Abstract
Multidrug-resistant organisms (MDROs) represent a continuing healthcare crisis with no definitive solution to date. An alternative to antibiotics is the development of therapies and vaccines using biocompatible physical methods such as ultrashort pulsed (USP) lasers, which have previously been shown to inactivate pathogens while minimizing collateral damage to human cells, blood proteins, and vaccine antigens. Here we demonstrate that visible USP laser treatment results in bactericidal effect (≥ 3-log load reduction) against clinically significant MDROs, including methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum beta-lactamase (ESBL)-producing Escherichia coli. Bacillus cereus endospores, which are highly resistant to conventional chemical and physical treatments, were also shown to be effectively inactivated by USP laser treatment, resulting in sporicidal (≥ 3-log load reduction) activity. Furthermore, we demonstrate that administration of USP laser-inactivated E. coli whole-cell vaccines at dosages as low as 105 cfu equivalents without adjuvant was able to protect 100% of mice against subsequent lethal challenge. Our findings open the possibility for application of USP lasers in disinfection of hospital environments, therapy of drug-resistant bacterial infections in skin or bloodstream via pheresis modalities, and in the production of potent bacterial vaccines.
Keywords: ultrashort pulsed laser, multidrug-resistant organisms, bacterial spores, pathogen inactivation, vaccines
Subject category: Prevention and therapy
Introduction
The emergence of multidrug-resistant organisms (MDROs) is among the gravest challenges in healthcare and a prominent cause of mortality in hospitalized patients worldwide. Bacterial evolution has outpaced the development of new antibiotics, with some bacterial pathogens now resistant to all available antibiotics1–3. In addition to MDROs, spore-forming bacteria such as Bacillus and Clostridium species represent another important source of highly transmissible nosocomial and community-acquired infectious agents which are resistant to common chemical and physical treatments and can persist in the environment for long periods of time4–7. Thus, there is an urgent need for new, orthogonal strategies to control these resistant pathogens.
An alternative approach to antibiotic development is the use of physical methods to inactivate pathogens in the context of therapy and vaccine production. Such a physical technique can ostensibly be applied to treat superficial infections or be coupled with a pheresis-like system to treat bloodstream infections8,9, while inactivated pathogens can serve as whole-cell vaccines10. Unfortunately, the existing physical methods including ultraviolet (UV) radiation, gamma-rays, X-rays, and heating cause extensive collateral damage to human proteins and nucleic acids, making them unsuitable for use in vivo11–18. The ideal physical method for clinical pathogen inactivation would be a simple one-step treatment process that inactivates a broad spectrum of pathogens, without the need to introduce chemical or biological agents. Furthermore, the method should not involve ionizing radiation or thermal heating which damage biomolecular structures. In these regards, ultrashort pulsed (USP) lasers represent a promising new approach for pathogen inactivation in the clinical setting8. Visible USP laser light has been shown to inactivate viruses, bacteria, mycoplasma, and fungi with minimal collateral damage to human cells and blood proteins8,10,19–27. The selective effect of USP lasers against microorganisms results from a unique inactivation mechanism, impulsive stimulated Raman scattering (ISRS), which kills microorganisms through forced mechanical vibration8,21,23. These properties make USP laser irradiation an attractive technology for clinical translation. In this report, we demonstrate the efficacy of USP laser treatment against clinically-important MDROs and against bacterial spores, for which few current therapies are effective. Furthermore, we demonstrate the potency and efficacy of a chemical-free USP laser-inactivated bacterial vaccine in mice.
Materials and Methods
Bacterial strains and culture.
