinically meaningful impact on the pathogenesis of CAVD47,48.
Two pivotal clinical trials investigating novel Lp(a)-lowering therapies are currently underway. The HORIZON phase 3 study is investigating the benefit of Lp(a)-lowering with pelacarsen (also termed IONIS-APO(a)-LRx, AKCEA-APO(a)-LRx, and TQJ230) on major cardiovascular outcomes of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and urgent coronary re-vascularization requiring hospitalization among individuals with established cardiovascular disease and elevated Lp(a) levels. Pelacarsen is a highly potent hepatocyte-directed antisense oligonucleotide targeting the LPA gene messenger RNA, inhibiting its transcription to apolipoprotein(a)49. Phase 2 study data showed that Pelacarsen safely and dose-dependently decreases Lp(a) and its associated OxPL levels by up to 80%; approximately 98% of patients treated with the highest-dose regimen achieved Lp(a) levels below 125 nmol/L (~50 mg/dL), the established threshold for Lp(a)-driven cardiovascular disease for individuals already on statin therapy50–52. A separate phase 2 randomized study is currently being conducted to evaluate the efficacy, safety, and tolerability of olpasiran (also known as AMG 890) in individuals with elevated levels of Lp(a). Olpasiran is a small interfering RNA (siRNA) molecule targeting hepatic expression of apolipoprotein(a). Phase 1 study outcomes showed that 90% reduction in Lp(a) levels could be observed with olpasiran treatment. Although no pre-specified CAVD endpoints have been assessed as part of trial designs for both pelacarsen and olpasiran, these investigations represent landmark trials In the Lp(a) field and may ultimately lead to novel therapeutics for the management of CAVD.
Conclusions
Although Lp(a) has been accepted as an important independent risk factor for both cardiovascular disease and CAVD, there are challenges to integration of Lp(a) levels into clinical decision making. One obstacle is that the measurement of Lp(a) is not yet standardized. The most common method for measuring Lp(a) are immunoassays which utilize polyclonal antibodies to target the apo(a) molecule. However, the large heterogeneity in apo(a) size between, as well as within individuals because of the heterozygosity of the apo(a) gene, can lead to inaccurate determination of Lp(a) plasma concentration. Because these polyclonal antibodies cross-react with the multiple KIV2 repeats, these assays can result in an overestimation of Lp(a) plasma concentrations in individuals with large isoforms and underestimation of Lp(a) levels in those with small isoforms53,54. For this reason, there may be a benefit in switching from the most commonly used total mass assays which report Lp(a) levels in mg/dL to measuring Lp(a) concentration in nmol/L17.
In addition, an important challenge in using Lp(a) as a biomarker to identify patients at higher risk for atherosclerotic cardiovascular disease (ASCVD) and CAVD is the lack of consensus on the target level of Lp(a). Based on the available studies, the National Lipid Association suggests using a universal cut point of ≥ 100 nmol/L, which approximates the 80th percentile in the Caucasian United States populations55. However, the 2019 American College of Cardiology (ACC)/American Heart Association (AHA) Cholesterol Guidelines recommend using Lp(a) concentration ≥ 125 nmol/L (> 50 mg/dL)56. These guidelines are likely to change as future studies take into account epidemiological differences based on risk, ethnicity, and comorbidities to determine the optimal cut-off levels.
Finally, integrating assessment of Lp(a) into current clinical care is not yet common practice. Measuring Lp(a) may be reasonable in patients at high risk for ASCVD, those with a family history of premature ASCVD, and for reclassification purposes in patients at borderline risk for ASCVD. However, current European and ACC/AHA recommendations offer less guidance in the assessment of Lp(a) among patients with known CAVD, with or without concurrent ASCVD56,57. Additionally, it remains unclear how Lp(a) might be used to identify those individuals at greater risk for CAVD and whether such information may guide timing of valvular intervention and imaging surveillance. Ultimately, clinical trials will be required to determine if new Lp(a)-lowering agents can slow the progression of CAVD. Such trials will require careful design with respect to enrolling patients who have elevated Lp(a) and aortic valve disease. However, it will also be important to identify patients who do not yet have severe disease, where the disease may still be modifiable. While there will be much more to learn, it is thought-provoking to know that Lp(a)-lowering agents may prove to be the first medical therapy that can modify the progression of CAVD.
