Abstract
The main reason for the growth of mucormycosis in people with Coronavirus disease-2019 (COVID-19) is mainly produced by Rhizopus spp. The infective mechanisms and issues recognized in Rhizopus spp. are the cell wall, germination proteins, and enzymes assisted to iron sequestration, CotH protein, and positive regulation of the GRP78 cell receptor. Mucormycosis is mainly caused by the Rhizopus spp. such as R. oryzae, R. microsporus, R. arrhizus, R. homothallicus, etc. that are gifted to numerous host defense mechanisms and attribute to the endothelium via specific receptors, GRP78 simplifying their endocytosis and angio-invasion. Factors such as hyperglycemia, elevated iron concentrations, and ketoacidosis have been shown to contribute to the pathogenesis in the tentative situation. The analytical data of ‘black fungus disease’ or ‘mucormycosis’, specify India reported for about 42.3% of published cases, followed by the USA about 16.9%, Iraq, Bangladesh, Iran, Paraguay, and 1 case each from Brazil, Mexico, Italy, UK, China, France, Uruguay, Turkey, and Austria. The COVID-19 infection is maybe a predisposing factor for mucormycosis and is related to a high mortality rate. Early recognition and restriction of hyperglycemia, liposomal amphotericin B, and surgical debridement are the bases in the successful managing of mucormycosis.
Keywords: Corticosteroid, COVID-19, Diabetics, Mucormycosis, Rhizopus
1. Introduction
Mucormycosis is an uncommon angio obtrusive disease principally perceived in immunocompromised patients which happens because of the growth of mucorales.1 The term ‘Mucormycosis’ was instituted by an American pathologist R. D. Baker and it can likewise be called Zygomycosis. Mucormycotina falls under the normal saprobes which are found in bad organic matter or soil. Infections are designated by instantaneous progression.2 The Mucorales are not demanding creatures, they develop at temperature ranges between 25 °C and 55 °C.1 Being ubiquitous organisms, Mucorales are dominant in commencing and accelerating the decay of organic materials. Since openness to spores of these growths is unavoidable, the uncommonness of the diseases is harmful and is a validation of an extremely basic inclination.3
The initially announced instance of mucormycosis traces back to 1885 when the German pathologist Paltauf depicted the primary case as Mycosis Mucorina.4 The pace of mucormycosis expanded mostly in immunocompromised individuals subsequently in the 1980s–1990s.2
Different types of mucormycosis that can be associated with COVID-19 infection are, rhino-cerebral mucormycosis, pulmonary mucormycosis, gastrointestinal mucormycosis, cutaneous mucormycosis, and miscellaneous. For the region of the head and neck, mucormycosis can be assorted into isolated nasal, rhino-orbital, or rhino-orbital-cerebral mucormycosis. In the case of sino-orbital mucormycosis, the mold mainly enters via the respiratory tract and is containing the nose and sinuses, into the orbital and intracranial structures with the possibility of further progression.5 , 6 Pulmonary mucormycosis is a lethal aggressive fungal infection. It typically infects immunocompromised patients. Transbronchial biopsies and Bronchial alveolar lavage (BAL) are usually explained as non-septated hyphae in the case of pulmonary mucormycosis.7 Mucormycosis in the gastrointestinal (GI) tract occurs due to the ingestion of the spores of the fungus. It is rarely reported in the COVID-19 patient.8 Patients with persistent skin maceration or skin barrier disruptions (catheter insertion, trauma, injections, burn) are suitable for increasing the risk of cutaneous mucormycosis.9 The fungus can invade into adjacent fat, fascia, muscle, and even bone, while hematogenous spread with secondary vascular invasion is fewer common.10 , 11 However, hematogenous dissemination with cutaneous mucormycosis has high fatality rates.12
From the perspective of disease to the immunocompromised people, mucormycosis likewise create a high danger for the patient determined to have serious COVID-19 pneumonia. This happens because of the hospitalized status, previous comorbidities, and treatment regimens comprising of steroids and generally anti-toxins.13 , 14 The predominance of mucormycosis in India is approximately 0.14 cases per 1000 populace, about multiple times the pervasiveness in different countries.15 COVID-19 contamination has been related to parasitic diseases.16 Globally, the most well-known danger factor related to mucormycosis is diabetes mellitus. In the prevalence of the COVID-19 pandemic, it is believed that this drop in resistance could be set off to these instances of mucormycosis.17
2. Mucormycosis as COVID-19's deadly companion
The aggravation of COVID-19 in 2020 has effectively crushed the entire world in its first wave, where an enormous number of cases have been noticed including deaths and deterioration. The destruction proceeds in 2021 in the period of second-wave, more in the most exceedingly terrible structure.18 The flood of COVID-19 in its subsequent wave has additionally left a path of infection and deaths, where the ‘black fungus disease’ or ‘mucormycosis’ went with. Mucormycosis is a rare but severe compilation of COVID-19, which may lead to a threat to life.19 , 20
Up to May of 2021, we have dissected around 59 instances of mucormycosis throughout the world, related to the second wave of COVID-19 (Fig. 1 ). The analytical data of “black fungus disease” specify India with a report of about 42.3% published cases (25/59), followed by the United States of America (10/59), Iraq (5/59), Bangladesh (4/59), Iran (4/59), Paraguay (2/59), and 1 case each from Brazil, Mexico, Italy, UK, China, France, Uruguay, Turkey, and Austria (Table 1 ). Most patients who torment this obstruction of mucormycosis had some significant comorbidity, by and large, diabetes mellitus, and diabetes ketoacidosis yet contaminations in immunocompetent patients have moreover been conceived.21 , 24
Fig. 1.
A global presentation of the number of published case reports of Coronavirus disease 2019 (COVID-19) associated mucormycosis (till May 2021). (a) The color gradient segment of the map indicates the number of absolute cases reported worldwide, where the dark-colored portion represents the higher number of cases, while the light-colored portion represents a smaller number. (b) A schematic presentation showing the variation of the number of reported cases in different countries.20, 21, 22, 23
Table 1.
A brief number of cases of Coronavirus disease 2019 (COVID-19) associated mucormycosis reported worldwide.
| Reported Area | Total No. of case | Age/Sex | Underlying Disease |
Disease Type | Verified COVID-19 | Medicine used for COVID-19 | Fungal culture | Clinical Outcomes | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| DM/DKA/T1DM/T2DM | Cancer | |||||||||
| India | 25 | 23–78 M-22 F-3 |
DM-24 (32–78) No- 67 M |
No All | Rhino-orbital: 23, 60 Rhino-orbital-cerebral: 40, 38, 51, 45, 56, 78, 67, 56, 37 Rhino-sinusitis: 43, 64, 49, 59 M, 59F Pulmonary: 55, 32, 43, 72 Sino-orbital: 38 Paranasal: 32 |
Confirmed | Steroid-51, 37, 43, 56, 78, 49, 60, 55, 38, 64, 60, 59F, 72 Tocilizumab-51, 37, 60 Remdisivir-32 M, 51, 37, 43, 56, 49, 55, 62 38, 67, 72, 38, 45 Not applied: 32F, 40, 23 |
Positive (Rhizopus spp.) | Expired-10 Recovered-13 Unchanged-2 |
5,25,26 |
| Bangladesh | 3 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Link 1 |
| USA | 10 | 33–79 M-8 F-2 |
DM-36, 48, 79, 68 DKA-36, 48, 33, 41 T1DM-60, 41 T2DM-44 No-49, 56 |
No- 9 (44F Yes) | Rhino-orbital: 33, 60 Rhino-orbital cerebral: 36, 48 Pulmonary: 44, 49, 79, 56 Rhino-cerebral: 41 Cutaneous: 68 |
Confirmed | Steroid- 36, 44, 48, 49, 60, 41, 79, 56, 68 Tocilizumab-33, 56 Remdesivir-36, 44, 48, 49, 60, 79 |
Positive all (79 M & 44F Aspergillus sp.) | Expired-6 Recovered-3 Unchanged-1 |
12,14,20,27, 28, 29, 30, 31, 32 |
| UK | 1 | 22 M-1 |
No | No | Pulmonary | Confirmed | Not applied | Positive | Expired | 33 |
| Brazil | 1 | 86 M-1 |
No | No | Gastrointestinal | Confirmed | Not applied | Positive (Rhizopus spp.) | Expired | 8 |
| Italy | 1 | 66 M-1 |
No | No | Pulmonary | Confirmed | Not applied | Positive (Rhizopus spp.) | Expired | 34 |
| France | 1 | 55 M-1 |
No | Yes | Pulmonary | Confirmed | Not applied | Positive (Aspergillus spp.) | Expired | 35 |
| Iran | 4 | 40–61 M-2 F-2 |
DM-44, 54 DKA-No T1DM-No No-40, 61 |
No-All | Rhino-orbital: 61, 54 Rhino-orbital cerebral: 40 Rhino-sinusitis: 44 |
Confirmed | Steroid- 40, 44, 54,61 Tocilizumab-No Remdesivir-40, 54 |
Positive (Rhizopus spp.) | Expired-2 Recovered-2 Unchanged-No |
24,36,37 |
| China | 1 | 32 F-1 |
No | No | Rhino-cerebral | Confirmed | Not applied | Positive | Expired | 23 |
| Mexico | 1 | 24 F-1 |
DM-No DKA-24 T1DM-No |
No | Rhino-orbital | Confirmed | Not applied | Positive (Rhizopus spp.) | Expired | 38 |
| Austria | 1 | 53 M-1 |
No | Yes | Pulmonary | Confirmed | Not applied | Positive (Rhizopus spp.) | Expired | 39 |
| Turkey | 1 | 56 F-1 |
DM-56 DKA-56 T1DM-No |
No | Rhino-orbital sinusitis | Confirmed | Steroid- 56 Tocilizumab-No Remdesivir-No |
Positive (Rhizopus spp.) | Expired | 40 |
| Uruguay | 1 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Recovered | Link 2 |
| Paraguay | 2 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Link 3 |
| Iraq | 5 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Link 4 |
| Chile | 1 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Link 5 |
M: Male, F: Female, DM: Diabetes mellitus, DKA: Diabetic ketoacidosis, T1DM: Type 1 diabetes mellitus, T2DM: Type 2 diabetes mellitus, COVID-19: Coronavirus disease 2019.
