Abstract
G protein-coupled receptors regulate a variety of cellular responses and have been considered as therapeutic targets for human diseases. Lysophosphatidic acid receptor 1 (LPA1) is a receptor for bioactive lysophospholipid, LPA. LPA/LPA1-mediated signaling contributes to inflammatory and fibrotic responses in lung diseases; thus understanding regulation of LPA1 stability is important for modulating LPA/LPA1 signaling. Our previous study has shown that LPA1 is degraded in the Nedd4 like (Nedd4L) E3 ubiquitin ligase-mediated ubiquitin-proteasome system. In the current study, we attempt to identify a peptide that stabilizes LPA1 through disrupting LPA1 association with Nedd4L. LPA treatment induces both endogenous and overexpressed LPA1 degradation, which is attenuated by a proteasome inhibitor, suggesting that LPA1 is degraded in the proteasome. LPA increases phosphorylation of extracellular signal-regulated kinase 1/2 (Erk1/2) and I-κB kinase in lung epithelial cells, and this effect is promoted by overexpression of a peptide (P1) that mimics C-terminal of LPA1. P1, not a control peptide, attenuates LPA-induced LPA1 ubiquitination and degradation, suggesting that P1 stabilizes LPA1. Further, P1 diminishes Nedd4L-mediated degradation of LPA1 and Nedd4L/LPA1 association. In addition to increasing LPA1 signaling, P1 enhances LPA-induced cell migration and gene expression of Elafin, matrix metallopeptidase 1, and serpin family B member 2 in lung epithelial cells. These data suggest that disruption of LPA1 interaction with Nedd4L by P1 increases LPA1 stability and LPA/LPA1 signaling.
Keywords: blocking peptide, degradation, E3 ubiquitin ligase, GPCR, LPA1, ubiquitination
1 |. INTRODUCTION
G-protein-coupled receptors (GPCRs) are a group of transmembrane receptors that couple with a heterotrimeric complex including a Gα protein and β/γ subunits.1,2 Depending on isotypes of Gα, GPCRs mediate a variety of signal transductions including cell proliferation, migration, differentiation, and immune responses.2–6 Lysophosphatidic acid (LPA) is a bioactive lipid that is increased in plasma from patients with ovarian cancer and organ fibrosis.7–11 LPA induces cellular responses through ligation to its receptors (LPA1-6) on the cell surface. Among the receptors, LPA1 is ubiquitously expressed in most tissues and plays a major role in transduction of LPA signaling.12–14 In addition to mediating LPA signaling through G-proteins, LPA1 has been reported that it could trans-activate tyrosine receptor kinases on the cell surface,15,16 such as epidermal growth factor receptor15,16 and TrkA.17 The cross-talk between LPA1 and c-Met downregulates HGF/c-Met signaling.18 Furthermore, LPA1 is involved in TLR4 receptor signaling through interaction with CD14.19 Inhibition or knockdown of LPA1 activation has been shown to diminish severity of experimental acute lung injury, pulmonary fibrosis, and sepsis.8,19,20 Taken together, LPA1 is a potential therapeutic target for human diseases. LPA1 is also a growth factor in the plasma and maintains homeostasis through its antiapoptotic property.21,22 Thus, complete inhibition of LPA1 by small molecule inhibitors may lead unexpected side effects.
Desensitization of GPCR response is an optimal strategy to downregulate GPCR signaling using a negative feedback mechanism without completely inhibiting its activity.23–25 Interaction with the ligand induces receptor conformational changes, leading to receptor activation. To avoid prolonged receptor activation and over-reaction, the receptor is desensitized through internalization and degradation.23–25 Protein ubiquitination is a posttranslational modification and regulates targeted protein degradation and intracellular trafficking.26,27 E3 ubiquitin ligases catalyze ubiquitination by conjugating one ubiquitin (mono-ubiquitination) or a chain of polyubiquitin (poly-ubiquitination) to targeted substrates.28–30 Our previous study has revealed that LPA1 is not stable in response to LPA ligation. LPA1 is degraded in the ubiquitin-proteasome system.31 Furthermore, we found that LPA1 is mono-ubiquitinated by Nedd4L, an E3 ubiquitin ligase. Increases in association between LPA1 and Nedd4L are responsible for LPA1 degradation after receptor activation. Overexpression of Nedd4L attenuates LPA/LPA1-mediated signaling and immune responses in lung epithelial cells.31
Disruption of association between E3 ubiquitin ligase and its substrates protects substrates from ubiquitination-mediated protein degradation. In this study, we designed a blocking peptide and determined the role of the blocking peptide on the association between LPA1 and Nedd4L. The current study shows that the blocking peptide stabilizes LPA1 and increases LPA/LPA1-mediated signaling and cellular responses through disruption of LPA1/Nedd4L association. This study provides a new strategy to modulate GPCR stability by controlling its interaction with its E3 ubiquitin ligase.
