Abstract
Purpose
Due to the biomedical applications universally, the Ag nanoparticles are one of the most commonly investigated nanoparticles (NPs). Curly kale (BroL) leaves contain numerous beneficial nutrients and phytochemicals. The aim of the current study is the fabrication of the Ag nanoparticles using the extracts of curly kale and to investigate their biological potentials.
Methods
The characterization of the generated BroLAgNPs was done through UV-Vis spectro study, Fourier-transform infrared spectro study, scanning electron microscope analysis, energy-dispersive X-ray study, distribution of size and zeta potential investigation, and X-ray powder diffraction study, and their biological effects were evaluated by antidiabetic, antioxidant, antibacterial and cytotoxicity effect.
Results
BroL-Ag nanoparticle displayed surface plasmon resonance at 432 nm. The Zeta potential of BroL (−26.6) AgNPs displayed a highly negative charge. In antidiabetic assay, BroL-AgNPs was highly effective with IC50 value 2.29 µg/mL at 1.0 µg/mL concentration. In cytotoxicity assay, BroL-AgNPs displayed strong activity at 10.0 µg/mL concentration. It showed inhibitory action against three food-borne pathogenic bacteria (9.29–11.44 mm inhibition zone) and displayed moderate antioxidant potential.
Conclusion
This study as a whole report an eco-friendly green synthesis of AgNPs using leafy vegetable aqueous extract and its multi-biological effects which could serve as a promising candidate in pharmacological and related industries.
Keywords: antidiabetic, antioxidant, antibacterial, cytotoxicity, curly kale, silver nanoparticle
Introduction
The green strategies for the synthesis of nanoparticles are adopted recently due to the adverse effects which arose due to the chemical synthesis processes, that resulted in the accumulation of toxic chemicals on the surface of the newly formed nanoparticles (NPs) that makes them unsuitable for clinical and biomedical applications.1–3 Plant-mediated green synthesis of metal NPs has gained popularity and attention of the public owing to the safe, economical, and eco-friendly nature.4–7 Among different metal nanoparticles, Au and Ag nanoparticles act a considerable part in various fields like pharmaceuticals, biomedicine, catalysis, biosensors, and nanomedicines.5,8–10 The process of formation of the metallic nanoparticle by plant extract is described commonly as three phases: the bioreduction of metal ions (activation phase), a combination of small particles with bigger ones (growth phase), and the end is defining the final shape of the synthesized nanoparticle (termination phase).11,12
During the plant and leaf extract mediated nanoparticle production, the extract of plant leaves and the metal solution (precursor) is mixed together at various reaction states. The factors which determine the states of the nanoparticle fabrication are the extract of plant leaves, as the phytochemicals type, concentration, and circumstance temperature, etc. are credited to regulate the rate of fabrication as well the yield and furthermore the stability of nanoparticles.12–14
The plant leaf extracts are considered as a tremendous source in the fabrication of metal nano-particle as it plays a substantial role in both reducing and stabilizing the nanoparticle in the process of synthesis.15–17 The plant extract composition is a significant element in the fabrication of NPs as diverse plant leaves contain different types with different concentrations of phytochemicals.5,18 The existing phytochemicals in plant extract have an amazing ability to reduce the metal ions within a very short period as compared to bacteria and fungi, which take longer incubation time. The primary phytochemicals present in plants such as aldehydes, ketones, carboxylic acids, flavonoids, sugars, and terpenoids are responsible for the bioreduction of the nanoparticle.
The phytochemicals like flavonoids comprise numerous functional groups which have an improved capability to reduce metal ions. Due to tautomeric transformation in flavonoids, the reactive hydrogen atom is released by which enol is changed into keto form. This method is understood by the metal ion reduction into metal NPs. In the production process of the biogenic silver nanoparticle, the transformation of enol form to keto form is the key element. In the plant extracts, existing glucose and fructose are also responsible for the fabrication of metallic NPs.19,20 Amino acids have various methods for metal ion reduction.12,21 Earlier, it was reported that amino acids like lysine, methionine, and cysteine remain capable of binding with Ag ions.22 The extracts of the plant are fabricated of proteins, carbohydrates, and biomolecules which act as a reducing agent to support the construction of metallic NPs.12,23 The functional groups like ‒C=O‒, ‒C=C‒, ‒C‒O‒ and‒C‒O‒C‒ etc. existing in the phytochemicals of plant extracts able to assist in the production of metallic NPs.5,12,24
The nanostructure AgNPs can be utilized in the health, food, textile, and cosmetic industry as a long-term antibacterial agent and various applications.25,26 In the previous centuries great importance has been given towards edible plants, which are extremely rich in phytochemicals, and these days there is a growing curiosity in the antioxidant activity of such active compounds present in the diet.27 Earlier various studies have stated green synthesis of AgNPs using various leafy vegetable extracts like Amaranthus dubius,28 Rumex acetosa,29 red spinach (Amaranthus tricolor L.),30 etc.
Curly kale is an economically significant and broadly consumed vegetable all over the globe. Leafy vegetables of the family Brassicaceae are an abundant source of phenolic acid, flavonols, tannins, flavones, anthocyanidins, coumarins, terpenoids, phytosterols, folic acid, β-carotene, α-tocopherol, ascorbic acid, calcium, copper, manganese, zinc, iron, and selenium, etc.31,32 These are known for their anticarcinogenic and antimicrobial potential.32,33
Commonly, the Curly kale (BroL) is highly recommended for its abundant quantity of nutrients, numerous dietary fibers, minerals, and various health-promoting bioactive compounds like vitamins, phenolic acids, flavonoids, antioxidative compounds, carotenoids, and glucosinolates, etc.34 It seats high owing to its nutrient density on the list of important vegetables and fruits from around the globe which are associated with decreasing the threat of chronic sicknesses like cancer and cardiovascular diseases.34 Kale is stated to possess high nutraceutical potential. It is reported to have a greater content of vitamin C, carotenoids, phenolic compounds, glucosinolates with antioxidant potential. Kale can be considered as an outstanding source of antioxidants.35 It was also reported that fermentation improves the nutraceutical properties of curly kale.36 Thirty-two phenolic compounds counting kaempferol, quercetin, ferulic, and derivatives of p-coumaric, caffeic acid, and sinapic were tentatively recognized in curly kale which was characterized and identified by HPLC analysis.37 Besides, it also contains calcium and potassium (Satheesh and Workneh Fanta, 2020). In kale, the calcium bioavailability is considerably high which is considered to be superior to milk. Also, the composition of amino acids is well balanced. It comprises less saturated fatty acid and higher unsaturated fatty acids.