As indicated by several reports till September of 2021, India has been accounted for by 45,435 instances of mucormycosis and is crumbling step by step. The black fungus cases are on the skyscraper in Gujarat alongside Maharashtra with around 7109 and 10,139 cases respectively and around 1336 deaths in Maharashtra, and about 708 deaths in Gujarat, brought about by this dark organism (Link 6). Telangana and Madhya Pradesh have been seen with 2638 and 2370 sequentially, where Madhya Pradesh has seen the expiration of 167 individuals. Besides, 1947 cases and 351 deaths in Delhi have been accounted for, close by Haryana with 1764 cases and 268 deaths. Uttar Pradesh, Karnataka, and Rajasthan are confronting the deficiency of liposomal amphotericin B, guaranteeing with around 2477, 3906, and 3621cases distributively, brought about by the verse growth of mucormycosis (Link 7; Link 9). The ongoing report uncovers the passageway of the dark parasite in West Bengal and Punjab with 179 and 158 cases approximately, where 11 deaths of individuals have been reported from West Bengal. Assam and Himachal Pradesh count with the least number of cases and may rise in the upcoming days (Fig. 2 ). The abrupt acceleration of dark organisms close by COVID-19 leads to the issues for the deficiency of liposomal amphotericin B in numerous states including Goa, Odisha, Kerala, and more. The ‘black fungus disease’ or ‘mucormycosis’ have been announced as an ‘epidemic’ by Rajasthan, Gujarat, and Odisha (Link 6; Link 7; Link 8; Link 9). However, various cases are expanding day by day which may lead towards another disturbance alongside COVID-19.21 , 22
Fig. 2.
An illustrative presentation on the number of cases of Coronavirus disease 2019 (COVID-19) associated mucormycosis reported in the different States of India (till September of 2021). (a) The colors provided in the different geographical area represents the variation in the number of cases. (b) A schematic presentation on the number of deaths in different States of India due to mucormycosis. (c) Up-to-date state-wise statistical indication of COVID-19 cases along with mucormycosis cases of India21,22 (Link 6; Link 7; Link 8).
A sudden escalation of mucormycosis is being reported in cases with COVID-19.22 Many cases reveal the affliction of mucormycosis even while undergoing treatment for COVID-19 (Table 2 ).
Table 2.
A precise of cases reported in India on Coronavirus disease 2019 (COVID-19) associated mucormycosis.
| Case No. | Age/Sex | Reported Area | Occurrence of fungal colonies during microscopy | Causative Agent | Disease Type | Underlying Disease | Infected internal body parts | Symptoms | Clinical outcomes | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Case-1 | 32/F | Mangalore | Positive | Rhizopus spp. | Paranasal Mucormycosis | Diabetes mellitus, left eye complete ptosis, facial problem | Sinus and orbit | Orbital apex syndrome Rapidly lost eye vision | Recovered but no improvement in vision | 41 |
| Case-2 | 60/M | Mumbai | Positive | Rhizopus spp. | Rhino-orbital Mucormycosis | Diabetes mellitus, Lung disease | Sinus and orbit | orbital swelling, headache, nosebleed | Expired | 42 |
| Case-3 | 38/M | Mumbai | Positive | Rhizopus oryzae | Sino-orbital Mucormycosis | Diabetes mellitus | Sinus and orbit | Swelling and pain in the left eye | Recovered | 5 |
| Case-4 | 72/M | Hyderabad | Positive | Rhizopus. oryzae | Pulmonary Mucormycosis | Diabetes mellitus, hypertension | Lungs | Streaky hemoptysis | The patient is not improving | 43 |
| Case-5 | 40/F | Mangalore | Positive | Rhizopus spp. | Rhino orbital cerebral Mucormycosis | Diabetes mellitus | Sinus, orbit, and CNS | Swelling of the left eye and facial pain, rhinitis | Recovered | 15 |
| Case-6 | 38/M | Bangalore | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes mellitus | Orbit, sinus | Right eye pain and chemosis | Expired | 26 |
| Case-7 | 51/F | Mumbai | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes, Hypothyroidism | Eye, sinus, and CNS | Left side facial pain, nose block, periorbital pain, and headache | Recovered | 26 |
| Case-8 | 45/M | Puducherry | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes mellitus, Hypertension, CKD | Eye damage, sinus, and CNS | Impairment of right eye vision | Recovered | 26 |
| Case-9 | 56/M | Bangalore | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | CKD, diabetes, hypertension, hyperthyroidism | Eye conjunctiva, brain | Right eye swelling | Expired | 26 |
| Case-10 | 78/M | Bangalore | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes and hypertension | Sinus, orbit, and CNS | Holocranial headache | Expired | 26 |
| Case-11 | 43/M | Bangalore | Positive | Rhizopus oryzae | Rhino-sinusitis Mucormycosi | Diabetes mellitus, CLD | Sinus, nasal passages, oral cavity, and brain | Dryness and cresting in the nasal cavity | Recovered | 26 |
| Case-12 | 60/M | Delhi | Positive | Rhizopus arrhizus | Rhino-sinusitis Mucormycosis | Diabetes mellitus, deranged kidney function | Sinus and brain | Periorbital swelling, chemosis, restricted eye movement | Expired | 44 |
| Case-13 | 64/M | Delhi | Positive | Rhizopus microsporus | Rhino-sinusitis Mucormycosis | Diabetes mellitus, renal function failure | Sinus, nasal passages, oral cavity, and brain | Proptosis of the eye with Periorbital discoloration, blackening of the middle turbinate. | Expired | 44 |
| Case-14 | 67/M | Not Reported | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Hypertension | Cornia, conjunctiva, eyelids, optic nerve damage | High fever, dizziness, blurred vision | Recovered | 45 |
| Case-15 | 49/M | Not Reported | Positive | Rhizopus homothallicus | Rhino-sinusitis Mucormycosis | Diabetes mellitus, problem in breathing | Sinus, brain, and nasal passages | High fever, facial swelling | Recovered | 45 |
| Case-16 | 23/M | Not Reported | Positive | Rhizopus oryzae | Rhino-orbital Mucormycosis | Diabetes mellitus, hypertension | Sinus and orbit | High fever, headache, periorbital pain, facial pain | Expired | 45 |
| Case-17 | 59/F | Delhi | Positive | Rhizopus arrhizus | Rhino-sinusitis Mucormycosis | Diabetes | Sinus and brain | High fever, facial swelling, blackening of turbinate | Recovered | 45 |
| Case-18 | 62/M | Not Reported | Positive | Rhizopus oryzae | Rhino orbital Mucormycosis | Diabetes mellitus, High pressure | Sinus and orbit | Periorbital pain, blurred vision, and headache | Expired | 45 |
| Case-19 | 43/M | Not Reported | Positive | Rhizopus oryzae | Pulmonary mucormycosis | Diabetes mellitus, problems in renal area | Lung | Facial swelling, infection in the lung, high fever | Recovered | 45 |
| Case 20 | 32/M | Hyderabad | Positive | Rhizopus arrhizus | Pulmonary Mucormycosis | Diabetes mellitus | Lung | High fever, nasal tract infection, headache, infection in lung | Recovered | 45 |
| Case-21 | 60/M | Mumbai | Positive | Rhizopus oryzae | Rhino orbital Mucormycosis | Diabetes mellitus | Sinus and orbit | Periorbital pain, blurred vision | Expired | 22 |
| Case-22 | 55/M | Chandigarh | Positive | Rhizopus spp. | Pulmonary Mucormycosis | Diabetes mellitus, End-stage kidney disease | Lung | Facial swelling, infection in the lung, high fever | Recovered | 22 |
| Case-23 | 59/M | Delhi | Positive | Rhizopus spp. | Rhino sinusitis Mucormycosis | Diabetes mellitus, High pressure, Coronary artery disease | Sinus and brain | High fever and facial swelling, blackening of turbinate | Expired | 25 |
| Case-24 | 56/M | Bangalore | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes mellitus | Cornia, conjunctiva, eyelids, optic nerve damage | Right eye pain and gradual loss of vision | Loss of follow up | 26 |
| Case-25 | 37/M | Not Reported | Positive | Rhizopus oryzae | Rhino orbital cerebral Mucormycosis | Diabetes mellitus | Cornia, conjunctiva, eyelids, optic nerve damage | Pain and bleeding from gums | Recovered | 26 |
M: Male, F: Female, CKD: Chronic kidney disease, CLD: Chronic liver disease, CNS: Central nervous system.
In general, from the referenced cases on mucormycosis related COVID-19 in India, the most effective type of mucormycosis, that is, the sort which holds the more detrimental rate is Rhino-orbital cerebral mucormycosis with about 36%, alongside Rhino-sinusitis mucormycosis with 24%. The Rhino-orbital mucormycosis and Pulmonary mucormycosis holds about 20% and 16% respectively, while the Paranasal type holds the least number of cases with 4% of viability46, 47, 48 (Fig. 3 ). Trigger off mucormycosis may prompt a deadly rise and could be fatal.
Fig. 3.