2 |. MATERIALS AND METHODS
2.1 |. Cell culture and reagents
Murine lung epithelial cells (MLE12) and human bronchial epithelial cells (HBEpCs) were purchased from American Type Culture Collection. MLE12 cells were cultured in Dulbecco’s modified Eagle’s medium/F12-based HITES medium. HBEpCs were cultured in EMBM-based medium with growth factor complements. Cells were incubated in a humanized cell culture incubator in 37°C and 5% CO2. Passages 3–5 of HBEpCs were used for experiments. V5 antibody and lipofectamine 2000 transfection reagent were purchased from Invitrogen (Life technologies). LPA1 antibody was purchased from Life-Span BioScience, Inc. LPA (18:1), cycloheximide (CHX), leupeptin, myc antibody, and β-actin antibody were from Sigma-Aldrich. MG-132 was purchased from EMD chemicals. p-Erk1/2, Erk1/2, p-IKK, immunobilized protein A/G beads were purchased from Santa Cruz Biotechnology. Nedd4L antibody and ubiquitin antibody were from Cell Signaling. LPA is diluted in 0.1% bovine serum albumin (BSA) solution. All materials used in the experiments are the highest grades and commercially available.
2.2 |. Plasmid transfection
Mammalian expression plasmids (Nedd4L-V5, LPA1-V5) were constructed based on pCDNA3.1/V5-His-Topo vector.31 LPA1-myc was constructed and inserted into pCDNA3.1 vector. Transfection of MLE12 cells with plasmids was performed using Lonza nucleofector system. Transfection of HBEpCs with plasmids was performed using lipofectamine 2000 transfection reagents. Cell culture medium was changed after 6 h of transfection. Experiments were performed after 48 h of transfection.
2.3 |. Immunoblotting and immunoprecipitation analysis
Cells were collected in cell lysis buffer followed by sonication for 12 s. Protein concentration of cell lysates was measured using the DC protein Assay kit (Bio-Rad Lab). For immunoprecipitation, total cell lysates (1 mg) were mixed with primary antibody overnight, followed by incubation with A/G beads for additional 2 h. After centrifugation, immunoprecipitated complex was washed with PBS 3 times and eluted by boiling in sodium dodecyl-sulfate (SDS) buffer. Cell lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. After incubation in 5% nonfat milk for 1 h, membrane was incubated with primary antibody for 2 h or overnight, followed by washing with Tris-buffered saline with Tween-20 (TBST) three times. The second antibody was then diluted in 5% BSA in TBST and incubated with the membrane for an additional 1 h. After washing three times, the immunoblotting image was developed using an Azure imaging system.
2.4 |. In vivo ubiquitination assay
Cells were collected with PBS, followed by a brief centrifugation to remove supernatant. Cell pellets were then suspended in 80 μl of 2% SDS lysis buffer containing ubiquitin aldehyde and N-ethylmaleimide. After mixing well and sonication, cell lysates were boiled for 5 min. The denatured samples were diluted with 600–800 μl of TBS. Equal amounts of protein (1 mg) were incubated with primary antibody overnight, followed by immunoprecipitation.
2.5 |. Cell wound healing assay
MLE12 cells were grown on six-well plates until 100% confluence. Cells were scratched using a pipette tip to generate a wound gap. Images at 0 h and 24 h of scratch area were taken by an EVOS cell imaging systems. Cell migration was calculated as % of wound healing (0 h scratch area–24 h scratch area)/0 h scratch area) × 100%.
2.6 |. Real-time polymerase chain reaction
Total RNA was extracted from cells using RNA extraction mini kit (IBI Scientific, IA) according to the manufacturer’s instruction. Complementary DNA (cDNA) was synthesized using iScript cDNA synthesis kit (Bio-Rad Laboratories). Real-time polymerase chain reaction (PCR) was performed using IQ SYBR Green Supermix in CFX96 real-time PCR system. Primers were designed based on human Elastin, MMP1, and Serpin B2 genes.
2.7 |. Statistical analysis
All results were subjected to statistical analysis using two-way analysis of variance and Student t test. Data are expressed as mean ± SD of triplicate samples from at least three independent experiments and p values less than .05 were considered statistically significant.