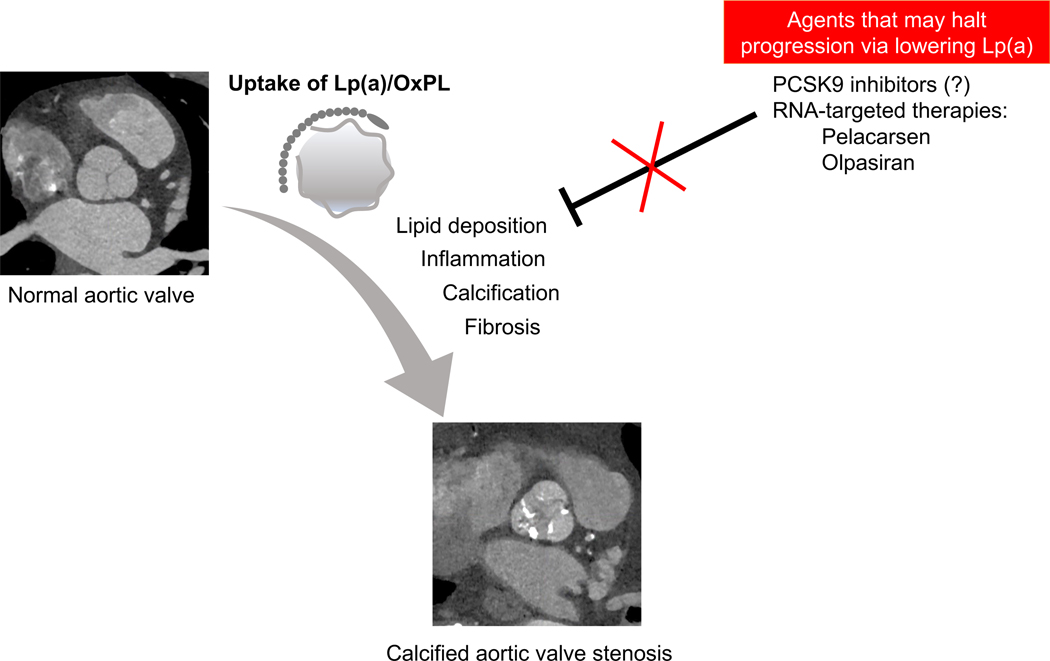
Figure 1.
Lipoprotein (a) mediates the progression of calcific aortic valvular disease. Upon endothelial damage, lipoprotein(a) and oxidized phospholipids (OxPL) accumulate within the valvular tissue, driving a feed-forward cycle of inflammation, calcification, and fibrosis. This ultimately results in calcified aortic valve stenosis. Currently, there are no approved medical therapies for aortic stenosis. However, emerging therapies to target Lp(a), including PCSK9 inhibitors and RNA-based therapeutics, may halt disease progression in aortic stenosis.
Key Points:
Lp(a) is associated with increased severity and increased progression of CAVD.
Lp(a) is a key mediator of calcific aortic stenosis via its delivery of OxPL to aortic valvular interstitial cells.
There are no approved therapies to lower Lp(a), but several agents are being evaluated in clinical trials.
Niacin and PCSK9 inhibitors can lower Lp(a), but the resulting modest reductions in Lp(a) may be insufficient to halt progression of CAVD.
Future studies will be required to determine whether emerging RNA-targeted therapeutics to lower Lp(a) may impact the progression of CAVD.