An evanescent theory on the types of mucormycosis presenting their efficacious side on the Coronavirus disease 2019 (COVID-19) associated mucormycosis patients. The diagram represents rhino-orbital cerebral (36%) mucormycosis is the highest reported type in India followed by the rhino orbital (20%), pulmonary (16%), and paranasal (4%).21,22
3. Rhizopus, the key player for COVID-19 associated mucormycosis
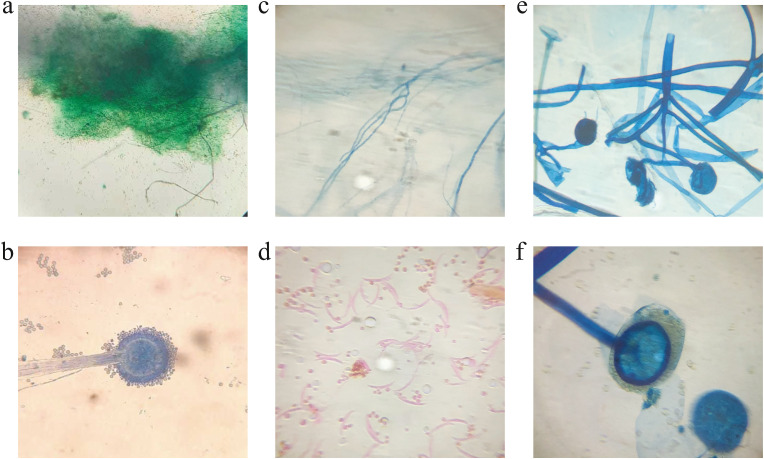
Mucormycosis is driving logically throughout the world, especially in India. The ruling fungal genera Mucorales, especially the Rhizopus species is the most well-known growths found in the patients of mucormycosis in both diabetic and non-diabetic COVID-19 patients. Rhizopus species appears differently concerning some others from the Mucorales family.21 Since it is aseptate and making sporangiophores, it is remarkably quick in making and spreading sorts of molds with blackish and a bit of the caramel or brownish sporangia49 (Fig. 4 ). Different aspiratory mucormycosis was perceived by going to the parasites with septate hyphae and sporangiophores through direct microscopy or despite fluorescent brighteners from clinical models like sputum, Bronchoalveolar Lavage Fluid (BALF), and so on also, by using the Lactophenol cotton blue (LCB) association in microscopy, the septate hyphal arrangement and the strain of hyphae were analyzed to see the microorganism.50 , 51 To confirm the assurance, non-pigmented hyphae showing tissue assault should show up in tissue sections stained with hematoxylin and eosin (HE) staining, Periodic Acid Schiff (PAS), or Grocott-methenamine-silver (GMS).14 , 50 The most notable species that cause mucormycosis after COVID-19 in India comprises Rhizopus oryzae, Rhizopus microsporous, Rhizopus arrhizus, Rhizopus homothallicus, and some different equally species. These developments now and again impact the immunocompetent, yet rather immunocompromised patients.21
Fig. 4.
Microscopic view of Rhizopus spp. under Lactophenol cotton blue (LCB) mount. (a–b) Compound microscopic view of Rhizopus sp. showing columella and brownish sporangia under ca.×100 and ca.× 450 magnification, respectively. (c) Compound microscopic view of Rhizopus sp. showing the hyphal region under ca.×100 magnification. (d) Compound microscopic view of Rhizopus sp. showing sporangiospores under ca.×450 magnification.
In patients with seriously controlled diabetes mellitus, the persistently expanded blood glucose levels will provoke the debilitated neutrophil measure.52 The parasites increase section through internal breath into the paranasal sinuses and may finally spread to be the sphenoid sinus and immense sinus. However most instances of mucormycosis are sporadic, and a sudden outburst of mucormycosis is ought to be lethal.53
3.1. Clinical expressions of the disease
Alongside COVID-19, the major cause for the increasing rate of mucormycosis triggered by Rhizopus spp. has been integrated with the upliftment of prevalence of diabetes mellitus (DM) and diabetic ketoacidosis (DKA). Infectious diseases hold up to 12% of all deaths in people with diabetes mellitus.54, 55, 56 DM is a classical fear element for mucormycosis, associated with high ailment and mortality rate in COVID-19, while DKA also stands as an ideal risk factor.57 , 58 In recent studies, euglycemic DKA is also being reported in COVID-19 patients.59 The pervasiveness of type 1 DM and DKA in COVID-19 were much higher compared to the type 2 DM and DKA in the general population.58 In addition, the utilization of immunosuppressive treatment like glucocorticoids and tocilizumab results in systemic immune adaptions by the infection that paved the way for mucormycosis contamination in patients during COVID-19.56
Mucormycosis would also be fatal for the patients who are seriously immunocompromised, likewise in cancer patients or AIDS patients.21 The infection of mucormycosis targets the region of the nose, sinuses, orbit, central nervous system (CNS), lung (pulmonary), gastrointestinal tract (GIT), skin, jawbones, joints, heart, kidney, mediastinum (invasive type), and abdominal portion.1 , 60 It is signalized by the appearance of hyphal invasion of sinus tissue in between a period of fewer than four weeks.61 , 62 Fever, headache, coughing, shortness of breath, bloody vomit and, altered mental state are all the primary symptoms of the disease. Moreover, congestion with the nasal release (blackish/bloody), confined pain on the cheekbone, partial facial pain with swelling, blackish discoloration above the bridge of nose or palate, loosening of teeth, diminished or double vision, skin lesion is the severe symptoms for the distinctive types of mucormycosis.9 , 21
In the first instance, the expression of rhino-cerebral mucormycosis is compatible with either sinusitis or periorbital cellulitis and includes eye or facial pain with numbness, followed by the onset of conjunctival suffusion, blurry vision, and soft tissue swelling.63, 64, 65, 66, 67 Fever is inconsistent and might be absent in up to half of cases; white blood cell counts are typically uplifted, as far as the patient has functioning bone marrow.64 , 67 Histological features include mycotic invasion of blood vessels, vasculitis with thrombosis, tissue localized necrosis, hemorrhage, and intense neutrophilic infiltrate.68
The clinical indications of pulmonary mucormycosis include cough with chest pain and dyspnea.69 This facilitates the result of inhalation or lymphatic spread. Patients with DKA can also thrive the disease, even though contamination in the patients is less conventional and less volatile than the infectious track that is typically seen in the patients with neutropenia.69 , 70 Otherwise, it also arises in the leukemic patients undergoing chemotherapy.9
Patients who are at an intense danger of creating cutaneous mucormycosis are those with interruption of the typical defensive cutaneous hindrance. Typically, the factors of mucormycosis are incapable of nauseating intact skin. In immunocompromised and diabetic patients, the cutaneous lesions may also rise due to catheter insertion and insulin injection sites.71 , 72 Infected surgical dressings have also been incriminated as a source of cutaneous mucormycosis.73 , 74 Mucormycosis in the gastrointestinal tract is rare. It mainly hinders malnourished patients (especially infants or children) and is thought to arise from the ingestion of fungi.75, 76, 77, 78, 79, 80 The most frequently involved sites include the stomach, ileum, and colon. The symptoms are varied and based on the site affected. Fever and hematochezia may also arise, along with lenient abdominal pain and distention related to nausea and vomiting are the best fitted well-known manifestations.81, 82, 83
4. Molecular mechanism: the panoramic story of COVID-19 associated mucormycosis
4.1. Exposition: Preface of the story
The attendance of Diabetes mellitus (DM), whether with or without Diabetic ketoacidosis (DKA), enhances the chance of acquiring mucormycosis, and DM is frequently linked to enhanced COVID-19 intensity. Meanwhile, corticosteroid use is regularly linked with uncontrolled hyperglycemia and the commencement of DKA. Acidosis causes a low pH, which is ideal for mucor spores to grow. Furthermore, use of steroid decreases the phagocytic nature of WBC (both first and second-line defensive mechanisms), impairs bronchoalveolar macrophage ingestion, migration, and phagolysosome fusion, and makes a diabetic patient more prone to mucormycosis.22
4.2. Crisis period of the story
According to a well-known and established hypothesis about the pathogenesis of DM, elevated levels of glucose in the muscle, and adipose tissue induce cellular hypoxia, endoplasmic reticulum (ER) stress, enhanced discharge of reactive oxygen species (ROS), free fatty acids (FFA), and cytokine production. Interleukin-1 (IL-1β) and tumor necrosis factor (TNF) are released by hypertrophic cells in adipose tissue, along with different chemokines. TNF-α recruits M1 macrophages, and its activation produces more pro-inflammatory cytokines (most notably IL-1β) that cause chronic inflammation and the employment of additional M1 macrophages. FFA is also detected by TLR in the tissue cells, initiating JNK-AP-I and IKK-NFKB signalling.84, 85, 86 The utterance and discharge of pro-inflammatory cytokines are enhanced consequently, which promotes the native inflammatory state. In diabetes individuals, M1 macrophages infiltrate the tissue, producing a pro-inflammatory M1 macrophage response rather than a regulating M2 macrophage response. Because M2 macrophages seem to be better able to trigger and then destroy fungal cells, penetration of diabetic tissue with M1 macrophages could provide to Rhizopus spp. impedance to phagocytosis.87
Several cellular level injuries like endothelial damage, endothelialitis, lymphopenia, thrombosis, and a drop-down in the degree of CD4+, CD8+, and T-cells levels are frequently caused by COVID-19 which is ultimately putting the patient at risk of secondary or opportunistic fungal infection.22
Proteins like ferritin and transferrin show excessive glycosylation due to the effect of hyperglycemia which ultimately reduces their iron affinity.88 Furthermore, the low pH environment in the blood vessels severely limits transferrin's ability to chelate iron in the presence of an acidotic state triggered by the generation of ketone bodies (e.g., β-hydroxybutyrate [BHB]).89 Thus, the availability of the free iron in the blood vessel is triggered and a combined effect of free iron, glucose, and BHB activates the hyphal expansion of the fungus.90 , 91
The fasting condition induced by a lack of insulin causes the catabolism of amino acids and triacylglycerols (TAGs), deposited in adipose tissue to become active as an energy source in diabetic patients. In serum, due to limited lipolysis, the concentrations of free fatty acids and glycerol, are much higher whereas the concentration of alanine is much higher due to muscle catabolism. Excess glucagon and insulin insufficiency stimulates gluconeogenesis, which uses those alanines and glycerol as substrates. Glucagon also advances the transformation of free fatty acids to ketones in the mitochondria. Insulin inhibits the transfer of the derivatives of free fatty acid to the matrix of mitochondria in normal conditions, but ketogenesis continues in the deficiency of insulin.85 Numerous ketone bodies are produced by virtue of TAG metabolism, influencing serum pH and causing the malfunction of numerous serum enzymes. Few instances, such as hemoglobin and transferrin, remain protonated and unable to transport Fe+3 at a pH of 6.88–7.3, resulting in a higher amount of Fe+3 accessible in serum in diabetic patients. Rhizopus has a ketone reductase enzyme that enables the fungus to develop in this acidic condition apart from using the free Fe+3 in these patients.92 , 93 The acidosis produced by Rhizopus spp. affects other host enzymes, which hold a direct impression on chemotaxis and phagocytosis. Reduced iron levels have also been shown to promote the M1 pro-inflammatory LPS-induced response, suggesting that additional mechanism contributes to the dissemination of an adverse feedback to fungal allowance.94