3 |. RESULTS
3.1 |. LPA1 is not stable in response to LPA stimulation
Ligand-induced receptor degradation is a negative feedback mechanism for desensitization of receptors.32 To investigate molecular regulation of LPA1 degradation, MLE12 cells were transfected with LPA1-V5 or LPA-myc plasmid for 48 h and then followed by LPA treatment for 0–2 h. Entoptic expressed LPA1 was determined by a V5 or myc antibody. LPA treatment decreased LPA1-V5 or LPA-myc levels in a time-dependent manner (Figure 1A). Endogenous LPA1 levels were also diminished after 1 and 2 h of LPA treatment (Figure 1B). The proteasome and lysosome system regulate protein degradation. To investigate which system mediates LPA1 degradation, MLE12 cells were pretreated with proteasome inhibitor (MG-132) or lysosome inhibitor (leupeptin) for 1 h, and then cells were treated with LPA. As shown in Figure 1B, MG-132, not leupeptin, attenuated LPA1 degradation, suggesting that LPA induces LPA1 degradation in the proteasome. This conclusion is consistent with our previous study.31
FIGURE 1.
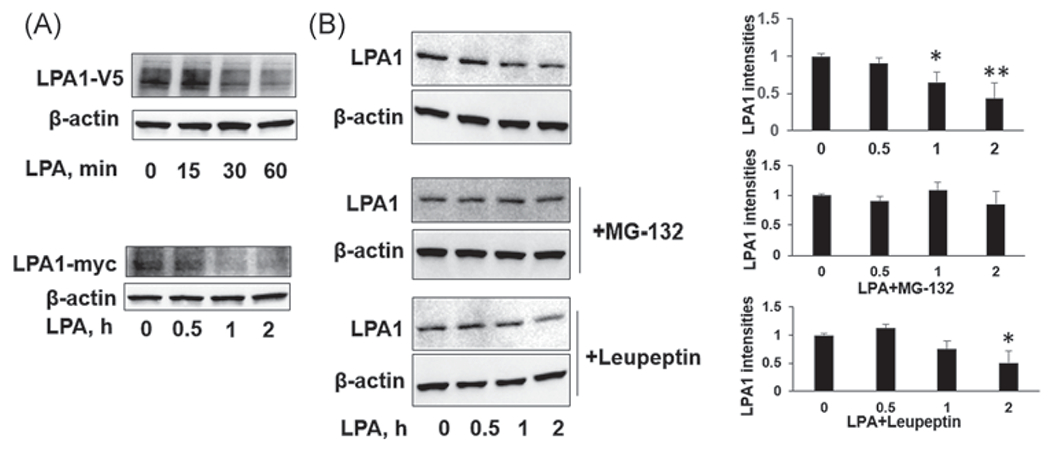
Lysophosphatidic acid receptor 1 (LPA1) is not stable and is degraded in the ubiquitin-proteasome system in MLE12 cells. (A) MLE12 cells were transfected with LPA1-V5 or LPA1-myc plasmid for 48 h. Cells were then treated with LPA (1 μM) for 0–2 h. LPA1-V5, LPA1-myc, and β-actin levels were examined by immunoblotting. (B) MLE12 cells were treated with MG-132 (20 μM) or leupeptin (100 μM) for 1 h, and then cells were treated with LPA (1 μM) for 0, 0.5, 1, and 2 h. LPA1 and β-actin levels were examined by immunoblotting. LPA1 intensities were analyzed by ImageJ. n = 3, *p < .05, compared to 0 h; **p < .01, compared to 0 h. Shown are representative blots from three independent experiments
3.2 |. A peptide (P1) enhances LPA-induced Erk1/2 and IKK phosphorylation
We have shown that Nedd4L is associated with LPA1 after LPA treatment.31 To determine whether a small molecule could modulate LPA1 stability and signaling, we designed a peptide (P1) based on C-terminus of LPA1 amino acid sequence. To express P1 in the cells, we constructed a mammalian plasmid containing a DNA fragment that can translate P1. MLE12 cells were transfected with P1 plasmid for 48 h, and then cells were treated with LPA for 10 min. LPA-induced phosphorylation of Erk1/2 and IKK were enhanced in P1-overexpressed cells (Figure 2), indicating that P1 may modulate LPA/LPA1 activation.
FIGURE 2.