There are limited studies regarding the health benefits of curly kale. Only very few in-vivo and in-vitro studies have well established the potential role of kale in controlling bilirubin metabolism, macular disease, antigenotoxic ability, gastroprotective activity, anti-inflammatory activity, inhibition of the carcinogenic compound’s development, defensive role in coronary artery disease, antimicrobial effect against particular microbes. It is established that kale is a prospective vegetable (leafy) for dietary recommendations for entire age groups and it has abundant perspective for health and food-based products.38
Referring to the above reports of previous studies, kale is a source of potential phytochemicals with abundant health benefits.31,32,34,37,38 As per the above evidence, it is an excellent candidate for the biosynthesis of Ag nanoparticles and it can be produced in a cost-effective technique. Thus, this study considers the usage of the BroL plant leaves for Phyto-mediated biosynthesis of silver NPs and study of its multiple biological effects by various activities such as antioxidant, antidiabetic, cytotoxicity, and antibacterial assays.
Materials and Methods
Preparation of BroL Extract
Fresh commonly edible curly kale (Brassica oleracea) was obtained from the certified fruits and vegetable outlets at the Goyangsi market, the Republic of Korea. The species was identified and a voucher specimen (RIILSEH No. 202103-01) is deposited in the e-herbarium, Dongguk University, Republic of Korea . The leaves were washed properly with sterilized water, dried, and cut into small pieces. The leaves (100 g) amount was immersed in DDH2O (500 mL) in a 1000 mL Erlenmeyer flasks, boiled with continuous stirring then cooled to normal room temperature by filtering with filter (Whatman filter paper no. 1) stored at 4°C in a bottle.39
The BroL Extract Phytochemical (Primary) Screening
The primary phytochemical analysis of the extract (BroL) was tested for the presence of saponins, terpenoids, flavonoids, carbohydrates, proteins, and amino acids.40–42
Synthesis and Characterization of AgNPs Using BroL Leave Extract
Concisely in two separate 500 mL volumes of Erlenmeyer flask, the BroL-AgNPs were taken. BroL extract was prepared by placing 100 mL AgNO3 (1 mM) aqueous solution and BroL extract (10 mL) was added dropwise to a flask at normal room temperature with constant stirring.43 The BroL-AgNPs biosynthesis was noticed by observing gradual changes in the color of the solution (reaction solution). The solution (reaction) was centrifuged for 30 min at (10,000 rpm) after complete synthesis. The generated AgNPs pellet was gently washed with sterilized H2O and centrifuged again. In the concluding step, the dehydrated pellets (55°C) put in vials and held in reserve in storage for further study.
The synthesized BroL-AgNPs were undergone through characterization by using UV-VIS spectroscopy, XRD, SEM, EDX, FTIR, DLS, and zeta potential analysis using specific devices and standard protocol detailed in the earlier published research articles.43–45
The UV-VIS spectrophotometer (Thermo Scientific, Multiskan GO; Waltham, MA, USA) was used to measure the spectra in between 300 and 700 nm range for 24 h. The XRD analysis of the BroL-AgNPs was performed by using (XRD machine X’Pert MRD; PANalytical, Almelo, Netherlands) setup at 30 kV and 40 mA with Cu Kα radians at an angle of 2θ by the following protocol by Iravani et al.44 The SEM and EDX analysis of BroL AgNPs was done through SEM (Hitachi, S-4200, Tokyo, Japan) analyzer connected with an EDX device (EDS; EDAX Inc., Mahwah, NJ, USA) by following the protocol of Zhou et al.46 The BroL-AgNPs FT-IR analysis was carried out by an FT-IR spectrophotometer (from 400 cm−1 to 4000 cm−1 Spectrum wavelengths; TwoTM FT-IR Spectrometer; PerkinElmer, Waltham, MA, USA) by using the protocol by Iravani et al.44
The Biological (Antidiabetic, Antibacterial, Antioxidant, and Cytotoxicity) Effect of BroL-AgNPs
The α-glucosidase inhibition activity was estimated by a standard process.47 The absorbance value of the sample was measured at a 405 nm wavelength using a plate reader. The test plates as well contained a positive control (standard) like buffer, enzyme, and substrate. The α-glucosidase inhibition percentage was estimated as follows:
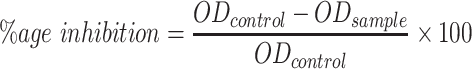 |
Where ODcontrol is the absorbance of the control, ODsample is the absorbance of tested sample, %age is the percentage.
In the BroL-Ag nanoparticle, the antibacterial effect was tested by following the disc diffusion (standard method) described by Naqvi et al48 against some pathogenic bacteria (foodborne) and it was active against three of them, ie, Enterococcus faecium DB01, Aeromonas hydrophila ATCC 7966 and Salmonella typhimurium KCTC 1925.