4.3. Rising period of the story
Free iron is another excellent resource for mucormycosis. According to several studies, iron plays a major function in Rhizopus and it is taken from the host via two methods, either siderophores (iron chelators) or high-affinity iron permeases.95, 96, 97, 98 Fungi battle with the host for the free iron in the siderophore system. Intrinsic and extrinsic siderophores are the two major types of fungi siderophores. Speaking of Rhizopus, both forms of siderophores are utilized. The major intrinsic siderophore, found in Rhizopus, is Rhizoferrin. It absorbs iron from outside the cell environment via a receptor-conciliated and energy-reliant method. Thirteen potential siderophore permeases are found after the genome-sequencing investigation of R. oryzae which could act as receptors for different siderophores. According to numerous protein crystallography experiments, rhizoferrin has a diaminobutane backbone connected to two citric acid residues with an R, R arrangement encircling a chiral centre.57 , 99
Another consideration for a better phagocytic response is reactive oxygen species (ROS). Owing to insulin resistance, hyperglycemia persists in people with diabetes, and in an attempt to lower glucose levels, glucose metabolism and secondary lipolysis are elevated via oxidative phosphorylation. Low pH in patients with diabetic ketoacidosis (DKA) makes more vulnerability to mucormycosis as a result of the summed-up oxidative climate which influences glutathione to remodel through the GSH/GSSG compound cycle. Advanced glycation end products (AGEs) and ROS produced by enhanced glucose metabolism cumulate in organs and tissues, causing typical micro and macrovascular changes in diabetic patients directing to an enlarged vulnerability to a Rhizopus infection.100 , 101 Due to inadequacy of the cofactor NADPH, downregulation of the major antioxidant system of glutathione (GSH/GSSG), which is the prime requirement for the reconstruction of reduced glutathione, ultimately reduces the ability of the patient to control the oxidative stress. The polyol route for glucose metabolism consumes NADPH quickly, resulting in a deficit of NADPH. Oxidative stress triggers inflammation through the NF-kB and TLR receptors, resulting in a long-term chronic inflammatory state.57 , 101
4.4. The climax of the story: The interaction between GRP78 and CotH3
In transformed fibroblasts, the production rate of a particular protein was increased when the reduction of glucose was caused. Later on, that particular protein was discovered as glucose-regulated proteins (GRPs). GRP78 or glucose-regulated protein has a molecular weight of 78-kDa, was first identified as a heat shock protein that has a role in stress-related responses.102 It is also known as immunoglobulin-binding protein (BiP) or HSP5a and is mostly found in the lumen of the endoplasmic reticulum (ER) and produced in mammalian cells. The HSP5a gene, which is found on chromosome 9q34, encodes GRP78. GRP78 is mostly found in the ER, although it has also been found in the cytoplasm, mitochondria, nucleus, plasma membrane, and secreted, even though it is primarily responsible for engaging endogenous cytoprotective mechanisms.103 The nucleotide-binding domain (NBD) or ATPase and substrate-binding domain (SBD) or protein/peptide-binding domains are the two main functional domains of GRP78.104, 105, 106 The function of this protein is controlled by the allosteric ATPase cycle in which the binding with ATP and hydrolyzation of ATP is performed by NBD whereas the SBD performs the job of bindings with polypeptides.104 , 105 , 107 GRP78 has long been believed to be a molecular chaperone having a place with the HSP70 family that directs the unfolded protein reaction (UPR) to control ER stress and assumes a critical part in protein collapsing and quality control, just as misformed protein degradation.108 , 109
GRP78 expression has recently gained importance due to its translocation to the cell membrane's surface (csGRP78) during ER stress,110 where it serves as a receptor and regulator in cell indicating by forming complexes with extracellular ligands and proteins attached to the cell surface.111, 112, 113 Recent Research reveals that hyperglycemia behaves like a stress trigger in ER which simultaneously initiates the overexpression of the GRP78 protein, based on the glucose concentration. MTJ-1 chaperone-mediated mechanism helps to translocate these GRP78 proteins from the ER to the cell surface.114 Likewise, overexpression of csGRP78 has been found to play a crucial role as an entrance receptor for various pathogens, including the Ebola virus, Dengue virus, Coxsackievirus, and the new SARS-CoV2 virus, and other viruses and Rhizopus spp. as well.115, 116, 117
Rhizopus spp. interact with various receptors of epithelial cells of alveolar and nasal origin. When Rhizopus spp. infect nasal epithelial cells, csGRP78 is overexpressed, but not in alveolar epithelial cells. In addition, it was discovered that Rhizopus spp. interrelate with alveolar epithelial cells by binding to integrin-1 rather than csGRP78. Subcellular factors, like iron, glucose, and DKA trigger the excessive production of csGRP78 only in nasal epithelial cells and subsequently enhance the pathogenicity of Rhizopus spp.57
Following the discovery of GRP78 as a required receptor for the invasion of the species of Mucorales,115 the hunt for a possible ligand led to the discovery of CotH in Mucorales.116 As a result, in a wide spectrum of Mucorales species, the utterance of the CotH1, CotH2, and CotH3 genes has been identified. Nonetheless, research data has suggested that CotH3 is mostly produced in R. oryzae germinations and has a better ability to attach and so penetrate endothelium and nasal epithelial cells in the DKA environment.95 , 116 , 118 , 119 On the other hand, CotH7 is the primary ligand that interrelates with integrin-1 of alveolar epithelial cells in the pulmonary mucormycosis, and it is not closely linked to CotH3 (50% amino acid identity).118
In Mucorales, csGRP78 binds particularly with spore coating homolog proteins (CotH), facilitating invasion and injury to endothelial cells.115 , 116 , 118 , 119 By nature, CotH protein is a type of protein kinase and a member of a vast family of spore coating proteins. It has diversified functions. It is essential for protein assembly in the inner layer of the spore-coat. During sporulation, this protein is produced and shows its activity. ATP-dependent autophosphorylation and successive phosphorylation of serine residues of CotG and CotB proteins regulate its activity. The half-life of CotH is only four to 5 h. Its concentration drops quickly when the structural gene's transcription is turned off. Recent findings show its essential role in spore germination of many human pathogens like spore-producing fungi such as Rhizopus oryzae and the expression of many bacterial strains like Bacillus anthracis.116 , 120 , 121
The appearance and interaction of GRP78 and CotH leads to increased fungal interference and consequent endothelial injury in vitro.91 , 115 As the iron chelation fused with pH reversal by sodium bicarbonate protects endothelial cells from Rhizopus-mediated invasion and injury,91 it emerges that BHB-related acidosis has a straight effect on both GRP78 and CotH expression and an indirect effect by compromising transferrin's ability to chelate iron. Importantly, host cells with higher BHB, produced as a result of DKA, have lower blood pH, higher accessible serum iron, higher GRP78 expression in focussed organs (e.g., lungs and sinuses), and are more susceptible to mucormycosis.91 , 115
Thus, the extraordinary affectability of DKA patients to mucormycosis is clarified by the special communications of GRP78 and CotH proteins, just as their expanded articulation under hyperglycemia and ketoacidosis. Treatment with anti-GRP78 or anti-CotH antibodies protects DKA and neutropenic mice against mucormycosis, emphasizing the relevance of GRP78/CotH protein interactions in the progress of mucormycosis.115 , 116 , 122 The discovery that reversing ketoacidosis in Rhizopus-infected animals by administering sodium bicarbonate (instead of insulin) enhances survival is also potentially clinically relevant.91 Reversal of accelerated fungal expansion, reconstruction of immune function, and terminating of fungal invasion of host tissues are thought to be the causes of this protection. The activity of GRP78/CotH interactions in the neutropenic host, the other main patient category prone to mucormycosis, is currently unknown.122 , 123
4.5. Falling action of the story
The processes that increase the interaction of invading fungus with endothelial/epithelial cells are beginning to gain a toehold, and they represent a key stage in the pathogenesis of diabetes-associated mucormycosis.115 , 116 , 118 Thus, the DKA environment, high glucose, iron, and Bhydroxy butyrate (BHB) as the vital ketone body promote fungal development by promoting CotH3 expression.124 The surface translocation of the GRP78 protein, which copes with endoplasmic reticulum stress occurred by hyperglycemia and an acid milieu, assists a tissue stage favorable to Rhizopus spp. establishment. Iron is released from sequestrated protein transferrin by glycosylation mechanisms in the same tissular niche. As a result, high glucose concentrations, free iron availability,125 and acerbic microenvironment boost CotH expression on the fungal cell surface facilitating GRP78/CotH3 contact for endothelial/epithelial invasion and fungal spread.124 The fungus must interrelate with its basement membrane after infecting the apparent nasal epithelium because the spores and stem cells from germ tubes adhere to extracellular matrix constituents. The scrutiny of Rhizopus spp. sticking to plates coated with collagen IV and laminin supports this theory.126
4.6. Resolution: The final consequences
Meanwhile, endothelial cells keep on creating GRP78 in all cubicles, and the hypha can connect with these proteins on the basal side where the existence of reticulin filaments is surpassed, permitting it to secure and outdo this region to later collaborate with GRP78 communicated on endothelial cells' luminal surface. When fungi become actualized in the lumen of blood vessels, they activate the extrinsic coagulation pathway, which causes cell injury and, as a result, the thrombus formed. This causes ischemia and prolonged hypoxia, resulting in tissue infarction and necrosis (Fig. 5 ). Finally, the microenvironment has changed and the disease has been established on the body.57
Fig. 5.