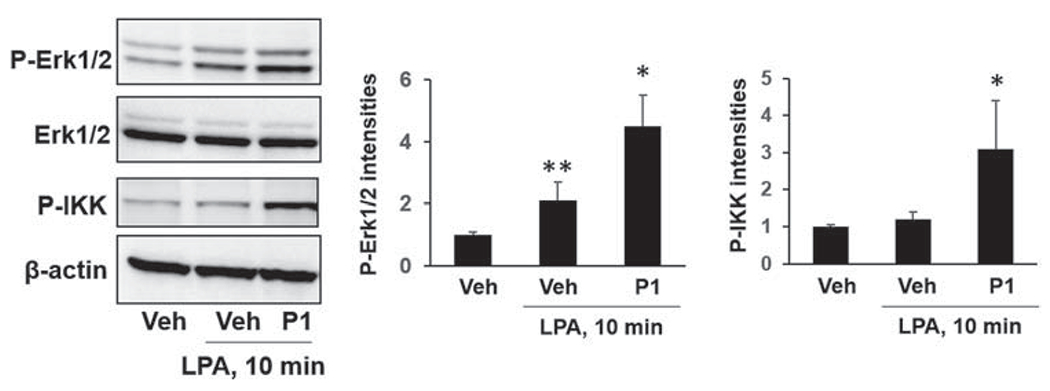
Peptide P1 promotes LPA-induced phosphorylation of Erk1/2 and I-κB kinase (IKK) in MLE12 cells. MLE12 cells were transfected with P1 plasmid for 48 h, and then cells were treated with LPA (1 μM) for additional 10 min. Phospho-Erk1/2, Erk1/2, p-IKK, and β-actin levels were examined by immunoblotting. Blots were analyzed with ImageJ. n = 3, *p < .05, compared to Veh; **p < .01, compared to Veh. Shown are representative blots from three independent experiments. LPA, lysophosphatidic acid receptor
3.3 |. P1 stabilizes LPA1 and reduces LPA1 ubiquitination
CHX is a protein synthesis inhibitor and is used to examine protein stability. MLE12 cells were cotransfected with LPA1-V5 plasmid with P1 or control peptide (Contp) plasmid, followed by CHX treatment. CHX treatment decreased LPA1-V5 in Contp-overexpressed cells, while LPA1-V5 levels remained no changes in P1-overexpressed cells (Figure 3A). This data suggests that P1 stabilizes LPA1. It has been shown that LPA1 is mono-ubiquitinated. We confirmed that LPA1 is mono-ubiquitinated (Figure 3B). Furthermore, mono-ubiquitination of LPA1 was attenuated in P1-overexpressed MLE12 cells. Contp overexpression had no effect on LPA1 mono-ubiquitination (Figure 3B). Taken together, the data indicate that P1 stabilizes LPA1 by preventing its mono-ubiquitination.
FIGURE 3.
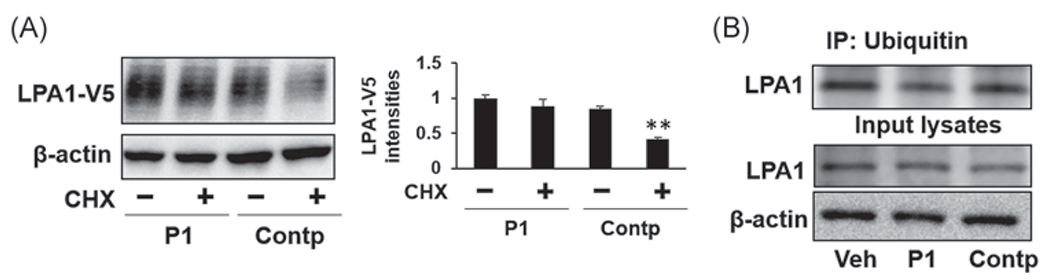
Peptide P1 increases LPA1 stability through mitigating its ubiquitination. (A) MLE12 cells were transfected with P1 or Control peptide (Contp) plasmid for 48 h, and then cells were treated with cycloheximide (CHX, 40 μg/ml) for 2 h. LPA1-V5 and β-actin levels were examined by immunoblotting. LPA1-V5 levels were analyzed by ImageJ. n = 3, **p < .01, compared to untreated cells. Shown are representative blots from three independent experiments. (B) MLE12 cells were transfected with P1 or Control peptide (Contp) plasmid for 48 h, and then cells were collected and subjected to in vivo ubiquitination assay with a modified co-immunoprecipitation protocol. Input lysates were analyzed by immunoblotting with a LPA1 antibody. Shown are representative blots from two independent experiments.