Acknowledgments
Financial support and sponsorship
None
Conflicts of interest
Dr. Blankstein receives research support from Amgen Inc. and Novartis Inc.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368(9540):1005–11. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135(10):e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenhek R, Binder T, Porenta G, et al. (2000) Predictors of out- come in severe, asymptomatic aortic stenosis. N Engl J Med 343(9):611–617. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira-Gonzalez I, Pinar-Sopena J, Ribera A, et al. Prevalence of calcific aortic valve disease in the elderly and associated risk factors: a population-based study in a Mediterranean area. Eur J Prev Cardiol 2013;20:1022–1030. [DOI] [PubMed] [Google Scholar]
- 5.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 6.Yutzey KE, Demer LL, Body SC, et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol 2014, 34, 2387–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohle ER, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [DOI] [PubMed] [Google Scholar]
- 8.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 2011;108: 1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HY, Engert JC, Thanassoulis G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes 2019, 26, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler. Thromb. Vasc. Biol 2014, 34, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong SS, Li L, Shen X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med 2019, 144, 266–278. [DOI] [PubMed] [Google Scholar]
- 12.Peeters RECM, Meex SJR, Dweck MR, et al. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J 2018, 39, 2618–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawade TA, Newby DE, Dweck MR. Calcification in Aortic Stenosis: The Skeleton Key. J Am Coll Cardiol. 2015; 66(5): 561–577. [DOI] [PubMed] [Google Scholar]
- 14.Blaser MC, Aikawa E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front. Cardiovasc. Med 2018, 5, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjortnaes J, Butcher J. Figueiredo JL, et al. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: A role for inflammation. Eur. Heart J 2010, 31, 1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater 2016, 15, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI working group recommendations to reduce Lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71(2):177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh T, Kuroda T, Yamasawa M, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol 1995, 76, 928–932. [DOI] [PubMed] [Google Scholar]
- 19.Thanassoulis G, Campbell CY, Owens DS, et al. CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013; 368: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic stenosis in the general population. J Am Coll Cardiol 2014;63(5):470–7. [DOI] [PubMed] [Google Scholar]
- 21. Bourgeois R, Devillers R, Perrot N, et al. Interaction of Autotaxin with Lipoprotein(a) in Patients with Calcific Aortic Valve Stenosis. J Am Coll Cardiol Basic Trans Science. 2020; 5(9):888–897. *This study demonstrates that autotaxin, an adipose tissue-derived phospholipase D that can help induce osteogenic differentiation of valvular interstitial cells, is preferentially transported by Lp(a) and could be used as a biomarker of CAVS.
- 22.Rao F, Schork AJ, Maihofer AX, et al. Heritability of Biomarkers of Oxidized Lipoproteins: Twin Pair Study. Arterioscler Thromb Vasc Biol 2015; 35:1704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015; 132:677–690. [DOI] [PubMed] [Google Scholar]
- 24. Capoulade R, Torzewski M, Mayr M, et al. ApoCIII-Lp(a) complexes in conjunction with Lp(a)-OxPL predict rapid progression of aortic stenosis. Heart. 2020. May;106(10):738–745. *This study shows that apolipoprotein CIII (ApoCIII) complexes with Lp(a) is associated with progression of CAVS. Elevated levels of ApoCIII-Lp(a) can identify patients with pre-existing mild-moderate AS who will go on to experience rapid progression of AS and higher rates of AVR/death.
- 25. Schlotter F, de Freitas RCC, Rogers MA, et al. Apoc-III is a novel inducer of calcification in human aortic valves. J Biol Chem. 2021;296:100193. *This study demonstrates that ApoCIII is not only associated with aortic valvular calcification, but actively stimulates calcification by promoting mitochondrial stress and inflammation-mediated calcification.
- 26.Que X, Hung MY, Yeang C, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018; 558, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noureen A, Fresser F, Uttermann G, Schmidt K. Sequence variation within the KIV-2 copy number polymorphism of the human LPA gene in African, Asian, and European populations. PLoS ONE 2015;10(d):e0121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Després AA, Perrot N, Poulin A, et al. Lipoprotein(a), Oxidized Phospholipids, and Aortic Valve Microcalcification Assessed by 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography. CJC Open 2019;1:131–40. **This study showed that elevated Lp(a) and OxPL levels are associated with CAVS in patients. Moreover, this study also demonstrated evidence of aortic valve microcalcification by 18F-NaF PET/CT imaging, even before the development of macroscopic valvular calcifications and clinically manifested CAVS. This suggests that it may be possible to detect early disease initiation in patients with elevated Lp(a) levels.
- 29.Zheng KH, Tsimikas S, Pawade T, et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J Am Coll Cardiol 2019;73:2150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capoulade R, Yeang C, Chan KL, et al. Association of Mild to Moderate Aortic Valve Stenosis Progression With Higher Lipoprotein(a) and Oxidized Phospholipid Levels: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol 2018;3:1212–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capoulade R, Chan KL, Yeang C, et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 2015;66:1236–46. [DOI] [PubMed] [Google Scholar]
- 32. Pérez de Isla L, Watts GF, Alonso R, et al. Lipoprotein(a), LDL-cholesterol, and hypertension: predictors of the need for aortic valve replacement in familial hypercholesterolaemia. Eur Heart J. 2021. Jan 12:ehaa1066. *SAFEHEART is a long-term prospective cohort study of a population with familial hypercholesterolemia. This study showed that the need for AVR due to CAVS is increased in these patients, particularly if they have elevated Lp(a).