Diagram pictured the planned mechanisms for the immunopathogenesis of COVID-19 assisted mucormycosis in the immunocompromised diabetic individual22,57,124(Created withBioRender.com). (a) In COVID-19 severity, (b) uncontrolled diabetes mellitus and overdrive of Corticosteroid drugs increases the vulnerability to Mucorales infection due to diabetic ketoacidosis (DKA) and hyperglycemia. (c) An elevation in the glucose level of the adipose tissue induces endoplasmic reticulum (ER) stress, cellular hypoxia, enhanced discharge of free fatty acids (FFA), reactive oxygen species (ROS), and generate cytokine storm. (d) A diversified range of the cytokines like interleukin-1 (IL-1β), tumor necrosis factor (TNF), and various types of chemokines are released to the cellular hypoxic environment. (e) These cytokines especially TNF-α recruits the proinflammatory M1 macrophages and (f) inhibits the activity of anti-inflammatory M2 macrophages. (g) The activated M1 macrophage again discharges more pro-inflammatory cytokines like IL-1β, FFA and generates ROS. (h) These FFA are also detected by TLR-4 in the tissue cells, initiating JNK-AP-I and IKK-NFkB (nuclear factor-kappa B) signalling. (i) Simultaneously, diabetic ketoacidosis (DKA) causes a low pH environment which ultimately enhances the cellular H+ ion level. (j) Due to the activity of hyperglycemia, iron-scavenging proteins like ferritin and transferrin show increased glycosylation in the blood vessel, which lowers their iron affinity. (k) Furthermore, in the attendance of an acidotic condition promoted by the creation of ketone bodies (e.g., β-hydroxybutyrate [BHB]), the low pH environment in the blood vessels substantially restricts transferrin's ability to chelate iron. (l) As a result, the accessibility of free iron in the blood vessel is stimulated whereas (m) the counts of IFN-γ, CD4+, CD8+, and T-cell are sharply declined. (n) A combination of free iron, glucose, and BHB triggers epithelial fungal adhesion and tissular hyphal growth or opportunistic fungal infection. (o) This combination causes a stress response in ER, which drives to overexpression of the GRP78 protein. (p) The MTJ-1 chaperone aids in the translocation of GRP78 proteins from ER to the cell surface. (q) Fungi battle with the host for the presence of iron in the siderophore system. (r) High glucose concentrations, free iron availability, and an acid microenvironment boost CotH expression on the fungal cell surface, facilitating GRP78/CotH3 contact for epithelial/endothelial invasion and fungal spread. (s) The connection between GRP78 and CotH is additionally aided by ROS, FFA, and cytokines. (t) Meanwhile, endothelial cells pursue to generate GRP78 in all partitions, and the hypha can connect with these proteins on the basal side and become internalized in the lumen of blood vessels. (u) They produce cell damage, thrombus formation, ischemia, prolonged hypoxia, tissue infarction, and finally necrosis by activating the external coagulation pathway.
5. Proposed modes of investigation for COVID-19 associated mucormycosis
To date, there are no pathognomonic hematologic changes. Elevated white blood cell counts and acute-phase reactant levels indicate the abnormalities that are found reflect underlying predisposing conditions (e.g., diabetic ketoacidosis) and general indications of fungal infection. Blood cultures are virtually always negative. Plain orbit or sinus radiography is not a reliable investigation for this disease.95 , 127
Computed Tomography (CT) analysis indicates the extent of orbital and cranial involvement and progression of the disease. Magnetic resonance imaging (MRI) is also helpful by showing T2-weighted MR images, which demonstrate intracerebral extension while on the other hand, contrast-enhanced MRI scans give us a demonstration of the perineural spread of disease.128
Angiography or surgical exploration is necessary for areas of anatomic complexity. Biopsy with histopathologic examination remains the most sensitive and specific modality for definitive diagnosis. Microscopic investigation shows that aseptate hyphal elements of the species belong to the order Mucorales are wide (ranging from 6 to 30 μm), thick-walled, ribbon-like, and showing branch at right angles,42 whereas the hyphae of Aspergillus and, Fusarium are comparatively thinner, highly septate and showing branch at acute angles (Fig. 6 ). The width of the fungus and its ribbon-like shape are the most distinctive characteristics for identifying mucormycosis.129 , 130 Schiff or hematoxylin and eosin staining can be used to better visualize the Mucorales; they do not stain as well as methenamine silver.130
Fig. 6.
Compound microscopic view of different types of fungal species. (a–b) Compound microscopic view of Aspergillus sp. showing perpendicular hyphal branching pattern under ca.×100 and ca.× 450 magnification, respectively. (c-d) Compound microscopic view of Fusarium sp. showing conidia with conidiospores and dichotomous hyphal branching pattern under ca.×100 and ca.× 450 magnification, respectively. (e-f) Compound microscopic view of Rhizopus sp. showing perpendicular hyphal branching pattern under ca.×100 and ca.× 450 magnification, respectively.
Histopathology is used to identify the Mucorales, but species identification is limited to culturing. Imaging techniques are used to investigate the condition's advancement and severity. For example, fungal sinusitis that is different from bacterial sinusitis is the most usual finding on CT or MRI scans of the head and sinuses of a patient with rhino-orbital mucormycosis. MRI is more sensitive (by approximately 80%) than CT in the detection of orbital and CNS disease.131 Nasal endoscopy is an excellent diagnostic method for determining the presence of mucormycosis, while the MRI findings are very useful and significant to show the spread of mucormycosis in different regions as a supportive example to make it clinically significant but these findings will be varying according to the case-by-case basis (Fig. 7 ).
Fig. 7.
Illustration of mucormycosis infection spreading to the nasal vestibules, maxillary sinus, and brain. (a) Nasal endoscopic view, showing the location of mucormycosis. (b) The magnetic resonance imaging (MRI) scan of the head and sinuses showing the location of pus accumulation and inflammation of the maxillary sinus due to mucormycosis (T1 weighted and T2 weighted MRI scans).
The polymerase chain reaction (PCR) is used as a current diagnostic tool in the research of mucormycosis,132 however, it has not yet been licensed by the U.S. Food and Drug Administration (FDA) for this purpose and it is a rare find.9
6. Current therapeutics for COVID-19 associated mucormycosis
As significant trouble, the prevalent COVID-19 spreads worldwide.25 , 133 While various treatment options are estimated, at that time systemic glucocorticoids are shown to enhance the survival rate of COVID-19.25 , 134 Glucocorticoids are not too expensive, available widely, and are shown to decrease fatality in COVID-19 patients with hypoxemia.9 , 25 Unfortunately, the extensive use of glucocorticoids can develop secondary fungal infections like mucormycosis.25 The medical diagnosis of mucormycosis requires treatment quickly, as the fungal invasion advances rapidly.50 , 135, 136, 137 Simultaneously, the therapeutics of mucormycosis should be established as soon as possible, to reverse underlying risk factors.138
Surgical debridement (FESS or Functional Endoscopic Sinus Surgery is a minimally invasive technique used to restore sinus ventilation and normal function and/or orbital exenteration) not only decreases the burden of the disease but also permits better percolation of intravenous medical drugs. It reduces further disease spreading and permits to allow intraoperative diagnosis of necrotic tissue with applicable characteristics to provide the sample for microbiological and histopathological confirmation.139 , 140 But prompt initiation of medication therapy and instant reversal of underlying risk factors are always the better alternatives to surgical debridement because it is crucial to maintain a high index of suspicion in patients who are at risk for mucormycosis at all times.138
Antifungals can also play an important role along with surgical debridement. The guidelines, accepted globally in 2019 for the management and diagnosis of mucormycosis by Mycoses Study Group Education and Research Consortium (MSGERC) and the European Confederation of Medical Mycology (ECMM) strongly prescribe surgical treatment if possible with the addition of systemic treatment of antifungals..141 Liposomal Amphotericin B, Posaconazole and Amphotericin B lipid complex oral suspension can be treated as first-line antifungal agent monotherapy and Isavuconazole can be assisted like salvage therapy.141 , 142 Irrigation of sinuses and orbit with(1 mg/ml) Amphotericin B improves the local drug concentration and is shown to enhance outcomes.143 Intra-orbital and Retrobulbar injection in respect to Amphotericin B are also given in those patients who have no ability for surgical debridement (the dose of anesthesia along with retrobulbar injection is 1 ml of three 3.5 mg/ml).139 , 143 , 144 The recent guideline of MSGERC and ECMM for mucormycosis management recommends liposomal amphotericin B (L-AMB), the dose is 5–10 mg/kg every day.141 , 145 Adults and children are often administered to treat mucormycosis at start-up doses of 1 mg/kg daily for Amphotericin B deoxycholate (d-AMB) and 5 mg/kg daily for L-AMB and Amphotericin B lipid complex (ABLC).127 The dose of 5 mg/kg is used to recommend when the implication of the nervous system is absent.25 , 141 Amphotericin B has potential renal toxicity so that the dosage should be adjusted between 0.5 mg/kg/day and 1.5 mg/kg/day by the condition of the patient as well as disease. Hyperbaric oxygen (HBO) therapy should also be used in case of aggressive infection.9
For the hyperglycemic patient, the early treatment of liposomal amphotericin B and if necessary surgical treatment is needed. Hyperglycemia is annoyed with COVID-19 effective therapy, namely glucocorticoids. Multi-organ dysfunction and co-existing Acute Respiratory Distress Syndrome (ARDS) prevent timely testing and diagnostic imaging.25 , 34 The hospitals are overburdened by patients of COVID-19, and diagnostics after those surgeries can be curtailed significantly.34 Hence, the mortality of COVID-19 associated mucormycosis may higher than non-COVID patients with mucormycosis because of the immunosuppressed condition of the patient, presence of acute hyperglycemia, and necessarily use of glucocorticoids to treat the severity of COVID-19.49 , 146 , 147 Thus, in moderate COVID-19 cases (absence of hyperglycemia), the huge dose of glucocorticoid utilization must be avoided. Hence, the judicial considerable use of glucocorticoids in COVID-19 cases is necessary because this aggravates the hyperglycemia condition and advances the formation of diabetes ketoacidosis. Apart from this, in COVID-19 treatment, there is also an increment of D-Dimer as a product of cross-linked fibrin (inappropriate blood clot or thrombus formation). To treat the inappropriate formation of thrombus, the immunomodulatory drug tocilizumab is using, which also unluckily, promotes the mucormycosis infection.68 Therefore, the use of drugs like tocilizumab which are targeting the immune pathways is discouraged without any transparent benefit.25 , 148 Moreover, the virus causes the dysregulation of the immune system and as a result, using consistent immunomodulatory medical drugs like tocilizumab can further raise this dreadful infection in the patients of COVID-19 disease.25 , 148 , 149 So, it is necessary to use judicially under intense monitoring of the patient to detect an early fungal infestation.138
7. Conclusion
The recurrence of mucormycosis, opportunistic microorganisms has expanded altogether in the previous twenty years. This study gives an overview of comparative cases of different countries, along with the implications of the disease. The rise in mucormycosis emerges to be the result of certain factors including diabetes, uncontrolled use of glucocorticoids (which raises blood glucose, free iron that advances the probable fungal infection), and COVID-19 infection (cytokine storm, neutropenia, endothelial cell surface injury). The involvement between the fungal species of Rhizopus and the endothelial cells has also been featured. The mechanism concerning the pathogenesis of the disease has been comprehended and would initiate a vital role in future elevation. Recent tentative regimens for the treatment of mucormycosis comprises the usage of Amphotericin B and Isavuconazole. The administration of therapeutic substances should be closely managed to obtain a therapeutic impact at the moderate possible dose and for the shortest possible duration under keen observation. In the future, an improved establishment of the criteria regarding the diagnosis for COVID-19 associated mucormycosis is required including the radiological patterns of COVID-19 and the difficulty of isolating Rhizopus spp. Finally, rapid diagnosis and surgical debridement are considered to be the keystone for this life-threatening disease.