The BroL-AgNPs free radical scavenging effect (antioxidant activity) was estimated by evaluating DPPH, ABTS, reducing power, and NOx radical scavenging analyses following the standard protocol.49 The BroL-AgNPs cytotoxicity potential was assessed against the HepG2 cancer cell lines (The Korea Cell Line Bank, Seoul, South Korea). In the test samples, BroL-AgNPs were diluted into Dulbecco, phosphate-buffered saline at 1 mg/mL and then filter sterilized using a syringe filter (0.22 µm filter; Millipore, Billerica, MA, USA). The morphology and dead cell percentage exposed to BroL-AgNPs were calculated through the trypan blue exclusion test method.50
Statistical Analysis
The data are presented as the mean ± standard deviation. ANOVA (One-way analysis of variance) was achieved at a 5% level of significance (P > 0.05) using Duncan’s test through SPSS software (SPSS, version 23.0, IBM Crop., Armonk, NY, USA, software).
Results and Discussion
The Leaf Extract Primary Phytochemical Screening
The metal NPs synthesis by the green method defeats the negative impact associated with the chemical method of NPs synthesis as it is eco-friendly and economical. The green method includes the oxidation or reduction process and is also catalyzed by plant phytochemicals or catalytic microbial enzymes. The major phytochemicals consist of terpenoids, aldehydes, flavones, and ketones, etc.29,51 As a natural bio-resource, the edible plant leaf extract is non-toxic and holds a group of bioactive compounds which helps in the process of reduction of Ag+ ions.
The phytochemical (primary) screening of BroL extract was accomplished and confirmed the existence of terpenoids, saponins, flavonoids, proteins, and amino acids, and carbohydrates, phytochemicals in the BroL extract, and the result is displayed in Table 1. The phyto-mediated biosynthesis and the stability process of the synthesized BroL-AgNPs are owing to the action of the phytochemicals particularly the terpenoids, flavonoids, and saponins.29,52
Table 1.
Phytochemicals Screening of Aqueous BroL Extract
| Name of Phytochemicals | BroL Extract |
|---|---|
| Flavonoids | + |
| Terpenoids | + |
| Saponins | + |
| Proteins & Amino acids | + |
| Carbohydrates | + |
Note: “+” means Present.
Green Synthesis of BroL-AgNPs
Among the natural active metabolites, polyphenols are immensely found in several foods and an extensive variety of leafy vegetables.53 Previously there are reports of Ag nanoparticles synthesized from various leafy vegetable extracts like Moringa oleifera, Rumex acetosa, Amaranthus dubius.28,54,55 Phytochemicals can be well utilized in the food and medicine industry for functional food development and medications.56 BroL-AgNPs were biosynthesized in the current investigation using the leafy vegetable (BroL) (Figure 1A). Synthesis of both BroL AgNPs was progressive and visually it was tracked and evidenced by the regular and continuous color change of the solution (reaction mix) in BroL-AgNPs from neutral to brownish red (Figure 1B).57
Figure 1.
(A) Brassica oleracea leaves (BroL); (B) gradual alteration of BroL extract color pigment in the course of the BroL-AgNPs synthesis (0–12 h); (C) UV-Vis absorbance spectra of the BroL-AgNPs.
The BroL-AgNPs Characterization
The green-synthesized BroL-Ag nanoparticle was characterized (through UV-Vis spectral study, XRD, SEM, EDX, FT-IR spectroscopy, DLS, and zeta potential analysis). Subsequently, the phyto-synthesis of BroL-AgNPs was evidenced through the UV-Vis spectral absorption consequence.
The BroL-Ag nanoparticles surface plasmon resonance absorbance value was noticed maximum at around 432 nm wavelength at 12 h (Figure 1C). This result is analogous to the previous AgNPs synthesis result.58 This outcome of the present investigation proposes that phytochemicals and secondary metabolites existing in plant food leafy vegetables in BroL extract works, like reducing agent and capping agents, are derived from leafy vegetables that are eco-friendly and non-toxic. Among the natural resource, plant leaf extracts are easily obtainable, harmless and generally non-toxic and it possesses numerous bioactive compounds which help in the Ag+ (ions) reduction process.54
FTIR
The FTIR study was carried out to detect the major functional groups in the BroL aqueous leaf extract and their promising contribution in the process of silver NPs synthesis and stabilization. As per the FT-IR analysis result, probably the peaks of BroL extract at 3308.21, 2126.11, 1628.38, 1092.95, and 668.75 cm−1 shifted to 3298.78, 2103.48, 1632.15, 1040.16, and 674.40 cm−1 correspondingly for BroL-AgNPs (Figure 2).
Figure 2.
FT-IR study absorbance peaks of BroL-extract and BroL-AgNPs.
The difference in the absorbance spectrum may propose their influence on the synthesis of BroL-AgNPs. The FT-IR outcomes of BroL showed a difference in peak value with changed stretching modes between BroL extract (Figure 2).
The observation peaks for BroL-AgNPs at 3298.78 cm−1 state the presence of broad and strong O–H stretch, H–bonded, and N–H stretch (bond) which belongs to the amines, phenols, alcohols, amides, and functional groups, respectively.59 For BroL-Ag nanoparticles, the absorbance peaks at 2103.48 cm−1 state the presence of C≡N stretch these belong to the nitriles functional group. Similarly, for BroL-Ag nanoparticles, the peaks at 1632.15 cm−1 state the N-H bend bond presence which comes under the functional group amines. Peaks at 1040.16 cm−1 (BroL-Ag nanoparticles) indicate the C–O stretch presence and it comes under the alcohols, carboxylic acids, and esters functional groups. The absorbance peak (at 674.40) cm−1 specifies the C–H bond functional group’s existence.59
The slight variation in the observed peaks of BroL silver nanoparticles can be accredited to the capping and stabilization processes of the synthesized Ag nanoparticles.57 According to the earlier reports, the FTIR absorption spectra peaks at 1622, 1606, 1077, and 1042 are due to the silver ion reduction to AgNPs by photophosphorylation (non-cyclic).12 The earlier research proves that the heterocyclic compounds and the biomolecules that exist in the extract of plants were responsible for the extracellular metallic nanoparticle synthesis by plant extracts.12
For identification of the morphology and chemical structure of the BroL silver nanoparticles further, SEM and EDX studies were carried out.