MRSA USA400 was received from Dr. Juliane Bubeck Wardenburg (Washington University School of Medicine, St Louis, MO). ESBL-producing E. coli ATCC 51446 was obtained from Sonora Quest Laboratories (Tempe, AZ, USA) and confirmed resistant to 11 antibiotics28. E. coli strain 25922 was purchased from the American Type Culture Collection (ATCC, Manassas, VA). MRSA and ESBL E. coli were cultured in trypticase soy broth and Luria-Bertani (LB) broth, respectively, to mid-logarithmic growth phase at 37°C. Glycerol stocks were generated by addition of 50% glycerol to bacterial samples after overnight culture and frozen at −80°C until use. Bacillus cereus ATCC 14579 cultures were grown with gentle agitation in nutrient broth for 24 h at 30°C. Saturated cultures were inoculated onto nutrient agar and incubated for 48 h at 37°C to induce sporulation29,30. The agar plates were flooded with 0.9% NaCl (saline), and spores were scraped from the agar surface, collected into microcentrifuge tubes, and stored at 4°C. Differential staining with malachite green and safranin and microscopic visualization were used to confirm sporulation. For experiments with E. coli 25922, bacteria were inoculated from frozen glycerol stocks into tubes containing LB media and grown at 37°C with shaking until an optical density (OD) of 0.5 was reached. Enumeration was performed via standard colony forming assays. Briefly, bacterial samples or aliquots thereof were plated on LB agar, inverted and incubated at 37°C for 18 hours. Colony counts were obtained manually. A standard curve was constructed to correlate OD with bacterial counts as determined by plating.
For USP laser treatment studies, MRSA and ESBL E. coli were pelleted, washed, and resuspended in sterile saline to cell densities of approximately 108 colony forming units (cfu)/mL. B. cereus was prepared in sterile saline with approximate concentrations of 108 spores/mL. Prior to USP laser treatment, bacterial and spore samples (100 μL volumes) were stored on ice.
Animals.
Six-week old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in designated animal facilities, fed ad libitum and inspected regularly. All animal studies were approved by the Washington University School of Medicine Animal Studies Committee (protocol number 19-0814) and performed in strict accordance with humane care and use of research animals.
USP laser treatment.
Inactivation of MDR bacteria and bacterial spores:
The excitation source used in this work was described previously9. A diode-pumped continuous wave (CW) mode-locked Ti-sapphire laser produced a continuous train of 60 fs pulses at a repetition rate of 80 MHz. The output of the second harmonic generation (SHG) system of the Ti-sapphire laser was used to treat each sample. The excitation laser operated at a wavelength of λ = 420 ± 5 nm and with an average power of approximately 150 mW. It has a pulse width of full-width at half maximum (FWHM) = 100 fs. An achromatic lens was used to focus the laser beam into a spot about 100 μm in diameter within the sample volume. Each bacterial or spore sample with a volume of about 0.1 ml, which was placed inside a glass vial, was subjected to gentle magnetic stirring and USP laser irradiation at room temperature (22°C), followed by incubation on ice. Control samples not subjected to USP laser treatment were similarly incubated at room temperature. Cell viability was determined by plating duplicate 10-fold serial dilutions for each sample on trypticase soy agar or LB agar for MRSA and ESBL E. coli, respectively, and enumerating cfu after incubating plates overnight at 37°C. For B. cereus, samples were subjected to duplicate 10-fold serial dilutions, inoculated onto nutrient agar, and incubated overnight at 30°C.
Generation of inactivated E. coli vaccines:
In order to expedite the production of large volumes of bacteria at high titers needed for vaccine experiments in mice, we employed a more powerful USP laser system which produces a train of ultrashort laser pulses with the pulse width, wavelength and repetition rate comparable with the one described above but with a much larger average power of 1.65 W. In addition, the experimental configuration was switched from the glass vial with a magnetic stirrer to a continuous-flow configuration, where the sample was passed through tubes via peristaltic pump to enable treatment by the laser beam. Samples of E. coli 25922 at 5×108 cfu/ml in PBS were inactivated by USP laser irradiation and stored at 4°C prior to immunization studies. Cell viability was determined by plating duplicate 10-fold serial dilutions for each sample on LB agar and enumerating cfu after incubating plates overnight at 37°C.
Immunization experiments.
Three groups of mice (n=3 per group) were immunized twice at 3-week intervals via i.p. injection of USP laser-inactivated E. coli 25922 bacteria at 107, 106, or 105 cfu equivalents suspended in 100 μL PBS, respectively, while a control group (n=5) was injected with placebo (100μL PBS). To establish the peritoneal infection model, 3 weeks after the last immunization the mice were infected via i.p. injection of 1×107 cfu/mouse live E. coli suspended in 100μL PBS. During the first 24 hours post-infection, mice were monitored approximately every 4 hours for signs of morbidity including behavioral changes associated with sepsis (agitation/lethargy, shivering, piloerection), ruffling of fur, and reduced responsiveness to stimuli. After the initial 24 hours, mice were inspected twice daily. Mice deemed to be terminally moribund according to these observed signs were considered having reached the mortality endpoint and were sacrificed by cervical dislocation under isoflurane anesthesia. Survival of mice over time was recorded and plotted via Kaplan-Meier analysis (GraphPad Prism).