IP, immunoprecipitation; LPA, lysophosphatidic acid receptor
3.4 |. P1 disrupts LPA1 association with Nedd4L
Nedd4L is identified as an E3 ubiquitin ligase that is responsible for LPA1 ubiquitination and degradation.31 To investigate whether the protective effect of P1 on LPA1 stability is related to activation of Nedd4L, we examined the effect of P1 on Nedd4L-induced LPA1 degradation. As shown in Figure 4A, ectopic expressed Nedd4L-V5 diminished LPA1-V5 levels in Contp-overexpressing cells, while Nedd4L-V5 had no effect on LPA-V5 levels in P1-overexpressing cells. This data suggests that the effect of P1 occurs through modulating Nedd4L activation. To further investigate the molecular mechanisms, we examined the effect of P1 on association between LPA1 and Nedd4L. P1, not Contp, disrupted LPA1/Nedd4L complex (Figure 4B), suggesting that P1 is a blocking peptide that could interrupt Nedd4L interaction with LPA1 and thus protects LPA1 from ubiquitination-mediated degradation.
FIGURE 4.
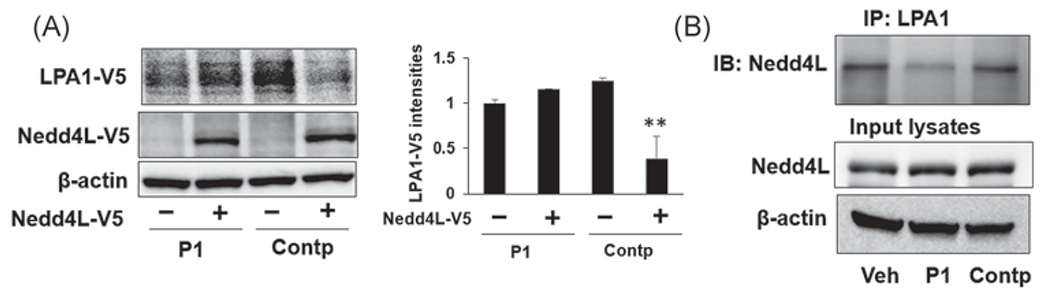
Peptide P1 attenuates Nedd4L-mediated LPA1 degradation and disrupts LPA1/Nedd4L association. (A) MLE12 cells were transfected with LPA1-V5, Nedd4L-V5, P1, or Contp plasmids for 48 h. LPA1-V5, Nedd4L-V5, and β-actin levels were examined by immunoblotting. LPA1-V5 levels were analyzed by ImageJ. n = 3, **p < .01, compared to untreated cells. Shown are representative blots from three independent experiments. (B) MLE12 cells were transfected with P1 or Control peptide (Contp) plasmid for 48 h, and then cell lysates were subjected to immunoprecipitation with a LPA1 antibody, followed by Nedd4L immunoblotting. Input lysates were analyzed by immunoblotting with Nedd4L and β-actin antibodies. Shown are representative blots from two independent experiments. IP, immunoprecipitation; LPA, lysophosphatidic acid receptor
3.5 |. P1 promotes LPA-induced cell migration and gene expression in lung epithelial cells
LPA has been shown to increase cell migration in lung epithelial cells.17,33 To further investigate if P1 affects LPA/LPA1-mediated cellular responses, we examined cell wound healing using a scratch assay. P1-overexpressed cells exhibited increased migration compared to cells treated with LPA alone (Figure 5). LPA is strong stimulator of transcriptional factors, such as nuclear factor-κB and AP-1, thereby increasing a variety of gene expression.33 A microarray analysis showed that LPA increases Elafin, MMP1, and Serpin B2 transcripts (data not shown). In Figure 6, we confirmed the data from microarray analysis by realtime PCR, and found that LPA increased Elafin, MMP1, and Serpin B2 gene expression, and that the effects were promoted in P1-overexpressed HBEpCs. These data suggest that P1 prevents Nedd4L interaction with LPA1, resulting in stabilization of LPA1 and elevation of LPA/LPA1-mediated signaling and cellular responses (Figure 7).
FIGURE 5.