Authors’ contributions
Conceptualization: [Joy Sarkar]; Methodology: [Joy Sarkar]; Formal analysis and investigation: [Joy Sarkar]; Writing – original draft preparation: [Deganta Ghosh], [Sagardeep Dey], [Himanko Chakraborty], [Ankita Halder], [Akash Sarkar], [Sneha Mukherjee], [Pallab Chakraborty], [Rajdeep Ghosh]; Writing – review and editing: [Joy Sarkar]; Funding acquisition: [N/A]; Resources: [N/A]; Supervision: [Joy Sarkar].
Declaration of competing interest
On behalf of all listed authors, the corresponding author declares that there is not any sort of financial and non-financial conflict of interest in the subject materials mentioned in this manuscript.
Acknowledgment
The authors like to acknowledge Mr. Debangan Chowdhury and Ms. Shreemoyee Palmal for providing us the microscopic images of Aspergillus sp. The authors like to thank Mr. Prithu Bhattacharyya for supplying us one of the microscopic images of Rhizopus sp. We also like to acknowledge BioRender.com for making the way suitable to get Fig. 5.
Deganta Ghosh, Sagardeep Dey, Himanko Chakraborty, Ankita Halder, Akash Sarkar, Sneha Mukherjee, Pallab Chakraborty, and Rajdeep Ghosh contributed equally to this article. We do not have any funding support from any organizational or institutional level.
References
- 1.Sugar A.M. Mucormycosis. Clin Infect Dis. 1992;14(1):126–129. doi: 10.1093/clinids/14.Supplement_1.S126. [DOI] [Google Scholar]
- 2.Nishanth G., Anitha N., Aravindha Babu N., Malathi L. Mucormycosis - a review. Eur J Mol Clin Med. 2020;7(3):1786–1791. [Google Scholar]
- 3.Kwon-Chung K.J. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(1):8–15. doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1) doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maini A., Tomar G., Khanna D., Kini Y., Mehta H., Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021;82(1) doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadi R., Nazeri M., Amin Sayedayn S.M., Ehteram H. A successful treatment of rhinocerebral mucormycosis due to Rhizopus Oryzae. J Res Med Sci. 2014;19(1):72. [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez J.F., Maselli D.J., Simpson T., Restrepo M.I. Pulmonary mucormycosis: what is the best strategy for therapy? Respir Care. 2013;58(5):60–63. doi: 10.4187/respcare.02106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do Monte E.S., Dos Santos M.E.L., Ribeiro I.B., et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53(6):746–749. doi: 10.5946/CE.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellberg B., Edwards J., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roden M.M., Zaoutis T.E., Buchanan W.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 11.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(1):23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 12.Khatri A., Chang K.M., Berlinrut I., Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – case report and review of literature. J Med Mycol. 2021;31(2) doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt K., Agolli A., Patel M H., et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9(1) doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan N., Gutierrez C.G., Martinez D.V., Proud K.C. A case report of COVID-19 associated pulmonary mucormycosis. Arch Clin Cases. 2020;7(3):46–51. doi: 10.22551/2020.28.0703.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revannavar S.M., Supriya P., Samaga L., Vineeth K. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14(4) doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S., Sarkar S., Das A., Das S., Chakraborty P., Sarkar J. A comprehensive review of various categories of face masks resistant to Covid-19. Clin Epidemiol Global Health. 2021;12:100835. doi: 10.1016/J.CEGH.2021.100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S., Grover M., Bhargava S., Samdani S., Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segrelles-Calvo G., De Saraújo G.R., Frases S. Systemic mycoses: a potential alert for complications in COVID-19 patients. Future Microbiol. 2020;15(14):1405–1413. doi: 10.2217/fmb-2020-0156. [DOI] [PubMed] [Google Scholar]
- 19.Satish D., Joy D., Ross A.B. Mucormycosis coinfection associated with global COVID-19: a case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021;7(5):815–820. doi: 10.18203/issn.2454-5929.ijohns20211574. [DOI] [Google Scholar]
- 20.Alekseyev K., Didenko L., Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12(3):85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo J.P., Mishra A.P., Pradhan P., Samal K.C. Misfortune never comes alone - the new “black fungus” accompanying COVID-19 wave. Biotica Res Today. 2021;3(5):318–320. [Google Scholar]
- 22.Singh AK, Singh R, Joshi SR, Mishra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes, Metab Syndrome: Clin Res Rev. Published online 2021:1-7. doi:10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed]
- 23.Yeo C.D., Kim J.S., Kwon S.H., et al. Rhinocerebral mucormycosis after functional endoscopic sinus surgery A case report. Medicine. 2018;97(51) doi: 10.1097/MD.0000000000013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadikia K., Hashemi S.J., Khodavaisy S., et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021:1–11. doi: 10.1111/myc.13256. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg D., Muthu V., Sehgal I.S., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra N., Mutya V.S.S., Thomas A., et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7(5):867–870. doi: 10.18203/issn.2454-5929.ijohns20211583. [DOI] [Google Scholar]
- 27.Dallalzadeh L.O., Ozzello D.J., Liu C.Y., Kikkawa D.O., Korn B.S. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2021:1–4. doi: 10.1080/01676830.2021.1903044. Published online. [DOI] [PubMed] [Google Scholar]
- 28.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. AJEM (Am J Emerg Med) 2021;(264):42. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020;15(11):2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):40–80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanwar A., Jordan A., Olewiler S., Wehberg K., Cortes M., Jackson B.R. A fatal case of rhizopus azygosporus pneumonia following covid-19. J Fungi. 2021;7(3):174. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley B., Naresh K.N., Roufosse C., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020;1(6):245–253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasero D., Sanna S., Liperi C., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellanger AP, Navellou JC, Lepiller Q, et al. Mixed Mold Infection with Aspergillus fumigatus and Rhizopus Microsporus in a Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patient. Infectious Diseases Now. Published online 2021. doi:10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed]
- 36.Karimi-Galougahi M., Arastou S., Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021;11(6):1029–1030. doi: 10.1002/alr.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veisi A., Bagheri A., Eshaghi M., Rikhtehgar M.H., Rezaei Kanavi M., Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021:1–6. doi: 10.1177/11206721211009450. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13(2) doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurl C., Hoenigl M., Schulz E., et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID-19 patient with underlying hematological malignancy. J Fungi. 2021;7(2):88. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sungurtekin H., Sargin F., Akbulut M., Karaduman S. Severe rhinocerebral mucormycosis case developed after COVID-19. J Bacteriol Parasitol 1. 2021;12(1):1–3. [Google Scholar]
- 41.Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. Published online 2021:1-4. doi:10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed]
- 42.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9) doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar Chennamchetty V., Adimulapu S., Patel Kola B., De Padua M., Ambika C., Raghavendra Rao M.D. Post-COVID pulmonary mucormycosis- A case report. IP Indian J Immunol Respir Med. 2021;6(1):62–66. doi: 10.18231/j.ijirm.2021.014. [DOI] [Google Scholar]
- 44.White P.L., Dhillon R., Cordey A., et al. Clinical Infectious Diseases; 2020. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar S., Gokhale T., Choudhury S., Deb A. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 47.Diwakar A., Dewan R.K., Chowdhary A., Randhawa H.S., Khanna G., Gaur S.N. Zygomycosis - a case report and overview of the disease in India. Mycoses. 2007;50(4):247–254. doi: 10.1111/j.1439-0507.2007.01382.x. [DOI] [PubMed] [Google Scholar]
- 48.Meis J.F., Chakrabarti A. Changing epidemiology of an emerging infection: Zygomycosis. Clin Microbiol Infect. 2009;15(5):10–14. doi: 10.1111/j.1469-0691.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 49.Patel A., Kaur H., Xess I., et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944. doi: 10.1016/j.cmi.2019.11.021. e9-944.e15. [DOI] [PubMed] [Google Scholar]
- 50.Skiada A., Lass-Floerl C., Klimko N., Ibrahim A., Roilides E., Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(1):93–101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kr P.K. Mucormycosis: a black fungus- post covid complications. J Regen Biol Med. 2021;3(4):1–8. doi: 10.37191/Mapsci-2582-385X-3(4)-078. [DOI] [Google Scholar]
- 52.Dadwal S.S., Kontoyiannis D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18(10):845–854. doi: 10.1080/14737159.2018.1522250. [DOI] [PubMed] [Google Scholar]
- 53.Song G., Liang G., Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020:1–8. doi: 10.1007/s11046-020-00462-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou C., Byard R.W. An analysis of the morbidity and mortality of diabetes mellitus in a forensic context. J Forensic Sci. 2018;63(4):1149–1154. doi: 10.1111/1556-4029.13674. [DOI] [PubMed] [Google Scholar]