Statistical analyses.
Differences between mean cfu of control vs. laser-treated bacteria were analyzed by unpaired Student’s t-test. A p value of <0.05 was used as a threshold for statistical significance. Survival of vaccinated vs. control mice subsequent to live bacterial challenge was compared by standard Kaplan-Meier analyses (GraphPad Prism).
Results
Prototypical MDROs are efficiently inactivated by USP laser treatment.
MRSA and ESBL-producing E. coli represent clinically significant and prevalent MDROs in healthcare settings today. We exposed these bacteria to visible USP laser treatment and assessed the effects of the laser treatment on bacterial viability. As shown in Fig. 1A and 1B, USP laser treatment resulted in significant ~3.5 log (p < 0.0001) and ~3 log (p = 0.0442) reductions in viable MRSA and beta-lactamase-producing E. coli, respectively, revealing bactericidal (≥ 99.9% reduction) activity.
Figure 1. Inactivation of MDROs by USP laser treatment.

Samples of MRSA USA400 and ESBL-producing E. coli ATCC 51446 were exposed to USP laser treatment for 90 min. Initial cfu (filled circles) were quantified prior to laser exposure. Control samples (filled squares) were incubated at similar temperatures in parallel with USP laser-treated samples (filled red triangles), but were not exposed to the USP laser. The dotted horizontal line represents the limit of detection. The solid horizontal bars represent the average of the respective data. The error bars represent the SEM from three experiments. *, p = 0.0442; ****, p < 0.0001; unpaired Student’s t-test.
Bacterial spores are efficiently inactivated by USP laser treatment.
B. cereus is a saprophytic, Gram-positive, spore-forming bacterium associated with toxin-mediated foodborne illnesses. To assess sporicidal inactivation, we exposed B. cereus endospores to visible USP laser treatment. Fig. 2 shows that USP laser treatment resulted in an average 3.8 log reduction (p = 0.0001) in B. cereus endospores, revealing sporicidal (≥ 99.9% reduction) activity.
Figure 2. Inactivation of bacterial endospores by USP laser treatment.
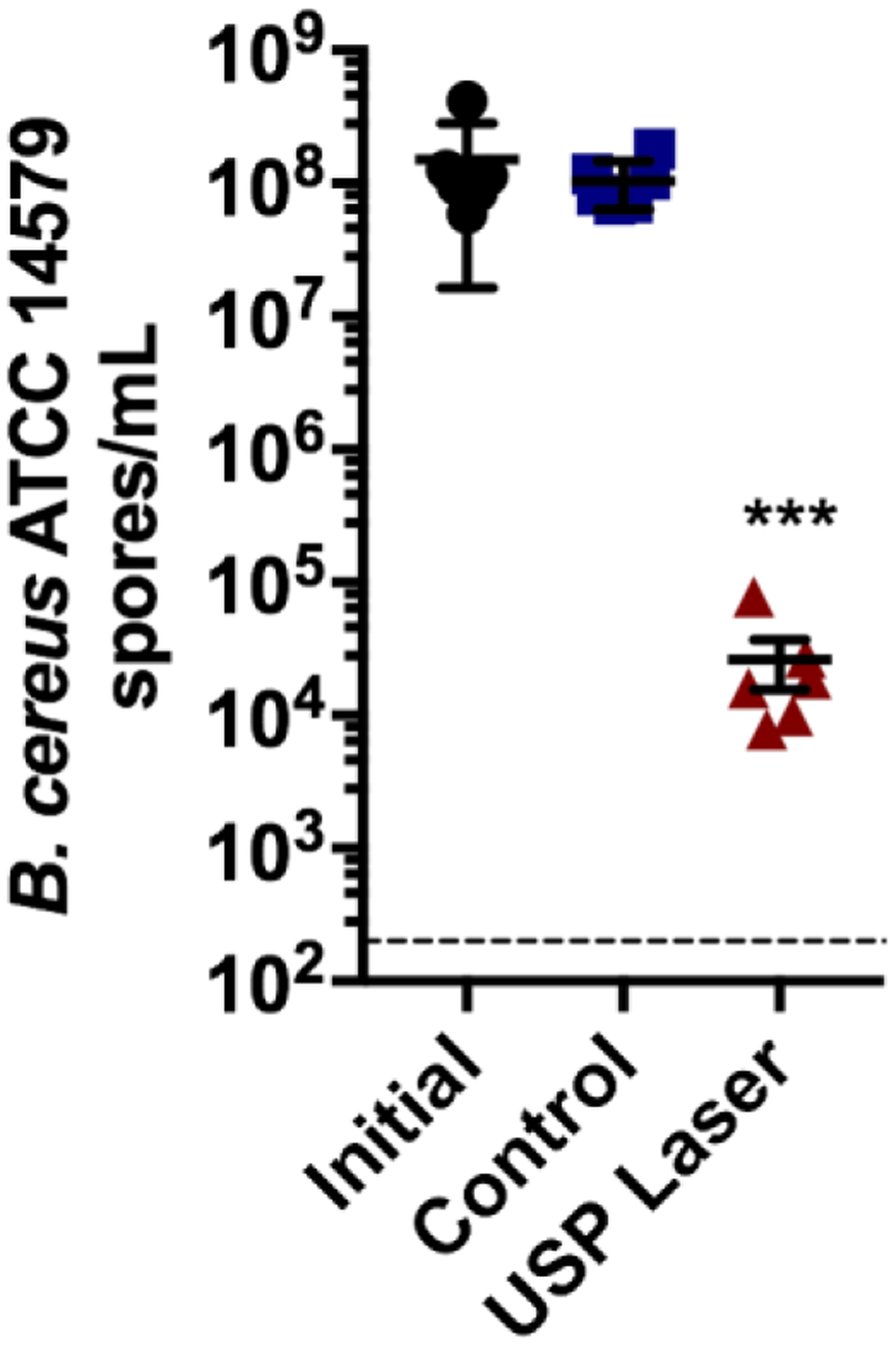
Samples of B. cereus ATCC 14579 spores were exposed to USP laser treatment for 90 min. Initial spores (filled black circles) were quantified prior to laser exposure. Control samples (filled blue squares) were incubated at similar temperatures in parallel with USP laser-treated samples (filled red triangles), but were not exposed to the USP laser. The dotted horizontal line represents the limit of detection. The solid horizontal bars represent the average of the respective data. The error bars represent the SEM from six experiments. ***, p = 0.0001; unpaired Student’s t-test.