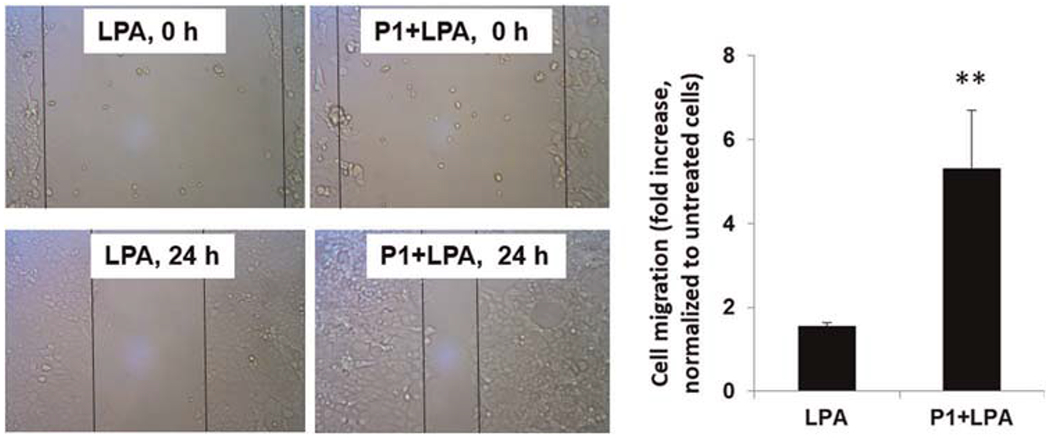
Peptide P1 promotes LPA-induced cell migration in MLE12 cells. MLE12 cells were transfected with P1 plasmid for 48 h, and then cells were scratched using a pipette tip, followed by LPA (1 μM) treatment. Images at 0 and 24 h were taken by microscope. Cell migration was calculated and normalized to untreated cells. n = 6, **p < .01, compared to cells treated with LPA alone. LPA, lysophosphatidic acid receptor
FIGURE 6.
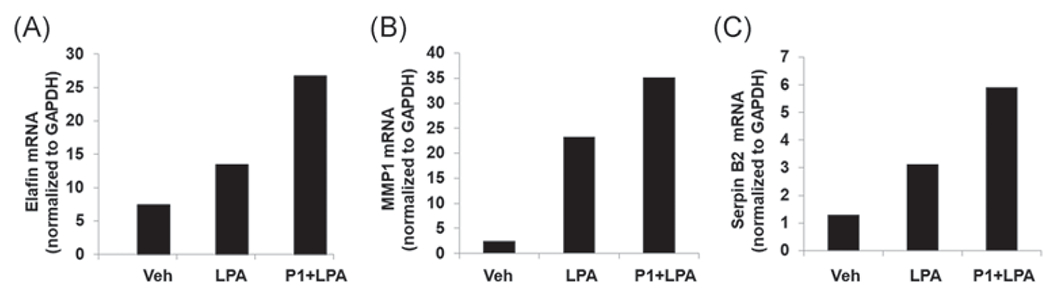
Peptide P1 promotes LPA-induced gene expression in MLE12 cells. HBEpCs were transfected with P1 plasmid for 48 h, and then cells were treated with LPA (1 μM) for additional 6 h. Total RNA was extracted and mRNA levels of (A) Elafin, (B) MMP1, (C) and Serpin B2 were analyzed by real-time PCR. n = 2. HBEpC, human bronchial epithelial cell; LPA, lysophosphatidic acid receptor; mRNA, messenger RNA; PCR, polymerase chain reaction
FIGURE 7.
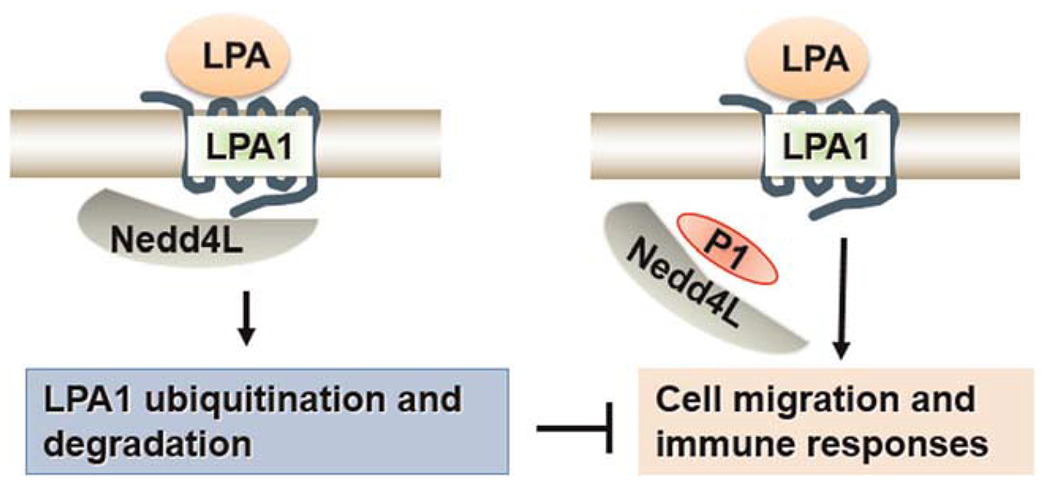
Peptide P1 stabilizes LPA1 and promotes LPA/LPA1 signaling and cellular responses through disruption of LPA1/Nedd4L association. LPA1, lysophosphatidic acid receptor 1
4 |. DISCUSSION
LPA1 is a major GPCR for LPA. It is highly expressed in most cell types. The pathogenesis role of LPA/LPA1 in various diseases has been revealed.7,8,12,12,33 LPA1 is a potential therapeutic target for human diseases. However, LPA is considered a growth factor that maintains homeostasis of cellular physiological functions.34–36 Complete knockout of lysophospholipid D, a key enzyme for LPA generation, leads to embryonic lethality,37 suggesting that complete inhibition of LPA/LPA1 pathway by small molecular inhibitors may result in unexpected side effects. Development of a strategy to precisely modulate LPA1 levels in a certain time frame or cell type will benefit development of new therapeutic medicine to treat human diseases. In the current study, we provide a new strategy to modulate LPA/LPA1-mediated signaling and cellular responses through regulation of LPA1 stability and desensitization. A blocking peptide may change LPA1 stability and regulate LPA-induced cell migration and gene expression.