SEM
The SEM analysis result revealed the almost spherical nature of the BroL silver nanoparticles in the nanometer range (Figure 3A). An analogous consequence was reported earlier.60
Figure 3.
(A) SEM photograph, (B) EDX data of BroL-AgNPs, (C) XRD configuration of BroL-AgNPs.
EDX
The BroL-AgNPs composition (elemental) was detected through the EDX study. This outcome may be well credited to the existing bioactive compounds in the extract of BroL in the green synthesis procedure which could have assisted in the BroL-AgNPs capping process.57 The percentage (atomic) of Ag was 30.84% for BroL-AgNPs. The higher percentage of (Ag) was detected in the generated AgNPs specifies that the maximum of particles was AgNPs (Figure 3B).
XRD
The XRD analysis results of BroL-AgNPs revealed four distinct peaks and also confirmed that the BroL-AgNPs were crystalline (Figure 3C). The diffraction peaks were noticed at 2 theta angles in the synthesized Ag nanoparticles at 38.11°, 46.24°, 64.43°, and 76.98°; these are correspondent to (111), (200), (220), and (311), respectively (Figure 3C).
The above-detected peaks (four) were corresponding to the fcc (face-centered cubic-phase) of the standard (Ag0) (JCPDS card No. 04–0783).57,61 A similar result was stated in earlier studies.57
DLS and Zeta Potential
The green synthesized BroL Ag nanoparticle size distributions (hydrodynamic diameter) and surface charges were estimated through DLS and Zeta potential analysis. The average size distribution (hydrodynamic diameter) of BroL-AgNPs is 276.35 d.nm (Figure 4A), similar results were reported earlier.62,63 The BroL-AgNPs zeta potential (−26.6) displayed a highly negative charge (Figure 4B) which supports the long period stability of synthesized NPs.45,64 It confirms the better and colloidal nature of the green synthesized nanoparticle.63 This result is similar to earlier published article results.2,62,65
Figure 4.
(A) BroL-Ag nanoparticle size distribution and (B) BroL-AgNPs zeta potential analysis.
Biological Activities of BroL-AgNPs
Antidiabetic Activity (α-Glucosidase Inhibition Effect)
Mostly, the metabolism of carbohydrate alteration of oligosaccharides into monosaccharides is assisted by intestinal α-glucosidase enzymes and pancreatic α-amylase.66,67 Diabetes (non-insulin) can manage to treat, by limiting the activity of α-glucosidase and α-amylase enzymes as it slows the release of glucose into blood.67,68 To decrease hyperglycemia, the α-amylase, and α-glucosidase (carbohydrate-digesting enzymes) require to be inhibited therapeutically, consequently limiting the breaking of the starches or carbohydrates into monosaccharides, which assists in elevation of blood sugar levels.69,70 Therefore, new compounds with a carbohydrate-hydrolyzing enzymes inhibitory potential could be beneficial to manage diabetes.
Herein, the BroL-AgNPs exhibited a significant antidiabetic effect in a dose-dependent approach. A relatively greater effect was exhibited by the BroL-AgNPs (18.28%) at the tested (lower) concentration of 1.0 µg/mL. Whereas at 2.5 µg/mL concentration the AgNPs displayed 80.44% inhibition. At the tested (higher) concentration (5.0 µg/mL) the BroL-AgNPs displayed 95.85% inhibition (Figure 5A). Analogous results have been stated for AgNPs against α-glucosidase in the earlier studied article.71–73 The α-glucosidase (inhibition) IC50 value of the BroL Ag nanoparticle was 2.29 µg/mL (Table 2) which confirms the antidiabetic effects of the synthesized AgNPs as the (IC50) value is considerably low (Table 2), and is an indication of its effectiveness in the control of diabetes.
Figure 5.
(A) α-glucosidase effect result of BroL-Ag nanoparticles, values with the dissimilar superscript letters (a-c) are significant at P> 0.05; (B) cytotoxicity activity of BroL-Ag nanoparticles (Dead cell %) against cancer (HepG2) cells; (C) images showing the cells treated with different concentrations of BroL-AgNPs, visualized by an inverted microscope.
Table 2.
BroL-AgNPs, Antioxidant and Antidiabetic Assays IC50 Values
| Factors | IC50 value BroL-AgNPs (µg/mL) | |
|---|---|---|
| Antioxidant test | DPPH | 098.22 |
| ABTS | 451.22 | |
| NOX | 039.76 | |
| Reducing (IC0.5 value) | 025.61 | |
| Antidiabetic test | α-gucosidase | 002.29 |
Cytotoxicity Activity
Various types of free radicals, organic hydroperoxides as well as oxygen radicals are stated to play a significant role in cytotoxicity.29,74 Synthetic antioxidants and natural vitamins are known to restrain carcinogenic chemicals through detoxification, and modifying their activation as well as their aptitude to scavenge the free radicals and reactive carcinogen metabolites.29,75 The structure of NPs makes them an excellent approach for targeting the scarce harsh cell development originated by cancer.76 For the detection and control of cancer syndrome and its related conditions, nano-based medications are greatly effective.3,77
The cytotoxicity effect of the BroL-Ag nanoparticle was tested against HepG2 cancer cells and the result signifies that the dead cells% of HepG2 (cancer) cell lines increased with the increased concentration of Brol-AgNPs (Figure 5B). The cells treated with the BroL-AgNPs, visualized by an inverted microscope, showed variable morphology for both the control and the treated cells. The control result displayed more quantity of live cells while in treatments (Brol-AgNPs), the percentage of the dead cells was progressively increased as the AgNPs concentrations increased (Figure 5C). Earlier, similar results are also reported by various researchers.77–80 The biosynthesized NPs exhibited a strong anticancer effect, but the BroL-AgNPs were more active than PL-AgNPs at lower (10 µg/mL) and higher (1000 µg/mL) tested concentrations. However, the BroL-AgNPs were greatly toxic to HepG2 cell lines at all tested (10–1000 µg/mL) concentrations with dead cell percentage 25–84% in case of BroL-AgNPs and 19–64% (Figure 5B).