- 33. Tsimikas S, Gordts PLSM, Nora C, et al. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24);2275–2284. **Previous reported data on the modulatory effects of statins on Lp(a) levels are inconsistent. However, this large meta-analysis of patients from six randomized trials (three statin versus placebo, and three statin versus statin trials) showed that statins significantly increase plasma Lp(a) levels. Elevated Lp(a) following statin therapy may contribute to residual cardiovascular risk.
- 34.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352(23):2389–97. [DOI] [PubMed] [Google Scholar]
- 35.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359(13):1343–56. [DOI] [PubMed] [Google Scholar]
- 36.Chan KL, Teo K, Dumesnil JG, et al. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121(2):306–14. [DOI] [PubMed] [Google Scholar]
- 37.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365(24):2255–67. [DOI] [PubMed] [Google Scholar]
- 38.Albers JJ, Slee A, O’Brien KD, et al. Relationship of Apolipoproteins A-1 and B, and Lipoprotein(a) to Cardiovascular Outcomes: The AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371(3):203–12. [DOI] [PubMed] [Google Scholar]
- 40.Thanassoulis G. Lipoprotein (a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. J Lipid Res 2016;57(6):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raal FJ, Giugliano RP, Sabatine MS, et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: an analysis of 10 clinical trials and the LDL receptor’s role. J Lipid Res. 2016;57:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713e1722. [DOI] [PubMed] [Google Scholar]
- 43.Wang WG, He YF, Chen YL, et al. Proprotein convertase subtilisin/kexin type 9 levels and aortic valve calcification: A prospective, cross sectional study. J Int Med Res 2016;44:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langsted A, Nordestgaard BG, Benn M, et al. PCSK9 R46L Loss-of-Function Mutation Reduces Lipoprotein(a), LDL Cholesterol, and Risk of Aortic Valve Stenosis. J Clin Endocrinol Metab 2016;101:3281–7. [DOI] [PubMed] [Google Scholar]
- 45.Poggio P, Songia P, Cavallotti L, et al. PCSK9 Involvement in Aortic Valve Calcification. J Am Coll Cardiol 2018;72:3225–7. [DOI] [PubMed] [Google Scholar]
- 46. Bergmark BA, O’Donoghue ML, Murphy SA, et al. An Exploratory Analysis of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition and Aortic Stenosis in the FOURIER Trial. JAMA Cardiol. 2020. 06 01; 5(6):709–713. *This post-hoc analysis showed that elevated Lp(a) levels, but not Lp(a)-corrected LDL-C levels are associated with higher risk of AS events (63 patients) and aortic valve replacement. Long-term therapy with the PCSK9 inhibitor, evolocumab, beyond one year was associated with a reduction in AS events, suggesting that PCSK9 inhibitor treatment might slow disease progression in CAVS.
- 47.Burgess S, Ference BA, Staley JR, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: A mendelian randomization analysis. JAMA Cardiol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamina C, Kronenberg F. Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol 2019;4:575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipo- protein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57:340e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsimikas S, Viney NJ, Hughes SG, et al. Antisense therapy targeting apolipo- protein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472e1483. [DOI] [PubMed] [Google Scholar]
- 51.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides tar- getting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388: 2239e2253. [DOI] [PubMed] [Google Scholar]
- 52. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382: 244e255. **This randomized controlled trial showed that treatment with APO(a)-LRx, a novel antisense oligonucleotide targeting Lp(a), reduced Lp(a) levels in a dose-dependent manner in patients. Importantly, treatment with this novel therapy resulted in 98% of patients achieving a Lp(a) levels of 50 mg/dL (125 nM) or lower, the target value supported by European and US guidelines.
- 53.Marcovina SM, Albers JJ, Gabel B, et al. Effect of the number of apoliprotein(a) kringle −4 domains on immunochemical measurements of lipoprotein(a). Clin Chem 1995; 41: 246–255. [PubMed] [Google Scholar]
- 54. Cegla J, France M, Marcovina SM, Neely RDG. Lp(a): When and how to measure it. Ann Clin Biochem. 2021. Jan;58(1):16–21. *This review highlights the challenges in the measurement of Lp(a) in the clinical laboratory setting.
- 55.Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–92. [DOI] [PubMed] [Google Scholar]
- 56.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS), European Heart Journal, Volume 41, Issue 1, 1 January 2020, Pages 111–188. **These updated dyslipidemia guidelines now support routine assessment of Lp(a) in each adult individual to identify those with very high inherited Lp(a) levels and to improve risk re-classification for those individuals who are borderline between moderate and high-risk. However, these guidelines have not yet incorporated the role of Lp(a) assessment in the management of CAVD.