Protein ubiquitination is mediated by a series of enzymatic reactions. E3 ubiquitin ligases conjugate ubiquitin to the substrates. It is common for one E3 ubiquitin ligase to target multiple substrates.29,30 This raises a concern that modulation of an E3 ubiquitin ligase by inhibitors or activators not only affects the targeted substrate, but may also cause unexpected phenomenon. A precise strategy is needed to regulate substrate stability. Action of E3 ubiquitin ligases is dependent on its association with substrates, thus preventing their association may protect substrates from ubiquitination and degradation. In contrast, enhancing their association may target a certain substrate for degradation. Proteolysis targeting chimeras are designed molecules that link an E3 ubiquitin ligase to substrate.38,39 In the current study, the P1 peptide prevents LPA1/Nedd4L association. The P1 sequence is the same as part of the C-terminus of LPA1 which may contain Nedd4L binding sites. It is possible that P1 binds to Nedd4L and prevents Nedd4L association with LPA. Thus, P1 exhibits a blocking peptide property. Notedly, P1 stabilizes LPA1, but it does not significantly increase LPA1 levels. It is possible that P1 may affect LPA1 activation by preventing inhibitory proteins interaction with LPA1. Key amino acids in P1 that are responsible for interaction with Nedd4L must be identified in the future. A shorter peptide can be generated based on discovery of key amino acids. Currently, we used a plasmid transfection to increase P1 levels in the cells. A cell permeable peptide needs to be synthesized and its role in LPA1 stability should be examined.
We have discussed that LPA1 is a pro-inflammatory and pro-fibrotic GPCR.8,19,33 Current P1 peptide increases LPA1 stability and promotes LPA/LPA1 signaling and cellular responses, thus the P1 peptide cannot be translated to treat human inflammatory and fibrotic diseases. However, our study provides a strategy to design a blocking peptide to interfere with E3 ubiquitin ligase or deubiquitinase interaction with substrates. Mono-ubiquitination of LPA1 is reversed by USP11, a deubiquitinase, which stabilizes LPA1.31 In the future, a new peptide will be designed to investigate if disruption of USP11 and LPA1 by the new blocking peptide could increase LPA1 ubiquitination and degradation, leading to precise downregulation of LPA1 and diminishing of LPA/LPA1-mediated signaling and proinflammatory, profibrotic, and protumor activation.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health, R01HL131665, HL112791, and HL136294 to Yutong Zhao, R01 GM115389 and R01HL151513 to Jing Zhao. All the authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
Funding information
National of Institutes of Health, Grant/Award Numbers: R01HL131665, HL112791, and HL136294 to Y.Z., R01 GM115389 and R01HL151513 to J.Z.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Tuteja N Signaling through G protein coupled receptors. Plant Signal Behav. 2009;4:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Eps N, Altenbach C, Caro LN, et al. Gi- and Gs-coupled GPCRs show different modes of G-protein binding. Proc Natl Acad Sci USA. 2018;115:2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol. 2014;27:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillenbrand M, Schori C, Schoppe J, Pluckthun A. Comprehensive analysis of heterotrimeric G-protein complex diversity and their interactions with GPCRs in solution. Proc Natl Acad Sci USA. 2015;112:E1181–E1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson EN, Druey KM. Heterotrimeric G protein signaling: role in asthma and allergic inflammation. J Allergy Clin Immunol. 2002;109:592–602. [DOI] [PubMed] [Google Scholar]
- 6.Koelle MR. Heterotrimeric G protein signaling: getting inside the cell. Cell. 2006;126:25–27. [DOI] [PubMed] [Google Scholar]
- 7.Pradere JP, Klein J, Gres S, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18: 3110–3118. [DOI] [PubMed] [Google Scholar]
- 8.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Wu X, Chen W, Liu J, Wang X. The lysophosphatidic acid (LPA) receptors their expression and significance in epithelial ovarian neoplasms. Gynecol Oncol. 2007;104:714–720. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Miyamoto S, Onoyama I, Sonoda K, Mekada E, Nakano H. Expression of lysophosphatidic acid receptors and vascular endothelial growth factor mediating lysophosphatidic acid in the development of human ovarian cancer. Cancer Lett. 2003;192:161–169. [DOI] [PubMed] [Google Scholar]