Antioxidant Activity
The antioxidant molecules reduce the free radical scavenging reaction against the oxidative stress and as a result slow down or hindrance the oxidative damage to the biomolecules like proteins, DNA, and lipids.67,81,82
The antioxidant activity consequence of the BroL-Ag nanoparticle was studied by ABTS, DPPH, NOx, and reducing power assays, at 25, 50, and 100 (µg/mL) concentrations, and the results were displayed in Figure 6. The bio-synthesized BroL-AgNPs nanoparticles overall revealed a good antioxidant effect (Figure 6, Table 2). The BroL-AgNPs, DPPH scavenging (free radical) percentage was in the range of 19.54 to 36.03% and in the case of BHT, it was 75.04 to 83.31% (Figure 6A). The ABTS potential was between 6.73 to 11.09% for BroL-AgNPs and for BHT it was between 35.58 to 94.07% (Figure 6B). The reducing power result of BroL-AgNPs was between the range of 0.0713 to 0.1651 and for BHT it was in between the range of 0.1632 to 0.3284 (Figure 6C). The NOx scavenging. The NOx scavenging result of BroL-AgNPs was between 49.23 to 82.58% and in the case of BHT, it was in between 93.08 to 98.41% range (Figure 6D). The antioxidant assays and IC50 values were estimated and displayed in Table 2. The above results could be due to the presence of numerous functional groups in the extract of BroL, which could have played a significant role in the coating and stabilization course throughout the production of BroL-AgNPs.17,57,83
Figure 6.
(A) Antioxidant effect consequences of the BroL-AgNPs. (B) DPPH scavenging effect ABTS scavenging effect. (C) Reducing power effect. (D) NOx scavenging effect. Values with dissimilar superscripts letters (a-f) are significant at P> 0.05.
Antibacterial Activity
Presently, the extension rate of the multidrug-resistant pathogen is common, which might have a hostile effect on human health.84,85 The Ag nanoparticle’s antibacterial effect is credited to one of the reasons as their ability to attach to the cell wall of bacteria and cause cavity and alter the permeability of the bacterial cell membrane and resulting in death of bacterial cells.29,86 The BroL-AgNPs displayed a positive antibacterial effect against three pathogenic bacteria (Table 3). BroL-AgNPs displayed 9.29–11.44 mm inhibition zone against the above three foodborne pathogenic bacteria. The standard Erythromycin exhibited inhibition against two of the pathogenic bacteria with 9.73 and 9.82 inhibition zone (Table 3). This consequence or result is similar to earlier reports.2,16,17,72 A hypothesis on the possible mode of action of the BroL-AgNPs could be that when the NPs interacted with the bacteria, it might have bind to the bacterial cell membrane and could have resulted in the modification of the chemical and physical nature of the bacterial cell membrane resulting in the malfunction of the normal biological process such as permeability, respiration, etc. of cells leading to the death of the bacteria.10,87
Table 3.
BroL-AgNPs Antibacterial Effect Against Foodborne Pathogenic Bacteria
| Bacterial Strains | BroL-AgNPs* | Erythromycin (Er) |
|---|---|---|
| Enterococcus faecium DB01 | 09.29d ±0.06** | 09.73c±0.19 |
| Aeromonas hydrophila ATCC 7966 | 11.44a ±0.22 | 09.82b±0.16 |
| Salmonella typhimurium KCTC 1925 | 09.68c ±0.05 | 00.00e±0.00 |
Notes: *Inhibition zone (in mm); **Values with the different superscript letters (a, b, c, d, e) are statistically significant at P> 0.05.
Conclusion
The BroL-AgNPs were successfully biosynthesized using the BroL leaf extract which is rich in phytochemicals with ample health benefits. The generated novel BroL-AgNPs showed substantial antidiabetic, antioxidant, and cytotoxicity potentials, together with a positive antibacterial effect. In overall conclusions, the BroL-AgNPs could be a favorable candidate in various fields including applications in controlling diseases such as cancer, diabetes, and for the preservation of food or as an antibacterial agent, etc. The supporting results may suggest green-synthesis of environmentally friendly Ag nanoparticles and their applications in pharmacological, food, cosmetics, textile, and other related industries. Further detailed research on drug delivery and drug formulation aspects is essential for its effective and potential commercial applications.
Acknowledgment
G Das, JK Patra, and H-S Shin are grateful to Dongguk University-Seoul, Republic of Korea, for the support. The authors also wish to thank all the helping hands in this research work. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), the Republic of Korea.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), the Republic of Korea.