USP laser-inactivated E. coli vaccines at low dosages protect mice against lethal challenge.
Samples of E. coli strain 25922 were inactivated by USP laser treatment and complete inactivation was confirmed via plating. The inactivated bacteria were administered intraperitoneally (i.p.) to C57BL/6 mice twice at a 3-week interval at dosages of 107, 106, or 105 cfu equivalents per mouse, while controls received placebo (PBS). Three weeks after the second vaccine dose, the mice were challenged i.p. with a lethal dose of live E. coli 25922. As shown in Fig. 3, all mice in the control (placebo) group exhibited terminal morbidity or mortality within 28 hours of challenge. In contrast, mice that received USP laser-inactivated vaccine showed complete protection against lethal challenge, even with vaccine dosages as low as 105 cfu equivalents/dose. Our additional experimental data indicate that, in order to completely protect the mouse against lethal challenge, two doses of vaccination with 1 × 105 cfu/dose is required. 1 × 105 cfu/dose is the lower limit for complete protection. Lower dosages such as 1 × 104 cfu/dose and 1 × 103 cfu/dose are inefficient. As a reference, bacterial vaccines have typically required dosages on the order of 108-109 cfu equivalents for protection using traditional inactivation methods31–33.
Figure 3. Protection of mice against E. coli infection by USP laser-inactivated E. coli vaccines.

Mice were immunized twice i.p. at 3-week intervals with different dosages of USP laser-inactivated E. coli 25922 as indicated (107, 106, or 105 cfu equivalents), or received placebo (control). Three weeks after the final vaccine dose, mice were challenged i.p. with 107 cfu live E. coli 25922. Survival of mice over time after live bacterial challenge is depicted by Kaplan-Meier analysis.
Discussion
The inactivation mechanism for viral pathogens via USP laser irradiation through ISRS-driven protein aggregation has been established previously8,21,23. ISRS process in a single USP laser configuration can excite Raman-active vibrational modes and break bonds in a molecule34. The electric field of the electromagnetic wave from the laser induces a polarization in the molecule (i.e., a protein or the capsid of a virus). The induced polarization can store energy which is like charging of a capacitor. It is the variation of this energy with configuration coordinate that produces an impulsive force that drives the damped harmonic oscillators in the molecule.