- 11.Ledein L, Leger B, Dees C, et al. Translational engagement of lysophosphatidic acid receptor 1 in skin fibrosis: from dermal fibroblasts of patients with scleroderma to tight skin 1 mouse. Br J Pharmacol. 2020;177:4296–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagida K, Ishii S. Non-Edg family LPA receptors: the cutting edge of LPA research. J Biochem. 2011;150:223–232. [DOI] [PubMed] [Google Scholar]
- 14.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santiskulvong C, Rozengurt E. Galardin (GM 6001), a broad-spectrum matrix metalloproteinase inhibitor, blocks bombesin- and LPA-induced EGF receptor transactivation and DNA synthesis in rat-1 cells. Exp Cell Res. 2003;290:437–446. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, He D, Saatian B, et al. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem. 2006;281:19501–19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nan L, Wei J, Jacko AM, et al. Cross-talk between lysophosphatidic acid receptor 1 and tropomyosin receptor kinase A promotes lung epithelial cell migration. Biochim Biophys Acta. 2016;1863:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, He D, Stern R, et al. Lysophosphatidic acid modulates c-Met redistribution and hepatocyte growth factor/c-Met signaling in human bronchial epithelial cells through PKC delta and E-cadherin. Cell Signal. 2007;19:2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, He D, Su Y, et al. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L547–L556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Wei J, Weathington N, et al. Lysophosphatidic acid receptor 1 antagonist ki16425 blunts abdominal and systemic inflammation in a mouse model of peritoneal sepsis. Transl Res. 2015;166:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries B, Matthijsen RA, van Bijnen AA, Wolfs TG, Buurman WA. Lysophosphatidic acid prevents renal ischemia-reperfusion injury by inhibition of apoptosis and complement activation. Am J Pathol. 2003;163:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Haney N, Kropp D, Kabore AF, Johnston JB, Gibson SB. Lysophosphatidic acid (LPA) protects primary chronic lymphocytic leukemia cells from apoptosis through LPA receptor activation of the anti-apoptotic protein AKT/PKB. J Biol Chem. 2005;280:9498–9508. [DOI] [PubMed] [Google Scholar]
- 23.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchese A, Trejo J. Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cell Signal. 2013;25:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopal S, Shenoy SK. GPCR desensitization: acute and prolonged phases. Cell Signal. 2018;41:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–1253. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Loh C, Chen J, Mainolfi N. Targeted protein degradation mechanisms. Drug Discov Today Technol. 2019;31: 53–60. [DOI] [PubMed] [Google Scholar]
- 28.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18: 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y Targeting E3 ubiquitin ligases for cancer therapy. Cancer Biol Ther. 2003;2:623–629. [PubMed] [Google Scholar]
- 30.Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–157. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Wei J, Dong S, et al. Destabilization of lysophosphatidic acid receptor 1 reduces cytokine release and protects against lung injury. EBioMedicine. 2016;10:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black JB, Premont RT, Daaka Y. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin Cell Dev Biol. 2016;50:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Natarajan V. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta. 2013;1831:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris AJ, Panchatcharam M, Cheng HY, et al. Regulation of blood and vascular cell function by bioactive lysophospholipids. J Thromb Haemost. 2009;7(Suppl 1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rancoule C, Dusaulcy R, Treguer K, Gres S, Attane C, Saulnier-Blache JS. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 2014;96:140–143. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J Lipid Res. 2002;43:2049–2055. [DOI] [PubMed] [Google Scholar]
- 37.van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith BE, Wang SL, Jaime-Figueroa S, et al. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun. 2019;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Mi D, Pei H, et al. In vivo target protein degradation induced by PROTACs based on E3 ligase DCAF15. Signal Transduct Target Ther. 2020;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]