Ethical Approval
The author declares that necessary approvals are exempted for conducting research on the said plant material according to institutional guidelines.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Metz KM, Sanders SE, Pender JP, et al. Green synthesis of metal nanoparticles via natural extracts: the biogenic nanoparticle corona and its effects on reactivity. ACS Sustain Chem Eng. 2015;3(7):1610–1617. doi: 10.1021/acssuschemeng.5b00304 [DOI] [Google Scholar]
- 2.Borah D, Das N, Das N, et al. Alga‐mediated facile green synthesis of silver nanoparticles: photophysical, catalytic and antibacterial activity. Appl Organomet Chem. 2020;34(5).e5597. 10.1002/aoc.5597 [DOI] [Google Scholar]
- 3.Jain N, Jain P, Rajput D, Patil UK. Green synthesized plant-based silver nanoparticles: therapeutic prospective for anticancer and antiviral activity. Micro Nano Systems Letters. 2021;9(1):5. doi: 10.1186/s40486-021-00131-6 [DOI] [Google Scholar]
- 4.Aritonang HF, Koleangan H, Wuntu AD. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int J Microbiol. 2019;2019. doi: 10.1155/2019/8642303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu MS, Mandal BK, Maddili SK. Biofabrication of size controllable silver nanoparticles – a green approach. J Photochem Photobiol B. 2017;167(236–241). [DOI] [PubMed] [Google Scholar]
- 6.Maddinedi SB, Mandal BK, Anna KK. Environment friendly approach for size controllable synthesis of biocompatible Silver nanoparticles using diastase. Environ Toxicol Pharmacol. 2017;49:131–136. doi: 10.1016/j.etap.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Maddinedi SB, Mandal BK, Anna KK. Tyrosine assisted size controlled synthesis of silver nanoparticles and their catalytic, in-vitro cytotoxicity evaluation. Environ Toxicol Pharmacol. 2017;51:23–29. doi: 10.1016/j.etap.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 8.Nasrollahzadeh M, Mahmoudi‐Gom Yek S, Motahharifar N, Ghafori Gorab M. Recent developments in the plant‐mediated green synthesis of Ag‐based nanoparticles for environmental and catalytic applications. Chem Record. 2019;19(12):2436–2479. doi: 10.1002/tcr.201800202 [DOI] [PubMed] [Google Scholar]
- 9.Maddinedi S, Mandal BK, Ranjan S, Dasgupta N. Diastase assisted green synthesis of size-controllable gold nanoparticles. RSC Adv. 2015;5(34):26727–26733. doi: 10.1039/C5RA03117F [DOI] [Google Scholar]
- 10.Riaz M, Mutreja V, Sareen S, et al. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci Rep. 2021;11(1):2866. doi: 10.1038/s41598-021-82555-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Si S, Mandal TK. Tryptophan‐based peptides to synthesize gold and silver nanoparticles: a mechanistic and kinetic study. Chem Eur J. 2007;13(11):3160–3168. doi: 10.1002/chem.200601492 [DOI] [PubMed] [Google Scholar]
- 12.Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P. ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology. 2018;16(1):1–24. doi: 10.1186/s12951-018-0408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–356. doi: 10.1016/j.biotechadv.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Devanesan S, AlSalhi MS. Green synthesis of silver nanoparticles using the flower extract of abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int J Nanomedicine. 2021;16:3343. doi: 10.2147/IJN.S307676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph J, Khor KZ, Moses EJ, Lim V, Aziz MY, Samad NA. In vitro anticancer effects of vernonia amygdalina leaf extract and green-synthesised silver nanoparticles. Int J Nanomedicine. 2021;16:3599. doi: 10.2147/IJN.S303921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novelles M, Ortega AR, Pita BA, et al. Biosynthesis of fluorescent silver nanoparticles from Leea coccinea leaves and their antibacterial potentialities against Xanthomonas phaseoli pv phaseoli. Bioresources Bioprocessing. 2021;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R, Hano C, Nath G, Sharma B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. Biomolecules. 2021;11(2):299. doi: 10.3390/biom11020299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabir MS, Saleh YM, Sulaiman GM, et al. green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials (Basel). 2021;11(2):384. doi: 10.3390/nano11020384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad N, Sharma S, Alam MK, et al. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces. 2010;81(1):81–86. doi: 10.1016/j.colsurfb.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 20.Gomaa IE, Gaber SAA, Bhatt S, et al. In vitro cytotoxicity and genotoxicity studies of gold nanoparticles-mediated photo-thermal therapy versus 5-fluorouracil. J Nanoparticle Res. 2015;17(2):1–11. doi: 10.1007/s11051-015-2912-x [DOI] [Google Scholar]
- 21.Gruen LC. Interaction of amino acids with silver (I) ions. Biochimica Et Biophysica Acta (BBA)-Protein Structure. 1975;386(1):270–274. doi: 10.1016/0005-2795(75)90268-8 [DOI] [PubMed] [Google Scholar]
- 22.Tan YN, Lee JY, Wang DI. Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc. 2010;132(16):5677–5686. doi: 10.1021/ja907454f [DOI] [PubMed] [Google Scholar]
- 23.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13(10):2638–2650. doi: 10.1039/c1gc15386b [DOI] [Google Scholar]
- 24.Li S, Shen Y, Xie A, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852–858. doi: 10.1039/b615357g [DOI] [Google Scholar]
- 25.Burdușel A-C, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8(9):681. doi: 10.3390/nano8090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wypij M, Jędrzejewski T, Trzcińska-Wencel J, Ostrowski M, Rai M, Golińska P. Green synthesized silver nanoparticles: antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front Microbiol. 2021;12:888. doi: 10.3389/fmicb.2021.632505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartea ME, Francisco M, Soengas P, Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16(1):251–280. doi: 10.3390/molecules16010251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigamoney M, Shaik S, Govender P, Krishna S. African leafy vegetables as bio-factories for silver nanoparticles: a case study on Amaranthus dubius C Mart. Ex Thell. South African J Botany. 2016;103:230–240. doi: 10.1016/j.sajb.2015.08.022 [DOI] [Google Scholar]