- 55.Yang W., Dall T.M., Beronjia K., et al. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe covid-19 converge: the perfect storm for mucormycosis. J Fungi. 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales-Franco B., Nava-Villalba M., Medina-Guerrero E.O., et al. Host-Pathogen molecular factors contribute to the pathogenesis of rhizopus spp. in diabetes mellitus. Curr Trop Med Rep. 2021:1–12. doi: 10.1007/s40475-020-00222-1. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman N., Fink D., Cai J., Lee Y.N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166(108291):1–7. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oriot P., Hermans M.P. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg: Int J Clin Lab Med. 2020:1–5. doi: 10.1080/17843286.2020.1780390. Published online. [DOI] [PubMed] [Google Scholar]
- 60.Lanternier F., Dannaoui E., Morizot G., et al. A global analysis of mucormycosis in France: the RetroZygo study (2005-2007) Clin Infect Dis. 2012;54(1):S35–S43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson B.J. Definitions of fungal rhinosinusitis. Otolaryngol Clin. 2000;33(2):227–235. doi: 10.1016/S0030-6665(00)80002-X. [DOI] [PubMed] [Google Scholar]
- 62.Chakrabarti A., Denning D.W., Ferguson B.J., et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119(9):1809–1818. doi: 10.1002/lary.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhiwakar M., Thakar A., Bahadur S. Improving outcomes in rhinocerebral mucormycosis - early diagnostic pointers and prognostic factors. J Laryngol Otol. 2003;117(11):861–865. doi: 10.1258/002221503322542854. [DOI] [PubMed] [Google Scholar]
- 64.Talmi Y.P., Goldschmied-Reouven A., Bakon M., et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127(1):22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 65.Khor B.S., Lee M.H., Leu H.S., Liu J.W. Rhinocerebral mucormycosis in Taiwan. J Microbiol Immunol Infect. 2003;36(4):266–269. [PubMed] [Google Scholar]
- 66.Peterson K.L., Wang M., Canalis R.F., Abemayor E. Rhinocerebral mucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107(7):855–862. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Thajeb P., Thajeb T., Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand J Infect Dis. 2004;36(9):643–648. doi: 10.1080/00365540410020794. [DOI] [PubMed] [Google Scholar]
- 68.Atallah B., El Nekidy W., Mallah S.I., et al. Thrombotic events following tocilizumab therapy in critically ill COVID-19 patients: a Façade for prognostic markers. Thromb J. 2020;18(22):1–6. doi: 10.1186/s12959-020-00236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tedder M., Spratt J.A., Anstadt M.P., Hegde S.S., Tedder S.D., Lowe J.E. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–1050. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 70.Rothstein R.D., Simon G.L. Subacute pulmonary mucormycosis. Med Mycol. 1986;24(5):391–394. doi: 10.1080/02681218680000591. [DOI] [PubMed] [Google Scholar]
- 71.Kerr O.A., Bong C., Wallis C., Tidman M.J. Primary cutaneous mucormycosis masquerading as pyoderma gangrenosum. Br J Dermatol. 2004;150(6):1212–1213. doi: 10.1111/j.1365-2133.2004.05826.x. [DOI] [PubMed] [Google Scholar]
- 72.Quinio D., Karam A., Leroy J.P., et al. Zygomycosis caused by Cunninghamella bertholletiae in a kidney transplant recipient. Med Mycol. 2004;42(2):177–1780. doi: 10.1080/13693780310001644644. [DOI] [PubMed] [Google Scholar]
- 73.Gartenberg G., Bottone E.J., Keusch G.T., Weitzman I. Hospital-acquired mucormycosis (rhizopus rhizopodiformis) of skin and subcutaneous tissue. N Engl J Med. 1978;299(20):1115–1118. doi: 10.1056/nejm197811162992007. [DOI] [PubMed] [Google Scholar]
- 74.Mead J.H. Cutaneous Rhizopus infection. Occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA: J Am Med Assoc. 1979;242(3):272–274. doi: 10.1001/jama.242.3.272. [DOI] [PubMed] [Google Scholar]
- 75.Amin S.B., Ryan R.M., Metlay L.A., Watson W.J. Absidia corymbifera infections in neonates. Clin Infect Dis. 1998;26(4):990–992. doi: 10.1086/513940. [DOI] [PubMed] [Google Scholar]
- 76.Craig N.M., Lueder F.L., Pensler J.M., et al. Disseminated rhizopus infection in a premature infant. Pediatr Dermatol. 1994;11(4):346–350. doi: 10.1111/j.1525-1470.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 77.Kecskes S., Reynolds G., Bennett G. Survival after gastrointestinal mucormycosis in a neonate. J Paediatr Child Health. 1997;33(4):356–359. doi: 10.1111/j.1440-1754.1997.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 78.Kline M.W. Mucormycosis in children: review of the literature and report of cases. Pediatr Infect Dis. 1985;4(6):672–676. doi: 10.1097/00006454-198511000-00015. [DOI] [PubMed] [Google Scholar]
- 79.Reimund E., Ramos A. Disseminated neonatal gastrointestinal mucormycosis: a case report and review of the literature. Fetal Pediatr Pathol. 1994;14(3):385–389. doi: 10.3109/15513819409024268. [DOI] [PubMed] [Google Scholar]
- 80.Sharma M.C., Gill S.S., Kashyap S., et al. Gastrointestinal mucormycosis--an uncommon isolated mucormycosis. Indian J Gastroenterol : official journal of the Indian Society of Gastroenterology. 1998;17(4):131–133. [PubMed] [Google Scholar]
- 81.Imhof A., Balajee S.A., Fredricks D.N., England J.A., Marr K.A. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743–746. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- 82.Marty F.M., Cosimi L.A., Baden L.R. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell Transplants. N Engl J Med. 2004;350(9):950–952. doi: 10.1056/nejm200402263500923. [DOI] [PubMed] [Google Scholar]
- 83.Siwek G.T., Dodgson K.J., De Magalhaes-Silverman M., et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39(4):584–587. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- 84.Kronsteiner B., Chaichana P., Sumonwiriya M., et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49(7):1092–1106. doi: 10.1002/eji.201848037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayelign B., Negash M., Genetu M., Wondmagegn T., Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res. 2019:1–8. doi: 10.1155/2019/6196532. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C., Feng X., Li Q., Wang Y., Li Q., Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi: 10.1016/j.cyto.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 87.Toniolo A., Cassani G., Puggioni A., et al. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol. 2019;30(1):1–17. doi: 10.1097/MRM.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ribes J.A., Vanover-Sams C.L., Baker D.J. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. doi: 10.1128/cmr.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Artis W.M., Fountain J.A., Delcher H.K., Jones H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31(12):1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 90.Fu Y., Lee H., Collins M., et al. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS (Fed Eur Microbiol Soc) Microbiol Lett. 2004;235(1):169–176. doi: 10.1016/j.femsle.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 91.Gebremariam T., Lin L., Liu M., et al. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest. 2016;126(6):2280–2294. doi: 10.1172/JCI82744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Navarro-Mendoza M.I., Pérez-Arques C., Murcia L., et al. Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-26051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrianaki A.M., Kyrmizi I., Thanopoulou K., et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat Commun. 2018;9(1):3333. doi: 10.1038/s41467-018-05820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agoro R., Taleb M., Quesniaux V.F.J., Mura C. Cell iron status influences macrophage polarization. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(1):S16–S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lax C., Pérez‐arques C., Navarro‐mendoza M.I., et al. Genes, pathways, and mechanisms involved in the virulence of mucorales. Genes. 2020;11(3):317. doi: 10.3390/genes11030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Petrikkos G., Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clin Therapeut. 2018;40(6):894–902. doi: 10.1016/j.clinthera.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 98.Farmakiotis D., Kontoyiannis D.P. Mucormycoses. Infect Dis Clin North Am. 2016;30(1):143–163. doi: 10.1016/j.idc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 99.Larcher G., Dias M., Razafimandimby B., Bomal D., Bouchara J.P. Siderophore production by pathogenic mucorales and uptake of deferoxamine B. Mycopathologia. 2013;176(5–6):319–328. doi: 10.1007/s11046-013-9693-5. [DOI] [PubMed] [Google Scholar]
- 100.Magdaleno F., Blajszczak C., Charles-Ni-o C., et al. Aminoguanidine reduces diabetes-associated cardiac fibrosis. Exp Ther Med. 2019;18(4):3125–3138. doi: 10.3892/etm.2019.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Melo L.G.P., Nunes S.O.V., Anderson G., et al. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuro Psychopharmacol Biol Psychiatr. 2017;78:34–50. doi: 10.1016/j.pnpbp.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 102.Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxidants Redox Signal. 2009;11(9):2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Casas C. GRP78 at the centre of the stage in cancer and neuroprotection. Front Neurosci. 2017;11(177):1–15. doi: 10.3389/fnins.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ting J., Lee A.S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- 105.Yang J., Nune M., Zong Y., Zhou L., Liu Q. Close and allosteric opening of the polypeptide-binding site in a human Hsp70 chaperone BiP. Structure. 2015;23(12):2191–2203. doi: 10.1016/j.str.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hughes S.J., Antoshchenko T., Chen Y., Lu H., Pizarro J.C., Park H.W. Probing the ATP site of GRP78 with nucleotide triphosphate analogs. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hendershot L.M., Valentine V.A., Lee A.S., Morris S.W., Shapiro D.N. Localization of the gene encoding human bip/grp78, the endoplasmic reticulum cognate of the hsp70 family, to chromosome 9q34. Genomics. 1994;20(2):281–284. doi: 10.1006/geno.1994.1166. [DOI] [PubMed] [Google Scholar]