When the photons in an ultrashort pulsed laser interact with a molecule such as a pathogen or a protein, they can excite low-frequency Raman-active vibrations on the molecule through ISRS process34. As the amplitude of vibration is sufficiently large, hydrogen bonds/hydrophobic contacts within the molecule will be broken. If there are no similar molecules close by, these broken hydrogen bonds/hydrophobic contacts will be reformed very rapidly to their original configuration following the passage of photons at room temperature. On the other hand, if there are similar excited molecules nearby, the broken hydrogen bonds/hydrophobic contacts in the molecules, instead of getting back to their original configurations, can become cross-linked and thus the molecules become aggregated. Therefore, the ISRS process can cause density-dependent aggregation of molecules.
For enveloped viruses, bacteria and fungi and their spores, a lot of proteins are very tightly packed within them. When such pathogens are excited with an USP laser through the ISRS process, there is a large probability that the broken hydrogen bonds/hydrophobic contacts in these proteins, instead of getting back to their original configurations, can become cross-linked and therefore the proteins are aggregated. These aggregated proteins will lose their functionality because the functionality of a protein is intimately related to its structure. This aggregation process induced by the USP laser irradiation leads to the inactivation of the enveloped viruses and bacteria23,35. The inactivated pathogens remain a whole particle due to inefficiency of the laser to disrupt the envelope – a layer primarily composed of fatty acids. Furthermore, the surface proteins of the pathogens are immune from this aggregation effect due to the fact that they are microscopically, relatively apart from each other.
For non-enveloped viruses, the protein aggregation effect for the enveloped viruses, bacteria and fungi described above will be active. In addition, there is one more effect induced by the ISRS process. The capsid, which is formed by connecting subunit proteins through weak hydrogen bonds, can be disrupted by the ISRS process, leading to the disintegration of the capsid and the inactivation of non-enveloped viruses27.
We now address the likely mechanisms of the observed inactivation of Staphylococcus aureus, Escherichia coli and bacterial spores by the USP laser irradiation, and provide an explanation for the observed high potency of the USP laser-generated bacterial vaccine.
Lu et al. previously reported the inhibition of Escherichia coli respiratory enzymes by ultrashort visible femtosecond laser irradiation35. Based on protein gel electrophoresis studies of Escherichia coli with or without USP laser treatment, they attributed the inactivation of Escherichia coli to protein aggregation induced by the USP laser irradiation, a phenomenon consistent with prior viral studies23. Gel electrophoresis experiments on bacteria have been performed for average laser powers: 150 mW and 1.65 W, respectively. The results (which are not shown) are qualitatively similar to that reported in Ref. 35, indicating that protein aggregation process prevails when bacteria are irradiated by femtosecond lasers under our experimental conditions.
As such, the most likely mechanism for the inactivation of Staphylococcus aureus and Escherichia coli observed in our work is protein aggregation within Staphylococcus aureus and Escherichia coli induced by the USP laser irradiation through the ISRS process. Within pathogens such as enveloped viruses, bacteria, and bacterial spores, there exist structures comprising proteins at high density (i.e. very tightly packed within confined spaces)8,23,35. When such pathogens are excited with an USP laser through the ISRS process, there is a high probability of protein aggregation leading to loss of protein function. The inactivated pathogens remain as a whole particle due to inability of the laser to disrupt the viral envelope or bacterial cell membrane, a layer primarily composed of lipids. Furthermore, soluble proteins and many of the surface proteins of pathogens are expected to have reduced susceptibility to the abovementioned aggregation effect due to the fact that they are separated by longer distances at the microscopic level.
A secondary mechanism which may contribute to the overall observed inactivation may arise due to the presence of light-absorbing chromophores (such as porphyrins) in certain species of bacteria. We previously reported the inactivation of Salmonella typhimurium by USP laser irradiation and found that the killing efficacy for Salmonella typhimurium with a deletion in a DNA-repair enzyme was slightly greater than that for Salmonella typhimurium without the deletion21. This suggested that DNA damage due to excitation of porphyrins might account partially for the bacterial inactivation effect by USP laser irradiation. DNA damage was not observed previously in USP laser-inactivated viral particles, which are expected to lack such chromophores23,24.