- 29.Kota S, Dumpala P, Anantha RK, Verma MK, Kandepu S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatimah I, Aftrid ZHVI. Characteristics and antibacterial activity of green synthesized silver nanoparticles using red spinach (Amaranthus tricolor L.) leaf extract. Green Chem Letters Rev. 2019;12(1):25–30. doi: 10.1080/17518253.2019.1569729 [DOI] [Google Scholar]
- 31.Singh J, Upadhyay A, Prasad K, Bahadur A, Rai M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J Food Composition Analysis. 2007;20(2):106–112. doi: 10.1016/j.jfca.2006.08.002 [DOI] [Google Scholar]
- 32.Major N, Prekalj B, Perković J, Ban D, Užila Z, Ban SG. The effect of different extraction protocols on Brassica oleracea var. acephala antioxidant activity, bioactive compounds, and sugar profile. Plants. 2020;9(12):1792. doi: 10.3390/plants9121792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayaz FA, Hayırlıoglu-Ayaz S, Alpay-Karaoglu S, et al. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008;107(1):19–25. doi: 10.1016/j.foodchem.2007.07.003 [DOI] [Google Scholar]
- 34.Michalak M, Gustaw K, Waśko A, Polak-Berecka M. Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation. Microbiol Res. 2018;206:121–130. doi: 10.1016/j.micres.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 35.Becerra-Moreno A, Alanís-Garza PA, Mora-Nieves JL, Mora-Mora JP, Jacobo-Velázquez DA. Kale: an excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. Cyta-J Food. 2014;12(3):298–303. doi: 10.1080/19476337.2013.850743 [DOI] [Google Scholar]
- 36.Michalak M, Szwajgier D, Paduch R, Kukula-Koch W, Waśko A, Polak-Berecka M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. J Funct Foods. 2020;67:103866. doi: 10.1016/j.jff.2020.103866 [DOI] [Google Scholar]
- 37.Olsen H, Aaby K, Borge GIA. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. convar. acephala var. sabellica) by HPLC-DAD-ESI-MS n. J Agric Food Chem. 2009;57(7):2816–2825. doi: 10.1021/jf803693t [DOI] [PubMed] [Google Scholar]
- 38.Satheesh N, Workneh Fanta S. Kale: review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agriculture. 2020;6(1):1811048. doi: 10.1080/23311932.2020.1811048 [DOI] [Google Scholar]
- 39.Patra JK, Das G, Shin H-S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. /Int J Nanomedicine. 2019;14:6679. doi: 10.2147/IJN.S212614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: John Willey Spectrum;1993. [Google Scholar]
- 41.Ravikumar V, Gopal V, Sudha T. Analysis of phytochemical constituents of stem bark extracts of Zanthoxylum tetraspermum wight & arn. Res J Pharmaceutical, Biol Chem Sci. 2012;3(4):391–402. [Google Scholar]
- 42.Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Scientific World J. 2017;2017. doi: 10.1155/2017/5873648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patra JK, Baek K-H. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;8:167. doi: 10.3389/fmicb.2017.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385. [PMC free article] [PubMed] [Google Scholar]
- 45.Raj S, Mali SC, Trivedi R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem Biophys Res Commun. 2018;503(4):2814–2819. doi: 10.1016/j.bbrc.2018.08.045 [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Itoh H, Uemura T, Naka K, Chujo Y, Preparation of π-conjugated polymer-protected gold nanoparticles in stable colloidal form. Chem Commun. 2001;7:613–614. doi: 10.1039/b100636n [DOI] [Google Scholar]
- 47.Butala MA, Kukkupuni SK, Venkatasubramanian P, Vishnuprasad CN. An ayurvedic anti‐diabetic formulation made from Curcuma longa L. and Emblica officinalis L. inhibits α‐Amylase, α‐Glucosidase, and starch digestion, in vitro. Starch‐Stärke. 2018;70(5–6):1700182. doi: 10.1002/star.201700182 [DOI] [Google Scholar]
- 48.Naqvi SZH, Kiran U, Ali MI, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomedicine. 2013;8:3187. doi: 10.2147/IJN.S49284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patra JK, Baek K-H. Comparative study of proteasome inhibitory, synergistic antibacterial, synergistic anticandidal, and antioxidant activities of gold nanoparticles biosynthesized using fruit waste materials. Int J Nanomedicine. 2016;11:4691. doi: 10.2147/IJN.S108920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faedmaleki F, Shirazi FH, Salarian -A-A, Ashtiani HA, Rastegar H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iranian J Pharm Res. 2014;13(1):235. [PMC free article] [PubMed] [Google Scholar]
- 51.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Letters. 2012;2(1):32. doi: 10.1186/2228-5326-2-32 [DOI] [Google Scholar]
- 52.Kulkarni N, Muddapur U. Biosynthesis of metal nanoparticles: a review. J Nanotechnol. 2014;2014:1–8. doi: 10.1155/2014/510246 [DOI] [Google Scholar]
- 53.Frond AD, Iuhas CI, Stirbu I, et al. Phytochemical characterization of five edible purple-reddish vegetables: anthocyanins, flavonoids, and phenolic acid derivatives. Molecules. 2019;24(8):1536. doi: 10.3390/molecules24081536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kota S, Dumpala P, Anantha RK, Verma MK, Kandepu S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of. Rumex Acetosa Sci Rep. 2017;7(1):11566. doi: 10.1038/s41598-017-11853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moodley JS, Krishna SBN, Pillay K, Govender P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv Nat Sci: Nanosci Nanotechnol. 2018;9(1):015011. [Google Scholar]
- 56.Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Comprehensive Revi Food Sci Food Safety. 2018;17(3):512–531. [DOI] [PubMed] [Google Scholar]