- 108.Roller C., Maddalo D. The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol. 2013;4(10):1–5. doi: 10.3389/fphar.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwon J.W., Jung I., Jee D. Glucose-regulated protein 78 in the aqueous humor in diabetic macular edema patients. Medicine. 2018;97(45) doi: 10.1097/MD.0000000000012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun F.C., Wei S., Li C.W., Chang Y.S., Chao C.C., Lai Y.K. Localization of GRP78 to mitochondria under the unfolded protein response. Biochem J. 2006;396(1):31–39. doi: 10.1042/BJ20051916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ni M., Zhang Y., Lee A.S. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434(2):181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Gronow M., Selim M.A., Papalas J., Pizzo S.V. GRP78: a multifunctional receptor on the cell surface. Antioxidants Redox Signal. 2009;11(9):2299–2306. doi: 10.1089/ars.2009.2568. [DOI] [PubMed] [Google Scholar]
- 113.Crane E.D., Al-Hashimi A.A., Chen J., et al. Anti-GRP78 autoantibodies induce endothelial cell activation and accelerate the development of atherosclerotic lesions. JCI insight. 2018;3(24) doi: 10.1172/jci.insight.99363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Krieken R., Mehta N., Wang T., et al. Cell surface expression of 78-kDa glucose-regulated protein (GRP78) mediates diabetic nephropathy. J Biol Chem. 2019;294(19):7755–7768. doi: 10.1074/jbc.ra118.006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu M., Spellberg B., Phan Q.T., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120(6):1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gebremariam T., Liu M., Luo G., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124(1):237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ha D.P., Van Krieken R., Carlos A.J., Lee A.S. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alqarihi A., Gebremariam T., Gu Y., et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio. 2020;11(3) doi: 10.1128/mBio.01087-20. e01087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shumilov E., Bacher U., Perske C., et al. In situ validation of the endothelial cell receptor GRP78 in a case of rhinocerebral mucormycosis. Antimicrob Agents Chemother. 2018;62(5) doi: 10.1128/AAC.00172-18. e00172-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Isticato R., Sirec T., Giglio R., et al. Flexibility of the prograamme of spore coat formation in Bacillus subtilis: bypass of CotE requirement by over-production of CotH. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nguyen K.B., Sreelatha A., Durrant E.S., et al. Phosphorylation of spore coat proteins by a family of atypical protein kinases. Proc Natl Acad Sci USA. 2016;113(25):E3482–E3491. doi: 10.1073/pnas.1605917113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gebremariam T., Alkhazraji S., Soliman S.S.M., et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci Adv. 2019;5(6) doi: 10.1126/sciadv.aaw1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J., Lee A. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 124.Baldin C., Ibrahim A.S. Molecular mechanisms of mucormycosis—the bitter and the sweet. PLoS Pathog. 2017;13(8):1–9. doi: 10.1371/journal.ppat.1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boelaert J.R., De Locht M., Van Cutsem J., et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection: in vitro and in vivo animal studies. J Clin Invest. 1993;91(5):1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bouchara J.P., Oumeziane N.A., Lissitzky J.C., Larcher G., Tronchin G., Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70(1):76–83. [PubMed] [Google Scholar]
- 127.Spellberg B., Walsh T.J., Kontoyiannis D.P., Edwards J.J., Ibrahim A.S. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Therakathu J., Prabhu S., Irodi A., Sudhakar S.V., Yadav V.K., Rupa V. Imaging features of rhinocerebral mucormycosis: a study of 43 patients. Egypt J Radiol Nucl Med. 2018;49(2):447–452. doi: 10.1016/j.ejrnm.2018.01.001. [DOI] [Google Scholar]
- 129.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heaton S.M., Weintrob A.C., Downing K., et al. Histopathological techniques for the diagnosis of combat-related invasive fungal wound infections. BMC Clin Pathol. 2016;16(11) doi: 10.1186/s12907-016-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herrera D., Dublin A., Ormsby E., Aminpour S., Howell L. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2008;19(2):117–125. doi: 10.1055/s-2008-1093099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baldin C., Soliman S.S.M., Jeon H.H., et al. PCR-based approach targeting mucorales-specific gene family for diagnosis of mucormycosis. J Clin Microbiol. 2018;56(10) doi: 10.1128/JCM.00746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veronese N., Demurtas J., Yang L., et al. Use of corticosteroids in Coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med. 2020;7(170) doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guinea J., Escribano P., Vena A., et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12(6):1–10. doi: 10.1371/journal.pone.0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tissot F., Agrawal S., Pagano L., et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Katragkou A., Walsh T.J., Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect. 2014;20(6):74–81. doi: 10.1111/1469-0691.12466. [DOI] [PubMed] [Google Scholar]
- 138.Spellberg B., Ibrahim A.S. Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010;12(6):423–429. doi: 10.1007/s11908-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sen M., Lahane S., Lahane T.P., Parekh R., Honavar S.G. Mucor in a viral land: a Tale of two pathogens. Indian J Ophthalmol. 2021;69(2):244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fox A., Janson B., Stiff H., et al. A multidisciplinary educational curriculum for the management of orbital compartment syndrome. AJEM (Am J Emerg Med) 2020;38(6):1278–1280. doi: 10.1016/j.ajem.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 141.Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical Mycology in cooperation with the mycoses study Group education and research Consortium. Lancet Infect Dis. 2019;19(12):405–421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rawson T.M., Moore L.S., Zhu N., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing | Clinical Infectious Diseases | Oxford Academic. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee A.S., Lee P.W.Y., Allworth A., Smith T., Sullivan T.J. Orbital mycoses in an adult subtropical population. Eye. 2020;34:1640–1647. doi: 10.1038/s41433-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirabayashi K.E., Kalin-Hajdu E., Brodie F.L., Kersten R.C., Russell M.S., Reza Vagefi M. Retrobulbar injection of amphotericin B for orbital mucormycosis. Ophthalmic Plast Reconstr Surg. 2017;33(4):e94–e97. doi: 10.1097/IOP.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 145.Handzel O., Landau Z., Halperin D. Liposomal amphotericin B treatment for rhinocerebral mucormycosis: how much is enough? Rhinology. 2003;41(3):184–186. [PubMed] [Google Scholar]
- 146.Muthu V., Agarwal R., Dhooria S., et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(4):538–549. doi: 10.1016/j.cmi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 147.Jeong W., Keighley C., Wolfe R., et al. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(5):589–597. doi: 10.1016/j.ijantimicag.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Kimmig L.M., Wu D., Gold M., et al. IL-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med. 2020;7(583897):1–7. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar G., Adams A., Hererra M., et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Related links
- Bangladesh reports 1st death by black fungus: https://www.aa.com.tr/en/asia-pacific/bangladesh-reports-1st-death-by-black-fungus/2253604.
- Uruguay reports "black fungus" in a patient recovered from COVID-19: https://amp.dw.com/es/uruguay-reporta-hongo-negro-en-paciente-recuperado-de-covid-19/a-57695374.
- Two cases of "black fungus" confirmed in Paraguay: https://www.rdn.com.py/2021/05/27/confirman-dos-casos-de-hongo-negro-en-paraguay/.
- Iraq detects five cases of the deadly “black fungus” among coronavirus patients: https://globelivemedia.com/world/iraq-detects-five-cases-of-the-deadly-black-fungus-among-coronavirus-patients/.
- First case of "black fungus" detected in Chile in a patient with Covid-19: it is the second reported in Latin America: https://m.elmostrador.cl/dia/2021/05/28/detectan-primer-caso-de-hongo-negro-en-chile-en-paciente-con-covid-19-es-el-segundo-reportado-en-latinoamerica/.
- 5,500 cases, 126 lives lost: Black fungus stalks states, Maharashtra worst-hit: https://m.timesofindia.com/india/black-fungus-stalks-states-5500-cases-126-lives-lost/amp_articleshow/82813528.cms.
- India faces black fungus epidemic as cases climb: https://www.hindustantimes.com/india-news/india-faces-black-fungus-epidemic-as-cases-climb-101621550432802-amp.html.
- Total Cases of Black Fungus In India Is 11717 State Gujarat And Maharashtra Has Maximum Number: https://newsbust.in/total-cases-of-black-fungus-in-india-is-11717-state-gujarat-and-maharashtra-has-maximum-number/amp/.
- Delhi faces acute shortage of Amphotericin B, drug used to treat black fungus, as cases rise in city: https://www.indiatoday.in/amp/cities/delhi/story/delhi-shortage-amphotericin-drug-black-fungus-1805752-2021-05-22.