The aggregation of specific intracellular proteins, and the preservation of whole bacterial particles after USP laser irradiation, provide a plausible explanation for the high potency of USP laser-inactivated bacterial vaccines. We previously showed that USP laser-inactivated influenza virus showed complete preservation of its spike protein, hemagglutinin, which serves as the major antigen for effective vaccine-elicited immune responses10. This USP laser-inactivated influenza virus vaccine was shown to have at least 10-fold greater potency relative to the formaldehyde-inactivated vaccine. Viral capsid and matrix proteins in the interior of enveloped virions have been shown to be aggregated by USP laser treatment23. Aggregation is well-known to increase the immunogenicity of proteins, a phenomenon that may lead to augmented immune responses elicited by such USP laser-generated vaccines36,37.
In order to get better insight on why the bacterial vaccine prepared by the USP laser treatment elicits strong immune reactions, experiments involving more mice per group and a comprehensive analysis of humoral and cellular immune response induced by the potent bacterial vaccine are being planned.
Conclusions
In this report we demonstrate that methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum beta-lactamase (ESBL)-producing Escherichia coli, as well as Bacillus cereus endospores which are highly resistant to conventional chemical and physical treatments, are effectively inactivated by USP laser treatment. These results open the possibility for USP laser disinfection of hospital environments, and superficial or pheresis-coupled bloodstream therapy of drug-resistant bacterial infections. We have also shown that immunization of mice with USP laser-inactivated E. coli vaccines was able to protect 100% of the mice against lethal challenge without the use of adjuvants. This finding paves the way for the production of chemical-free and highly potent bacterial vaccines via USP laser irradiation.
ACKNOWLEDGEMENTS
We would like to thank Dr. Juliane Bubeck Wardenburg of Washington University School of Medicine for providing MRSA USA400 bacteria. This research was supported by ASU investigator incentive funding to S.E.H.
Footnotes
CONFLICTS OF INTEREST
SDT and KT hold patents on “System and method for inactivating microorganisms with a femtosecond laser” (publication no. US20080299636 A1).
ETHICAL STATEMENT
This manuscript complies with the Committee on Publication Ethics (COPE) Code of Conduct and with the originality and authorship requirements of this Journal.
REFERENCES
- 1.Falagas ME & Bliziotis IA Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents 29, 630–636, doi: 10.1016/j.ijantimicag.2006.12.012 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Martens E & Demain AL The antibiotic resistance crisis, with a focus on the United States. J Antibiot (Tokyo) 70, 520–526, doi: 10.1038/ja.2017.30 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Brown ED & Wright GD Antibacterial drug discovery in the resistance era. Nature 529, 336–343, doi: 10.1038/nature17042 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Salton MRJ & Kim KS in Medical Microbiology (ed Baron S) (University of Texas Medical Branch at Galveston, 1996). [PubMed] [Google Scholar]
- 5.Setlow P Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101, 514–525, doi: 10.1111/j.1365-2672.2005.02736.x (2006). [DOI] [PubMed] [Google Scholar]
- 6.Russell AD Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev 3, 99–119, doi: 10.1128/cmr.3.2.99 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagripanti JL & Bonifacino A Bacterial spores survive treatment with commercial sterilants and disinfectants. Appl Environ Microbiol 65, 4255–4260 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsen SW & Tsen KT Selective photonic disinfection: a ray of hope in the war against pathogens. (Morgan & Claypool Publishers, 2016). [Google Scholar]
- 9.Tsen SW, Wu TC, Kiang JG & Tsen KT Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation. J Biomed Sci 19, 62, doi: 10.1186/1423-0127-19-62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsen SW et al. Chemical-free inactivated whole influenza virus vaccine prepared by ultrashort pulsed laser treatment. Journal of biomedical optics 20, 051008, doi: 10.1117/1.JBO.20.5.051008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douki T & Cadet J Individual determination of the yield of the main UV-induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions. Biochemistry 40, 2495–2501 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Gaber MH Effect of gamma-irradiation on the molecular properties of bovine serum albumin. Journal of bioscience and bioengineering 100, 203–206, doi: 10.1263/jbb.100.203 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Seo JH et al. Ovalbumin modified by gamma irradiation alters its immunological functions and allergic responses. International immunopharmacology 7, 464–472, doi: 10.1016/j.intimp.2006.11.012 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Terpstra FG et al. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion 48, 304–313 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Wei H, Cai Q, Rahn R & Zhang X Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free radical biology & medicine 23, 148–154 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Burnouf T & Radosevich M Reducing the risk of infection from plasma products: specific preventative strategies. Blood reviews 14, 94–110, doi: 10.1054/blre.2000.0129 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Chan HL et al. Proteomic analysis of UVC irradiation-induced damage of plasma proteins: Serum amyloid P component as a major target of photolysis. FEBS letters 580, 3229–3236 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Verhaar R et al. UV-C irradiation disrupts platelet surface disulfide bonds and activates the platelet integrin IIb 3. Blood 112, 4935 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsen K, Tsen SWD, Hung CF, Wu T & Kiang JG Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses. Journal of Physics: Condensed Matter 20, 252205 (2008). [Google Scholar]
- 20.Tsen KT et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. Journal of biomedical optics 14, 064042, doi: 10.1117/1.3275477 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Tsen KT et al. Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation mechanisms. Journal of biomedical optics 16, 078003, doi: 10.1117/1.3600771 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Tsen KT, Tsen SWD, Sankey OF & Kiang JG Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses. Journal of Physics: Condensed Matter 19, 472201 (2007). [Google Scholar]
- 23.Tsen SW et al. Inactivation of enveloped virus by laser-driven protein aggregation. Journal of biomedical optics 17, 128002, doi: 10.1117/1.JBO.17.12.128002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsen SW et al. Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus. Antiviral research 110, 70–76, doi: 10.1016/j.antiviral.2014.07.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsen SW et al. Selective photonic disinfection of cell culture using a visible ultrashort pulsed laser. Journal of Selected Topics in Quantum Electronics In Press (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsen SW et al. Pathogen reduction in human plasma using an ultrashort pulsed laser. PloS one 9, e111673, doi: 10.1371/journal.pone.0111673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsen SW et al. Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser. Virology journal 11, 20, doi: 10.1186/1743-422X-11-20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haydel SE, Remenih CM & Williams LB Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. J Antimicrob Chemother 61, 353–361, doi: 10.1093/jac/dkm468 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman WH, Zhang P, Li YQ & Setlow P Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50, 507–514, doi: 10.1111/j.1472-765X.2010.02827.x (2010). [DOI] [PubMed] [Google Scholar]
- 30.Raso J, Barbosa-Canovas G & Swanson BG Sporulation temperature affects initiation of germination and inactivation by high hydrostatic pressure of Bacillus cereus. J Appl Microbiol 85, 17–24, doi: 10.1046/j.1365-2672.1998.00460.x (1998). [DOI] [PubMed] [Google Scholar]
- 31.Arshadi N, Mousavi SL, Amani J & Nazarian S Immunogenic Potency of Formalin and Heat Inactivated E. coli O157:H7 in Mouse Model Administered by Different Routes. Avicenna J Med Biotechnol 12, 194–200 (2020). [PMC free article] [PubMed] [Google Scholar]
- 32.Baqar S, Applebee LA & Bourgeois AL Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun 63, 3731–3735, doi: 10.1128/iai.63.9.3731-3735.1995 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gohar A, Abdeltawab NF, Fahmy A & Amin MA Development of safe, effective and immunogenic vaccine candidate for diarrheagenic Escherichia coli main pathotypes in a mouse model. BMC Res Notes 9, 80, doi: 10.1186/s13104-016-1891-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Y-X, Gamble EB & Nelson KA Impulsive stimulated scattering: General importance in femtosecond laser pulse interactions with matter, and spectroscopic applications. J Chem Phys 83 (1985). [Google Scholar]
- 35.Lu C-H, Lin K-H, Hsu Y-Y, Tsen KT & Kuan Y-S Inhibition of Escherichia Coli respiratory enzymes by short visible femtosecond laser irradiation. Journal of Physics D: Applied Physics 47, 315402 (2014). [Google Scholar]
- 36.Moussa EM et al. Immunogenicity of Therapeutic Protein Aggregates. J Pharm Sci 105, 417–430, doi: 10.1016/j.xphs.2015.11.002 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Ratanji KD, Derrick JP, Dearman RJ & Kimber I Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol 11, 99–109, doi: 10.3109/1547691X.2013.821564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