- 57.He Y, Wei F, Ma Z, et al. Green synthesis of silver nanoparticles using seed extract of Alpinia katsumadai, and their antioxidant, cytotoxicity, and antibacterial activities. RSC Adv. 2017;7(63):39842–39851. doi: 10.1039/C7RA05286C [DOI] [Google Scholar]
- 58.Upadhyay P, Mishra SK, Purohit S, Dubey G, Singh Chauhan B, Srikrishna S. Antioxidant, antimicrobial and cytotoxic potential of silver nanoparticles synthesized using flavonoid rich alcoholic leaves extract of Reinwardtia indica. Drug Chem Toxicol. 2019;42(1):65–75. doi: 10.1080/01480545.2018.1488859 [DOI] [PubMed] [Google Scholar]
- 59.Kalaiyarasu T, Karthi N, Sharmila GV, Manju V. In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J Pharm Clin Res. 2016;9(1). [Google Scholar]
- 60.Ismail M, Gul S, Khan M, Khan MA, Asiri AM, Khan SB. Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange. Green Processing Synthesis. 2019;8(1):118–127. doi: 10.1515/gps-2018-0030 [DOI] [Google Scholar]
- 61.Jagtap U, Bapat V. Biosynthesis, characterization and antibacterial activity of silver nanoparticles by aqueous Annona squamosa L. leaf extract at room temperature. J Plant Biochem Biotechnol. 2013;22(4):434–440. doi: 10.1007/s13562-012-0172-8 [DOI] [Google Scholar]
- 62.Singh P, Pandit S, Beshay M, et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):S886–S899. doi: 10.1080/21691401.2018.1518909 [DOI] [PubMed] [Google Scholar]
- 63.Vadlapudi V, Amanchy R. Phytofabrication of silver nanoparticles using Myriostachya wightiana as a novel bioresource, and evaluation of their biological activities. Brazilian Arch Biol Technol. 2017;60. doi: 10.1590/1678-4324-2017160329 [DOI] [Google Scholar]
- 64.Singh H, Du J, Yi T-H. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells, Nanomed Biotechnol. 2017;45(7):1310–1316. doi: 10.1080/21691401.2016.1228663 [DOI] [PubMed] [Google Scholar]
- 65.Skandalis N, Dimopoulou A, Georgopoulou A, et al. The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo, on their antibacterial efficacy. Nanomaterials. 2017;7(7):178. doi: 10.3390/nano7070178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287(3):360–372. doi: 10.1001/jama.287.3.360 [DOI] [PubMed] [Google Scholar]
- 67.Chinnasamy G, Chandrasekharan S, Bhatnagar S. Biosynthesis of silver nanoparticles from Melia azedarach: enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int J Nanomedicine. 2019;14:9823. doi: 10.2147/IJN.S231340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Podsedek A, Majewska I, Redzynia M, Sosnowska D, Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J Agric Food Chem. 2014;62(20):4610–4617. doi: 10.1021/jf5008264 [DOI] [PubMed] [Google Scholar]
- 69.Nickavar B, Abolhasani L. Bioactivity-guided separation of an α-amylase inhibitor flavonoid from. Salvia Virgata Iranian J Pharm Res. 2013;12(1):57. [PMC free article] [PubMed] [Google Scholar]
- 70.Ullah S, Shah SWA, Qureshi MT, et al. Antidiabetic and hypolipidemic potential of green AgNPs against diabetic mice. ACS Appl Bio Mater. 2021;4(4):3433–3442. doi: 10.1021/acsabm.1c00005 [DOI] [PubMed] [Google Scholar]
- 71.Badmus J, Oyemomi S, Adedosu O, et al. Photo-assisted bio-fabrication of silver nanoparticles using Annona muricata leaf extract: exploring the antioxidant, anti-diabetic, antimicrobial, and cytotoxic activities. Heliyon. 2020;6(11):e05413. doi: 10.1016/j.heliyon.2020.e05413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Govindappa M, Hemashekhar B, Arthikala M-K, Rai VR, Ramachandra Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Physics. 2018;9:400–408. doi: 10.1016/j.rinp.2018.02.049 [DOI] [Google Scholar]
- 73.Balan K, Qing W, Wang Y, et al. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016;6(46):40162–40168. doi: 10.1039/C5RA24391B [DOI] [Google Scholar]
- 74.Kensler TW, Trush MA. Role of oxygen radicals in tumor promotion. Environ Mutagen. 1984;6(4):593–616. doi: 10.1002/em.2860060412 [DOI] [PubMed] [Google Scholar]
- 75.Kahl R. The dual role of antioxidants in the modification of chemical carcinogenesis. J Environ Sci Health Part C. 1986;4(1):47–92. [Google Scholar]
- 76.Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–111. doi: 10.1016/j.ijpharm.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 77.Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 78.Bhatnagar S, Kobori T, Ganesh D, Ogawa K, Aoyagi H. Biosynthesis of silver nanoparticles mediated by extracellular pigment from Talaromyces purpurogenus and their biomedical applications. Nanomaterials. 2019;9(7):1042. doi: 10.3390/nano9071042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Shan K, Shao X, et al. Nanotoxic effects of silver nanoparticles on normal HEK-293 cells in comparison to cancerous HeLa cell line. Int J Nanomedicine. 2021;16:753–761. doi: 10.2147/IJN.S289008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lashin I, Fouda A, Gobouri AA, Azab E, Mohammedsaleh ZM, Makharita RR. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules. 2021;11(3):341. doi: 10.3390/biom11030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139 [DOI] [PubMed] [Google Scholar]
- 82.Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites. 2009;60(4):405–412. doi: 10.3989/gya.010109 [DOI] [Google Scholar]
- 83.El-Seedi HR, El-Shabasy RM, Khalifa SA, et al. Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv. 2019;9(42):24539–24559. doi: 10.1039/C9RA02225B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajeshkumar S, Malarkodi C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorg Chem Appl. 2014;2014. doi: 10.1155/2014/581890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pugazhendhi A, Kumar SS, Manikandan M, Saravanan M. Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb Pathog. 2018;122:84–89. doi: 10.1016/j.micpath.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 86.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 87.Salomoni R, Léo P, Rodrigues M. Antibacterial activity of silver nanoparticles (AgNPs) in Staphylococcus aureus and cytotoxicity effect in mammalian cells. Substance. 2015;17:18. [Google Scholar]








